Abstract
Ralstonia sp. strain CH34 is resistant to nickel and cobalt cations. Resistance is mediated by the cnr determinant located on plasmid pMOL28. The cnr genes are organized in two clusters, cnrYXH and cnrCBA. As revealed by reverse transcriptase PCR and primer extension, transcription from these operons is initiated from promoters located upstream of the cnrY and cnrC genes. These two promoters exhibit conserved sequences at the −10 (CCGTATA) and −35 (CRAGGGGRAG) regions. The CnrH gene product, which is required for expression of both operons, is a sigma factor belonging to the sigma L family, whose activity seems to be governed by the membrane-bound CnrY and CnrX gene products in response to Ni2+. Half-maximal activation from the cnrCBA operon was determined by using appropriate lacZ gene fusions and was shown to occur at an Ni2+ concentration of about 50 μM.
Ralstonia sp. strain CH34 (formerly Alcaligenes eutrophus strain CH34 [3]) contains at least seven determinants encoding resistances to toxic heavy metals, located either on the bacterial chromosome or on one of the two indigenous plasmids pMOL28 (180 kb [37]) and pMOL30 (238 kb [7, 20]). The cnr determinant of plasmid pMOL28 mediates inducible resistance to Co2+ and Ni2+ in Ralstonia sp. strain CH34 (15). The cnr determinant is similar to ncc (nickel-cobalt-cadmium resistance) of Alcaligenes xylosoxidans 31A (34) and czc (cobalt-zinc-cadmium resistance) on plasmid pMOL30 of Ralstonia sp. strain CH34 (28). All three resistances are based on cation efflux, which is best characterized for Czc (13, 24, 29). In analogy to Czc (9, 25, 28, 32), the products of the genes cnrA, cnrB, and cnrC are likely to form a membrane-bound protein complex catalyzing an energy-dependent efflux of Ni2+ and Co2+, and the mechanism of action of the CnrCBA complex may be that of a proton/cation antiporter.
Three regulators seem to control Cnr, an extracellular function (ECF) sigma factor (CnrH) and the products of two additional genes with unknown precise functions (CnrX and CnrY products, respectively) (15, 16). The genes cnrYXH are located upstream of cnrCBA and have the same direction of transcription. Transposon Tn5 insertion upstream of cnrH led to a constitutive expression of nickel resistance and to low zinc resistance as well (4, 15). However, this may be a polar effect and, additionally, a readthrough from a transposon promoter. As shown with Tn5-lacZ fusions (31), cnr is best induced by 128 μM Ni2+. Other metals serve as less efficient inducers; however, this experiment was done with nickel-sensitive cnr-lacZ transposon-insertion mutants. In this study an improved cnrCBA-lacZ operon fusion was constructed, which mediates full nickel resistance and which was used to evaluate the physiology of cnr regulation.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Tris-buffered mineral salts medium (20) containing 2 g of sodium gluconate/liter was used to cultivate Ralstonia strains (Table 1). Solid Tris-buffered medium contained 20 g of agar/liter. β-Galactosidase activity in permeabilized cells was determined as published previously (26), with 1 U defined as the activity forming 1 nmol of o-nitrophenol per min at 30°C.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| A. xylosoxidans 31A | ncc determinant | 34 |
| E. coli strains | ||
| CC118 | phoA | 17 |
| S17/1 | RP4 mobilization system | 35 |
| Ralstonia sp. strains | ||
| AE104 | No megaplasmid, metal sensitive | 20 |
| AE128(pMOL28) | cnr on megaplasmid pMOL28 | 20 |
| DN176(pMOL28-1) | cnrY1(Con), constitutive expression of cnr | This publication |
| DN177(pMOL28-2) | Φ(cnrCBA-lacZ) | This publication |
| DN190(pMOL28-3) | Φ(cnrCBA-lacZ) ΔcnrYXH | This publication |
| DN195(pMOL28-4) | cnrY1(Con) Φ(cnrCBA-lacZ) | This publication |
| DN410(pMOL28-5) | pMOL28-2 Ω(2100 bp::pECD581) Φ(cnrCBA-lacZ) | This publication |
| Plasmids | ||
| pDNA291 | cnrYXH in pVDZ′2 | This publication |
| pDNA298 | Φ(cnrHp-lacZ) in pVDZ′2 | This publication |
| pDNA299 | Φ(cnrCp-lacZ) in pVDZ′2 | This publication |
| pDNA300 | Φ(cnrYp-lacZ) in pVDZ′2 | This publication |
| pECD500 | phoA fusion vector | 32 |
| pECD581 | pLO2 with bp 2100–2400 of cnrH′C′ | This publication |
| pGEM T-Easy | PCR cloning vector | Promega |
| pLO2 | Broad-host-range vector for recombination in Ralstonia | 14 |
| pMC1871 | lacZ gene without promoter | Pharmacia, Freiburg, Germany |
| pVDZ′2 | IncP1, broad-host-range Ralstonia vector | 6 |
Genetic techniques.
Standard molecular genetic techniques were used (24, 33). For conjugative gene transfer, overnight cultures of donor strain Escherichia coli S17/1 (35) and of the Ralstonia recipient strains grown at 30°C in complex medium were mixed (1:1) and plated onto nutrient broth agar. After overnight growth, the bacteria were suspended in saline (9 g of NaCl/liter), diluted, and plated onto selective medium as previously described (24). DNA sequences were obtained with an A.L.F. sequencer (Pharmacia, Uppsala, Sweden). Total RNA of Ralstonia strains was isolated as described previously (11, 30).
Reporter protein fusions.
The complete cnrY and cnrX genes were cloned into fusion vector pECD500 (phoA fusions) (32) in E. coli CC118 (17). Specific activity of alkaline phosphatase (17) was determined in triplicate as described previously (27).
Construction of a Φ(cnrCBA-lacZ) transcriptional fusion.
To construct the lacZ reporter strain DN177(pMOL28-2), the 300 bp upstream of the cnrA stop codon were PCR amplified as a PstI-XbaI fragment from megaplasmid DNA of Ralstonia sp. strain AE126(pMOL28). Similarly, the 300 bp downstream of the cnrA stop codon were amplified as an XbaI-PstI fragment. These fragments were digested with XbaI, but not with PstI, and both fragments were cloned into vector plasmid pGEM T-Easy (Promega, Madison, Wis.) in one step. As confirmed by control sequencing, this led to a plasmid harboring a 600-bp cnr fragment with an XbaI site located directly downstream of the stop codon of cnrA, mutating the sequence TGA8025GTTTGCGA (the TGA stop codon of cnrA is in boldface) to TGAGTTTCTAGA (numbering is according to reference 15). The promoterless lacZ gene of plasmid pMC1871 (Pharmacia, Freiburg, Germany) was inserted into the XbaI site of this plasmid, and the fragment containing cnr-lacZ was cloned as a PstI fragment into plasmid pLO2 (14). Finally, the pLO2 hybrid plasmid with Φ(cnrCBA-lacZ) was used in a double-recombination event to insert the lacZ gene downstream of cnrA on megaplasmid pMOL28 as described previously (11). The correct insertion and orientation of lacZ in strain DN177(pMOL28-2) was verified by PCR and restriction endonuclease digestion (Fig. 1).
