Abstract
Aims
We aimed to examine the hypothesis that circulating trimethylamine-N-oxide (TMAO) levels serve as a biomarker in pulmonary arterial hypertension (PAH), and to determine whether 3,3-dimethyl-1-butanol (DMB), a TMAO inhibitor, exerted a protective effect in monocrotaline (MCT)-induced PAH rats.
Methods and results
In-patients with PAH were prospectively recruited from the Fuwai Hospital. Fasting blood samples were obtained to assess the TMAO levels and other laboratory values during the initial and second hospitalization. In a MCT-induced PAH rat, a normal diet and water supplemented with or without 1% DMB were administered for 4 weeks. The TMAO levels, haemodynamic examinations, changes in organ-tissue, and molecular levels were evaluated. In total, 124 patients with PAH were enrolled in this study. High TMAO levels were correlated with increased disease severity and poor prognosis even after adjusting for confounders. The TMAO levels in the rats decreased in the MCT + DMB group, accompanied by improved haemodynamic parameters, decreased right ventricular hypertrophy, and amelioration of pulmonary vascular remodelling. The decrease in abnormal apoptosis, excessive cell proliferation, transforming growth factor-β expression, and restoration of endothelial nitric oxide synthase after DMB treatment further explained the amelioration of PAH.
Conclusion
Increased TMAO levels were associated with poor prognosis in patients with PAH, and DMB played a protective effect in MCT-induced PAH rat.
Keywords: Pulmonary arterial hypertension, Gut microbiota, TMAO, Metabolite, Prognosis, Pulmonary vascular remodelling
Graphical Abstract
Graphical Abstract.
TMAO’s role in pulmonary arterial hypertension.
Translational perspective
Elevated circulating levels of trimethylamine-N-oxide (TMAO) are associated with poor prognosis in patients with pulmonary arterial hypertension (PAH). 3,3-Dimethyl-1-butanol (DMB), a TMAO inhibitor, decreases circulating TMAO levels and attenuates the effects of the disease at the system, organ-tissue, and molecular levels in rats with monocrotaline-induced PAH, suggesting the protective role of DMB against PAH. Here, we first demonstrate the potential value of circulating TMAO as a biomarker and reveal the beneficial effects of DMB in PAH. Our findings, if further confirmed, will provide significant insights into the management and treatment strategy of PAH and can help potentially improve the prognosis of patients in the future.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive and frequently fatal disease that leads to progressive pulmonary vascular resistance, right heart failure, and even death. Risk stratification for PAH is used to guide patient management and therapeutic strategies.1,2 A comprehensive assessment is required since no single variable provides sufficient diagnostic and prognostic information. However, these assessments require experienced medical personnel, and are expensive and time-consuming. It has been widely recognized that the detection of measurable and reproducible biomarkers contributes to the improved prognostic evaluation of patients with PAH. However, brain natriuretic peptide (BNP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) remain the only plasma biomarkers currently used.2
Trimethylamine-N-oxide (TMAO) is an intestinal microbiota-dependent metabolite. Some commensal microbes in the gut metabolize phosphatidylcholine, choline, and l-carnitine into trimethylamine (TMA) using enzymes such as Cut C/D. This then enters the portal circulation and is converted into TMAO by hepatic flavin monooxygenases in the liver.3 3,3-Dimethyl-1-butanol (DMB) acts as an analogue of choline and reduces the plasma TMAO level by inhibiting the activity of microbial choline TMA lyase Cut C to restrain the TMA synthesis. Our previous studies showed that TMAO can promote brain ageing and cognitive impairment in mice and may accelerate endothelial cell senescence and vascular ageing via oxidative stress.4,5 Moreover, accumulating evidence shows the positive associations between the elevated levels of TMAO and cardiovascular diseases, including hypertension,6,7 atherosclerosis,8 and heart failure9,10 highlighting its promising potential role as a biomarker in this field.
A recent study by Kim et al.11 revealed a unique gut microbiome profile in patients with PAH by shotgun metagenomics. The study focused on the alteration of the microbiome, suggesting a significant increase in TMA/TMAO producing taxa including Collinsella, Desulfovibrio, Enterobacter, and Clostridium, while the reverse is true in those negatively associated with TMA/TMAO production such as Bvulgatus, Bcellulosilyticus, and Amuciniphila. However, the changes in plasma TMAO levels accompanied by microbiome alteration and the association with disease severity and prognosis in patients with PAH have not been explored yet. Based on this knowledge, the present study aimed to examine the hypothesis that TMAO served as a biomarker for PAH. In addition, we also investigate whether DMB, a TMAO inhibitor, exerted a protective effect in MCT-induced PAH rats.
Methods
Study population
This clinical study investigated the value of TMAO in patients with PAH. The study implementation, data analyses, and re-analyses have been approved by the ethics committee of Fuwai Hospital (Approval No. 2018-1063). The procedures performed adhered to the tenets of the Declaration of Helsinki and written informed consent was obtained from all patients.
In-patients with PAH in Fuwai Hospital Pulmonary Vascular Ward were recruited from March 2019 to March 2020. The diagnosis of PAH was confirmed based on a mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg, pulmonary arterial wedge pressure (PAWP) ≤ 15 mmHg, and pulmonary vascular resistance (PVR) > 3 Wood units by right heart catheterization. The following comprehensive evaluations were performed to determine the pathogenesis and functional severity of the patients' condition: electrocardiography, chest radiography, pulmonary function tests, arterial blood gas analyses, echocardiography, ventilation/perfusion lung scans, high-resolution computed tomography, contrast-enhanced computed tomography, right heart catheterization, and other additional tests. The exclusion criteria were as follows: (i) connective tissue disease-related PAH and/or immune disease; (ii) coronary heart disease, active infection, malignancy, heart failure, diabetes, and/or chronic kidney disease; (iii) intestinal disease; (iv) individuals who have received antibiotics, immunologic drugs, and/or bowel surgery; and (v) disagreement to participate or loss to follow-up.
Clinical data collection
Fasting blood samples were obtained to assess TMAO levels and other laboratory values during the initial and second hospitalizations. Haemodynamic evaluations were performed via right heart catheterization to obtain the mean right atrial pressure (mRAP), mPAP, PAWP, PVR, and cardiac output index. A symptom-limited cardiopulmonary exercise test was conducted using a cycle ergometer (10 W/min increments) to measure peak oxygen consumption and other indicators of exercise capacity. The test was considered maximal if the peak respiratory exchange ratio was >1.1. Demographic characteristics, body mass index (BMI, calculated as weight/height2), World Health Organization functional class (WHO-FC), 6-min walk distance (6-MWD), and echocardiographic data were also collected.
Study endpoint and follow-up
Plasma TMAO levels were measured during the initial hospitalization and subsequent hospital visit within 18.3 months. The endpoints of this study were composite outcome events defined as mortality, re-hospitalization due to heart failure, escalation of the targeted medication including the addition of at least one more targeted drug according to the disease condition and/or starting treating with treprostinil, deterioration of PAH including worsening symptoms, higher WHO-FC compared with the baseline, or at least 15% decreased 6-MWD from the baseline.12
When exploring the prognostic value of the baseline TMAO levels, the follow-up duration was defined as the time from the first TMAO examination to the occurrence of outcomes or end of the study. The mean duration was 18.1 (12.8, 23.2) months. When investigating the implications in the changes in TMAO, the follow-up duration was calculated from the second TMAO examination to the occurrence of outcome or the end of the follow-up. The mean duration was 12.1 (8.1, 16.6) months.
Experimental animals
We also designed an animal study to explore the effects of low TMAO on PAH. DMB, a TMAO inhibitor, is a structural analogue of choline, exerting a non-lethal inhibitory effect on TMA formation resulting in a decreased concentration of TMAO.13 It has been recognized as a drug that decreases TMAO levels which was used regularly to explore the effects of low TMAO in diseases.14–17 Therefore, in this pre-clinical study, DMB was used for the exploration of the low TMAO effect in vivo.
