Abstract
Aims
A group of heart centres in the Netherlands have been at the forefront internationally to implement the principles of value-based healthcare. This study aims to give an up-to-date assessment of outcome-based quality improvement in 2020 at a national level in Dutch heart care.
Methods and results
Physicians and healthcare professionals for each participating hospital filled out a questionnaire with 26 detailed questions on quality improvement and organization of care. In total, 20 hospitals participated; 11 heart centres with thoracic surgery and 9 without thoracic surgery. Results show that outcome reports are actively used within the heart centres to support quality improvement initiatives. In 50% of the centres, apart from physicians, also nurses and hospital management are involved. For 60% of the heart centres, outcome measurement is embedded in strategy and annual plans. The stage of development of supporting IT infrastructure (outcome measurement in the Electronic Health Record and dashboards) is very diverse. A wide range of different learning strategies supports outcome-based quality improvement.
Conclusion
Health outcomes have become a relevant element in quality improvement and organization of Dutch heart centres. Earlier research shows that in 2012–2016 heart centres focused mainly on measuring outcomes. Now in 2020, heart centres are more able to actually use the acquired insights based on these measurements to initiate improvement projects. The diversity in how this is done indicates that this field is still strongly developing and shows potential for heart centres to share best practices in the implementation of value-based healthcare.
Keywords: Value-based healthcare, Quality improvement, Outcome assessment
Graphical Abstract
Graphical Abstract.
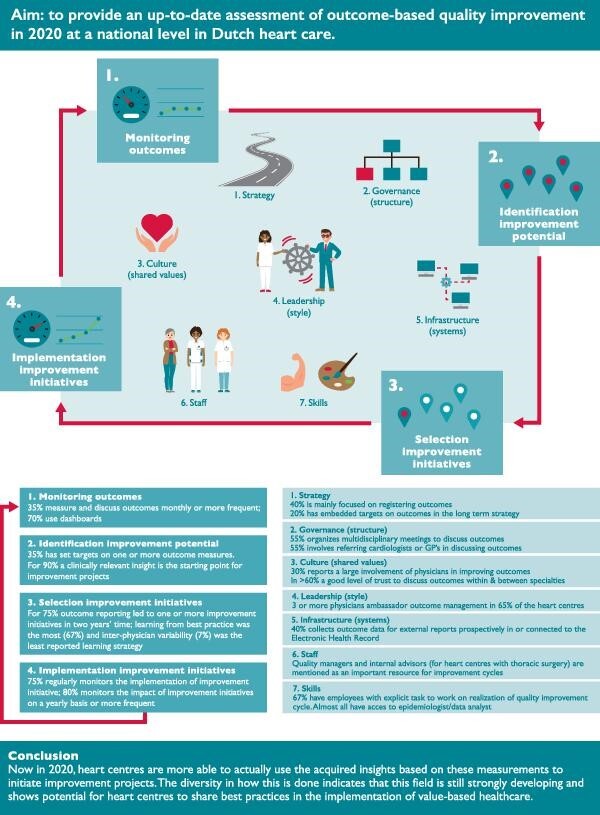
The 2020 national-level assessment of Dutch heart care on the 11 steps of the Health Outcomes Management Evaluation model (Steps I-IV of the Outcome-based improvement cycle and Steps 1-7 of the Organizational Context).
Introduction
Worldwide, professionals working in the healthcare sector are trying to accomplish a transition of their national healthcare systems from volume-based to value-based. Most active in this field are the USA and countries in Western Europe.1 A central role is played by ‘value-based healthcare (VBHC)’ as defined by Porter and Teisberg. They defined value as a set of outcomes, for specific medical conditions over the full care delivery chain with respect to the costs of care delivery.2,3 Recent publications show that different perspectives exist on VBHC. Within the USA, the ‘payment perspective’ is dominant, focusing on moving from fee-for-service to forms of value-based payment. Within Europe, the ‘quality improvement perspective’ is dominant.4 In European countries, the focus is on the use of outcomes to support quality improvement, the use of outcomes to support shared decision-making, integrating care across facilities in networks, and setting up multidisciplinary teams or Integrated Practice Units for specific medical conditions. It should be noted, that even within European countries, different perspectives exist on VBHC.5
However, independent of these different perspectives, measuring and improving outcomes is widely considered a sine qua non for the implementation of VBHC.6 This requires selecting those outcomes that truly matter to patients and providing clear definitions for these outcomes. For this purpose, standard sets of outcomes have been developed for many medical conditions since the introduction of VBHC in 2006. One of the main organizations developing standard sets specifically to support VBHC is the International Consortium for Health Outcomes Measurement (ICHOM).