FIG. 1.
RT-PCR experiments and physical map of the cnr region. (A) Physical map of the cnr region, with rectangles indicating the genes. The size marker is in base pairs (15). Triangles mark the two promoters cnrYp and cnrCp; double-headed arrows indicate positive RT-PCR results, with the 5′ positions of the primer pair given adjacently in base pairs. The crossed arrows indicate negative RT-PCR results, with the primer positions given on the left. E, EcoRI restriction site between cnr and the adjacent chromate resistance region chr (22, 23), which would be left of cnr in this figure. (B) Physical map of derivatives of the cnr region on plasmid pMOL28. In all plasmids, the lacZ gene (large black arrows) was inserted between cnrA and orf104ff. In plasmid pMOL28-3, cnrYXH were additionally deleted in frame. In plasmid pMOL28-4, the cnrY1(Con) gene carries a 14-bp insertion after position 1111; the open reading frame continues out of frame thereafter (white box following the truncated cnrY symbol). This cnrY1 frameshift mutation leads to constitutive expression of Cnr. In plasmid pMOL28-5, insertion of plasmid pECD581 leads to a separation of cnrYXH and cnrCBA. The vector plasmid of pECD581, pLO2 (14), is shown as a thick line.
Construction of other bacterial strains.
The control region cnrYXH was deleted from megaplasmid pMOL28-2 as previously described (11), leading to strain DN190(pMOL28-3), which is Φ(cnrCBA-lacZ) ΔcnrYXH (Fig. 1). Briefly, PCR was used to amplify a 600-bp fragment from plasmid pMOL28. This fragment contains the 300 bp upstream of cnrY, including the first 24 bases of this gene (up to position G1006), and the 300 bp downstream of cnrH starting from C2261, with the last 24 bases of that gene. Both fragments were joined using a MunI site. With double recombination using a pLO2 derivative, the wild-type fragment on plasmid pMOL28-2 was exchanged for this mutated fragment.
To separate cnrYXH from cnrCBA, the cnr region from bp 2100 to 2400 (15) was PCR amplified and cloned as an XbaI-PstI fragment into pLO2 (14). The resulting plasmid, pECD581, was integrated into plasmid pMOL28-2, leading to plasmid pMOL28-5 in strain DN410(pMOL28-5) Ω(2100 bp::pECD581) Φ(cnrCBA-lacZ) (Fig. 1). (Note that the region between bp 2100 and 2400 is duplicated and flanks the integrated vector plasmid pLO2 on megaplasmid pMOL28-5 [Fig. 1].)
When plasmids used for complementation assays were generated, the respective cnr genes were amplified from plasmid pMOL28 by PCR (which introduced suitable restriction sites), cloned into pGEM T-Easy (Promega), sequenced, and subcloned into the broad-host-range vector pVDZ′2 (6). To measure the activity of the cnr promoters, cnrYp (bp 698 to 1284), cnrHp (bp 1516 to 1740), and cnrCp (bp 2250 to 2360) were fused upstream to a promoterless lacZ gene and into plasmid pVDZ′2 in the orientation opposite to that of the lac promoter located on this vector plasmid (Table 1).
Primer extension and RT-PCR experiments.
Primer extension analysis was performed with a modification of a standard protocol (33) using fluorescein-labeled oligonucleotides and an automated A.L.F. DNA Sequencer (Pharmacia, Uppsala, Sweden) as described previously (11, 39). The fluorescein-labeled 3′ antisense primers (5′-GGCGCAGCAGATGGCACG for cnrYp and 5′-GGCTCAACGCAACGGG for cnrCp) were complementary to the corresponding gene regions. After phenol-chloroform extraction, the cDNA was precipitated with ethanol, vacuum dried, and suspended in 4 μl of H2O and 4 μl of A.L.F. stop solution (Pharmacia, Uppsala, Sweden). Following heat denaturation, the sample was loaded on a 7% polyacrylamide sequencing gel. In parallel, a sequencing reaction was performed with the same fluorescein-labeled primer and a DNA fragment containing the respective gene region. The transcription start site was determined by comparison of the retention time of the primer extension reaction with that of the sequencing reaction. Primer extension experiments with total RNA from plasmid-free AE104 cells and a control reaction carried out without reverse transcriptase were used as negative controls. Reverse transcriptase PCR (RT-PCR) was carried out as described previously (11) using various primer pairs (Fig. 1) and a variety of negative control reactions, such as those with RNA isolated from the plasmid-free strain AE104, with no RNA template, and with no reverse transcriptase, as described previously (11).
RESULTS
Structures of the cnr mRNAs and the cnr promoters.
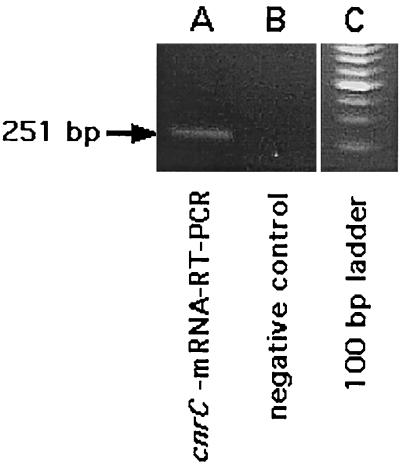
After induction with 300 μM Ni2+ (10 min, 30°C, Tris-buffered mineral salts medium), total RNA was isolated from cells of strain AE126(pMOL28). Northern mRNA-DNA hybridization experiments with cnr-specific probes did not yield clear signals, probably due to highly unstable cnr messages (data not shown). Using RT-PCR, however, signals in the regions of cnrA (the RT-PCR product contained bp 7734 to 8028 of cnr [Fig. 1]), cnrC (bp 2360 to 2610 [Fig. 2]), and cnrH (bp 2086 to 2393 and 1934 to 2393 [Fig. 1]) indicated the presence of transcripts. Additionally, RT-PCR revealed continuous transcripts from cnrA into the incomplete orf104ff following cnrA (bp 7736 to 8333) but no continuous transcript upstream of cnrY (bp 698 to 1059 and 698 to 1204 [Fig. 1]). There were no signals in the negative controls (RNA from the plasmid-free strain AE104, given for cnrC in Fig. 2, lane B).
FIG. 2.