Seven-week-old Sprague–Dawley rats weighing 230–250 g (Charles River, Beijing) were used in this study. The rats were housed in a specific pathogen-free environment and maintained on a standard rat diet, under a 12-h light/dark cycle in a room with a constant temperature (22–24°C) and humidity (45–55%) in the State Key Laboratory of Cardiovascular Disease (Beijing, China). After a 1-week adaptation period, the animals were randomly divided into three groups: (i) a control group (n = 8) that received a standard rat diet and water for 4 weeks; (ii) a monocrotaline (MCT) group (n = 8) that received a subcutaneous injection of 60 mg/kg of MCT18 (Sigma-Aldrich, St Louis, MO, USA) and a standard rat diet and water for 4 weeks; and (iii) a MCT + DMB group (n = 6) that was administered with MCT and subsequently received a standard rat diet and water containing 1% DMB13,16 (Sigma-Aldrich, St Louis, MO, USA) for 4 weeks. All animal procedures were approved by the Institutional Animal Care and Use Committee of Fuwai Hospital.
Haemodynamic measurements and sample collection
All rats were anesthetized and underwent right heart catheterization for haemodynamic examination. A pre-curved catheter was inserted through the right jugular vein to obtain mRAP, right ventricular systolic pressure (RVSP), and mPAP. The haemodynamic data were recorded and analysed using the PowerLab data acquisition system. After haemodynamic examination, blood samples were collected for TMAO detection. The hearts and lungs were flushed with saline to remove residual blood. The right ventricle (RV) was dissected and weighed to calculate the index of RV hypertrophy (RVH), which was calculated as {RV/[left ventricle (LV) plus the septum]}. The left lung tissues were fixed in 4% paraformaldehyde and paraffin-embedded for histological analysis, while right lung tissues were frozen in liquid nitrogen for further molecular study, including Western blot.
Morphological assay
Paraffin-embedded lung tissues were cut into 5-μm thick sections. The slices were stained with haematoxylin and eosin (H&E) and Elastic-Van Gieson (EVG) to examine the lung histology. Peripheral vessels 50–150 µm in diameter were chosen and examined for morphological analysis. The wall thickness percentage (WT%) and wall area percentage (WA%) of 10–15 arterioles per rat were measured to evaluate the pulmonary vascular remodelling, expressed as WT% = [(external diameter − internal diameter)/external diameter] × 100 and WA% = [(total area − luminal area)/total area] × 100. The pathological nature of the pulmonary arterioles was evaluated by a senior pathologist, and pulmonary vascular remodelling calculations were implemented independently by two researchers (Y.Y. and Q.Z.).
Immunohistochemical and immunofluorescent staining
The paraffin-embedded lung slices were deparaffinized using xylene, ethanol, and demineralized water. Heat-mediated antigen retrieval was performed using 1 mM EDTA (pH 9.0) before commencing with the immunohistochemical (IHC) and immunofluorescent (IF) staining protocol. Subsequently, 3% hydrogen peroxide was used for 20 min to eliminate peroxidase. After the sections were washed using phosphate-buffered saline, suitable serum albumin was used to incubate the lung slices for 1 h at room temperature to block non-specific interactions.
Slices were incubated with primary antibodies against cleaved caspase 3 (CST, 9661S, 1:800), caspase 3 (CST, 9662S, 1:10000), proliferating cell nuclear antigen (PCNA, CST, 13110, 1:10000), endothelial nitric oxide synthase (eNOS, Abcam, ab5589, 1:100), and transforming growth factor-β (TGF-β, Abcam, ab215715, 1:1000) overnight at 4°C and incubated with EnVision Mouse or Rabbit conjugate (Dako/Agilent) for 30 min at 37°C. The colour reaction was induced using a 3,3-diaminobenzidine (DAB)-positive substrate in accordance with the manufacturer's instructions. Haematoxylin was used as a counterstain. At 400× magnification (Olympus BX61), the pulmonary arterioles were observed, and ImageJ 1.46 was used to quantify the integrated density and positive area of each pulmonary arteriole. The average optical density (AOD) was calculated as the integrated option density/area. The ratio of positive cell count per vessel was also quantified. A minimum of 10 pulmonary arterioles were selected from each image and used for calculation.19
The slices were incubated with primary antibodies against CD31 (Abcam, 281583, 1:3000), alpha-smooth muscle antibody (α-SMA, Beyotime, AA132, 1:2000), and cleaved caspase 3/caspase 3/PCNA. Suitable secondary antibodies were used for incubation, and the nuclei were counterstained in blue with 4,6-diamidino-2-phenylindole (DAPI, Molecular Probes).
Western blot analysis
Radioimmunoprecipitation lysis buffer was used for lung protein extraction and the bicinchoninic acid method for the determination of protein concentration. The protein was denatured at 95°C for 5 min, separated using the sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to the polyvinylidene difluoride membranes. The blots were incubated with primary antibodies against cleaved caspase3, caspase 3, eNOS, PCNA, and β-actin (Proteintech, 66009-1, 1:300000) at 4°C overnight after being blocked with 5% milk. A suitable secondary antibody was used for 1 h incubation after the blots were washed thrice. The blots were visualized using chemiluminescence (ThermoFisher, 2450). ImageJ 1.46 was used to quantify the density of the bands.
Enzyme-linked immunosorbent assay
The rat plasma levels of TGF-β were determined using an enzyme-linked immunosorbent assay (ELISA) kit (MSKBIO, China) according to the manufacturer’s instructions.
Quantification of trimethylamine-N-oxide
As demonstrated previously, the TMAO levels were quantified using stable isotope dilution liquid chromatography–tandem mass spectrometry (LC/MS/MS). The specific measurement procedures have been described in previous works.4,20
Statistical analysis
A restricted cubic spline was used to determine the non-linear or linear association between TMAO and the clinical endpoints. The differences between the groups were analysed using Student’s t-tests, paired Student’s t-tests, or one-way analyses of variance followed by Tukey’s multiple comparison tests for continuous variables and χ2 tests for categorical variables. The correlations were assessed using Pearson’s or Spearman’s correlation (two-tailed) analyses. Logistic and linear regression models were used to determine the correlations between TMAO and clinical markers of disease severity. Kaplan–Meier (KM) analysis and Cox proportional hazards regression were used to evaluate the hazard ratios (HRs) and 95% confidence intervals (CIs) per 1-SD increase in TMAO. The multivariate Cox model was validated by bootstrapping with 500 repetitions. Statistical significance was defined as a two-sided P-value of <0.05. Images were obtained using a Leica laser confocal microscope (TCS SP2 AOBS). The statistical analyses were performed using R 2.8.0 (Vienna, Austria) and IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA).
Results
Population characteristics at baseline
The study enrolled 124 patients with PAH, including 40 idiopathic/heritable PAH cases, 82 PAH associated with congenital heart disease cases, and 2 with pulmonary veno-occlusive disease cases. After the follow-up, 102 patients survived without clinical outcomes, 9 patients were hospitalized again due to the progression of PAH, 8 were hospitalized for the escalation of the targeted medication, 3 for heart failure, and 2 patients died.
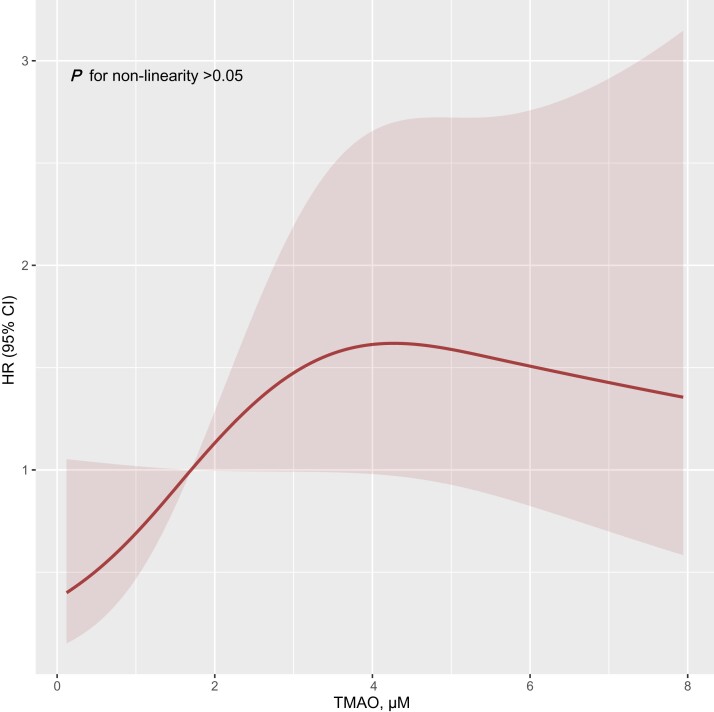
The patients were divided into two cohorts stratified by the 50th percentile of TMAO levels (Table 1). Restricted cubic spline analyses revealed the linear effect of TMAO on clinical outcomes (non-linear, P > 0.050. Figure 1). The mean levels of TMAO in the total cohort, high TMAO, and low TMAO groups were 1.7 (interquartile range: 0.9–3.5) μM, 3.3 (interquartile range: 2.3–5.3) μM, and 0.9 (interquartile range: 0.4–1.2) μM, respectively. The patients with high plasma TMAO levels had more severe WHO-FC, higher NT-proBNP, lower tricuspid annular plane systolic excursion, and cardiac output index than those with low plasma TMAO levels (see Supplementary material online, Figure S1A–D).
