Abstract
Introduction:
Sickle cell disease (SCD) is the most common hemoglobinopathy in the world. Over 90% of those born with SCD live in low- and middle-income countries (LMICs), yet individuals in these settings have much poorer outcomes compared to those in high-income countries.
Areas Covered:
This manuscript provides an in-depth review of the cornerstones of basic SCD care, the barriers to implementing these in LMICs, and strategies to increase access in these regions. Publications in English language, peer-reviewed, and edited from 2000 to 2021 were identified on PubMed. Google search was used for gray literature.
Expert Opinion:
Outcomes for patients with SCD in high-income countries have improved over the last few decades due to the implementation of universal newborn screening programs and use of routine antimicrobial prophylaxis, increase in therapeutic and curative options, and the adoption of specific measures to decrease risk of stroke. This success has not translated to LMICs due to several reasons including resource constraints. A combination of several strategies is needed to increase access to basic SCD care for patients in these settings.
Keywords: Global health, sickle cell disease, low- and middle-income countries, newborn screening, penicillin, hydroxyurea, stroke, voxelotor, crizanlizumab, l-glutamine
1. Introduction
SCD is a monogenic red blood cell (RBC) disorder, where normal hemoglobin (HbA) is replaced by sickle hemoglobin (HbS). It is inherited as an autosomal codominant trait [1], and common types of SCD include homozygous hemoglobin SS disease, hemoglobin SC disease, and sickle beta-thalassemia [1]. It is a chronic and debilitating condition characterized by hemolytic anemia and endothelial dysfunction, with findings of vaso-occlusive crises, acute chest syndrome, increased risk of stroke, and cumulative multiorgan damage. Children with SCD are also at heightened risk of morbidity and mortality from specific infections due to functional asplenia. There are approximately 300,000–400,000 babies born with SCD globally, with over 75% of them born in Africa [1]. Nigeria, India, and the Democratic Republic of the Congo alone account for over 50% of patients with SCD [2]. This geographic distribution is attributed to the ‘malaria hypothesis’ that the HbS carrier state is protective against malaria infection, which was substantiated by the coexistence of high HbS carrier rates and malaria infections in Africa [3].
SCD was first described in published literature in 1910 and was noted to be frequently fatal in childhood until a few decades ago [4]. Dramatic improvements have been noted in the survival of patients beyond childhood in high-income countries, but 50–90% of children with SCD in sub-Saharan Africa (SSA) die before the age of five [5]. Estimated life expectancy of patients in other LMICs with high prevalence of SCD, such as India, is not known [6]. Reduction in mortality for patients in high-income countries is mainly attributed to universal newborn screening and our subsequent ability to protect young patients against life-threatening infections with antimicrobial prophylaxis and immunizations. The use of hydroxyurea and other disease modifying therapies, as well as the use of transcranial doppler screening to monitor risk of stroke, has significantly ameliorated outcomes for patients with SCD. These aforementioned interventions represent standard of care for patients with SCD in high-income countries; however, they are mostly inaccessible to patients in LMICs due to insufficient resources.
In this review, we will discuss the components of basic SCD care and elaborate on the barriers faced by patients in accessing SCD care in LMICs. We will then describe strategies to address some of these hurdles. Given the high burden of SCD in the region, the review will primarily focus on SSA. Peer-reviewed publications in English, edited between 2000 and 2021, were identified on PubMed, and supplemented with literature known to the authors. Boolean terms were used with the following search terms: sickle cell anemia/disease, low- and middle-income countries, access, newborn screening, antimicrobials, prophylaxis, immunizations, hydroxyurea, transcranial doppler screening, cost, health system, treatment, hydroxyurea. We augmented these publications with technical reports and other gray literature found through Google web search.
2. Newborn screening (NBS)
2.1. Benefits of NBS
Children with homozygous SCD (hemoglobin SS) are at increased risk for bacterial infections and septicemia; especially Streptococcus pneumoniae. In 1981, the Cooperative Study for Sickle Cell Disease in the United States of America (USA) documented a pneumococcal septicemia rate for infants with SCD under three years old at ‘10 per 100 person-years, with a 30% case fatality rate [7].’ Those stark statistics prompted the group to initiate a double-blind, randomized, placebo-controlled clinical trial with the hypothesis that administering oral penicillin twice a day to infants with SCD between three months and three years of age would reduce the rate of septicemia. The study was terminated early because S. pneumoniae infection was reduced by 84% in the group receiving penicillin compared to the control group, and there were no deaths in the penicillin group compared to three deaths in the control group [8]. These results prompted the National Institutes of Health (NIH) consensus panel to recommend NBS for SCD, and prophylactic penicillin for infants with homozygous SCD by three months of age. This was the advent of NBS for SCD [9,10].
Effective management for SCD begins with early diagnosis, genetic counseling, initiation of prophylactic penicillin, and comprehensive follow-up care [11–14]. Since the initiation of NBS in the USA, 95% of children with SCD live to 18 years of age [15]. However, in LMICs, it is a different story.
2.2. Barriers to NBS in LMICs
Nigeria has the greatest burden of SCD in the world with about 150,000 children born yearly with SCD, and it is estimated that this figure will increase by 100% by the year 2050 in the absence of effective control measures [16]. Even though more than two-thirds of children with SCD are born in SSA, there are no national universal NBS programs for SCD [17–19]. One of the greatest barriers to NBS in SSA and other LMICs is cost. In high-income countries, hemoglobin identification for NBS is performed by high-performance liquid chromatography (HPLC), isoelectric focusing, or tandem mass spectrometry [20,21]. These techniques are cost and resource intensive, and most LMICs do not have the resources to replicate and sustain a NBS model using these technologies [22]. Alkaline hemoglobin electrophoresis is the most commonly used and available method of diagnosing hemoglobin phenotypes in Nigeria and other LMICs, but it has its limitations [23]. Several partnerships between high-income and LMICs have proved successful in establishing NBS for SCD, but only a few have proved sustainable (lasting more than five years), and none have achieved national universal NBS.
Poverty, comorbidities, lack of transportation, competing health-care priorities, social stigma, lack of knowledge and perceived benefits, and other complex cultural issues further hinder NBS implementation and follow-up in LMICs. While genetic counseling is seemingly straightforward, cultural factors must be weighed carefully when developing a NBS program in LMICs [24–26]. Many LMICs have high rates of consanguinity (25–70%), and practice polygyny [27]. Also, significant gender inequality exists in many LMICs. SCD genetic counseling advocates must be aware of cultural norms and ensure that identification of females ‘at-risk’ of having a child with SCD does not result in punitive or negative sanctions [28].
Prophylactic penicillin and immunizations are the hallmarks of a successful NBS follow-up program for SCD. Additionally, genetic counseling is also an important component of NBS as families are informed early about SCD and its complications, as well as the misconceptions attached to this inherited blood disorder. However, most NBS programs in LMICs are hosted by academic or top-tier hospitals where patients (or other organizations) pay for their medications [29,30]. Additionally, childhood immunization rates in SSA and other LMICs lag far behind those in high-income countries. In the US, >90% of infants are fully vaccinated by 24 months of age compared to approximately 76% of infants in Africa [31,32].
2.3. Strategies to promote NBS in LMICs
Learning collaboratives with shared resources and knowledge can improve disease outcomes in LMICs; that is, HIV/AIDS organizations have been performing POC testing in the field for years to track disease progression, and the World Health Organization (WHO) has had an initiative to increase immunizations in LMICs since 1974 [31,33]. These techniques can be adapted to SCD to improve healthcare access. Point of care (POC) testing has been used in LMICs as an alternative hemoglobin identification method for SCD. The benefits of POC testing include instantaneous results, and low overall cost since it is cheap and trained lay people can perform the test. Pilot studies have proved that POC testing for SCD is reliable, cost effective, and feasible in LMICs [34–36].
Partnering with non-government organizations (NGOs) with complimentary missions is another way to leverage resources, that is, H20 for Life and Drop in the Bucket are two organizations dedicated to improving access to clean water and sanitary conditions in LMICs. Many health-care facilities in LMICs do not have access to clean water, yet handwashing is one of the best methods to prevent infection in SCD [37], and infection is one of the leading causes of death in infants with SCD in LMICs.