The Netherlands is at the forefront of the implementation of VBHC.1 For example, the Netherlands has been leading several of the initiatives to develop standard sets of outcome measures to complement and accelerate the efforts of ICHOM.7–11 Within the Netherlands, heart care has been leading this change since 2010.12 Standard sets of outcomes have been developed for the main heart conditions, and are being measured and reported annually.13 The standard set for Coronary Artery Disease (CAD) has been aligned with the international standard set of ICHOM, showing feasibility of measuring these sets and providing an international benchmark.7 Outcomes are registered within the Netherlands Heart Registration (NHR) in a joint effort between the national associations of cardiologists and thoracic surgeons. The NHR covers all cardiac procedures performed across the Netherlands and about 80 000 procedures are registered per year. Baseline, procedural and outcome data within the NHR is over 98% complete for almost all interventions. Apart from collecting and reporting outcome data, for 25 out of 30 heart centres the patient-relevant outcomes are publically reported. In addition, the NHR facilitates registration committees in which physicians from the participating hospitals discuss outcomes and processes of healthcare delivery. In this manner, a learning platform is created that actively supports best practices in care delivery to be identified and shared among heart centres.12,14 For example, a preincision safety check (Isala Safety Check) was implemented in other heart centres after the observation of risk-adjusted reduced mortality within one centre.15
Measuring outcomes is the first step in the transition toward VBHC. To effectively support outcome-based quality improvement, outcome measurement needs to be implemented within quality improvement cycles in the hospitals.16 However, it has been shown that the use of outcome measures from clinical registries to implement and monitor quality improvement initiatives remains limited.17 Earlier research among a selection of six large Dutch heart centres shows that in 2016 outcome measurements played a limited role to support quality improvement in these centres, even though high-quality data on health outcomes were available for the majority of cardiac diseases.18 Four barriers were identified that explained this limited role: (1) insufficient data infrastructure, (2) lack of a systematic approach to identifying and implementing improvement initiatives, (3) governance and specifically the lack of formalization of roles and responsibilities regarding outcome improvement, and (4) the lack of the implementation of outcomes within the hospital strategy, policy documents, and planning and control cycle.
Efforts to measure outcomes and implement VBHC have been ongoing in Dutch heart care since this 2016 study. Outcome measurement has gained in importance, due to a national-level agreement between the Ministry of health welfare and sports, patient organizations, hospitals, and healthcare insurance companies to reach reporting on outcomes for 50% of hospital care by 2022.19 In addition, the coverage, quality, and completeness of outcome reporting in heart care further improved at a national level and more centres joined specific VBHC projects of the NHR.
The aim of this research is to reevaluate at a larger (national) scale the status of outcome-based quality improvement in Dutch heart centres.
Methods
Outcome-based quality improvement was assessed using the same framework as before and has been extensively described elsewhere.18 The framework is based on the Plan-Do-Study-Act (PDSA) model and the 7S model of McKinsey. The original questionnaire in which aforementioned framework was incorporated consists of 42 detailed questions divided over 11 (4 + 7) steps. The original questionnaire was evaluated and shortened to 26 detailed questions (Table 1)], to increase participation rates and eliminate questions that turned out to be less relevant. Hereafter, this framework consisting of 11 steps and related questionnaire is referred to as the Health Outcomes Management Evaluation (HOME) model.
Table 1.