Table 1.
Characteristics of patients stratified by the 50th percentile of trimethylamine-N-oxide
| Variables | Total PAH patients n = 124 |
High TMAO group n = 61 |
Low TMAO group n = 63 |
P-value |
|---|---|---|---|---|
| Age, years | 34.0 ± 13.1 | 35.5 ± 14.0 | 32.5 ± 12.0 | 0.212 |
| Female sex, n (%) | 89 (71.8) | 43 (68.3) | 46 (75.4) | 0.428 |
| BMI, kg/m2 | 20.9 ± 3.8 | 21.3 ± 4.3 | 20.5 ± 3.1 | 0.257 |
| WHO-FC, n (%) | ||||
| I–II | 86 (69.4) | 31 (49.2) | 55 (90.2) | <0.001 |
| III–IV | 38 (30.6) | 32 (50.8) | 6 (9.8) | <0.001 |
| Laboratories | ||||
| TMAO, µmol/L | 1.7 (0.9, 3.5) | 3.3 (2.3, 5.3) | 0.9 (0.4, 1.2) | <0.001 |
| NT-proBNP, pg/mL | 439.3 (150.7, 1302.0) | 700.7 (269.1, 2426.0) | 297.2 (111.0, 574.5) | 0.001 |
| Albumin, g | 43.1 ± 4.6 | 43.0 ± 4.4 | 43.3 ± 4.9 | 0.714 |
| Creatinine, µmol/L | 74.2 ± 17.5 | 77.4 ± 17.7 | 70.8 ± 16.7 | 0.034 |
| Total cholesterol, mmol/L | 4.1 ± 1.0 | 4.0 ± 1.0 | 4.1 ± 1.0 | 0.601 |
| Serum iron, µmol/L | 15.5 ± 8.1 | 14.8 ± 8.7 | 16.2 ± 7.4 | 0.373 |
| Echocardiography | ||||
| LVEF, % | 65.3 ± 6.2 | 64.8 ± 6.5 | 65.8 ± 5.9 | 0.367 |
| RVD, mm | 32.3 ± 7.1 | 33.6 ± 7.1 | 31.3 ± 7.3 | 0.078 |
| TAPSE, mm | 16.0 ± 3.9 | 15.0 ± 3.8 | 17.1 ± 3.7 | 0.003 |
| Exercise capacity | ||||
| PeakVO2, mL/min/kg | 14.6 ± 3.7 | 14.4 ± 3.6 | 14.8 ± 3.9 | 0.553 |
| VO2% | 1.6 ± 0.4 | 1.6 ± 0.3 | 1.6 ± 0.4 | 0.900 |
| VCO2% | 1.5 ± 0.4 | 1.5 ± 0.3 | 1.5 ± 0.4 | 0.983 |
| 6-MWD, m | 425.6 ± 88.8 | 426.2 ± 92.6 | 425.1 ± 86.0 | 0.949 |
| Haemodynamics | ||||
| mRAP, mmHg | 5.8 ± 4.3 | 6.5 ± 4.8 | 4.9 ± 3.0 | 0.056 |
| mPAP, mmHg | 66.1 ± 16.8 | 67.4 ± 15.6 | 64.9 ± 17.9 | 0.476 |
| Cardiac output index, L/min×m2 | 3.2 ± 1.0 | 3.0 ± 1.0 | 3.4 ± 0.9 | 0.046 |
| PAWP, mmHg | 8.3 ± 5.8 | 9.0 ± 7.4 | 7.5 ± 2.8 | 0.318 |
| PVR, WU | 12.9 ± 6.3 | 12.7 ± 5.9 | 13.0 ± 6.9 | 0.865 |
| Targeted drugs | ||||
| Monotherapy (ERAs or prostacyclins or NO pathway drug), n (%) | 23 (18.5) | 13 (20.6) | 10 (16.4) | 0.646 |
| Dual therapy, n (%) | 86 (69.4) | 43 (68.3) | 43 (70.5) | 0.847 |
| Triple therapy, n (%) | 7 (5.6) | 4 (6.3) | 3 (4.9) | 0.731 |
The χ2 test was conducted for categorical variables and Student’s t-tests or non-parametric tests were conducted for continuous variables with normal or skewed distribution, respectively. Bolded P-values in the table indicate statistical significance.
BMI, body mass index; WHO-FC, World Health Organization function class; NT-proBNP, N-terminal pro-brain natriuretic peptide; LVEF, left ventricular ejection fraction; RVD, right ventricular diameter; TAPSE, tricuspid annular plane systolic excursion; 6-MWD, 6-minute walk distance; mRAP, mean right atrial pressure; mPAP, mean pulmonary atrial pressure; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; ERAs, endothelin receptor agonists; NO, nitric oxide.
Figure 1.
Restricted cubic spline result of trimethylamine-N-oxide levels in relation to hazard ratio for the risk of clinical outcomes (n = 124). HR, hazard ratio; TMAO, trimethylamine-N-oxide.
Correlation of high plasma trimethylamine-N-oxide with worse World Health Organization function class, higher N-terminal pro-brain natriuretic peptide, and lower cardiac output index (vs. low plasma trimethylamine-N-oxide)
Supplementary material online, Table S1 shows the correlation between TMAO and the clinical characteristics. Following adjustments for confounders including age, sex, BMI, creatinine, and hypertension, elevated TMAO was still related to worse WHO-FC [Model 1: odds ratio (OR) = 1.395, P = 0.002; Model 2: OR = 1.545, P = 0.004; Model 3: OR = 2.072, P < 0.001], higher NT-proBNP (Model 1: OR = 1.248, P = 0.036; Model 2: OR = 1.531, P = 0.016), and lower cardiac output index (β = −0.272, P = 0.029) (Table 2).
Table 2.
The associations between trimethylamine-N-oxide levels and World Health Organization function class, N-terminal pro-brain natriuretic peptide, tricuspid annular plane systolic excursion, and cardiac output index after adjusting for confounders
| Logistics analysis | OR | 95% CI | P-value |
|---|---|---|---|
| Model 1: WHO-FC | |||
| Unadjusted | 1.380 | 1.145–1.663 | 0.001 |
| Adjusteda | 1.395 | 1.133–1.719 | 0.002 |
| Adjustedb | 1.545 | 1.139–2.094 | 0.004 |
| Adjustedc | 2.072 | 1.389–3.090 | <0.001 |
| Model 2: NT-proBNP (categorical variable) | |||
| Unadjusted | 1.242 | 1.030–1.496 | 0.023 |
| Adjusteda | 1.248 | 1.014–1.535 | 0.036 |
| Adjustedc | 1.531 | 1.082–2.168 | 0.016 |
| Model 3: TAPSE (categorical variable) | |||
| Unadjusted | 1.147 | 0.986–1.334 | 0.075 |
| Adjusteda | 1.171 | 0.989–1.387 | 0.066 |
| Linear regression | Beta | 95% CI | P-value |
| Model 4: Cardiac output index (continuous variable) | |||
| Unadjusted | −0.322 | −0.155 to 0.035 | 0.002 |
| Adjusteda | −0.272 | −0.153 to 0.008 | 0.029 |
TMAO was put into the model as a continuous variable. Bolded P-values in the table indicate statistical significance.