Community health workers (CHWs) have been used for years to support disease management in LMICs [38]. Using CHWs can help address barriers of NBS in SCD; CHWs are inexpensive and can engender trust within the community [39]. Social media is another method to increase community acceptance of SCD. NBS programs in LMICs can employ community influencers and adapt social media techniques for use in SCD. For example, Sierra Leone engaged community and faith-based leaders and used social mobilization to successfully combat vaccine hesitancy and increase Diphtheria-Pertussis-Tetanus (DPT) immunization uptake [40].
Integrating NBS into public health programs like immunizations and antenatal care packages is yet another strategy that can help establish NBS program in LMICs. Figure 1 provides an overview of the aforementioned barriers and alternative strategies for implementing NBS in LMICs.
Figure 1..
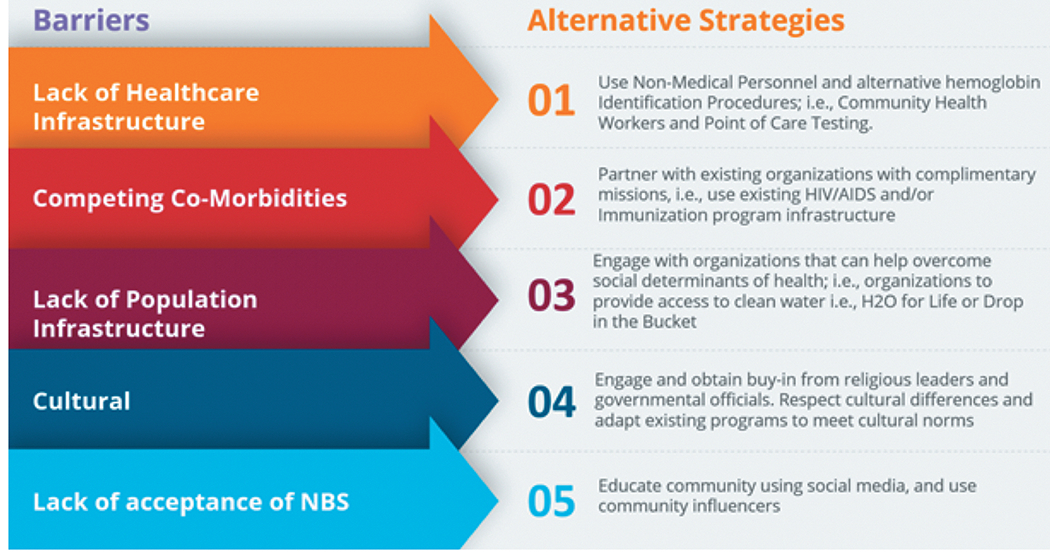
Overview of major barriers and alternative strategies for NBS in LMICs.
Establishing universal NBS for SCD in LMICs is challenging. However, it is incumbent upon high-income countries and global organizations to partner with LMICs to develop NBS for SCD. Early diagnosis and treatment are essential to decreasing mortality and morbidity for children with SCD.
3. Antimicrobial prophylaxis and vaccine implementation
3.1. Immune dysfunction in SCD
SCD is associated with increased susceptibility to infections, which is partly due to autosplenectomy and functional hyposplenism resulting from recurrent vaso-occlusive infarcts within the spleen [41]. Several other factors that predispose patients with SCD to infections have also been reported. These include abnormalities of opsonization, antibody production, alternate complement pathway, leukocyte function, and cell-mediated immunity [42–44]. Neutrophilia is a major hematological manifestation of SCD [45]; however, it does not protect patients against infections because of the functional defect in neutrophils in patients with SCD [46].
The range of immune abnormalities in SCD determines the pattern of susceptibility to microbial agents to a large extent. Hyposplenism predisposes to severe infections with malaria and encapsulated organisms including Haemophilus influenzae and Streptococcus pneumoniae. Low serum IgM levels, impaired opsonization, and abnormality of the complement pathway further increase susceptibility to other common infective agents, including Mycoplasma pneumoniae, Salmonella typhimurium, Staphylococcus aureus, and Escherichia coli[41,47]. In addition to immunological dysfunction, recurrent tissue infarcts also increase susceptibility to bacterial infection in patients with SCD. Tissue infarcts provide potential primary foci for infections that are easily propagated within the context of pre-existing immunological dysfunction associated with SCD [48]. Tissue infarcts are particularly supportive of microaerophilic bacteria species, such as salmonella, which is thus a very common infective agent in patients with SCD [49].
It is abundantly reported that malaria, other bacterial infections, and non-bacterial infections are associated with crises, exacerbation of SCD morbidities, and poor survival among patients with SCD [16,50,51]. Unfortunately, SCD patients are at high risk of acquiring both transfusion and non-transfusion transmissible infections [49]. The risk is especially high in Africa and other tropical regions, which carry the heaviest global burden of both SCD and infectious diseases [8,50–52]. Hence, the need for prophylactic measures against infections among patients with SCD cannot be overemphasized.
3.2. Prevention of infection in SCD Patients: chemoprophylaxis and vaccinations
The incidence of bacterial infections can be reduced by penicillin prophylaxis and vaccinations against encapsulated organisms. The introduction of the pneumococcal polysaccharide vaccine has markedly reduced the risk of invasive pneumococcal infections in children with SCD receiving daily prophylactic penicillin [52,53]. The heptavalent pneumococcal conjugate vaccine (PCV7) introduced in the year 2000 led to a further 70% reduction in the incidence of invasive pneumococcal infections [54], while the 13-valent pneumococcal vaccine (PCV13) vaccine introduced in 2010 also decreased the incidence of serious pneumococcal infections among a large cohort of patients with SCD in North America [54,55]. The pneumococcal conjugate vaccine (PCV) and Haemophilus influenza type b (Hib) vaccine are both administered during early infancy under the Expanded Program on Immunization in African countries [8,56]. In 2012, Nigeria joined other African countries to launch the Pentavalent vaccine (which contains the Hib vaccine) and in 2014, the 10-valent pneumococcal conjugate vaccine (PCV10) was also launched as part of its routine immunization schedule. Before the launch of the immunization program, few families could afford to vaccinate their children.
Current guidelines from the National Heart, Lung, and Blood Institute (NHLBI) have endorsed oral penicillin-V prophylaxis twice daily in all children with SCD [57]. The guidelines recommend the discontinuation of penicillin prophylaxis at five years of age if there is no history of invasive pneumococcal infection or surgical splenectomy as long as pneumococcal vaccinations have been given [57]. It still remains uncertain whether penicillin prophylaxis should be continued throughout childhood and adulthood [16]. However, the majority of pediatric hematologists recommend termination of prophylaxis at five years of age [43], though some pediatricians may elect to continue it for a longer period of time [43]. Therefore, standard practice for preventing bacterial disease in SCD should include the initiation of daily prophylactic penicillin by two months of age and the completion of the pneumococcal vaccine series (consisting of both PCV13 and PCV23) by five years of age, after which prophylactic penicillin can be discontinued [16]. In addition to pneumococcal vaccines, salmonella, and meningococcal vaccines may be useful in patients with SCD, especially in tropical settings where these diseases are endemic [58,59]. However, the availability of these vaccinations is limited and routine penicillin prophylaxis is unavailable in the majority of medical centers in SSA. In a recent study conducted across 18 SCD clinics in Nigeria, only eight of them routinely gave prophylactic penicillin [60]. This is in contrast to a study conducted in Brazil where 76.1% of patients with SCD received penicillin prophylaxis [61].
Malaria infection is the most common and potent trigger of vaso-occlusive crisis (VOC) in SCD patients living in malaria-endemic countries [62]. This underscores the importance of preventing malaria in patients with SCD living in these settings [63,64]. The WHO recommends that SCD patients in endemic areas should receive antimalarial prophylaxis [65] since prophylaxis is effective at reducing malaria infection [65–67]. Moreover, patients with SCD in tropical countries should receive a complete set of local routine immunizations [68,69]. There are several options for malaria prophylaxis regimens, including proguanil, chloroquine, pyrimethamine, sulfadoxine-pyrimethamine. Proguanil is recommended based on its lower side effect profile and the observation that adherence to once daily dosing is better than multiple times daily dosing.
3.3. Barriers to use of antimicrobial prophylaxis in LMICs
Although the NHLBI has recommended oral penicillin prophylaxis for children with SCD, the majority of patients in SSA still do not have access to this life-saving medication [57,60]. There are several challenges to widespread and efficient use of antimicrobial prophylaxis in low resource settings, particularly in SSA. Lack of affordable healthcare is a major hindrance to the effective management of SCD. While inexpensive, prophylactic penicillin is still beyond the financial reach of most people in LMICs. The unique challenge of limited resources and out-of-pocket health expenditures with no health insurance coverage needs to be addressed in order to improve access to preventive therapies in LMICs.