RT-PCR of the cnrC region. After induction with 300 μM Ni2+, total RNA was isolated from cells of strain AE126(pMOL28). RT-PCR experiments were performed, and the products were separated on an agarose gel which was stained with ethidium bromide. Lane A, cnrC mRNA RT-PCR product (positions 2360 to 2610 [Fig. 1]); lanes B, respective negative control (RNA from the plasmid-free strain AE104); lane C, 100-bp ladder starting at bp 200. The original photograph was scanned using Ofoto 2.0 (Light Source Computer Images, Inc.) and processed using Photoshop 3.0 (Adobe Systems, Inc.).
The start points of the cnrYXH and the cnrCBA mRNAs were studied with primer extension (Fig. 3). Both mRNAs started about 20 bp upstream of the respective ATG start codons (Fig. 4). Regions which were highly conserved between cnrYp and cnrCp as well as between the respective promoters of the nickel-cobalt-cadmium resistance determinant, nccYp and nccCp, were found upstream of these start sites. In contrast, no signal was found when a primer extension experiment was done with a primer located in the cnrH gene (Fig. 3).
FIG. 3.
Primer extension experiments. After induction with 300 μM Ni2+, total RNA was isolated from cells of strain AE126(pMOL28). Primer extension analyses were performed on an automated fluorescent DNA sequencer using this total RNA and fluorescently labeled oligonucleotides as primers (mRNA traces). The primers were located in the 5′ end of cnrY (A), cnrH (B), or cnrC (C). Dideoxy sequencing was run as a size marker with the same primer. The raw data output is shown below the retention time (in minutes), with traces A, C, G, and T representing the successively detected dideoxy nucleotide sequencing reaction products. This sequence corresponds to bp 924 to 987 (A), 1653 to 1726 (B), and 2302 to 2364 (C) (base numbering is according to reference 15). The arrows give the initiation sites for cnrY and cnrC.
FIG. 4.
Start sites of cnrYp and cnrCp. (A) The transcriptional start sites of the cnrY- and the cnrC-specific mRNAs were determined by primer extension analysis and are given in capital boldface letters (A and G, respectively). These are the results of several experiments. The a following those two sites indicates the one nonreproducible result. The predicted −35 region craggggrag and the predicted −10 region ccgtata are in boldface (consensus sequences are below), and the predicted ribosome binding sites upstream of the ATG codons of both genes are underlined. (B) Conserved sequences upstream of nccY and nccC (34) which might be NccH-dependent promoters. For optimal homology between CnrC and NccC, a start site of A1701TG (190 bp downstream of the 3′ end of cnrH) instead of G1464TG (in the cnrH gene) for nccC has been assumed. Both proteins have now a size of 418 aa. (C) ECF promoters sigWp from Bacillus subtilis (12) and MtP2 from Mycobacterium smegmatis (8).
Activities of the cnr promoters cnrYp and cnrCp.
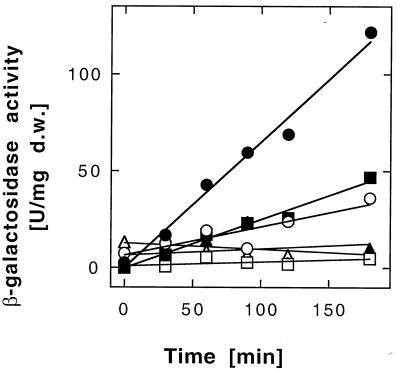
The lacZ gene was cloned together with the respective cnr promoter regions upstream into plasmid pVDZ′2 (6), leading to plasmid pDNA300 Φ(cnrYp-lacZ), pDNA299 Φ(cnrCp-lacZ), or pDNA298 Φ(cnrHp-lacZ). The last plasmid contained a possible ECF sigma factor recognition site upstream of cnrH, but no nickel induction of β-galactosidase activity could be observed in strain AE126(pMOL28) with plasmid pDNA298 Φ(cnrHp-lacZ) in trans (Table 2; Fig. 5). None of the three plasmids expressed nickel-inducible β-galactosidase activity in the megaplasmid-free strain AE104 (data not shown). However, the plasmids with the cnrYp and cnrCp promoters were nickel inducible in AE126(pMOL28) (Fig. 5). The activity of cnrYp was about twofold that of cnrCp, and there was also some induction when the cells started to grow in fresh medium without nickel (Table 2).
TABLE 2.
Expression of cnrCBA-lacZ in various AE126 mutant strainsa
| Megaplasmid | In trans | 0 mM Ni2+
|
0.5 mM Ni2+
|
||
|---|---|---|---|---|---|
| β-Galactosidase activityb before induction | Increase in β-galactosidase activityc | β-Galactosidase activity before induction | Increase in β-galactosidase activity | ||
| pMOL28d | Φ(cnrHp-lacZ) | 11.1 ± 3.7 | 0 | 10.8 ± 8.6 | 0 |
| Φ(cnrYp-lacZ) | 6.43 ± 1.49 | 8.76 | 0.06 ± 0.01 | 39 | |
| Φ(cnrCp-lacZ) | 0.87 ± 0.52 | 1.26 | 0 | 15 | |
| pMOL28-2, Φ(cnrCBA-lacZ) | None | 62.2 ± 6.8 | 0 | 64 ± 5 | 86.4 |
| nccYXH | 40.0 ± 4.5 | 0 | 46.1 ± 4.7 | 0 | |
| pMOL28-5e Φ(cnrCBA-lacZ) Ω(cnrHC::pECD581) | None | 121 ± 5 | 0 | 102.6 ± 0.4 | 90.7 |
| pMOL28-3, Φ(cnrCBA-lacZ) ΔcnrYXH | None | 28.1 ± 2.5 | 0 | 26.6 ± 1.0 | 0 |
| cnrYXH | 36.0 ± 5.1 | 0 | 48.5 ± 1.8 | 24.8 | |
| cnrY | 39.9 ± 3.3 | 0 | 33.2 ± 3.3 | 0 | |
| cnrX | 34.6 ± 6.0 | 0 | 36.7 ± 3.7 | 0 | |
| cnrH | 937 ± 106 | 0 | 770 ± 52 | 0 | |
| cnrYX | 31.6 ± 3.6 | 0 | 35.0 ± 4.8 | 0 | |
| cnrYH | 1,650 ± 93 | 0 | 1,590 ± 140 | 0 | |
| cnrXH | 900 ± 195 | 0 | 1,025 ± 320 | 0 | |
| cnrY1(Con) cnrXH | 1,350 ± 60 | 0 | 1,380 ± 97 | 0 | |
| nccYXH | 39.9 ± 4.2 | 0 | 47.9 ± 1.3 | 0 | |
| pMOL28-4, Φ(cnrCBA-lacZ) cnrY1(Con) | None | 1,025 ± 107 | 0 | 1,095 ± 88 | 0 |
| cnrYXH | 63.1 ± 5.9 | 18.4 | 61.3 ± 1.2 | 44.5 | |
| cnrY | 114 ± 4 | 0 | 97 ± 2 | 26.0 | |
| cnrX | 687 ± 53 | 0 | 896 ± 19 | 0 | |
| cnrH | 678 ± 97 | 0 | 785 ± 64 | 0 | |
| cnrYX | 151 ± 9 | 0 | 155 ± 19 | 0 | |
| cnrYH | 1,010 ± 68 | 0 | 1,050 ± 176 | 0 | |
| cnrXH | 1,226 ± 254 | 0 | 1,190 ± 176 | 0 | |
| cnrY1(Con) cnrXH | 1,180 ± 55 | 0 | 1,450 ± 123 | 0 | |
| nccYXH | 53.0 ± 6.3 | 0 | 59.1 ± 5.3 | 0 | |
Ni2+ (0.5 or 0 mM) was added to cells of various Ralstonia strains which were growing in the early exponential phase (0 h). The β-galactosidase activity was determined for at least 3 h and plotted against time.