BMI, body mass index; WHO-FC, World Health Organization function class; NT-proBNP, N-terminal pro-brain natriuretic peptide; TAPSE, tricuspid annular plane systolic excursion.
Adjusted for sex, age, BMI, creatinine (continuous variable), and hypertension (categorical variable).
Adjusted for sex, age, BMI, NT-proBNP (categorical variable), creatinine (continuous variable), and hypertension (categorical variable).
Adjusted for sex, age, BMI, creatinine (continuous variable), cardiac output index (continuous variable), and TAPSE (categorical variable).
Response of plasma trimethylamine-N-oxide levels to clinical non-deterioration or deterioration
During the follow-up, all patients underwent treatment that included targeted drugs and supportive therapy, in accordance with the recent guidelines.1 After a mean follow-up period of 5.5 months, fasting blood samples were collected again to assess the TMAO levels and other clinical indicators in each patient. The patients were divided into clinical non-deterioration and deterioration groups during the second hospitalization to explore the effects of TMAO changes (ΔTMAO). Clinical deterioration was defined as a decrease of >20% in 6-MWD and/or an increase in WHO-FC.12,21
In this study, ΔTMAO, ΔNT-proBNP, and ΔmPAP were calculated as the value during the second admission visit minus the baseline. After treatment, the plasma TMAO levels decreased relative to the baseline in the full cohort [ΔTMAO = −0.25 (−1.5, 0.7) μM, P = 0.021] and clinical non-deterioration cohort [ΔTMAO = −0.39 (−1.78, 0.49) μM, P = 0.008]. However, the TMAO levels increased in the deterioration group [ΔTMAO = 0.69 (−0.26, 1.13) μM, P = 0.021, Supplementary material online, Figure S2]. Moreover, ΔTMAO was positively correlated with ΔNT-proBNP and ΔmPAP (Supplementary material online, Figure S3). The KM analysis indicated patients with ΔTMAO > 0 had a poor prognosis (P = 0.011, see Supplementary material online, Figure S4).
Prediction of poor prognosis in patients with pulmonary arterial hypertension based on high plasma trimethylamine-N-oxide
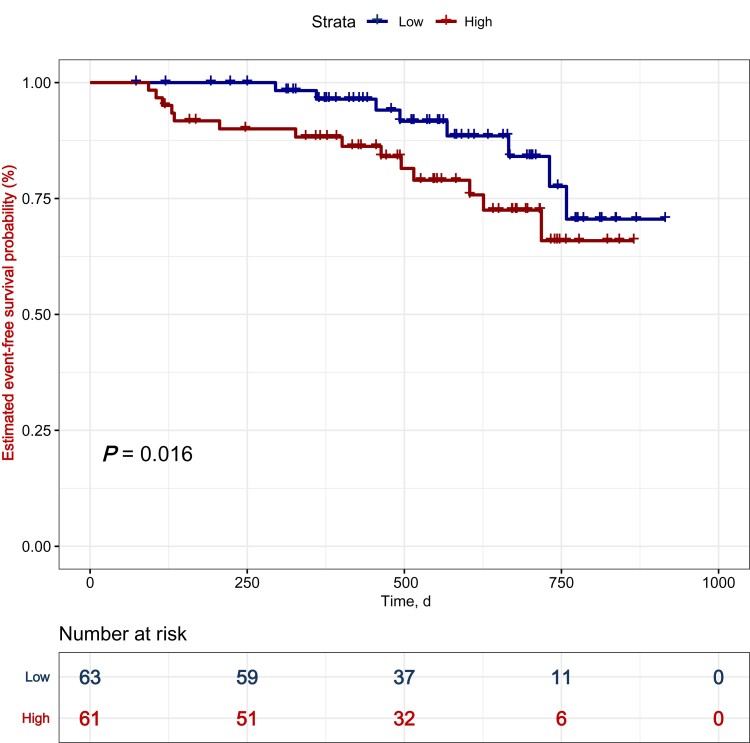
The proportional hazards assumption was examined using the Schoenfeld residual. No variables violated the proportional hazards assumption. KM analysis revealed that a higher TMAO level indicated a poor prognosis in patients with PAH (Figure 2, P = 0.016). The results of the univariate Cox regression analysis are shown in Supplementary material online, Table S2. Multivariate Cox analysis revealed that elevated TMAO was associated with adverse events among patients with PAH after adjusting for confounders (HR = 5.368, 95% CI: 1.096–26.287), P = 0.038, Table 3. Furthermore, TMAO had the highest predictive value (C statistic = 0.78, 95% CI: 0.61–0.96) with a C statistic over 0.7 after optimism correction (Supplementary material online, Table S3).
Figure 2.
Kaplan–Meier analysis for the incidence of composite outcome events in patients with high and low trimethylamine-N-oxide levels. A total of 124 patients were analysed (n = 61 in high trimethylamine-N-oxide group; n = 63 in low trimethylamine-N-oxide group). The P-value is calculated by the log-rank test. TMAO, trimethylamine-N-oxide.
Table 3.
Multivariate Cox analysis of trimethylamine-N-oxide and clinical outcome
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| TMAO (categorical variable) | 5.368 | 1.096–26.287 | 0.038 |
| BMI (continuous variable) | 0.861 | 0.704–1.054 | 0.148 |
| WHO-FC | 1.428 | 0.348–5.866 | 0.621 |
| LVEF (continuous variable) | 1.053 | 0.960–1.155 | 0.277 |
| mRAP (continuous variable) | 1.147 | 1.022–1.286 | 0.019 |
The continuous variable of TMAO is converted into a categorical variable with a boundary of 1.66 μM. Bolded P-values in the table indicate statistical significance.
TMAO, trimethylamine-N-oxide; BMI, body mass index; WHO-FC, World Health Organization function class; LVEF, left ventricular ejection fractions; mRAP, mean right atrial pressure.
Plasma trimethylamine-N-oxide levels in rats
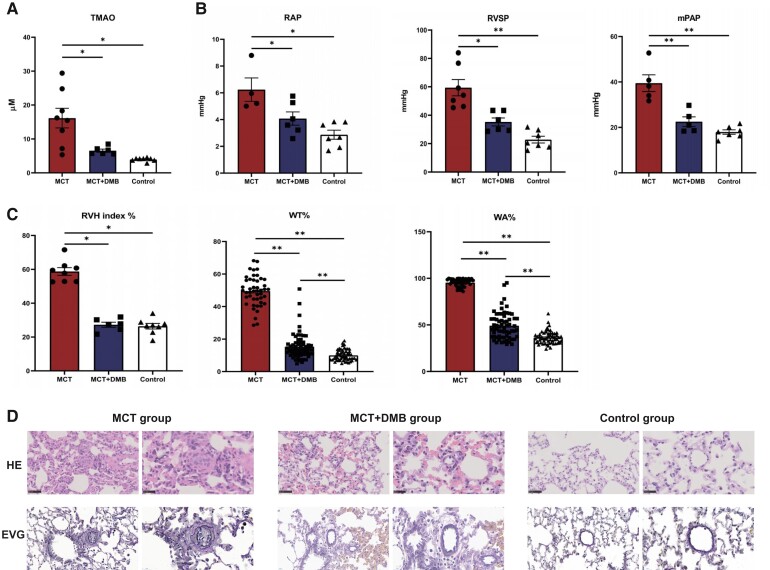
The plasma TMAO levels were significantly increased in the MCT group compared with that of the control group (13.6 ± 9.5 μM vs. 4.0 ± 0.5 μM, P = 0.003). After DMB treatment, the plasma TMAO levels markedly decreased in the PAH rats (6.6 ± 1.1 μM, P = 0.035) (Figure 3A).
Figure 3.