Aside from cost, barriers to the use of penicillin prophylaxis include poor adherence, non-prescription of penicillin, and unavailability of penicillin. Oral penicillin suspension is often not available in health facilities in LMICs and even when available, refrigeration of oral penicillin suspension is a challenge.
Non-availability of vaccines in some medical centers in SSA is a major barrier to its use. Even when the vaccines are available, they are underutilized. Galadanci et al. noted that only 13.2% and 32.3% of screened participants in their trial had received the entire PCV-13 and Hib series respectively[70]. The implementation of childhood immunization schedules, including universal PCV and Hib conjugate vaccination, could substantially improve the survival of children with SCD living in LMICs.
3.4. Strategies to improve access to infection prophylaxis in LMICs
Evidence-based interventions (EBI) targeted at SCD management must address patient, provider, and system-level barriers of care delivery. Findings from a recent Cochrane systematic review indicate that effective strategies for implementation of EBI in LMICs are those that involve a multi-level approach and are tailored to the context of the built-environment [71]. At the systems level, availability of therapy at a low cost will ensure that patients are able to afford medication. At the provider-level, training physicians (including non-hematologists) and other health-care providers (e.g. nurses) in the appropriate use and management of side effects of therapies may improve prescription practices [72,73]. This would also enable some aspects of SCD management such as health maintenance to be carried out at the primary care level, thereby increasing access to care. At the patient-level, educating caregivers around the importance of antimicrobial prophylaxis, routine immunizations, and knowledge on when families should seek urgent care (such as in the setting of fever) can improve patients’ condition [74]. Community leaders can utilize social media to increase immunization uptake, as was done with the DPT vaccine in Sierra Leone [40].
4. Use of hydroxyurea
4.1. Clinical benefits of hydroxyurea
Hydroxyurea (hydroxycarbamide) is a potent antimetabolite that selectively inhibits ribonucleoside diphosphate reductase [75]. In SCD, hydroxyurea increases RBC hemoglobin F (HbF) levels and RBC water content, improves deformability of sickled RBCs, and alters adhesion of RBCs to the endothelium [76].
Hydroxyurea decreases the frequency of acute and chronic complications of SCD, including fewer episodes of pain and acute chest syndrome, decreased hemolysis, and prolongs life overall [75,77]. In high-income countries, the use of hydroxyurea is standard of care as the primary disease modifying therapy for patients with SCD regardless of clinical severity. The NIH, the American Society of Hematology, and the British Society of Hematology recommend offering hydroxyurea to all patients with hemoglobin SS/Sβ0-thalassemia type SCD aged nine months and older [76,78]. Despite the overwhelming evidence supporting the benefits of hydroxyurea for patients with SCD in high-income countries, the majority of patients with SCD (who live in LMICs) still do not have access to this life-saving medication [76,79].
4.2. Toxicity/tolerability of hydroxyurea
Hydroxyurea side effects include dose-related neutropenia, thrombocytopenia, and reticulocytopenia [75]. Long-term studies on patients taking hydroxyurea have demonstrated the impressive safety profile of hydroxyurea and debunked theoretical concerns of infertility, teratogenicity, mutagenicity, or carcinogenicity [76,77].
4.3. Evidence supporting hydroxyurea use in LMICs
Until recently, there were no randomized clinical trials evaluating the role of hydroxyurea in SSA. Table 1 summarizes key clinical trials over the past decade evaluating the efficacy of hydroxyurea in SSA. The studies demonstrate evidence of hydroxyurea efficacy with expected decreases in SCD adverse events (AEs) including frequency of vaso-occlusive pain episodes, blood transfusions, and deaths. Recent studies in SSA demonstrated that hydroxyurea use does not increase the risk of severe malaria or other infections [80,81].
Table 1.
Summary of randomized clinical trials evaluating role of hydroxyurea in management of SCD in SSA.
| Study | Objectives | Endpoints | Key findings | References |
|---|---|---|---|---|
| REACH (Realizing Effectiveness Across Continents with Hydroxyurea) Study sites: Angola, Democratic Republic of the Congo, Kenya, Uganda |
Investigate the feasibility, safety, and benefits of hydroxyurea treatment for children with SCD living in SSA | Feasibility (enrollment, retention and adherence), Safety (dose levels, toxic effects, malaria), Benefits (lab values, SCD related events, transfusions, and survival) | (1) Study retention rate 94.2% at 3 years (2) Hydroxyurea increased total hemoglobin and HbF (3) Rates of clinical AEs of SCD decreased with HU use (44.6 vs 98.3 events per 100 patient-years) (4) Fewer non-malarial infections, fewer malaria infections, fewer deaths |
[80,81] |
| NOHARM Novel use Of Hydroxyurea in an African Region with Malaria Study site: Uganda |
Determine the safety and efficacy of hydroxyurea in a malaria-endemic region | Incidence of clinical malaria, SCD-related AEs, clinical and laboratory effects, and hematological toxicities | (1) No difference in malaria incidence between children on hydroxyurea vs. placebo (hydroxyurea/placebo malaria incidence rate ratio was 0.7 ([0.2, 2.7]; P = .61). Time to infection also did not differ significantly between treatment arms (2) SCD-related clinical events (vaso-occlusive painful crisis, dactylitis, acute chest syndrome, splenic sequestration, or blood transfusion) were less frequent with hydroxyurea (45%) than placebo (69%; P = .001) (3) Children receiving hydroxyurea had significantly increased hemoglobin concentration and fetal hemoglobin, with decreased leukocytes and reticulocytes (4) Serious AEs, sepsis episodes, and dose-limiting toxicities were similar between treatment arms |
[82] |
| NOHARM MTD Study site: Uganda |
Compare risks and benefits of hydroxyurea dosing to determine dosing standards for children with SCD in SSA | Hemoglobin 9 g/dl or more Or HbF 20% or more after 24 months | (1) Hydroxyurea with dose escalation had superior clinical efficacy to that of fixed dose hydroxyurea, with equivalent clinical safety | [83] |
| SPIN Stroke Prevention In Nigeria Study site: Nigeria |
Determine the adherence rate to daily hydroxyurea therapy in children with SCD and elevated TCD measurements Assess the safety of hydroxyurea therapy, as it relates to infection associated with hospitalization and mortality | Feasibility, Safety, Benefits | (1) Initial management of abnormal TCD measurements with moderate fixed-dose hydroxyurea (~20 mg/kg/day) was comparable to initial management with regular blood transfusion in the STOP trial and was superior to no treatment (2) Monthly CBC assessments for myelosuppression have limited clinical utility in LMICs with moderate-fixed-dose hydroxyurea (3) High enrollment and retention rate (100% in treatment group) noted (4) High adherence to hydroxyurea noted |
[70,95] |
| SPRING Hydroxyurea for primary stroke prevention in children with sickle cell anemia in Nigeria Study site: Nigeria |
Determine the incidence of stroke in children with SCA receiving moderate-dose hydroxyurea compared to those on low-dose hydroxyurea | Incidence of initial stroke or transient ischemic attack, All-cause hospitalization rate | (1) Equivalent stroke incidence rates noted in children with SCA with abnormal TCD velocities on moderate-dose and low-dose hydroxyurea (2) Decreased rate of in-hospital vaso-occlusive episodes and acute pain events at home noted when hydroxyurea dose was increased to moderate-dose from low-dose hydroxyurea |
[84] |
There is evidence to support the use of hydroxyurea in LMICs outside SSA as well. In a double-blind randomized controlled study in central India with 60 children, low-fixed dose (10 mg/kg/day) hydroxyurea demonstrated effectiveness with significant reductions in frequency of vaso-occlusive crises, hospitalizations, and blood transfusions [85]. An open label observational study in eastern India also had similar findings using low-fixed dose hydroxyurea, with significant decrease in frequency of painful crises in both pediatric and adult groups, with no significant adverse events [86].
4.4. Barriers to use of hydroxyurea in LMICs
Although the WHO currently lists hydroxycarbamide as an essential medicine for hemoglobinopathies, majority of patients with SCD in SSA still do not have access to this life-saving medication [79,87]. There are still several challenges to widespread and efficient use of hydroxyurea in low resource settings, particularly in SSA, which has a disproportionate burden of SCD (Table 2). Many LMICs utilize a self-pay system for hydroxyurea therapy and subsequent laboratory monitoring, which is cost-prohibitive for majority of families in these settings [70].