Means ± standard deviations. Values are units per milligram (dry weight). If no increase occurred, the mean values of all data points of the respective experiment are given.
Values are units per hour per milligram (dry weight).
The lacZ gene is under control of a cnr promoter on a pVDZ′2 plasmid complementing in trans.
Carries an insertion of pLO2 flanked by the duplicated region at bp 2100 to 2400. This separates cnrYXH from cnrCBA (Fig. 1).
FIG. 5.
Induction of β-galactosidase activity with cnr promoters upstream of lacZ. Cells of Ralstonia sp. strain AE126(pMOL28) with plasmid pDNA300 Φ(cnrYp-lacZ) (● and ○), pDNA299 Φ(cnrCp-lacZ) (■ and □), or pDNA298 Φ(cnrHp-lacZ) (38) (▴ and ▵) were diluted 15-fold to a cell density of 30 Klett units into fresh medium containing no added heavy metal (○, □, and ▵) or with 0.5 mM Ni2+ (●, ■, and ▴) added after 4 h. Incubation was continued with shaking at 30°C, and the specific β-galactosidase activity was measured. d.w., dry weight.
Induction of a Φ(cnrCBA-lacZ) operon fusion by heavy metal cations.
To study induction of cnr in a fully nickel-resistant bacterial cell, the lacZ gene was inserted immediately downstream of cnrCBA on plasmid pMOL28, leading to lacZ reporter strain DN177(pMOL28-2). The metal resistance of this strain was identical to the resistance of AE126(pMOL28) as shown by determination of the MICs of Ni2+ and Co2+ on agar plates (Table 3) and in liquid medium (data not shown).
TABLE 3.
MICs of nickel and cobalt in various derivatives of Ralstonia sp. strain AE126(pMOL28)
| Bacterial strain | Relevant genotype | MIC (mM)a
|
|
|---|---|---|---|
| Ni2+ | Co2+ | ||
| AE126(pMOL28) | Wild type | 4.0 | 5.0 |
| AE104 | Plasmid-free control | 0.3 | 0.3 |
| DN177(pMOL28-2) | Φ(cnrCBA-lacZ) | 4.0 | 5.0 |
| DN177(pMOL28-2)b | Φ(cnrCBA-lacZ) | 10 | |
| DN177(pMOL28-2, pDNA291) | Φ(cnrCBA-lacZ), cnrYXH in trans | 5.0 | 3.0 |
| DN190(pMOL28-3) | ΔcnrYXH Φ(cnrCBA-lacZ) | 0.4 | 0.3 |
| DN190(pMOL28-3, pDNA291) | ΔcnrYXH, cnrYXH in trans | 4.0 | 5.0 |
| DN195(pMOL28-4) | cnrY1(Con) | 7.0 | 10 |
| DN195(pMOL28-4, pDNA291) | cnrY1(Con), cnrYXH in trans | 7.0 | 10 |
The MIC is defined as the minimal concentration of heavy metal cation inhibiting growth at 30°C for 3 days. Occurrence of single colonies was not counted as growth. Each determination was repeated twice with identical results.
Cultivated in the presence of cobalt plus 1 mM Ni2+ for induction.
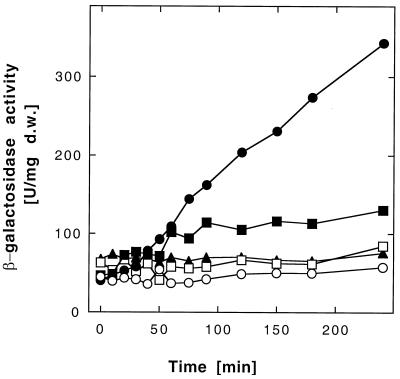
Nickel (0.5 mM Ni2+) was the best inducer of β-galactosidase activity in DN177(pMOL28-2). Co2+ (0.5 mM), Zn2+ (0.3 mM), and chromate (0.1 mM) induced only slightly (Fig. 6); due to the toxic effects on AE126 strains, zinc and chromate concentrations lower than the nickel concentration had to be used for induction. The increase in β-galactosidase activity after nickel induction was linear for at least 4 h. At up to 0.5 mM Ni2+, the slope of this increase was a function of the nickel concentration (data not shown). The increase in β-galactosidase activity could be described using saturation kinetics in a Lineweaver-Burk plot (data not shown). The regression coefficient was 1.00, the maximum increase in β-galactosidase activity after nickel induction was 94.8 U/h · mg (dry weight), and the nickel concentration required for half-maximal induction was 49 μM. Induction of Φ(cnr-lacZ) expression with 2 mM Ni2+, however, was significantly lower than induction with all of the other Ni2+ concentrations used, probably due to the toxic effect of a nickel concentration close to the MIC.
FIG. 6.
Induction of β-galactosidase activity in a cnrCBA-lacZ strain. Cells of Ralstonia sp. strain DN177(pMOL28-2) containing a Φ(cnrCBA-lacZ) operon fusion on plasmid pMOL28-2 were diluted 15-fold to a cell density of 30 Klett units into fresh medium containing no added heavy metal (○) or were induced after 4 h with 0.5 mM Ni2+ (●), 0.5 mM Co2+ (■), 0.3 mM Zn2+ (□), or 0.1 mM potassium chromate (▴). Incubation was continued with shaking at 30°C, and the β-galactosidase activity was determined. d.w., dry weight.