Plasma trimethylamine-N-oxide levels, haemodynamic parameters obtained by right heart catheterization, and pulmonary vascular morphology in rats among the different groups. (A) Effects of 3,3-dimethyl-1-butanol on trimethylamine-N-oxide levels (monocrotaline group: n = 8; monocrotaline + 3,3-dimethyl-1-butanol group: n = 6; control group: n = 8). After 3,3-dimethyl-1-butanol treatment, the level of plasma trimethylamine-N-oxide was decreased significantly in vivo. (B) Haemodynamic parameters obtained by right heart catheterization included mean right atrial pressure (monocrotaline group: n = 4; monocrotaline + 3,3-dimethyl-1-butanol group: n = 6; control group: n = 7), right ventricular systolic pressure (monocrotaline group: n = 7; monocrotaline + 3,3-dimethyl-1-butanol group: n = 6; control group: n = 7), and mean pulmonary atrial pressure (monocrotaline group: n = 5; monocrotaline + 3,3-dimethyl-1-butanol group: n = 5; control group: n = 7). After 3,3-dimethyl-1-butanol administration, mean right atrial pressure, right ventricular systolic pressure, and mean pulmonary atrial pressure were significantly decreased, indicating the amelioration of pulmonary arterial hypertension. (C) Right heart hypertrophy and quantitative analysis of pulmonary vascular remodelling in rats. The right ventricle hypertrophy index, performed as right ventricle/(left ventricle plus the septum), was used to evaluate the right heart load and reflect the severity of pulmonary arterial hypertension. The WT% and WA% of 10–15 arterioles per rat were measured to analyse the pulmonary vascular remodelling, expressed as WT% = [(external diameter − internal diameter)/external diameter] × 100 and WA% = [(total area − luminal area)/total area] × 100 (monocrotaline group: n = 8; monocrotaline + 3,3-dimethyl-1-butanol group: n = 6; control group: n = 8). In the monocrotaline + 3,3-dimethyl-1-butanol group, the right ventricle hypertrophy, WT%, and WA% were all decreased compared with the monocrotaline group. (D) Representative images of haematoxylin and eosin staining and Elastic-Van Gieson staining of the pulmonary vessels 50–150 µm in diameter in different groups, scale bars, 50 and 20 µm. After 3,3-dimethyl-1-butanol treatment, pulmonary vascular remodelling was ameliorated significantly (pointed by the black arrow). Continuous variables were represented as mean ± SEM. Group comparisons were performed using one-way analysis of variance. *P < 0.05, **P < 0.001. TMAO, trimethylamine-N-oxide; RHC, right heart catheterization; MCT, monocrotaline; DMB,3,3-dimethyl-1-butanol; mRAP, mean right atrial pressure; RVSP, right ventricle systolic pressure; mPAP, mean pulmonary atrial pressure; RVH, right ventricle hypertrophy; WT%, wall thickness percentage; WA%, wall area percentage.
Improvement in haemodynamic parameters after 3,3-dimethyl-1-butanol treatment in rats
The mRAP (4.1 ± 1.2 mmHg vs. 6.2 ± 1.8 mmHg, P = 0.017), RVSP (35.3 ± 6.9 mmHg vs. 53.7 ± 21.3 mmHg, P = 0.032), and mPAP (22.5 ± 4.8 mmHg vs. 39.5 ± 8.2 mmHg, P < 0.001) were significantly lower in the MCT + DMB group than that in the MCT group, suggesting that low plasma TMAO levels were associated with improved haemodynamics in MCT-induced PAH rat models (Figure 3B).
Amelioration of cardiac hypertrophy and pulmonary vascular remodelling after trimethylamine-N-oxide decrease in rats
The RVH index was used to explore the hypertrophy of the RV, which indirectly reflects the severity of PAH. In the MCT + DMB group, the RVH index was significantly reduced when compared with that in the MCT group (0.27 ± 0.04 0.59 ± 0.06, P < 0.001; Figure 3C). In addition, the rats treated with DMB exhibited decreased WT% and WA% values, indicating the amelioration of pulmonary vascular remodelling (Figure 3C). H&E and EVG staining demonstrated the muscularization and occlusion of the small pulmonary arteries in MCT group and the relative medial thickness of the pulmonary arteries decreased significantly after DMB treatment (Figure 3D).
Decreases in cleaved caspase 3, caspase 3, proliferating cell nuclear antigen, and transforming growth factor-β expression and restored endothelium nitric oxide synthase pathway after 3,3-dimethyl-1-butanol treatment in rats
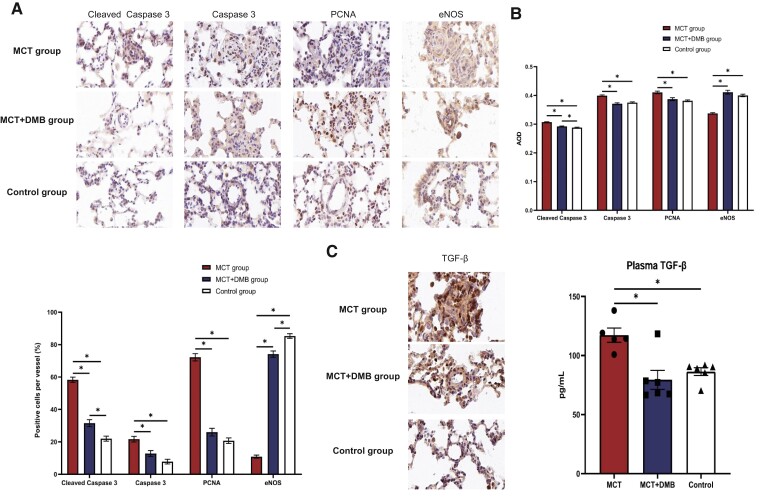
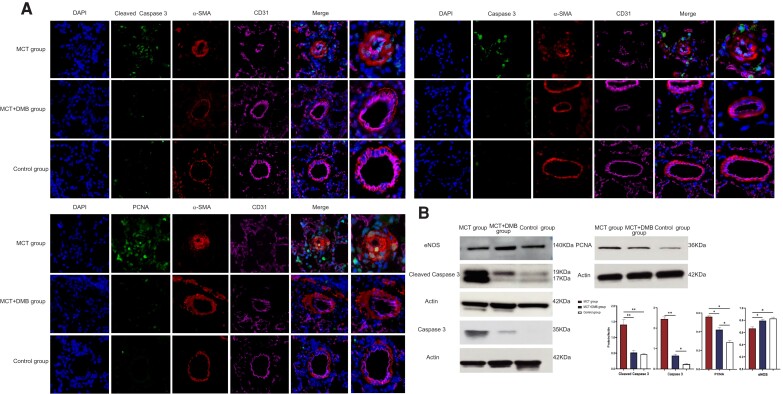
IHC staining revealed differences in the levels of molecular expression, including cleaved caspase 3, caspase 3, PCNA, eNOS, and TGF-β, among the three groups (Figure 4A). Compared with the control group, the MCT-induced PAH rats exhibited increased levels of cleaved caspase 3, caspase 3, and PCNA in the pulmonary arterioles, while these increases were attenuated after DMB treatment. In contrast, the eNOS expression in the pulmonary arterioles was reduced in the PAH model, and this change was reversed after DMB administration. The results of the AOD analysis and the proportion of positive cells per vessel for cleaved caspase 3, caspase 3, PCNA, and eNOS are shown in Figure 4B. Moreover, the expression of TGF-β in the lungs and plasma was elevated in the MCT-induced rats while the levels were decreased after DMB administration (Figure 4C). Triple IF staining with cell-specific marker (CD31 for the endothelial cells, α-SMA for the smooth muscle cells) was used to display cell-type-specific changes and targeted protein localization, which directly demonstrated the decrease in the pulmonary artery smooth muscle cells proliferation and amelioration of the endothelial cells in MCT + DMB group compared with the MCT group. The localization of cleaved caspase 3, caspase 3, and PCNA expressions in the pulmonary arterioles is shown in Figure 5A. Western blot of the lung homogenates was performed for quantitative analysis consistently indicating that the cleaved caspase 3, caspase 3, and PCNA levels were decreased, and eNOS was increased in the MCT + DMB group (Figure 5B).
Figure 4.