Table 2.
Barriers limiting widespread use of hydroxyurea in LMICs.
| Lack of availability, affordability, and accessibility |
| Limited education about hydroxyurea use and side effects |
| Limited knowledge about hydroxyurea dosing and drug monitoring |
| Lack of public health priority |
| Limited implementation infrastructure |
4.5. Strategies to improve access to hydroxyurea in LMICs
Hydroxyurea is a proven inexpensive and efficacious disease modifying treatment for SCD and has been shown to be scalable for use in low resource settings. It is critical to address barriers against the use of hydroxyurea in LMICs to ensure that all patients with SCD have access to this life-saving medication. Power-Hays and Ware [76] outlined the critical steps required to improve hydroxyurea use in LMICs, including increasing awareness about hydroxyurea use and effectiveness, measures to improve acceptance of medication, and facilitating increased uptake and affordability [76]. There is an urgent need to include hydroxyurea on national essential drug lists and improve local health systems by facilitating partnerships among governmental and NGOs, academic institutions, and pharmaceutical industries to improve access to hydroxyurea therapy in LMICs [76,79]. Hydroxyurea was recently approved for the use of SCD in India following partnerships between research institutions, a local pharmaceutical company, and the central governmental drug approval committee [88]. This will lead to expanded access for patients using hydroxyurea at standard dosing, and will also enable development of different formulations at smaller doses, thereby increasing access for younger children who are often excluded due to unavailability of age-appropriate formulations [88]. Local production of essential medicines may be another strategy to increase access in LMICs. For example, the SPIN trial employed the use of hydroxyurea that was produced locally in Nigeria, which led to decreased costs compared to hydroxyurea produced elsewhere [70]. Creating region-specific dosing and monitoring guidelines may also increase affordability of hydroxyurea. There is some evidence to suggest that fixed low-dose hydroxyurea may be sufficiently effective for patients in India, though larger studies would be required to elucidate this finding [85,86]. If confirmed, this would reduce resource utilization and costs in comparison to the use of hydroxyurea at maximum-tolerated dose.
5. Transcranial Doppler (TCD) Ultrasound Screening
5.1. Description of process
Approximately 11% of unscreened and untreated children with SCD are at increased risk of stroke and will have at least one stroke by 17 years of age [89]. In high-income countries, evidence-based practices (EBP) for primary stroke prevention in children with SCD involves screening for abnormal TCD velocity (>200 cm/s). The ultrasound is performed over the temporal area on a child’s head to measure the speed of blood through the middle cerebral artery. Patients with abnormal TCD velocity receive blood transfusion therapy for at least one year followed by treatment with hydroxyurea [90,91]. This EBP decreases the risk of stroke by 92% [92], leading to a 10-fold drop in stroke incidence from 0.67 to 0.06 strokes per 100-patient-years [92,93].
5.2. Barriers in LMICs
Unfortunately, this well-established EBP for stroke prevention in children with SCD is not implemented in low-resource settings like Nigeria where more than 50% of the world’s 300,000 children with SCD are born [16,94]. This is due to unavailability of safe blood for regular blood transfusions, and TCD screening services including trained personnel certified in performing TCD ultrasounds and shortages of TCD machines dedicated to primary stroke prevention [60,95].
5.3. Guidelines in LMICs
Following a feasibility trial on primary stroke prevention using hydroxyurea in children with SCD in Nigeria [95], the National Institute of Neurological Disorders and Stroke funded a multicenter randomized controlled trial for primary stroke prevention, randomly allocating low dose (10 mg/kg/day) and moderate dose (20 mg/kg/day) hydroxyurea to children with SCD in northern Nigeria [95,96]. Prior to these trials, there was no standard of care TCD screening for children with SCD in most regions of SSA. After completion of the trials, stroke prevention teams were established at the trial sites (academic centers) and few other hospitals in the region. The outcome of these trials led to the recent American Society of Hematology (ASH) Central Nervous System Guidelines that recommend moderate-dose hydroxyurea (20 mg/kg) to children with SCD with abnormal TCD measurements living in resource-constrained settings where regular blood transfusions are not readily available [97].
5.4. How Nigeria has addressed these barriers
Despite the high stroke burden within the region, the coverage of TCD screening is still very low. Three years into establishing the stroke prevention teams, in northern Nigeria the reach for TCD, which is defined as ‘the proportion of children with SCD that had TCD screening divided by the number of children with SCD eligible for TCD screening,’ was only 14.7% (471/3200) [98]. As highlighted by Galadanci et al., significant hindrances to stroke prevention strategies include a lack of TCD services in Nigeria [60], mainly due to lack of trained personnel certified in performing TCD, shortages of TCD machines dedicated to primary stroke prevention, and accessibility to blood [60,95]. Conservatively, it is estimated that there are 300 certified radiologists in Nigeria, a country with a population of 175 million people, that is, ~one radiologist per 700,000 people [98–100]. Suffice to say, even if all the radiologists in Nigeria are trained to interpret TCD ultrasounds, there would still not be a sufficient number of radiologists to cover the demand for TCD screening in the country.
To address the lack of TCD screening services, the research leadership of the SPIN/SPRING trial provided TCD machines and organized training sessions on the conduct of TCD and stroke detection. Given the shortage of radiologists in the hospital, initially non-radiologist medical officers were trained to conduct TCD screening. Subsequently, nurses were included in this ‘task-shifting’ process, making this a more sustainable option than only training the radiologists. Additionally, TCD screenings were done on clinic days, allowing for children with abnormal TCD values to be evaluated by the pediatrician on the same day. This eased the burden on families by reducing the costs associated with a second visit, such as transportation costs, missed work hours for parents, and missed school days for the children.
Having recognized the knowledge gap regarding increased stroke risk among families of children with SCD, culturally relevant general education materials were developed on SCD with emphasis on the signs and symptoms of a stroke. These included videos and pamphlets in Hausa, the region’s indigenous language. Additionally, radio shows were aired on local stations describing stroke as a complication of SCD, stroke prevention methods, and the availability of TCD screening at designated hospitals with established stroke prevention programs. Also, to ensure that children with SCD and abnormal TCD velocities had hydroxyurea, the governments of the three northern Nigerian states of Kano, Kaduna, and Katsina committed to providing free hydroxyurea to affected children.
6. Expert commentary
There have been significant strides in therapeutic options for the management of SCD over the last few years. Prior to 2017, hydroxyurea and blood transfusions were the only commonly used disease-modifying therapies [101]. However, as noted above, these therapies still remain vastly inaccessible in areas with significant prevalence of SCD, where their use is limited to small subsets of patients at higher risk of poor outcomes. In 2017, the U.S. Food and Drug Administration (FDA) approved L-Glutamine, a medication intended to decrease vaso-occlusive crises by reducing oxidative stress in sickled RBCs [102]. Crizanlizumab, an antibody against p-selectin, was approved in 2019 with the same goal of reducing vaso-occlusive crises [103]. Voxelotor, an inhibitor of hemoglobin S polymerization, was approved shortly after, with the intention of increasing baseline hemoglobin concentrations in patients with SCD [104]. While the introduction of these new disease-modifying therapies has changed the landscape of SCD management for many patients living in high-income countries, the significant costs of these therapies, ranging from 40,000 to 132,000 USD annually [105], are inhibitory in LMICs without government-supported health insurance [105]. For example, in Nigeria, the country with the highest number of patients with SCD in the world [106], 40% of the population lives on one USD per day [106,107]. Therefore, the use of costly therapies in LMICs is often dependent on public–private partnerships. One such example is the partnership between the government of Ghana, Novartis, and other stakeholders. As a result of this partnership, Novartis has supplied hydroxyurea to thousands of patients, and implemented a Crizanlizumab clinical trial in Ghana [108], thereby providing access to a medication that was previously inaccessible to patients there [108].