The chrH′C′ region (bp 2100 to 2400 [Fig. 1]) was cloned into plasmid pLO2 (14), leading to plasmid pECD581. Integration of this plasmid into plasmid pMOL28-2 led to segregation of the cnrCBA operon from the cnrYXH operon on the resulting plasmid, pMOL28-5 (Fig. 1). The β-galactosidase level before induction was slightly higher in DN410(pMOL28-5) (103 U/mg [dry weight]) than in the control DN177(pMOL28-2) (64 U/mg [dry weight]), probably due to some initiation of transcription from unknown promoters located on plasmid pLO2, e.g., that of the kanamycin resistance gene. After induction with 0.5 mM Ni2+, the increase in the β-galactosidase activity of Φ(cnrCBA-lacZ) in pMOL28-5 was 90.7 U/h · mg (dry weight), which was similar to the control value with plasmid pMOL28-2 (86.4 U/h · mg [dry weight] [Table 2]). Thus, the activity of cnrCp is sufficient to explain the observed induction of Φ(cnrCBA-lacZ) by nickel.
Deletion of cnrYXH almost abolishes cnr induction by nickel.
To study the influence of cnrXYH on cnr induction, these three genes were deleted from pMOL28-2, leading to strain DN190(pMOL28-3) Φ(cnrCBA-lacZ) ΔcnrYXH (Fig. 1). Both promoters, cnrYp and cnrCp, were still present on plasmid pMOL28-3. Strain DN190(pMOL28-3) showed a drastic reduction in nickel resistance when compared to strain DN177(pMOL28-2) and to strain AE126(pMOL28); however, DN190(pMOL28-3) was slightly more nickel resistant than the plasmid-free strain AE104 (Table 3).
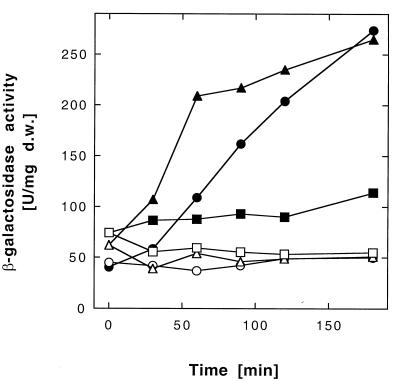
There was some slight induction of β-galactosidase activity in the ΔcnrYXH deletion strain DN190(pMOL28-3) (Fig. 7) by 0.5 mM Ni2+, a level similar to the induction level reached by DN177(pMOL28-2) after induction with low concentrations of Co2+ (Fig. 6). To test if cobalt resistance encoded by cnr is limited by insufficient induction of cnrCBA, strain DN177(pMOL28-2) was incubated in the presence of Co2+ and inducing concentrations of Ni2+ (Table 3), and indeed, the MIC of cobalt increased when Ni2+ induced Cnr. The MICs of Zn2+ and Cd2+, however, did not change with Ni2+ induction (data not shown).
FIG. 7.
Induction of β-galactosidase activity in a cnrCBA-lacZ ΔcnrYXH strain. Cells of Ralstonia sp. strain DN177(pMOL28-2) containing a Φ(cnrCBA-lacZ) operon fusion on plasmid pMOL28-2 (● and ○), of strain DN190(pMOL28-3) with an additional deletion of the cnrYXH regulatory genes (■ and □), and of strain DN190(pMOL28-3, pDNA291) with cnrYXH supplied in trans to this deletion (▴ and ▵) were diluted 15-fold to a cell density of 30 Klett units into fresh medium containing no added heavy metal (○, □, and ▵) or were induced after 4 h with 0.5 mM Ni2+ (●, ■, and ▴). Incubation was continued with shaking at 30°C, and the β-galactosidase activity was measured.
To demonstrate in trans complementation of the cnrYXH deletion, cnrYXH were PCR cloned into plasmid pVDZ′2 (6), leading to plasmid pDNA291. Strain DN190(pMOL28-3, pDNA291) was fully resistant to Ni2+ (Table 3), and nickel was again able to induce the Φ(cnrCBA-lacZ) fusion in this strain (Fig. 7); however, the time-dependent increase in β-galactosidase activity was no longer linear. Thus, a strain carrying a deletion of cnrYXH had lost the nickel-specific induction of Cnr, and this mutation could be complemented in trans.
A variety of DN190(pMOL28-3) derivatives were constructed which contained all combinations of cnrY, cnrX, and cnrH in trans on the pVDZ′2 vector (6) (Table 2). In these strains, any complementing plasmid harboring cnrH (cnrH alone or the combination cnrXH or cnrYH) led to expression of β-galactosidase activity at a high constitutive level. Complementation with cnrYH yielded the highest levels of cnrCBA-lacZ expression observed in all experiments. All strains without cnrH (cnrX alone, cnrY, or cnrYX) were not inducible and remained at a constitutive low level of β-galactosidase expression. Thus, CnrH alone is able to activate cnr expression, and both CnrY and CnrX are needed for nickel control of CnrH.
Constitutive expression of cnr.
To learn more about the interaction of the Cnr regulators, a mutant of strain AE126(pMOL28) which expressed Cnr constitutively was isolated. This strain, DN176(pMOL28-1), was isolated by a published procedure as a AE126 derivative able to grow in the presence of 1 mM Zn2+ (4), leading to DN176(pMOL28-1) cnrY1(Con). Into pMOL28-1, lacZ was inserted downstream of cnrCBA, leading to DN195(pMOL28-4) cnrY1(Con) Φ(cnrCBA-lacZ). Strain DN195(pMOL28-4) was resistant to a higher level of Ni2+ than DN177(pMOL28-2) and reached the same level of Co2+ resistance as nickel-induced cells of DN177(pMOL28-2) (Table 3).
The Φ(cnrCBA-lacZ) operon was expressed at a high constitutive level in DN195(pMOL28-4) (Table 2). Again, nickel induced Φ(cnrCBA-lacZ) when cnrYXH was supplied in trans in strain DN195(pMOL28-4, pDNA191) (Table 2). However, this strain did not loose its higher metal resistance (Table 3). The cnrY1(Con) cnrXH region, PCR cloned from plasmid pMOL28-4, led to constitutive expression of β-galactosidase when located in trans to ΔcnrYXH in strain DN190(pMOL28-3) (Table 2). Thus, the mutation leading to the constitutive phenotype must be located in one of the three regulator genes. When strain DN195(pMOL28-4) was complemented with single regulator genes or combinations thereof, cnrX, cnrH, or cnrXH did not restore nickel regulation of cnr, but cnrY did (Table 2). However, for a full complementation, cnrH and cnrX had to be present; cnrYX repressed induction of cnr but did not yield inducibility, while cnrYH did not complement at all.