Expression of cleaved caspase 3, caspase 3, proliferating cell nuclear antigen, endothelium nitric oxide synthase, and transforming growth factor-β among the different groups shown by immunohistochemistry and/or serology detection. (A) Representative immunohistochemistry staining of cleaved caspase 3, caspase 3, proliferating cell nuclear antigen, and endothelium nitric oxide synthase in the pulmonary arterioles. Scale bars, 20 µm. Compared with the monocrotaline group, the expressions of cleaved caspase 3, caspase 3, and proliferating cell nuclear antigen were decreased while the endothelium nitric oxide synthase expression was restored after 3,3-dimethyl-1-butanol administration. (B) Quantitative analysis of protein expression by average optical density values and proportion of positive cells per vessel. The average optical density values, calculated as integrated option density/area, were used to quantify the integrated density and positive area of each pulmonary arteriole. Quantifying the positive cell count per vessel was also used to analyse the changes in protein expressions. (C) Changes in transforming growth factor-β level in rats. Immunohistochemistry showed that the expression of transforming growth factor-β was increased in monocrotaline-induced pulmonary arterial hypertension rats while it was reduced by 3,3-dimethyl-1-butanol involvement. A similar trend was also discovered in the plasma by ELISA. Continuous variables were represented as mean ± SEM. Group comparisons were made with one-way analysis of variance. * P < 0.05. PCNA, proliferating cell nuclear antigen; eNOS, endothelium nitric oxide synthase; TGF-β, transforming growth factor-β; IHC, immunohistochemistry; MCT, monocrotaline; DMT, 3,3-dimethyl-1-butanol; AOD, average optical density.
Figure 5.
The localization of cleaved caspase 3, caspase 3, and proliferating cell nuclear antigen expressions in the pulmonary arterioles by triple immunofluorescence staining and the quantitative results of the expression of cleaved caspase 3, caspase 3, proliferating cell nuclear antigen, and endothelium nitric oxide synthase among different groups. (A) Triple immunofluorescence staining with cell and targeted protein-specific markers. 4′,6-Diamidino-2-phenylindole and antibodies including CD31 and alpha-smooth muscle antibody were used for the demonstration of the nucleus, endothelial cells (pink) and smooth muscle cells (red) in the pulmonary vessels, respectively. The green colour represents targeted proteins such as cleaved caspase 3, caspase 3, and proliferating cell nuclear antigen. The result showed the proliferation of the smooth muscle cells in the monocrotaline group while it was reversed by 3,3-dimethyl-1-butanol. Cleaved caspase 3, caspase 3, and proliferating cell nuclear antigen were mainly expressed in the nucleus, and the dynamic changes were consistent with immunohistochemistry. (B) Western blot analysis of the lung homogenates showed that the expression of cleaved caspase 3, caspase 3, and proliferating cell nuclear antigen was reduced, and that of endothelium nitric oxide synthase was increased, in the monocrotaline + 3,3-dimethyl-1-butanol group when compared with that in the monocrotaline group. The optical density of targeted protein and β-actin were calculated by Image J and the optical density ratios (targeted protein/β-actin) were represented as mean ± SEM (n = 4 for each group). Group comparisons were made with one-way analysis of variance. *P < 0.05, **P < 0.001. PCNA, proliferating cell nuclear antigen; eNOS, endothelium nitric oxide synthase; α-SMA, alpha-smooth muscle antibody; MCT, monocrotaline; DMB, 3,3-dimethyl-1-butanol.
Discussion
PAH is a severe, progressive, and frequently fatal disease. Recent studies have demonstrated the association between the circulating gut microbiota-dependent metabolites and cardiovascular diseases. The role of TMAO as a biomarker in cardiovascular diseases related to the left heart has been established; however, its value in right heart-related diseases remains to be elucidated. Since the microbiome producing TMAO was dysfunctional in PAH, we conducted a study to assess the implications of this alteration, specifically to explore the role of plasma TMAO levels in PAH. To the best of our knowledge, this is the first clinical and translational study to investigate the effects of plasma TMAO in this field. Our findings revealed that elevated circulating levels of TMAO were associated with increaseddisease severity and poor prognosis in patients with PAH, highlighting its promising role as a novel biomarker in PAH. Moreover, the protective effect of DMB, a type of TMAO inhibitor, was also evaluated in the MCT-induced PAH model.
WHO-FC, NT-proBNP, and cardiac output index are regarded as essential indicators for clinical evaluationssince right-sided cardiac dysfunction is an important predictor of dismal prognosis in patients with PAH.2 After adjusting for confounders, individuals with a high TMAO level were still associated with poor cardiac function indicated by higher level of WHO-FC, increased NT-proBNP, and decreased cardiac output index as reported,9,10 and thereby worse disease severity in patients with PAH. We noticed no difference in the exercise capacity between the two groups in our study. This may be attributed to the fact that patients with high TMAO levels tend to exhibited worse severity. Compared with those in the low TMAO group, a larger proportion of patients in high TMAO group who were intolerant to the exercise testing may result in the loss of data. Furthermore, evidence shows that treprostinil, a prostaglandin analogue, could significantly improve the exercise capacity of patients.22,23 Intravenous Treprostinil, such as remodulin, was administered in patients with advanced PAH and was used more commonly in the high TMAO group, which may improve the exercise capacity of those who were tolerant of exercise evaluations, leading to the further narrowing of the gap between these groups. The seemingly tighter association between TMAO and RV function but not PVR and mPAP could be attributed to the higher sensitivity of indicators related to RV function, better reflecting the dynamic changes of the disease rather than haemodynamic parameters. The amelioration of RV dysfunction occurred generally prior to the improvement in haemodynamic parameters (PVR, mPAP), which was reflected by an overt association between TMAO and the indicators of RV function (WHO-FC, NT-proBNP, and cardiac output index). In addition, most of the subjects enrolled in this study were prevalent cases that have been undergoing targeted treatment, which probably resulted in no significant difference in mPAP and PVR at baseline. However, a trend towards poorer results with the increase of plasma TMAO levels was consistently present in PAH-related haemodynamic data, especially mRAP, albeit without reaching statistical significance and remains to be confirmed in a larger cohort study.
The results of our study revealed corresponding dynamic changes in the plasma TMAO levels with the alterations of disease conditions after further treatment, which suggested its promising role in PAH management. The decrease in TMAO levels was consistent with the reduction in NT-proBNP, indicating that the dynamic changes in the metabolite may be attributed to improved cardiac function. In addition, medical therapies for PAH induce systemic vasodilation, including those in the intestine, which may ameliorate hypoperfusion and congestion of the gut and contribute to decreased TMAO levels. However, the explicit mechanisms require further exploration.
High plasma concentrations of TMAO have been correlated with unfavourable cardiovascular outcomes, serving as an independent marker of all-cause mortality.24 Likewise, our findings suggested that elevated TMAO is an independent biomarker of poor prognosis in patients with PAH, which was confirmed by internal validation. Though well accepted in cardiovascular disease, the exact role of TMAO remains vaguely defined. However, the correlation reported between elevated TMAO and adverse clinical outcome held true regardless of its role as an active mediator or an innocent bystander in this study. Similar to our results, Brunt et al.16 reported that TMAO could damage the vascular endothelium by augmenting superoxide-driven oxidative stress, which subsequently prompted a reduction in NO bioavailability. In addition, TMAO may served as an inflammatory inducer that activated multiple inflammation-related pathways and facilitated the adhesion of monocytes to the endothelium, resulting in increased pro-inflammatory cytokines and endothelial dysfunction.25–27 Due to the extensive overlap in the pathogenetic effects between TMAO and other established causative factors in PAH, it is plausible to assume that TMAO may have a contributory role in the onset and development of PAH and exert an adverse effect on the prognosis. However, the plasma TMAO levels may be increased as a consequence of the activation of compensatory mechanism in response to the homeostatic disturbances induced by PAH. Progressive PAH disrupts haemodynamic homeostasis, causing splanchnic congestion and gut hypoperfusion. The subsequent microbiome dysbiosis in the gut and increased intestinal permeability to TMA, the precursor of TMAO, eventually lead to an elevation of the plasma TMAO level. Given these contradictory effects, further studies are required to determine the direction of causality of the association between TMAO and PAH. In addition, the prognostic value of TMAO precursors, including choline, betaine, and trimethyllysine,28,29 has also gained increasing attention, with its correlations to cardiovascular disease being elaborated to various extents, whose role in PAH need further investigation.