At present, allogeneic hematopoietic stem cell transplant (HSCT) is the only curative therapeutic option for patients with SCD. The current standard of care involves the use of HLA-matched sibling donors, which restricts the usability of this option since less than 20% of patients have a healthy fully matched sibling donor [109]. HSCT is also an expensive, resource-intensive, and very specialized procedure [110], making transplant an unattainable option for most patients in low-income settings given the lack of capacity to support such procedures [110]. Gene therapy, which includes gene editing and gene addition techniques, is currently being investigated as a curative option in laboratory and very limited clinical settings. A significant advantage to gene therapy is that a matched sibling donor is not needed for this procedure as it would involve the alteration of the patient’s own genetic material. While the cost and intensity of resources required for gene therapy have not been evaluated at this time, the increased accessibility and expected long-term savings following curative therapy may make this an attractive option for patients in LMICs. However, this will need to be studied if and when gene therapy is approved for clinical use over the next few years.
Since 80–90% of children with SCD die before their fifth birthday in SSA, the impact of improved therapeutic options in LMICs will only be significant when progress is made in identifying patients with SCD at a young age. The cost associated with programs, such as NBS has been the reason universal screening has not been implemented in many LMICs. A report by the Economist Intelligence Unit in 2020 estimated that universal NBS for SCD in Ghana would cost 6.8 million USD per year, which is almost 1% of its health-care budget [111]. Public–private partnerships, involving academia, industry, and governmental organizations, have been established to begin building capacity towards universal NBS. In Angola, screening laboratories have been set up and lab personnel have received training through such a partnership [112]. In 2020, the American Society of Hematology funded the Consortium on Newborn Screening in Africa, which will screen over ten thousand babies per year over five years in seven African countries [113]. Such efforts are crucial, but we remain in the infancy of the journey to improving outcomes of individuals with SCD in LMICs. It is imperative to continually engage local governments and demonstrate the long-term cost-effectiveness of screening and infection prophylaxis programs to sustain ongoing progress.
7. Five year view
Three new disease-modifying therapies for SCD have been approved over the last five years, with multiple new therapeutic targets being developed currently. Hematopoietic stem cell transplants with haploidentical donors are being performed in investigational settings at limited centers. Over the next few years, we foresee increased success with using haploidentical donors, reduced conditioning regimens, and improved management of graft-versus-host disease, leading to higher utilization of hematopoietic stem cell transplants in high and potentially middle-income settings [101].
Looking ahead, we hope for increased access to basic SCD care in LMICs, with strong emphasis placed on establishing universal NBS programs, consistent provision of infectious prophylaxis, and monitoring of patients for poor outcomes with tools, such as TCD screening. Building capacity at multiple levels will also improve EBI adoption for SCD management in LMICs. We envision further development of public–private partnerships that increase access to disease-modifying therapies, such as hydroxyurea and also invest in the development of infrastructure to bring potential curative therapies, such as gene therapy to the forefront of the management of SCD in LMICs.
Article Highlights.
Over 300,000 babies are born with SCD annually. Approximately 90% of these births take place in LMICs.
The highest incidence of disease is seen in SSA, where 50–90% of children with SCD die before they reach age five.
Effective management for SCD begins with early diagnosis via NBS. However, the areas with the highest burden of SCD often lack universal NBS programs.
SCD is associated with increased susceptibility to infection secondary to encapsulated bacteria. Current recommendations include the use of prophylactic antimicrobials and the completion of the pneumococcal vaccine series. However, the availability of immunizations and penicillin is limited in LMICs.
Hydroxyurea decreases the frequency of acute and chronic complications of SCD and is standard of care for patients with SCD in high-income countries. The majority of patients in LMICs still do not have access to this life-saving medication.
Children with SCD are at increased risk of ischemic stroke. In high-income countries, EBP for primary stroke prevention includes screening for abnormal transcranial Doppler ultrasound velocity and use of blood transfusion therapy for repeated abnormal velocities. Unfortunately, this can often not be implemented in low-resource settings.
HLA-matched sibling donor hematopoietic stem cell transplantation is standard of care curative therapy for SCD. However, this is an expensive and resource-intensive procedure that is not accessible for most patients in LMICs.
Many strategies are being used to increase access to care for SCD patients in LMICs. Strategies include establishing public–private partnerships, training CHW to support SCD management, engaging local government officials and religious leaders to develop culturally competent programs, and developing locally relevant educational materials for patients and families.
Declarations of Interest
JH Estepp receives consultant fees from Daiichi Sankyo, Global Blood Therapeutics, Emmanus Life Sciences, and Agios Pharmaceuticals and research support from Global Blood Therapeutics, Forma Therapeutics, Pfizer, Eli Lily and Company, the American Society of Hematology Scholar Program, and the NIH.
H Bello-Manga is supported by the NIH (K43TW011583) and is an investigator for a Global Blood Therapeutics trial (Hope Kids2).
YM Carroll receives consultant fees from Forma Therapeutics and Global Blood Therapeutics.
AA King is supported by the NIH (1K24HL148305, 5U01HL133994) and HRSA (6U1EMC27865). AA King also receives research funding from Global Blood Therapeutics.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. Epub 2018/03/16. doi: 10.1038/nrdp.2018.10. Epub 2018/03/16. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–151. doi: 10.1016/S0140-6736(12)61229-X Epub 2012/10/30. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piel FB, Patil AP, Howes RE, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun. 2010;1(1):104. doi: 10.1038/ncomms1104 Epub 2010/11/04. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangla A, Ehsan M, Agarwal N, et al. Sickle Cell Anemia. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2021; [Google Scholar]
- 5.Grosse SD, Odame I, Atrash HK, et al. Sickle cell disease in Africa: a neglected cause of early childhood mortality.Am J Prev Med.2011;41(6 Suppl 4):S398–405;Epub 2011/12/07. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rees DC, Brousse VA. Sickle cell disease: status with particular reference to India.Indian J Med Res.2016;143(6):675–677;Epub 2016/10/18. doi: 10.4103/0971-5916.191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rankine-Mullings AE, Owusu-Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database Syst Rev. 2017;10. CD003427. Epub 2017/10/11. doi: 10.1002/14651858.CD003427.pub4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314(25):1593–1599. doi: 10.1056/NEJM198606193142501. Epub 1986/06/19. doi: . [DOI] [PubMed] [Google Scholar]; • This article provides the results from the Prophylaxis with Oral Penicillin in Children with Sickle Cell Disease (PROPS) study that was the basis of the recommendation of universal penicillin prophylaxis for newborns with SCD in the USA. The placebo group has a 84% higher incidence of infection compared to the penicillin prophylaxis group, and 3 deaths compared to no deaths in the prophylaxis group.