Results of the questionnaire used to assess the status of outcome-based quality improvement in heart centres in the Netherlands
| Heart centres with thoracic surgery | Heart centres without thoracic surgery | Total | |||
|---|---|---|---|---|---|
| No. | Questions | Answer options | N = 11 | N = 9 | N = 20 |
| 1. Monitoring outcomes | |||||
| 1 | How frequent are health outcomes measured and discussed within the heart centre? (for one or more medical conditions) | Not | 0% | 0% | 0% |
| Less than once a year | 0% | 0% | 0% | ||
| Once a year | 0% | 11% | 5% | ||
| Between once a year and quarterly | 0% | 22% | 10% | ||
| Quarterly | 18% | 33% | 25% | ||
| Between quarterly and monthly | 46% | 0% | 25% | ||
| Monthly | 18% | 22% | 20% | ||
| More often than monthly | 18% | 11% | 15% | ||
| 2 | Which specialties are involved in the periodic measurement and discussions of health outcomes (in joint or separate meetings). More than one answer possible | Cardiology | 100% | 100% | 100% |
| Thoracic surgery | 100% | n.a. | 100% | ||
| Anesthesiology | 55% | n.a. | 50% | ||
| Multidisciplinary team | 82% | 22% | 52% | ||
| Management | 55% | 89% | 67% | ||
| These meetings do not take place | 0% | 0% | 0% | ||
| 3 | How many physicians take active knowledge of the health outcomes of your hospital (for instance through meetings in which the annually reported outcomes are discussed)? | 0% | 0% | 0% | 0% |
| 1–25% | 36% | 33% | 35% | ||
| 25–50% | 18% | 22% | 20% | ||
| 50–75% | 9% | 22% | 15% | ||
| >75% | 36% | 22% | 30% | ||
| 4 | At what level within the organization and/or care chain are health outcomes discussed? (more than one answer possible) | Not | 0% | 0% | 0 |
| Among physicians (doctor's units, departments) | 100% | 100% | 100% | ||
| Support staff | 82% | 0% | 45% | ||
| Multidisciplinary | 73% | 22% | 50% | ||
| Patients | 9% | 11% | 10% | ||
| Nursing ward | 18% | 22% | 20% | ||
| Hospital management | 45% | 67% | 55% | ||
| Medical board | 9% | 33% | 20% | ||
| Board of directors | 45% | 44% | 45% | ||
| General practitioner | 9% | 11% | 10% | ||
| Referring hospitals | 45% | 11% | 30% | ||
| 5 | Are quality dashboards (or other tools) used to monitor outcomes of heart care? | No | 18% | 44% | 30% |
| Yes | 82% | 56% | 70% | ||
| 2. Identification improvement potential | |||||
| 6 | When do outcome reports (such as national benchmarks) lead to improvement initiatives within your hospital? (more than one answer possible) | Never | 0% | 0% | 0% |
| Only when the hospital is performing significantly worse than the average of other hospitals | 64% | 44% | 55% | ||
| When the report leads to clinically relevant insights that can be starting point for improvements (for instance a negative trend in the data or performance of subgroups within the patient population) | 91% | 89% | 90% | ||
| When one or more (other) hospitals are performing significantly better than average | 64% | 33% | 50% | ||
| Other (please specify) | 18% | 0% | 10% | ||
| 7 | Does the heart centre look at trends in the data on health outcomes periodically (based on all available tables, figures, etc.)? | No | 0% | 0% | 0% |
| No, only the comparison between centres is looked at (using funnel plots) | 9% | 0% | 5% | ||
| Partially, the comparison between centre and dependencies of outcomes on risk factors is looked at | 55% | 33% | 45% | ||
| Yes, all figures and tables available are looked at | 36% | 67% | 50% | ||
| 8 | Have targets been set for all outcome measures provided in outcome reports (e.g. 30-day mortality < 0.5%)? | No | 55% | 78% | 65% |
| Yes, for one or some outcome measures for all heart care provided by the heart centre | 18% | 0% | 10% | ||
| Yes, for one or some outcome measures for each of the medical conditions for which outcomes are available (e.g. coronary artery disease, atrial fibrillation) | 27% | 11% | 20% | ||