Moreover, we conducted animal experiments to further evaluate the effects of DMB, a TMAO inhibitor, in MCT-induced PAH models. In line with the report by Wang et al.13 that DMB could reduce the production of TMA and the subsequent TMAO by efficiently inhibiting distinct microbial TMA lyases, our study clearly demonstrated that application of DMB led to a reduction in the plasma level of TMAO along with a significant improvement in the PAH condition, reflected by improved haemodynamic parameters compared with the MCT group. The inconsistency in the effect of TMAO on PAH-related parameters between clinical and animal studies may mainly be due to the difference in the disease course. In contrast to the long disease duration with complicated situations in PAH patients, lesions in the pulmonary vasculature in the rodent model were newly induced by MCT injection and were more susceptible to interventions. Moreover, a markedly milder degree of cardiac hypertrophy and pulmonary vascular remodelling were also observed after DMB treatment, indicating an essential mitigation at the tissue level. It is worth noting that in addition to improved haemodynamics and reduced right heart preload, a decreased toxic effect from the lowering of TMAO concentrations may also contribute to the attenuation of cardiac hypertrophy in rats with DMB treatment.30
Abnormal apoptosis, excessive cell proliferation, and dysfunctional vasodilation are well-recognized pathogenesis of PAH. DMB might contribute to the restoration of the above dysfunctions, leading to the amelioration of PAH. Caspase-3 is an apoptosis-related protease that is up-regulated and cleaved to generate cleaved caspase-3 during the onset of PAH.31 The expression of PCNA increases with cell proliferation, and its overexpression results in extensively active proliferation of the smooth muscle cells in pulmonary arterioles, leading to the narrowing or occlusion of these arteries. We found arterial muscularization and media thickening in pulmonary arteries were followed by up-regulated caspase 3 and PCNA expression in the nucleus of smooth muscle cells of the PAH rats in the morphological study. The subsequently reduced expression of caspase 3 and PCNA after DMB treatment indicated suppressed abnormal cell apoptosis and proliferation, which was in accordance with the observed reversal of vascular muscularization at the tissue level in the MCT + DMB group. Abnormal overexpression of the TGF-β pathway in the lung tissue is a characteristic of MCT-induced PAH in the rodent model,32 which was reversed following the reduction of plasma TMAO via DMB treatment. The ensuing decrease in NO production following downregulated eNOS expression impairs the functional vascular reactivity and shifts the balance of the vascular tone towards vasoconstriction, resulting in increased vascular resistance in PAH.33 The restoration of eNOS expression after DMB treatment suggested the improvement of vasodilation, which might also contribute to PAH amelioration.
Most recently, Huang et al.34 explored the underlying molecular mechanisms of TMAO in PAH. After the exogenous addition of TMAO in drinking water, PAH mice exhibited a higher RVSP and greater pulmonary vascular remodelling. Further investigation revealed that TMAO increased the expression of pro-inflammatory factors in rat bone marrow derived macrophage. After adding the conditioned medium from macrophage treated with TMAO, pulmonary artery smooth muscle cells presented with both enhanced proliferation and migration abilities, characteristic of the pathogenesis in PAH in vivo. The study demonstrated that gut microbiota metabolite TMAO aggravated PAH probably via activating macrophage/monocyte to promote the release of chemokines and cytokines.
However, there are still many questions awaiting further investigations in this field. A major issue requiring clarification is the seemingly conflicting results regarding the effect of TMAO on cardiovascular disease. Videja et al.35 studied the role of increased TMAO in MCT-induced right heart failure in a rodent model, which reached an opposite conclusion stating the protective effect of elevated TMAO. Presumably, the discrepancy may arise from several aspects, such as differences in the concentration of TMAO exogenously added in the experimental settings. Whether the opponent effects of TMAO in PAH attributed to the different pathogenesis of disease remained to be further elucidated in future research. Another explanation may be the heterogeneous response of rats to MCT treatment. Different to the result of Videja et al.,35 the plasma TMAO level in the MCT group was significantly higher than that of the control 4 weeks after MCT injection in both our and Huang’s study, whereas no intergroup difference was observed in the former. To date, our appreciation of the effect that TMAO exerts on PAH has been only a tip of the iceberg. More collaborative research efforts are required to fully unmask the whole molecular landscape and address the conflicting evidence existing on this issue. Further investigations, including the specificity of DMB’s effect and its efficacy in the presence of various TMAO levels in MCT-induced PAH rats, are underway to further elucidate these issues.
Another limitation includes the single-centre design of the study that may limit the generalization of our findings. However, internal validation using the bootstrap method was conducted to test the stability and reliability of our model, which confirmed its superior performance. Moreover, more patients with PAH are recruited to validate our findings and to overcome the limitation of the small sample size. In addition, we failed to obtain the dietary history of the patients and could not rule out the impact of diet on TMAO levels in these patients.
Conclusions
Our clinical study demonstrated that a high level of plasma TMAO was associated with poor disease severity and prognosis in PAH, which indicated its promising role as a biomarker in this field. The pre-clinical study revealed that DMB enabled decreasing the TMAO level and reversing the progression of PAH by normalizing apoptosis, proliferation, and vasodilation pathways. Our findings indicate that collaborative research efforts are needed to develop innovative treatment strategies for PAH over the next decade.
Supplementary Material
Acknowledgements
We are very grateful to Professor Zeneng Wang (Department of Cellular & Molecular Medicine, Cleveland Clinic, Cleveland, OH 44195, USA) for his academic insights in our work.
Contributor Information
Yicheng Yang, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Qixian Zeng, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Jianing Gao, The Institute of Cardiovascular Sciences and Institute of Systems Biomedicine, School of Basic Medical Sciences, Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, NHC Key Laboratory of Cardiovascular Molecular Biology and Regulatory Peptides, Health Science Center, Peking University, Beijing, China.
Beilan Yang, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Jingjing Zhou, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Ke Li, China National Clinical Research Center for Neurological Diseases, Tiantan Hospital, Advanced Innovation Center for Human Brain Protection, The Capital Medical University, Beijing, China.
Li Li, Department of Pathology, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Anxin Wang, China National Clinical Research Center for Neurological Diseases, Tiantan Hospital, Advanced Innovation Center for Human Brain Protection, The Capital Medical University, Beijing, China.
Xin Li, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Zhihong Liu, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Qin Luo, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Zhihui Zhao, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Bingyang Liu, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Jing Xue, China National Clinical Research Center for Neurological Diseases, Tiantan Hospital, Advanced Innovation Center for Human Brain Protection, The Capital Medical University, Beijing, China.
Xue Jiang, China National Clinical Research Center for Neurological Diseases, Tiantan Hospital, Advanced Innovation Center for Human Brain Protection, The Capital Medical University, Beijing, China.
Matthew C Konerman, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Institute for Health Care Policy and Innovation, University of Michigan, Ann Arbor, MI, USA; VA Center for Clinical Management Research, Ann Arbor, MI, USA.
Lemin Zheng, The Institute of Cardiovascular Sciences and Institute of Systems Biomedicine, School of Basic Medical Sciences, Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, NHC Key Laboratory of Cardiovascular Molecular Biology and Regulatory Peptides, Health Science Center, Peking University, Beijing, China; China National Clinical Research Center for Neurological Diseases, Tiantan Hospital, Advanced Innovation Center for Human Brain Protection, The Capital Medical University, Beijing, China.
Changming Xiong, Center of Pulmonary Vascular Disease, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Lead author biography
 Yicheng Yang, MD, is a postgraduate student in Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. His clinical and research interests include metabolites in pulmonary hypertension and biomarkers for pulmonary hypertension and cardiovascular diseases.
Yicheng Yang, MD, is a postgraduate student in Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. His clinical and research interests include metabolites in pulmonary hypertension and biomarkers for pulmonary hypertension and cardiovascular diseases.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
This work was supported by the Natural Science Foundation of China (grant number no. 91639108, no. 81770272, and no. 81970425), the Capital Funds for Health Improvement and Research (grant number no. 2020-2-4035), and the CAMS Innovation Fund for Medical Sciences (CIFMS, 2021-I2M-C&T-A-009).
Conflict of interest
The authors declare that there is no conflict of interest.
Author contributions
Y.Y., Q.Z., J.G., and B.Y. were involved in the conception and wrote the article. Y.Y., Q.Z., J.G., X.L., and B.L. analysed the data. L.L. and J.Z. were responsible for pathological guidance. Y.Y., K.L., and A.W. prepared the figures and tables. L.Z., Q.L., Z.Z., X.J., J.X., L.Z., and C.X. checked and revised the manuscript. M.C.K. helped for research ideas and language polishing. L.Z. and C.X. provided funding support. All authors read and approved the manuscript.