- 9.Kolata G Panel urges newborn sickle cell screening: gale academic onefile. Science. 1987;236(4799):259–60. doi: 10.1126/science.3563504 [DOI] [PubMed] [Google Scholar]
- 10.Conference C Consensus conference. Newborn screening for sickle cell disease and other hemoglobinopathies. JAMA. 1987;258(9):1205–1209. Epub 1987/09/04. [PubMed] [Google Scholar]
- 11.Benson JM, Therrell BL Jr. History and current status of newborn screening for hemoglobinopathies.Semin Perinatol.2010;34 (2):134–144;Epub 2010/03/09. doi: 10.1053/j.semperi.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Therrell BL Jr., Lloyd-Puryear MA, Eckman JR, et al. Newborn screening for sickle cell diseases in the United States: a review of data spanning 2 decades.Semin Perinatol.2015;39(3):238–251;Epub 2015/05/17. doi: 10.1053/j.semperi.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Lobitz S, Telfer P, Cela E, et al. Newborn screening for sickle cell disease in Europe: recommendations from a Pan-European Consensus Conference. Br J Haematol. 2018;183(4):648–660. doi: 10.1111/bjh.15600 Epub 2018/10/20. doi: . [DOI] [PubMed] [Google Scholar]
- 14.Institute for Quality and Efficiency in Health Care (IQWiG). Newborn screening for sickle cell disease (SCD): IQWiG Reports – Commission No. S18-01 [Internet]. Institute for Quality and Efficiency in Health Care: Extracts. Cologne (Germany): Institute for Quality and Efficiency in Health Care (IQWiG); 2019. [PubMed] [Google Scholar]
- 15.ZS MMD, Rogers ZR, Buchanan GR, et al. Survival into adulthood in sickle cell disease from the Dallas newborn cohort. Blood. 2014;124 (21):599. DOI: 10.1182/BLOOD.V124.21.559.559 [DOI] [Google Scholar]
- 16.Piel FB, Hay SI, Gupta S, et al. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions.PLoS Med.2013;10(7):e1001484;Epub 2013/07/23. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diallo D, Tchernia G. Sickle cell disease in Africa. Curr Opin Hematol. 2002;9(2):111–116;Epub 2002/02/15. doi: 10.1097/00062752-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Hsu L, Nnodu OE, Brown BJ, et al. White paper: pathways to progress in newborn screening for sickle cell disease in Sub-Saharan Africa. J Trop Dis Public Health. 2018;6(2):260. doi: 10.4172/2329-891X.1000260 Epub 2018/12/07. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segbefia CI, Goka B, Welbeck J, et al. Implementing newborn screening for sickle cell disease in Korle Bu Teaching Hospital, Accra: results and lessons learned. Pediatr Blood Cancer. 2021;68(7):e29068. doi: 10.1002/pbc.29068 Epub 2021/04/24. doi: . [DOI] [PubMed] [Google Scholar]
- 20.Boemer F, Ketelslegers O, Minon JM, et al. Newborn screening for sickle cell disease using tandem mass spectrometry.Clin Chem.2008;54(12):2036–2041;Epub 2008/10/04. doi: 10.1373/clinchem.2008.106369. [DOI] [PubMed] [Google Scholar]
- 21.Frommel C Newborn Screening for Sickle Cell Disease and Other Hemoglobinopathies: a Short Review on Classical Laboratory Methods-Isoelectric Focusing, HPLC, and Capillary Electrophoresis. Int J Neonatal Screen. 2018;4(4):39;Epub 2018/12/05. doi: 10.3390/ijns4040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padilla CD, Krotoski D, Therrell BL Jr. Newborn screening progress in developing countries-overcoming internal barriers. Semin Perinatol. 2010;34(2):145–155;Epub 2010/03/09. doi: 10.1053/j.sem-peri.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Obaro SK, Daniel Y, Lawson JO, et al. Sickle-Cell Disease in Nigerian Children: parental Knowledge and Laboratory Results. Public Health Genomics. 2016;19(2):102–107. doi: 10.1159/000444475 Epub 2016/03/19. doi: . [DOI] [PubMed] [Google Scholar]
- 24.Marsh VM, Kamuya DM, Molyneux SS. ‘All her children are born that way’: gendered experiences of stigma in families affected by sickle cell disorder in rural Kenya. Ethn Health. 2011;16(4–5):343–359; Epub 2011/07/30. doi: 10.1080/13557858.2010.541903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinda EM, Rajkumar AP, Enemark U. Association between gender inequality index and child mortality rates: a cross-national study of 138 countries. BMC Public Health. 2015;15(1):97;Epub 2015/04/18. doi: 10.1186/s12889-015-1449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong A, Darren B, Loiseau B, et al. Ethical, social, and cultural issues related to clinical genetic testing and counseling in low- and middle-income countries: a systematic review. Genet Med. 2018;23(12):2270–2280. doi: 10.1038/s41436-018-0090-9 Epub 2018/08/04. doi: . [DOI] [PubMed] [Google Scholar]
- 27.Saadallah AA, Rashed MS. Newborn screening: experiences in the Middle East and North Africa. J Inherit Metab Dis. 2007;30(4):482–489;Epub 2007/08/19. doi: 10.1007/s10545-007-0660-5. [DOI] [PubMed] [Google Scholar]
- 28.Weber AM, Cislaghi B, Meausoone V, et al. Gender norms and health: insights from global survey data. Lancet. 2019;393(10189):2455–2468. doi: 10.1016/S0140-6736(19)30765-2 Epub 2019/06/04. doi: . [DOI] [PubMed] [Google Scholar]
- 29.Jain DL, Sarathi V, Upadhye D, et al. Newborn screening shows a high incidence of sickle cell anemia in Central India. Hemoglobin. 2012;36(4):316–322. doi: 10.3109/03630269.2012.691434 Epub 2012/06/21. doi: . [DOI] [PubMed] [Google Scholar]
- 30.McGann PT, Ferris MG, Ramamurthy U, et al. A prospective newborn screening and treatment program for sickle cell anemia in Luanda, Angola. Am J Hematol. 2013;88(12):984–989. doi: 10.1002/ajh.23578 Epub 2013/09/17. doi: [DOI] [PubMed] [Google Scholar]
- 31.Machingaidze S, Wiysonge CS, Hussey GD. Strengthening the expanded programme on immunization in Africa: looking beyond 2015.PLoS Med.2013;10(3):e1001405;Epub 2013/03/26. doi: 10.1371/journal.pmed.1001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance: part 1: childhood vaccinations. P T. 2016;41(7):426–436. Epub 2016/07/14. [PMC free article] [PubMed] [Google Scholar]
- 33.Reid SD, Fidler SJ, Cooke GS. Tracking the progress of HIV: the impact of point-of-care tests on antiretroviral therapy. Clin Epidemiol. 2013;5:387–396. Epub 2013/10/15. doi: 10.2147/CLEP.S37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez OA, Hustace T, Voltaire M, et al. Newborn screening for sickle cell disease using point-of-care testing in low-income setting. Pediatrics. 2019;144(4). doi: 10.1542/peds.2018-4105 Epub 2019/09/19. doi: [DOI] [PubMed] [Google Scholar]
- 35.Ilyas S, Simonson AE, Asghar W. Emerging point-of-care technologies for sickle cell disease diagnostics. Clin Chim Acta. 2020;501:85–91. Epub 2019/11/05. doi: 10.1016/j.cca.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Nnodu OE, Sopekan A, Nnebe-Agumadu U, et al. Implementing newborn screening for sickle cell disease as part of immunisation programmes in Nigeria: a feasibility study. Lancet Haematol. 2020;7(7):e534–e40. doi: 10.1016/S2352-3026(20)30143-5 Epub 2020/06/27. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chawla SS, Gupta S, Onchiri FM, et al. Water availability at hospitals in low- and middle-income countries: implications for improving access to safe surgical care.J Surg Res.2016;205(1):169–178;Epub 2016/09/14. doi: 10.1016/j.jss.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 38.Hand T, Rosseau NA, Stiles CE, et al. The global role, impact, and limitations of Community Health Workers (CHWs) in breast cancer screening: a scoping review and recommendations to promote health equity for all. Glob Health Action. 2021;14(1):1883336. doi: 10.1080/16549716.2021.1883336 Epub 2021/04/27. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.PR KAK, Chami T, Hejjaji V, et al. The effectiveness of community health workers for CVD prevention in LMIC. Glob Heart. 2017;12(3):233–43 e6. [DOI] [PubMed] [Google Scholar]
- 40.Jalloh MF, Wilhelm E, Abad N, et al. Mobilize to vaccinate: lessons learned from social mobilization for immunization in low and middle-income countries.Hum Vaccin Immunother.2020;16(5):1208–1214;Epub 2019/08/30. doi: 10.1080/21645515.2019.1661206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.William BM, Corazza GR. Hyposplenism: a comprehensive review. Part I: basic concepts and causes.Hematology.2007;12(1):1–13;Epub 2007/03/17. doi: 10.1080/10245330600938422. [DOI] [PubMed] [Google Scholar]
- 42.Salawu LOE, Durosinmi MA. Immunohaematological characteristics of Nigerian sickle cell disease patients in asymptomatic steady state. Eur J Gen Med. 2009;6:170–174. [Google Scholar]