DNA sequence analysis (data not shown) indicated an insertion of the sequence CGCGACGCGTCGCGCGC at position 1111 of the cnr sequence (15). Moreover, the wild-type sequence as published (15) has to be corrected. The published sequence is 1108-CGCCGCCGC-1117, but here the sequence was determined to be only CGCCGC. The sequence of the insertion in the cnrY1(Con) mutant gene contains a nearly complete duplication of the sequence CGCGACGCGTgGCGtGC (nonidentical base pairs are shown in lowercase) located directly downstream of the 14-bp insertion. Although the site of this putative target duplication is different from that of the insertion of IS1087 reported in the accompanying study (38), this duplication is a strong hint that an insertion sequence element was also responsible for the cnrY1(Con) mutation in strain DN195(pMOL28-4). The mutation leads to a frameshift resulting in the expression of a 134-amino-acid (aa) mutant protein which is identical in its amino-terminal 46 aa to CnrY but continues in another reading frame thereafter (15, 38). In contrast to CnrY, the mutant protein does not contain a possible transmembrane α-helix.
The carboxy-terminal parts of CnrY and CnrX are located in the periplasm.
With phoA fusions of the genes cnrY and cnrX, specific activities of 32.4 ± 3.3 U/mg (dry weight) for cnrY and of 57.6 ± 2.2 U/mg (dry weight) for cnrX were determined. As a control, the leader sequence of the β-lactamase gene of plasmid pUC19 (40) was cloned upstream of the phoA gene in plasmid pECD500 (32; T. Pribyl and D. H. Nies, unpublished data), which leads to a specific PhoA activity of 41.6 ± 7.6 U/mg (dry weight). When the leader sequence of the β-lactamase gene was deleted (Pribyl and Nies, unpublished data), the specific activity decreased to 1.4 ± 1.3 U/mg (dry weight) (all data are triplicate determinations done twice independently and are not shown). Thus, since the complete genes were cloned into plasmid pECD500, the carboxy termini of both proteins are located in the periplasm.
Interaction between NccYXH and CnrYXH.
To study a possible interaction between cnr and the highly related nickel-cobalt-cadmium resistance determinant ncc (34), the regulatory regions nccYXH and nccN were PCR cloned from A. xylosoxidans 31A into pVDZ′2 (6). In the ΔcnrYXH strain DN190(pMOL28-3), expression of nccYXH in trans did not mediate nickel-inducible expression of cnr (Table 2). Thus, NccYXH did not activate cnr promoters. NccN did not have any influence on nickel resistance in DN177(pMOL28-2) (data not shown).
Surprisingly, when nccYXH were expressed in trans in DN177(pMOL28-2), almost no induction of cnr by nickel was observed (Table 2). The same down-regulation was even obtained with the constitutive mutant strain DN195(pMOL28-4). Thus, the Ncc regulators are able to prevent induction of Cnr by its own regulators.
DISCUSSION
The cnr and the chr resistance determinants on megaplasmid pMOL28 of Ralstonia sp. strain CH34 might be required for survival against combined nickel, cobalt, and chromate toxicities, e.g., in serpentine-like soils (1; S. Juhnke, N. Peitzsch, and D. H. Nies, unpublished data). The cnr determinant is composed of at least six genes, encoding products with regulatory functions (cnrY, cnrX, and cnrH) or the subunits of the Co2+/Ni2+ efflux pump (cnrC, cnrB, and cnrA). Downstream of cnrA (Fig. 1) starts an open reading frame (orf104ff) which is not complete in the published DNA sequence (15). The predicted 104-aa product shows homology to MTH841, a 343-aa transporter-like protein from Methanobacterium thermoautotrophicum with unknown function (36). It is not clear if orf104ff, which was not disturbed by the insertion of lacZ downstream of cnrA in plasmid pMOL28-2, is involved in nickel resistance. It was not essential in the initial cloning experiments (15), but transcription from cnrCBA may continue into this open reading frame (Fig. 1).
Translation of cnrYXH and translation of cnrCBA seem to be closely coupled. The stop codons of the respective upstream genes overlap with the start codons of the following genes: ATGATGA for cnrYX, GTGA for cnrXH, ATGATGA for cnrCB, and ATGA for cnrBA. A comparable tight translational coupling has been shown to be important for regulation of other ECF sigma factor-controlled operons, e.g., the car operon from Myxococcus xanthus (10). This fact, the positions of the two identified promoters cnrYp and cnrCp, and the RT-PCR experiments indicate two possible tricistronic mRNAs as transcripts of cnrYXH and cnrCBA.
Deletion and complementation results indicate that CnrY, CnrX, and CnrH are essential as well as sufficient for cnr regulation, which is based on regulation of transcription. High-level constitutive expression of cnr was observed when CnrH was present in Ralstonia cells but CnrY and CnrX were not. Thus, nickel-dependent regulation of cnr depends on the presence of CnrY and CnrX. These data fit the fact that CnrH is a sigma factor (16) of the ECF family. In many examples from gram-negative and gram-positive bacteria (5, 10, 12, 18, 21), ECF sigma factors control operons encoding products which deal with environmental stimuli. Most of these ECF sigma factors are regulated by membrane-bound anti-sigma factors. If a stress condition is sensed by this anti-sigma factor, which might interact with a sensing protein, the sigma factor is released and is free to initiate transcription.
The PhoA data clearly demonstrate that the carboxy termini of CnrX and CnrY are in the periplasm. CnrH is indeed a sigma factor as shown by gel retardation assays (38) and runoff transcription (G. Grass, S. Kühnemund, and D. H. Nies, unpublished data). Thus, it is possible that CnrH is controlled by a CnrYX transmembrane anti-sigma factor complex which binds CnrH in the absence of Ni2+. If Ni2+ appears in the periplasm, it may be bound by CnrX; the signal then would be transmitted by CnrY into the cytoplasm and CnrH would be released. Since periplasmic nickel is probably the inducer of Cnr, this explains why induction of Cnr, as judged by the increase in β-galactosidase activity, continues for at least 4 h, although the CnrCBA complex, which detoxifies the cytoplasm, should have been synthesized in the meantime.
The MIC for the cnr-free derivatives of Ralstonia sp. is 300 μM Ni2+. Thus, with half-maximum induction of cnr at about 50 μM, cnr is strongly expressed at toxic Ni2+ concentrations. On the other hand, Ni2+ is an essential trace element for Ralstonia, at least required for synthesis of hydrogenases (2, 20) at a concentration of about 10 nM. At this concentration, synthesis of CnrCBA-LacZ is slower than dilution of these proteins by cell doubling. Thus, regulation of Cnr guarantees a sufficient supply of Ni2+ as a trace element and at the same time efficient detoxification at higher concentrations.
The Ncc system mediates resistance to Ni2+, Co2+, and Cd2+ and is composed of seven proteins. Three are the subunits of the efflux pump, NccCBA, and three are the regulators NccY, NccX, and NccH. NccN is related to CzcN; however, no function has been assigned to these proteins yet (11, 34). Homology between the Cnr and the Ncc proteins indicates that ncc is also regulated by an NccYX sensory complex and the ECF sigma factor NccH. Upstream of nccY and nccC, sequences with strong similarity to the consensus sequences of the CnrH-dependent promoters (Fig. 4B) were found. The common consensus motifs for both systems are CGAGGGGGAG (−35) and CCGTAT (−10). The −35 motifs lack an AAC motif, which was proposed to be a consensus motif for all EFC sigma factors (19). In other ECF sigma factor-dependent promoters (Fig. 4, MtP2), however, AAC is also lacking.