| Yes, for all outcome measures | 0% | 11% | 5% | ||
| 3. Selection improvement initiatives | |||||
| 9 | Have additional data analyses been performed in 2018 or 2019 based on the outcome reports? (aiming to better understand results and possibly to suggest improvement initiatives) | None | 9% | 33% | 20% |
| 1 | 0% | 22% | 10% | ||
| 2–4 | 55% | 33% | 45% | ||
| 5 or more | 36% | 11% | 25% | ||
| 10 | How many improvement initiatives have monitoring of outcomes resulted in, in 2018 and 2019 (e.g. NHR report, internal quality dashboards, etc.)? | None | 18% | 33% | 25% |
| 1 | 18% | 33% | 25% | ||
| 2–3 | 46% | 11% | 30% | ||
| 4 or more | 18% | 22% | 20% | ||
| 11 | Which learning strategies are used to initiate improvement initiatives?* (more than one answers possible) | None | 0% | 0% | 0% |
| Best practice | 67% | 67% | 67% | ||
| Process analysis | 67% | 50% | 60% | ||
| File review | 56% | 50% | 53% | ||
| Scientific literature | 67% | 33% | 53% | ||
| Guidelines | 33% | 17% | 27% | ||
| (Ad-hoc) initiatives based on clinical experience | 44% | 0% | 27% | ||
| (Structural) learning environment with other hospitals | 33% | 0% | 20% | ||
| Inter-physician variability | 11% | 0% | 7% | ||
| Benchmarking | 78% | 17% | 53% | ||
| Other (please specify) | 22% | 0% | 13% | ||
| 4. Implementation improvement initiatives | |||||
| 12 | Is it standard practice to monitor the implementation of improvement initiatives? (e.g. do you check if improvements are implemented correctly and for all eligible patients?) | No, never | 0% | 11% | 5% |
| No, most of the time not | 27% | 11% | 20% | ||
| Yes, most of the time | 64% | 56% | 60% | ||
| Yes, always | 9% | 22% | 15% | ||
| 13 | Is the effect of improvement initiatives monitored? (impact on outcomes or intermediate outcomes) | No | 9% | 33% | 20% |
| Yes, annually using the outcome reports | 36% | 33% | 35% | ||
| Yes, more often than annually, during regular team meetings. | 55% | 33% | 45% | ||
| 1. Strategy | |||||
| 14 | To what extent is measuring and improving outcomes using outcome measures part of the strategy and annual plans of the heart centre? | The heart centre is now mainly focusing on registering outcome measures. | 27% | 56% | 40% |
| The heart centre has set clear targets in the annual plan (or annual plans of the individual departments) aiming to improve outcomes of specific patient groups. | 46% | 33% | 40% | ||
| Performance on outcomes is a central part of the long-term strategy of the heart centre. This results in specific annual targets that are monitored using outcome measures. | 27% | 11% | 20% | ||
| 2. Governance (Structure) | |||||
| 15 | Is there a multidisciplinary meeting of the involved specialties in which outcomes of care are discussed (e.g. involving cardiology, thoracic surgery, and anesthesiology for coronary artery disease)? | No | 18% | 78% | 45% |
| Yes | 82% | 22% | 55% | ||
| 16 | Who are involved in the regular meetings in which the outcomes of care and improvement initiatives are discussed?** (more than one answer possible) | This does not take place | 0% | 0% | 0% |
| Only physicians | 100% | 50% | 91% | ||
| Nurses | 33% | 50% | 36% | ||
| Team leaders | 44% | 50% | 45% | ||
| Specialist nurses | 33% | 50% | 36% | ||
| Physicians from referring hospitals | 0% | 0% | 0% | ||
| General practitioners | 0% | 50% | 9% | ||
| Data manager/Data analyst | 67% | 100% | 73% | ||
| Department management | 89% | 100% | 91% | ||
| Hospital management | 22% | 50% | 27% | ||
| Patients or patient representatives | 11% | 0% | 9% | ||
| Support staff from the quality department | 67% | 50% | 64% | ||
| Other (please specify) | 33% | 0% | 27% | ||
| 17 | Are outcomes discussed with and are joint improvement initiatives started with partners in the care chain? (e.g. referring hospitals, general practitioners) | No | 46% | 44% | 45% |