References
- 1. Yang Y, Lin F, Xiao Z, Sun B, Wei Z, Liu B, Xue L, Xiong C. Investigational pharmacotherapy and immunotherapy of pulmonary arterial hypertension: an update. Biomed Pharmacother 2020;129:110355. [DOI] [PubMed] [Google Scholar]
- 2. Galiè N, Humbert M, Vachiery J, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez M, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard L, Trindade P, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 3. Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021;70:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ke Y, Li D, Zhao M, Liu C, Liu J, Zeng A, Shi X, Cheng S, Pan B, Zheng L, Hong H. Gut flora-dependent metabolite trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic Biol Med 2018;116:88–100. [DOI] [PubMed] [Google Scholar]
- 5. Li D, Ke Y, Zhan R, Liu C, Zhao M, Zeng A, Shi X, Ji L, Cheng S, Pan B, Zheng L, Hong H. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018;17:e12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang S, Shui Y, Cui Y, Tang C, Wang X, Qiu X, Hu W, Fei L, Li Y, Zhang S, Zhao L, Xu N, Dong F, Ren X, Liu R, Persson PB, Patzak A, Lai EY, Wei Q, Zheng Z. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol 2021;46:102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brunt VE, Casso AG, Gioscia-Ryan RA, Sapinsley ZJ, Ziemba BP, Clayton ZS, Bazzoni AE, VanDongen NS, Richey JJ, Hutton DA, Zigler MC, Neilson AP, Davy KP, Seals DR. Gut microbiome-derived metabolite trimethylamine n-oxide induces aortic stiffening and increases systolic blood pressure with aging in mice and humans. Hypertension 2021;78:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, Wang Z, Hazen SL, Tang WH. Plasma trimethylamine n-oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol 2016;67:2620–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjørndal B, Halvorsen B, Karlsen TH, Aukrust P, Gullestad L, Berge RK, Yndestad A. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 2015;277:717–726. [DOI] [PubMed] [Google Scholar]
- 10. Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 2014;64:1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim S, Rigatto K, Gazzana MB, Knorst MM, Richards EM, Pepine CJ, Raizada MK. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension 2020;75:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pulido T, Adzerikho I, Channick RN, Delcroix M, Galiè N, Ghofrani HA, Jansa P, Jing ZC, Le Brun FO, Mehta S, Mittelholzer CM, Perchenet L, Sastry BK, Sitbon O, Souza R, Torbicki A, Zeng X, Rubin LJ, Simonneau G. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013;369:809–818. [DOI] [PubMed] [Google Scholar]
- 13. Wang Z, Roberts A, Buffa J, Levison B, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley M, DiDonato A, Fu X, Hazen J, Krajcik D, DiDonato J, Lusis A, Hazen S. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu K, Yuan Y, Yu H, Dai X, Wang S, Sun Z, Wang F, Fei H, Lin Q, Jiang H, Chen T. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood 2020;136:501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Zeng Q, Xiong Z, Xian G, Liu Z, Zhan Q, Lai W, Ao L, Meng X, Ren H, Xu D. Trimethylamine-N-oxide induces osteogenic responses in human aortic valve interstitial cells in vitro and aggravates aortic valve lesions in mice. Cardiovasc Res 2021;cvab243. [DOI] [PubMed] [Google Scholar]
- 16. Brunt V, Gioscia-Ryan R, Casso A, VanDongen N, Ziemba B, Sapinsley Z, Richey J, Zigler M, Neilson A, Davy K, Seals D. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 2020;76:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, Barrington WT, Russell MW, Reed JM, Duzan A, Lang JM, Fu X, Li L, Myers AJ, Rachakonda S, DiDonato JA, Brown JM, Gogonea V, Lusis AJ, Garcia-Garcia JC, Hazen SL. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med 2018;24:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Savale L, Akagi S, Tu L, Cumont A, Thuillet R, Phan C, Le Vely B, Berrebeh N, Huertas A, Jaïs X, Cottin V, Chaouat A, Tromeur C, Boucly A, Jutant E, Mercier O, Fadel E, Montani D, Sitbon O, Humbert M, Tamura Y, Guignabert C. Serum and pulmonary uric acid in pulmonary arterial hypertension. Eur Respir J 2021;58:2000332. [DOI] [PubMed] [Google Scholar]
- 19. Zhou J, Li H, Li L, Li Y, Wang P, Meng X, He J. CYLD mediates human pulmonary artery smooth muscle cell dysfunction in congenital heart disease-associated pulmonary arterial hypertension. J Cell Physiol 2021;236:6297–6311. [DOI] [PubMed] [Google Scholar]
- 20. Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Romanov A, Cherniavskiy A, Novikova N, Edemskiy A, Ponomarev D, Shabanov V, Losik D, Elesin D, Stenin I, Mikheenko I, Zhizhov R, Kretov E, Pokushalov E, Po SS, Martynyuk TV, Steinberg JS. Pulmonary artery denervation for patients with residual pulmonary hypertension after pulmonary endarterectomy. J Am Coll Cardiol 2020;76:916–926. [DOI] [PubMed] [Google Scholar]
- 22. Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, Torbicki A, Xu KF, Yehle D, Laliberte K, Arneson C, Rubin LJ. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013;127:624–633. [DOI] [PubMed] [Google Scholar]
- 23. Hiremath J, Thanikachalam S, Parikh K, Shanmugasundaram S, Bangera S, Shapiro L, Pott GB, Vnencak-Jones CL, Arneson C, Wade M, White RJ. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo-controlled trial. J Heart Lung Transplant 2010;29:137–149. [DOI] [PubMed] [Google Scholar]
- 24. Stubbs J, House J, Ocque A, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore J, Nolin T, Spertus J, Yu A. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 2016;27:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abbasi J. TMAO and heart disease: the new red meat risk? JAMA 2019;321:2149–2151. [DOI] [PubMed] [Google Scholar]
- 26. Seldin M, Meng Y, Qi H, Zhu W, Wang Z, Hazen S, Lusis A, Shih D. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc 2016;5:e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma G, Pan B, Chen Y, Guo C, Zhao M, Zheng L, Chen B. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017;37:BSR20160244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 2014;35:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bjørnestad EØ, Dhar I, Svingen GFT, Pedersen ER, Svenningsson MM, Tell GS, Ueland PM, Ørn S, Sulo G, Laaksonen R, Nygård O, Carrero J-J. Trimethyllysine predicts all-cause and cardiovascular mortality in community-dwelling adults and patients with coronary heart disease. Eur Heart J 2021;1:oeab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, Ou C, Chen M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest 2019;99:346–357. [DOI] [PubMed] [Google Scholar]
- 31. White K, Dempsie Y, Caruso P, Wallace E, McDonald R, Stevens H, Hatley M, Van Rooij E, Morrell N, MacLean M, Baker A. Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension 2014;64:185–194. [DOI] [PubMed] [Google Scholar]
- 32. Sanada TJ, Sun XQ, Happé C, Guignabert C, Tu L, Schalij I, Bogaard HJ, Goumans MJ, Kurakula K. Altered TGFβ/SMAD signaling in human and rat models of pulmonary hypertension: an old target needs attention. Cells 2021;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaul P. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 2002;64:749–774. [DOI] [PubMed] [Google Scholar]
- 34. Huang Y, Lin F, Tang R, Bao C, Zhou Q, Ye K, Shen Y, Liu C, Hong C, Yang K, Tang H, Wang J, Lu W, Wang T. Gut microbial metabolite trimethylamine N-oxide aggravates pulmonary hypertension. Am J Respir Cell Mol Biol 2022. [DOI] [PubMed] [Google Scholar]
- 35. Videja M, Vilskersts R, Korzh S, Cirule H, Sevostjanovs E, Dambrova M, Makrecka-Kuka M. Microbiota-derived metabolite trimethylamine N-oxide protects mitochondrial energy metabolism and cardiac functionality in a rat model of right ventricle heart failure. Front Cell Dev Biol 2021;8:622741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study.