Despite the similarity between the two systems, the Ncc regulators were not able to complement a ΔcnrYXH mutant. On the contrary, NccYXH repressed induction of Cnr by Ni2+, even in the constitutive mutant. One possible explanation could be complete sequestration of NccH and, if present, CnrH by a putative NccYX complex and no release of a sigma factor in the presence of Ni2+. However, a better explanation of the tight repression observed is that NccH-RNA polymerase holoenzyme might form tight closed complexes at the cnr promoters but is not able to convert into the open complex for transcription initiation. This explanation fits the differences between the conserved motifs of the cnr and the ncc promoters; however, further studies are required to solve this question.
The assumption of the existence of the two Cnr promoters cnrYp and cnrCp is based on (i) the homology between the cnrY-nccY and the cnrC-nccC upstream regions (Fig. 4), (ii) primer extension data (Fig. 4), (iii) induction of Φ(cnrYp-lacZ) and Φ(cnrCp-lacZ) constructs by nickel (Fig. 5), and (iv) induction of Φ(cnrCBA-lacZ) by nickel under conditions when cnrYXH and Φ(cnrCBA-lacZ) were separated by insertion of plasmid pECD581 (Table 2). We found only evidence for the absence of the proposed promoter cnrHp (38); there were no results in the primer extension experiments (Fig. 3), no induction of Φ(cnrHp-lacZ) by nickel (Table 2), and no similarity between the upstream regions of cnrH and nccH with respect to an ECF promoter motif. The assumption of cnrHp is based on binding of the CnrH-containing RNA polymerase holoenzyme to a DNA sequence upstream of cnrH and on the loss of induction in a strain carrying a mutation in the cnrHp promoter region (38). However, mutation of the cnrHp promoter region may have changed the activity or expression level of CnrX, which would also explain the observed loss of induction. Binding of the CnrH-RNA polymerase to a region upstream of cnrH may be another element of Cnr regulation; e.g., it may be required to prevent too-high expression levels of CnrH.
Since the Φ(cnrCBA-lacZ) fusion located on plasmid pMOL28-5 can still be induced by nickel but cnrYXH and cnrCBA are separated by the insertion of plasmid pECD581 into plasmid pMOL28-5, cnrHp has no influence on the expression of Φ(cnrCBA-lacZ). Moreover, the reporter system used in this study is an insertion of lacZ directly downstream of cnrA. Thus, expression of cnrCBA was studied in a fully metal-resistant bacterial strain and with a cnrCBA copy number identical to the copy number in the wild-type situation. In contrast, the accompanying study has used the luciferase reporter system situated on vector plasmids which probably were present in higher copy numbers. Although both studies agree on the existence of promoter cnrYp, no induction of Φ(cnrYp-lux) was observed (38). Thus, the fact that no induction was measured with Φ(cnrHp-lux) and Φ(cnrCp-lux) is no evidence against the existence of cnrHp or cnrCp.
Despite these differences, both studies outline the following model of Cnr regulation. A periplasmic protein complex composed of CnrX and the carboxy-terminal part of CnrY senses nickel. This information is probably transmitted by the transmembrane protein CnrY to the ECF sigma factor CnrH, perhaps by release of CnrH from the amino-terminal part of CnrY when nickel is bound to CnrX. CnrH binds to RNA polymerase core, and transcription is initiated from the cnrYp promoter and at least a second promoter which is located upstream or downstream of cnrH.
ACKNOWLEDGMENTS
We thank Grit Schleuder and Ute Lindenstrauß for skillful technical assistance. Niels van der Lelie is acknowledged for fruitful and cooperative discussion.
This work was supported by Forschungsmittel des Landes Sachsen-Anhalt and by Fonds der Chemischen Industrie.
Footnotes
This publication is dedicated to Hans G. Schlegel, who started the cnr work, on his 75th birthday.
REFERENCES
- 1.Baker A J M. Metal tolerance in plants. New Phytol. 1987;106:93–111. [Google Scholar]
- 2.Bartha R, Ordal E J. Nickel-dependent chemolithotrophic growth of two Hydrogenomonas strains. J Bacteriol. 1965;89:1015–1019. doi: 10.1128/jb.89.4.1015-1019.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brim H, Heyndrickx M, de Vos P, Wilmotte A, Springael D, Schlegel H G, Mergeay M. Amplified rDNA restriction analysis and further genotypic characterisation of metal-resistant soil bacteria and related facultative hydrogenotrophs. Syst Appl Microbiol. 1999;22:258–268. doi: 10.1016/S0723-2020(99)80073-3. [DOI] [PubMed] [Google Scholar]
- 4.Collard J-M, Provoost A, Taghavi S, Mergeay M. A new type of Alcaligenes eutrophus CH34 zinc resistance generated by mutations affecting regulation of the cnr cobalt-nickel resistance system. J Bacteriol. 1993;175:779–784. doi: 10.1128/jb.175.3.779-784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Las Penas A, Connolly L, Gross C A. The sigmaE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of sigmaE. Mol Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 6.Deretic V, Chandrasekharappa S, Gill J F, Chatterjee D K, Chakrabarty A. A set of cassettes and improved vectors for genetic and biochemical characterization of Pseudomonas genes. Gene. 1987;57:61–72. doi: 10.1016/0378-1119(87)90177-6. [DOI] [PubMed] [Google Scholar]
- 7.Dressler C, Kües U, Nies D H, Friedrich B. Determinants encoding multiple metal resistance in newly isolated copper-resistant bacteria. Appl Environ Microbiol. 1991;57:3079–3085. doi: 10.1128/aem.57.11.3079-3085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes N D, Wu Q-L, KOng D, Puyang X, Garg S, Husson R N. A mycobacterial extracytoplasmic sigma factor involved in survival following heat shock and oxidative stress. J Bacteriol. 1999;181:4266–4274. doi: 10.1128/jb.181.14.4266-4274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg M, Pribyl T, Juhnke S, Nies D H. Energetics and topology of CzcA, a cation/proton antiporter of the RND protein family. J Biol Chem. 1999;274:26065–26070. doi: 10.1074/jbc.274.37.26065. [DOI] [PubMed] [Google Scholar]
- 10.Gorham H C, McGowan S J, Robson P R H, Hodgson D A. Light-induced carotenogenesis in Myxococcus xanthus: light-dependent membrane sequestration of EFC sigma factor CarQ by anti-sigma factor CarR. Mol Microbiol. 1996;19:171–186. doi: 10.1046/j.1365-2958.1996.360888.x. [DOI] [PubMed] [Google Scholar]