- 43.Falcao RP, Donadi EA. Infection and immunity in sickle cell disease. AMB Rev Assoc Med Bras. 1989;35(2):70–74. Epub 1989/03/01. [PubMed] [Google Scholar]
- 44.Overturf GD. Infections and immunizations of children with sickle cell disease. Adv Pediatr Infect Dis. 1999;14:191–218. Epub 1999/03/18. [PubMed] [Google Scholar]
- 45.Bunn HF, Epstein FH. Pathogenesis and treatment of sickle cell disease.N Engl J Med.1997;337(11):762–769;Epub 1997/09/11. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 46.Zhang D, Xu C, Manwani D, et al. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology.Blood.2016;127(7):801–809;Epub 2016/01/14. doi: 10.1182/blood-2015-09-618538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dieye TN, Ndiaye O, Ndiaye AB, et al. Complement and serum immunoglobulins in homozygous and heterozygous sickle cell anemia in Senegal. Dakar Med. 1999;44(2):175–179. Epub 2002/04/18. [PubMed] [Google Scholar]
- 48.Blacksin MF, Finzel KC, Benevenia J. Osteomyelitis originating in and around bone infarcts: giant sequestrum phenomena. AJR Am J Roentgenol. 2001;176(2):387–391;Epub 2001/02/13. doi: 10.2214/ajr.176.2.1760387. [DOI] [PubMed] [Google Scholar]
- 49.Elbashier AM, Al-Salem AH, Aljama A. Salmonella as a causative organism of various infections in patients with sickle cell disease. Ann Saudi Med. 2003;23(6):358–362;Epub 2006/07/27. doi: 10.5144/0256-4947.2003.358. [DOI] [PubMed] [Google Scholar]
- 50.Bhutta ZA, Sommerfeld J, Lassi ZS, et al. Global burden, distribution, and interventions for infectious diseases of poverty.Infect Dis Poverty.2014;3(1):21;Epub 2014/08/12. doi: 10.1186/2049-9957-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans C, Orf K, Horvath E, et al. Impairment of neutrophil oxidative burst in children with sickle cell disease is associated with heme oxygenase-1. Haematologica. 2015;100(12):1508–1516. doi: 10.3324/haematol.2015.128777 Epub 2015/09/01. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ochocinski D, Dalal M, Black LV, et al. Life-threatening infectious complications in sickle cell disease: a concise narrative review. Front Pediatr. 2020;8:38. Epub 2020/03/11. doi: 10.3389/fped.2020.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oron AP, Chao DL, Ezeanolue EE, et al. Caring for Africa’s sickle cell children: will we rise to the challenge? BMC Med. 2020;18(1):92. doi: 10.1186/s12916-020-01557-2 Epub 2020/04/29. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esposito S, Principi N. Impacts of the 13-valent pneumococcal conjugate vaccine in children. J Immunol Res. 2015;2015:591580. Epub 2015/09/10. doi: 10.1155/2015/591580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428–1433. doi: 10.1086/516781 Epub 2007/05/08. doi: [DOI] [PubMed] [Google Scholar]
- 56.McCavit TL, Gilbert M, Buchanan GR. Prophylactic penicillin after 5 years of age in patients with sickle cell disease: a survey of sickle cell disease experts. Pediatr Blood Cancer. 2013;60(6):935–939;Epub 2012/11/30. doi: 10.1002/pbc.24395. [DOI] [PubMed] [Google Scholar]
- 57.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517 Epub 2014/09/10. doi: [DOI] [PubMed] [Google Scholar]
- 58.Odey F, Okomo U, Oyo-Ita A. Vaccines for preventing invasive salmonella infections in people with sickle cell disease. Cochrane Database Syst Rev. 2018;12. CD006975. Epub 2018/12/07. doi: 10.1002/14651858.CD006975.pub4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cannas G, Merazga S, Virot E. Sickle cell disease and infections in high- and low-income countries. Mediterr J Hematol Infect Dis.2019;11(1):e2019042;Epub 2019/07/17. doi: 10.4084/MJHID.2019.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galadanci N, Wudil BJ, Balogun TM, et al. Current sickle cell disease management practices in Nigeria. Int Health. 2014;6(1):23–28. doi: 10.1093/inthealth/iht022 Epub 2013/10/12. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandes TA, Medeiros TM, Alves JJ, et al. Socioeconomic and demographic characteristics of sickle cell disease patients from a low-income region of northeastern Brazil. Rev Bras Hematol Hemoter. 2015;37(3):172–177. doi: 10.1016/j.bjhh.2015.03.013 Epub 2015/06/05. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolarinwa RAAN, Aboderin OA, Durosinmi MA. The role of malaria in vaso-occlusive crisis of adult patients with sickle cell disease. J Med Med Sci. 2010;1:407–411. [Google Scholar]
- 63.Oniyangi O, Omari AA. Malaria chemoprophylaxis in sickle cell disease. Cochrane Database Syst Rev. 2019;(11): [DOI] [PubMed] [Google Scholar]
- 64.Ahmed SG. The role of infection in the pathogenesis of vaso-occlusive crisis in patients with sickle cell disease. Mediterr J Hematol Infect Dis. 2011;3(1):e2011028;Epub 2011/08/27. doi: 10.4084/MJHID.2011.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.WHO. Sickle-cell disease: a strategy for the who African region report of the regional director. Malabo: Equatorial Guinea. 2010;Sixtieth Session:30. [Google Scholar]
- 66.Diop S, Soudre F, Seck M, et al. Sickle-cell disease and malaria: evaluation of seasonal intermittent preventive treatment with sulfadoxine-pyrimethamine in Senegalese patients-a randomized placebo-controlled trial. Ann Hematol. 2011;90(1):23–27. doi: 10.1007/s00277-010-1040-z Epub 2010/08/10. doi: [DOI] [PubMed] [Google Scholar]
- 67.Eke FU, Anochie I. Effects of pyrimethamine versus proguanil in malarial chemoprophylaxis in children with sickle cell disease: a randomized, placebo-controlled, open-label study. Curr Ther Res Clin Exp. 2003;64(8):616–625;Epub 2003/09/01. doi: 10.1016/j.curtheres.2003.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serjeant G Management of sickle cell disease: challenges and risks of transfusion. Int J Clin Transfusion Med. 2016;4:109–119. [Google Scholar]
- 69.Ahmed SG, Bukar AA, Jolayemi B. Hematological indices of sickle cell anaemia patients with pulmonary tuberculosis in northern Nigeria. Mediterr J Hematol Infect Dis. 2010;2(1):e2010014;Epub 2010/01/01. doi: 10.4084/MJHID.2010.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galadanci NA, Abdullahi SU, Ali Abubakar S, et al. Moderate fixed-dose hydroxyurea for primary prevention of strokes in Nigerian children with sickle cell disease: final results of the SPIN trial. Am J Hematol. 2020;95(9):E247–E50. doi: 10.1002/ajh.25900 Epub 2020/06/09. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pantoja T, Opiyo N, Lewin S, et al. Implementation strategies for health systems in low-income countries: an overview of systematic reviews. Cochrane Database Syst Rev. 2017;9:CD011086. Epub 2017/09/13. doi: 10.1002/14651858.CD011086.pub2. CD011086. Epub 2017/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Evidence based interventions (EBI) targeted at SCD management in LMICs should involve amulti-level approach, which addresses the patient-level, provider-level, and system-level barriers of care delivery.
- 72.Adewoyin AS. Management of sickle cell disease: a review for physician education in Nigeria (sub-saharan Africa). Anemia. 2015;2015:791498. Epub 2015/02/11. doi: 10.1155/2015/791498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adewoyin AS, Alagbe AE, Adedokun BO, et al. Knowledge, attitude and control practices of sickle cell disease among youth corps members in Benin City, Nigeria. Ann Ib Postgrad Med. 2015;13(2):100–107. Epub 2016/05/11. [PMC free article] [PubMed] [Google Scholar]
- 74.Adeodu OO, Alimi T, Adekile AD. A comparative study of perception of sickle cell anaemia by married Nigeria rural and urban women. West Afr J Med. 2000;19(1):1–5. Epub 2000/05/23. [PubMed] [Google Scholar]
- 75.McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert Opin Drug Saf. 2015;14(11):1749–1758;Epub 2015/09/15. doi: 10.1517/14740338.2015.1088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Power-Hays A, Ware RE. Effective use of hydroxyurea for sickle cell anemia in low-resource countries. Curr Opin Hematol. 2020;27(3):172–180;Epub 2020/03/25. doi: 10.1097/MOH.0000000000000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: a 17.5 year follow-up. Am J Hematol. 2010;85(6):403–408. doi: 10.1002/ajh.21699 Epub 2010/06/01. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.NIH. Evidence-based management of sickle cell disease: expert panel report. 2014.