| Yes | 55% | 56% | 55% | ||
| 3. Culture (Shared values) | |||||
| 18 | What is the involvement of physicians in the measurement and improvement of outcome measures? | No involvement | 0% | 0% | 0% |
| Small. One physician has responsibility for data delivery to external stakeholders. Apart from that no physicians are involved. | 0% | 0% | 0% | ||
| Reasonable. Some physicians are involved. | 64% | 78% | 70% | ||
| Large. There is a wide involvement. | 36% | 22% | 30% | ||
| Very large. All physicians are involved. | 0% | 0% | 0% | ||
| 19 | What level of trust exists within specialties to discuss outcomes openly (e.g. variance between physicians)? | Poor | 0% | 0% | 0% |
| Moderate | 9% | 11% | 10% | ||
| Fair | 27% | 22% | 25% | ||
| Good | 36% | 44% | 40% | ||
| Very good | 27% | 22% | 25% | ||
| 20 | What level of trust exists between specialties to discuss outcomes openly (e.g. between thoracic surgery, cardiology, and anesthesiology)? | Poor | 0% | 0% | 0% |
| Moderate | 9% | 13% | 11% | ||
| Fair | 27% | 25% | 26% | ||
| Good | 36% | 50% | 42% | ||
| Very good | 27% | 13% | 21% | ||
| 4. Leadership (Style) | |||||
| 21 | How many physicians are ambassadors of measuring and using outcome measures? (i.e. physicians with a leadership role to stimulate development of the hospital in this area and who are able to get colleagues along) | None | 9% | 44% | 25% |
| 1 | 9% | 0% | 5% | ||
| 2 | 9% | 0% | 5% | ||
| 3 | 18% | 22% | 20% | ||
| More than 3 | 55% | 33% | 45% | ||
| 22 | At which level(s) in the organization is initiative taken to realize an outcome-based improvement cycle within the heart centre? (more than one answer possible) | Physicians | 100% | 89% | 95% |
| Management of the department or heart centre | 73% | 56% | 65% | ||
| Hospital management | 18% | 56% | 35% | ||
| Nurses | 27% | 11% | 20% | ||
| Hospital quality department | 55% | 56% | 55% | ||
| Board of directors | 18% | 22% | 20% | ||
| Medical board | 9% | 11% | 10% | ||
| Other (please specify) | 18% | 0% | 10% | ||
| 5. Infrastructure (Systems) | |||||
| 23 | How is outcome data for external reports collected (excluding follow-up data)? Please select what best matches the current situation. | Not | 0% | 0% | 0% |
| Retrospectively by combing several sources. Involving still a lot of manual work | 18% | 44% | 30% | ||
| Prospectively build in a separate quality database | 18% | 11% | 15% | ||
| Prospectively build in a separate quality database and connected to the EHR | 27% | 22% | 25% | ||
| Prospectively build in the EHR | 9% | 22% | 15% | ||
| Other (please specify) | 27% | 0% | 15% | ||
| 6. Staff | |||||
| 24 | Who gets time to work on realization of an outcome-based improvement cycle? (more than one answer possible) | Physicians (FTE) (average) | 0.19 | 0.12 | 0.15 |
| Quality managers (FTE) (average) | 0.28 | 0.43 | 0.36 | ||
| Internal advisors (FTE) (average) | 0.50 | 0.02 | 0.20 | ||
| Medical management (FTE) (average) | 0.17 | 0.25 | 0.21 | ||
| Department management (FTE) (average) | 0.00 | 0.28 | 0.18 | ||
| Others (FTE) (average) | 1.20 | 0.36 | 0.68 | ||
| 7. Skills | |||||
| 25 | Are there employees within the hospital with the explicit task as part of their job to work on the realization of an outcome-based quality improvement cycle (e.g. manager value-based healthcare, advisor) | Yes (FTE) | 73% (3.00 FTE average) | 67% (1.27 FTE average) | 70% (2.26 FTE average) |
| No | 27% | 33% | 30% | ||
| 26 | How many physicians in the heart centre have expertise and affinity with data management and data analysis? | 0 | 9% | 0% | 5% |
| 1 | 0% | 33% | 15% | ||
| 2–3 | 55% | 56% | 55% | ||
| 4–5 | 18% | 0% | 10% | ||
| More than 5 | 18% | 11% | 15% | ||
EHR, Electronic Health Record; FTE, Full time equivalent ; n.a., Not applicable; 1 FTE in Dutch healthcare equals 36 h/wk.