- 11.Große C, Grass G, Anton A, Franke S, Navarrete Santos A, Lawley B, Brown N L, Nies D H. Transcriptional organization of the czc heavy metal homoeostasis determinant from Alcaligenes eutrophus. J Bacteriol. 1999;181:2385–2393. doi: 10.1128/jb.181.8.2385-2393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Gaballa A, Cao M, Helmann J D. Identification of target promoters for the Bacillus subtilis extracytoplasmic function ς factor, ςW. Mol Microbiol. 1999;31:361–371. doi: 10.1046/j.1365-2958.1999.01180.x. [DOI] [PubMed] [Google Scholar]
- 13.Kunito T, Kusano T, Oyaizu H, Senoo K, Kanazawa S, Matsumoto S. Cloning and sequence analysis of czc genes in Alcaligenes sp. strain CT14. Biosci Biotechnol Biochem. 1996;60:699–704. doi: 10.1271/bbb.60.699. [DOI] [PubMed] [Google Scholar]
- 14.Lenz O, Schwartz E, Dernedde J, Eitinger T, Friedrich B. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J Bacteriol. 1994;176:4385–4393. doi: 10.1128/jb.176.14.4385-4393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liesegang H, Lemke K, Siddiqui R A, Schlegel H-G. Characterization of the inducible nickel and cobalt resistance determinant cnr from pMOL28 of Alcaligenes eutrophus CH34. J Bacteriol. 1993;175:767–778. doi: 10.1128/jb.175.3.767-778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigF gene reveals the existence of a subfamily of eubacterial RNA polymerase ς factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoil C. Analysis of protein localization by use of gene fusions with complementary properties. J Bacteriol. 1990;172:1035–1042. doi: 10.1128/jb.172.2.1035-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin D W, Schurr M J, Deretic V. Analysis of promoters controlled by the putative sigma factor AlgU regulating conversion to mucoidy in Pseudomonas aeruginosa: relationship to ςE and stress response. J Bacteriol. 1994;176:6688–6696. doi: 10.1128/jb.176.21.6688-6696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinéz-Argudo I, Ruiz-Vázquez R M, Murillo F J. The structure of an ECF-ς-dependent, light-inducible promoter from the bacterium Myxococcus xanthus. Mol Microbiol. 1998;30:883–893. doi: 10.1046/j.1365-2958.1998.01129.x. [DOI] [PubMed] [Google Scholar]
- 20.Mergeay M, Nies D, Schlegel H G, Gerits J, Charles P, van Gijsegem F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bacteriol. 1985;162:328–334. doi: 10.1128/jb.162.1.328-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Missiakas D, Raina S. The extracytoplasmic function sigma factors: role and regulation. Mol Microbiol. 1998;28:1059–1066. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 22.Nies A, Nies D H, Silver S. Cloning and expression of plasmid genes encoding resistances to chromate and cobalt in Alcaligenes eutrophus. J Bacteriol. 1989;171:5065–5070. doi: 10.1128/jb.171.9.5065-5070.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nies A, Nies D H, Silver S. Nucleotide sequence and expression of a plasmid-encoded chromate resistance determinant from Alcaligenes eutrophus. J Biol Chem. 1990;265:5648–5653. [PubMed] [Google Scholar]
- 24.Nies D, Mergeay M, Friedrich B, Schlegel H G. Cloning of plasmid genes encoding resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus CH34. J Bacteriol. 1987;169:4865–4868. doi: 10.1128/jb.169.10.4865-4868.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nies D H. The cobalt, zinc, and cadmium efflux system CzcABC from Alcaligenes eutrophus functions as a cation-proton antiporter in Escherichia coli. J Bacteriol. 1995;177:2707–2712. doi: 10.1128/jb.177.10.2707-2712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nies D H. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J Bacteriol. 1992;174:8102–8110. doi: 10.1128/jb.174.24.8102-8110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nies D H, Koch S, Wachi S, Peitzsch N, Saier M H J. CHR, a novel family of prokaryotic proton motive force-driven transporters probably containing chromate/sulfate transporters. J Bacteriol. 1998;180:5799–5802. doi: 10.1128/jb.180.21.5799-5802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nies D H, Nies A, Chu L, Silver S. Expression and nucleotide sequence of a plasmid-determined divalent cation efflux system from Alcaligenes eutrophus. Proc Natl Acad Sci USA. 1989;86:7351–7355. doi: 10.1073/pnas.86.19.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nies D H, Silver S. Plasmid-determined inducible efflux is responsible for resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus. J Bacteriol. 1989;171:896–900. doi: 10.1128/jb.171.2.896-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oelmüller U, Krüger N, Steinbüchel A, Friedrich C G. Isolation of prokaryotic RNA and detection of specific mRNA with biotinylated probes. J Microbiol Methods. 1990;11:73–84. [Google Scholar]
- 31.Peitzsch N, Eberz G, Nies D H. Alcaligenes eutrophus as a bacterial chromate sensor. Appl Environ Microbiol. 1998;64:453–458. doi: 10.1128/aem.64.2.453-458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rensing C, Pribyl T, Nies D H. New functions for the three subunits of the CzcCBA cation-proton antiporter. J Bacteriol. 1997;179:6871–6879. doi: 10.1128/jb.179.22.6871-6879.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 34.Schmidt T, Schlegel H G. Combined nickel-cobalt-cadmium resistance encoded by the ncc locus of Alcaligenes xylosoxidans 31A. J Bacteriol. 1994;176:7045–7054. doi: 10.1128/jb.176.22.7045-7054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 36.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, Reeve J N, et al. Complete genome sequence of Methanobacterium thermoautotrophicum. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taghavi S, Mergeay M, van der Lelie D. Genetic and physical map of the Alcaligenes eutrophus CH34 megaplasmid pMOL28 and it derivative pMOL50 obtained after temperature induced mutagenesis and mortality. Plasmid. 1997;37:22–34. doi: 10.1006/plas.1996.1274. [DOI] [PubMed] [Google Scholar]
- 38.Tibazarwa C, Wuertz S, Mergeay M, Wyns L, van der Lelie D. Regulation of the cnr cobalt and nickel resistance determinant of Ralstonia eutropha (Alcaligenes eutrophus) CH34. J Bacteriol. 2000;182:1399–1409. doi: 10.1128/jb.182.5.1399-1409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voss H, Wirkner U, Jakobi R, Hewitt N A, Schwager C, Zimmermann J, Ansorge W, Pyerin W. Structure of the gene encoding human casein kinase II subunit β. J Biol Chem. 1991;266:13706–13711. [PubMed] [Google Scholar]
- 40.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]