- 79.Adepoju P Ghana takes on sickle-cell disease. Lancet. 2020;395(10222):402;Epub 2020/02/10. doi: 10.1016/S0140-6736(20)30293-2. [DOI] [PubMed] [Google Scholar]
- 80.McGann PT, Williams TN, Olupot-Olupot P, et al. Realizing effectiveness across continents with hydroxyurea: enrollment and baseline characteristics of the multicenter REACH study in Sub-Saharan Africa. Am J Hematol. 2018;93(4):537–545. doi: 10.1002/ajh.25034 Epub 2018/01/11. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tshilolo L, Tomlinson G, Williams TN, et al. Hydroxyurea for children with sickle cell Anemia in Sub-Saharan Africa. N Engl J Med. 2019;380(2):121–131. doi: 10.1056/NEJMoa1813598 Epub 2018/12/07. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Opoka RO, Ndugwa CM, Latham TS, et al. Novel use Of Hydroxyurea in an African Region with Malaria (NOHARM): a trial for children with sickle cell anemia. Blood. 2017;130(24):2585–2593. doi: 10.1182/blood-2017-06-788935 Epub 2017/10/21. doi: [DOI] [PubMed] [Google Scholar]
- 83.John CC, Opoka RO, Latham TS, et al. Hydroxyurea dose escalation for sickle cell Anemia in Sub-Saharan Africa. N Engl J Med. 2020;382(26):2524–2533. doi: 10.1056/NEJMoa2000146 Epub 2020/06/25. doi: [DOI] [PubMed] [Google Scholar]
- 84.Abdullahi SU, Jibir BW, Bello-Manga H, et al. Hydroxyurea for primary stroke prevention in children with sickle cell anaemia in Nigeria (SPRING): a double-blind, multicentre, randomised, phase 3 trial. Lancet Haematol. 2022;9(1):e26–e37. doi: 10.1016/S2352-3026(21)00368-9 Epub 2022/01/01. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jain DL, Sarathi V, Desai S, et al. Low fixed-dose hydroxyurea in severely affected Indian children with sickle cell disease. Hemoglobin.2012;36(4):323–332;Epub 2012/06/28. doi: 10.3109/03630269.2012.697948 [DOI] [PubMed] [Google Scholar]
- 86.Patel DK, Mashon RS, Patel S, et al. Low dose hydroxyurea is effective in reducing the incidence of painful crisis and frequency of blood transfusion in sickle cell anemia patients from eastern India.Hemoglobin.2012;36(5):409–420;Epub 2012/08/14. doi: 10.3109/03630269.2012.709897. [DOI] [PubMed] [Google Scholar]
- 87.Costa E, Tibalinda P, Sterzi E, et al. Making hydroxyurea affordable for sickle cell disease in Tanzania is essential (HASTE): how to meet major health needs at a reasonable cost. Am J Hematol. 2021;96(1):E2–E5. doi: 10.1002/ajh.26007 Epub 2020/09/26. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Janyala S CDSCO approves marketing of hydroxyurea tablets for sickle cell anemia treatment. The Indian Express. 2021. December 23. Available from: https://indianexpress.com/article/cities/hyderabad/cdsco-hydroxyurea-sickle-cell-anemia-treatment-7687532/. [Google Scholar]
- 89.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. Epub 1998/02/07. [PubMed] [Google Scholar]
- 90.Adams RJ. Lessons from the stroke prevention trial in sickle cell Anemia (STOP) study. J Child Neurol. 2000;15(5):344–349;Epub 2000/06/01. doi: 10.1177/088307380001500511. [DOI] [PubMed] [Google Scholar]
- 91.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial Doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661–670. doi: 10.1016/S0140-6736(15)01041-7 Epub 2015/12/17. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102 Epub 1998/07/02. doi: [DOI] [PubMed] [Google Scholar]
- 93.Enninful-Eghan H, Moore RH, Ichord R, et al. Transcranial Doppler ultrasonography and prophylactic transfusion program is effective in preventing overt stroke in children with sickle cell disease. J Pediatr.2010;157(3):479–484;Epub 2010/05/04. doi: 10.1016/j.jpeds.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anie KA, Egunjobi FE, Akinyanju OO. Psychosocial impact of sickle cell disorder: perspectives from a Nigerian setting. Global Health. 2010;6(1):2;Epub 2010/02/23. doi: 10.1186/1744-8603-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galadanci NA, Umar Abdullahi S, Vance LD, et al. Feasibility trial for primary stroke prevention in children with sickle cell anemia in Nigeria (SPIN trial). Am J Hematol. 2017;92(8):780–788. doi: 10.1002/ajh.24770 Epub 2017/04/26. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abdullahi SU , Jibir BW, Bello-Manga H, et al. Hydroxyurea for primary stroke prevention in children with sickle cell anaemia in Nigeria (SPRING): a double-blind, multicentre, randomised, phase 3 trial. Lancet Haematol. 2022;9(1):e26–e37. doi: 10.1016/S2352-3026(21)00368-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DeBaun MR, Jordan LC, King AA, et al. American society of Hematology 2020 guidelines for sickle cell disease: prevention, diagnosis, and treatment of cerebrovascular disease in children and adults. Blood Adv. 2020;4(8):1554–1588. doi: 10.1182/blood-advances.2019001142. Epub 2020/04/17. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]; •• These guidelines provide a paradigm shift in the prevention of stroke in children with SCD and abnormal TCD velocities living in LMICs where blood transfusions and iron chelation are not available or affordable. The recommendation is the use of fixed-dose hydroxyurea (20 mg/kg/day) or maximum tolerated dose.
- 98.Ghafuri DL, Abdullahi SU, Dambatta AH, et al. Establishing Sickle Cell Disease Stroke Prevention Teams in Africa is Feasible: program Evaluation Using the RE-AIM Framework. J Pediatr Hematol Oncol. 2021. Epub 2021/05/19. doi: 10.1097/MPH.0000000000002179. Epub 2021/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study highlights the importance of stakeholder involvement in ensuring sustainability of stroke prevention programs.
- 99.Idowu BO, Okedere TA. Diagnostic radiology in Nigeria: a country report. J Glob Radiol. 2020;6(1):1072. [Google Scholar]
- 100.HA SGNH, Khan S. Nigeria country report: RAD-AID.org. 2015.
- 101.Odame I, Jain D. Sickle cell disease: progress made & challenges ahead. Indian J Med Res. 2020;151(6):505–508;Epub 2020/07/29. doi: 10.4103/ijmr.IJMR_2064_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Niihara Y, Miller ST, Kanter J, et al. A phase 3 trial of l-Glutamine in sickle cell disease. N Engl J Med. 2018;379(3):226–235. doi: 10.1056/NEJMoa1715971 Epub 2018/07/19. doi: [DOI] [PubMed] [Google Scholar]
- 103.Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–439. doi: 10.1056/NEJMoa1611770 Epub 2016/12/14. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vichinsky E, Hoppe CC, Ataga KI, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509–519. doi: 10.1056/NEJMoa1903212 Epub 2019/06/15. doi: [DOI] [PubMed] [Google Scholar]
- 105.SE BP, Synnott PG, Chapman R, et al. Crizanlizumab Voxelotor, and L-Glutamine for sickle cell disease: effectiveness and value January 23, 2020. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_SCD_Evidence-Report_031220-FOR-PUBLICATION.PDF.
- 106.Nnodu OE, Oron AP, Sopekan A, et al. Child mortality from sickle cell disease in Nigeria: a model-estimated, population-level analysis of data from the 2018 demographic and health survey.Lancet Haematol.2021;8(10):e723–e31;Epub 2021/09/06. doi: 10.1016/S2352-3026(21)00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bank TW. Nigeria releases new report on poverty and inequality in country. May 28, 2020.
- 108.Novartis. New Novartis medicine Adkveo (crizanlizumab) approved by FDA to reduce frequency of pain crises in individuals living with sickle cell disease. Available from: https://www.novartis.com/news/media-releases/new-novartis-medicine-adakveo-crizanlizumab-approved-fda-reduce-frequency-pain-crises-individuals-living-sickle-cell-disease. [Accessed: November 15, 2019].
- 109.Demirci S, Uchida N, Tisdale JF. Gene therapy for sickle cell disease: an update. Cytotherapy. 2018;20(7):899–910;Epub 2018/06/04. doi: 10.1016/j.jcyt.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Broder MS, Quock TP, Chang E, et al. The cost of hematopoietic stem-cell transplantation in the United States. Am Health Drug Benefits. 2017;10(7):366–374. Epub 2017/12/22. [PMC free article] [PubMed] [Google Scholar]
- 111.Nature) LN. Screening saves lives, so why don’t governments fund it? Nature. 2021);596(7873):S6–S7. [Google Scholar]
- 112.Perkin E Battling sickle cell disease at its source. Available from: https://blog.perkinelmer.com/posts/battling-sickle-cell-disease-at-its-source/. [Accessed: March 25, 2019].
- 113.(ASH). ASoH. consortium on newborn screening in Africa. Available frpm: https://www.hematology.org/global-initiatives/consortium-on-newborn-screening-in-africa#:~:text=The%20Consortium%20on%20Newborn%20Screening,)%20in%20sub%2DSaharan%20Africa.