Percentages might not add up to 100 due to rounding or in case of multiple choice.
*applicable if one or more improvement initiatives were initiated (question 10)–9 heart centres with thoracic surgery, 6 heart centres without thoracic surgery.
**applicable if regular meetings in which the outcomes of care and improvement initiatives are discussed are organised (question 15)–9 heart centres with thoracic surgery, 2 heart centres without thoracic surgery.
All 30 heart centres (16 with and 14 without thoracic surgery onsite) in the Netherlands were invited in October 2019 to fill out the questionnaire. The questionnaire was sent to the NHR related project leader within each centre using an online survey tool. Project leaders were asked to fill out the questionnaire together with healthcare professionals working within each centre. The data underlying this article are available in the article.
Results
Twenty-one of all 30 heart centres in the Netherlands participated in the study and filled out the questionnaire between October 2019 and March 2020. Of the total group that participated, 11 heart centres with thoracic surgery and 9 heart centres without thoracic surgery were included.
The results are described for each of the 11 steps of the HOME model. Detailed answers to each of the 26 questions are provided in Table 1.
Outcome-based improvement cycle
Monitoring outcomes. Quality dashboards and health outcomes are discussed on a monthly basis or more frequently in one-third of the heart centres, 70% use quality dashboards. In half of the heart centres, a multidisciplinary setting is created to discuss outcomes. For approximately half of the heart centres (45%), more than 50% of the physicians know or discuss the health outcomes. Management is involved when health outcomes are discussed, in half of the heart centres.
Identification of improvement potential. All heart centres use outcome reports (such as national benchmarks on health outcomes) for identification of improvement initiatives. The most common use of outcome reports is to identify clinically relevant insights as a starting point for improvement (for instance a negative trend in the data or performance of subgroups within the patient population). About one-third (35%) of the heart centres has set targets on one or more outcome measures. This percentage seems slightly higher for centres with thoracic surgery (45%) compared to heart centres without thoracic surgery (22%).
Selection of improvement initiatives. Within the majority of heart centres monitoring of outcomes resulted in one or more additional data analyses and one or more improvement initiatives in the past 2 years (80% and 75%, respectively). Two or more additional analyses were performed in 45% of the heart centres, and for 20% of the heart centres 4 or more improvement initiatives were initiated. A wide range of different learning strategies supports outcome-based quality improvement. Sharing of best practices and process analysis were reportedly the most often used learning strategies (67% and 60%, respectively), while analysis of inter-physician variability was the least reported learning strategy (7%).
Implementation of improvement initiatives. The majority of the heart centres monitor the implementation of improvement initiatives and monitor the effect of the improvement initiatives (75% and 80%, respectively).
Organizational context
Strategy. For most heart centres (60%), outcome measurement is embedded in annual plans or long-term strategy. However, on average 40% of the heart centres report that they are still mainly focusing on registering outcome measures. Specifically, heart centres without thoracic surgery (56%).
Governance. Approximately half of the heart centres have a multidisciplinary meeting to discuss outcomes. For those heart centres that involve other professionals, there is a wide variety in who is involved. A relatively strong involvement is seen of department management and data managers. Only one centre involves patients or patient representatives in the discussion of outcomes and four centres involve nurses. Also, only one centre involves general practitioners and none involve physicians from referring hospitals in the discussion of outcomes. About half (55%) of the heart centres discuss outcomes or initiate improvement initiatives in collaboration with partner care providers in the care chain.
Culture. All heart centres report a reasonable or large involvement of physicians in their centre in the measurement and improvement of outcome measures. The reported level of trust to openly discuss outcomes within and between specialties are approximately the same; good (40% and 42%, respectively) and very good (25% and 20%, respectively).
Leadership. Heart centres with thoracic surgery reported almost always (91%) the presence of ambassadors (i.e. physicians with a leadership role to stimulate development of the hospital in outcome-based quality improvement and who are able to get colleagues along). For heart centres without thoracic surgery, strong medical leadership was either, strongly present with 3 or more (55%), or completely absent (44%). Initiative to set up an outcome-based quality improvement cycle within the heart centre is mostly taken by physicians, hospital quality departments or management of the department, or heart centre.
Infrastructure. The stage of development of supporting IT infrastructure [data collection in the Electronic Health Record (EHR) and dashboards] is very diverse. In 40% of the heart centres, outcome data for external reports are prospectively collected in or connected to the EHR. Other hospitals collect data manually or combine several data sources, and use databases separated from the EHR for quality measurement.
Staff. A wide variety of personnel is involved in setting up an outcome-based quality improvement cycle; physicians, management, and advisors. On average, each of these types of professionals has between 1 and 2 days a week available for this task. Predominantly quality managers and internal advisors (for heart centres with thoracic surgery) are mentioned as an important resource to realize outcome-based quality improvement cycles.
Skills. Over two-third of the heart centres reported that there are employees within their centre with the explicit task, as part of their job, to work on realization of an outcome-based quality improvement cycle. On average 2 FTE is available for this task. Almost all heart centres stated to have access to data analysts and/or epidemiologists to extract and analyse outcome data. In most heart centres (80%), two or more physicians have expertise and affinity with data-management and data-analysis.
Discussion
A status quo of implementation of health outcomes in Dutch heart centres to support quality improvement has been presented. The study shows that outcome measurement is actively used by the majority of the heart centres to support quality improvement and that outcome measurement is embedded within the organization. At the same time large differences between heart centres are present in all elements of the HOME model; both in the four steps of the quality improvement cycle and in the seven elements of outcome-based organization.
Among the total group of heart centres, experience exists with each of the four steps of the improvement cycle. However, 40% is mainly focusing on data collection and there are still quite some hospitals (25%) for which over 2 years’ time outcome reports did not lead to any improvement initiative. There can be several reasons for this. From the current study it is not possible to judge if this is: (1) caused by the hospital performance (all outcomes are considered to be good enough, i.e. no reason to improve), or (2) too little effort was put in identifying improvement potential and improvement initiatives, or (3) improvement potential was identified, but underlying causes or improvement initiatives were not found. The interviews in the earlier study suggest that a combination of these three reasons is likely.18
Hospitals are investing in outcome-based quality improvement on each of the seven elements of outcome-based organization. A valuable insight is that outcome-based quality improvement is still mainly an effort of physicians. Even though physicians discuss outcomes multidisciplinary, it is striking to see that nurses and patients are hardly involved at all. This might reflect the fact that general standard sets of outcome measures do not include clinical outcome measures and relevant outcome measures closer to the work of nurses, such as delirium, falls, incidents, and malnutrition. Even so, including nurses and patients in the discussions of outcomes and improvement initiatives could be an obvious next step for all heart centres, in line with the concept of integrated practice units within VBHC.20
A variety of approaches is used in the heart centres to discuss outcomes and use insights to improve value for patients. However, the optimal organizational model to effectively improve outcomes is still unknown. Because information about the impact of the improvement initiatives on clinical outcomes is still lacking, the added value of the different approaches applied by the heart centres cannot yet be established. Also, in literature no best practices have been described. Within heart centres different patient groups are served by subspecialized teams. It is unclear if different teams should use different strategies, for instance because cardiothoracic surgeons work more often in a multidisciplinary setting compared to electrophysiologists. Although earlier research has indicated that inter-physician variation can have significant impact on quality of healthcare delivery, inter-physician variability was the least reported learning strategy.21 Using this learning strategy as a standard part of outcome-based quality improvement might be an important next step for heart centres in the Netherlands.
The survey shows the relevance of a multicentre or national quality registry. First, because of the relatively high percentage of heart centres that report that they initiate improvement actions when outcomes appear to be worse than outcomes of peers in the benchmark. Secondly, many hospitals use dashboards to monitor outcomes. A national quality registry can develop and provide these dashboards online in an efficient manner and using nationwide trends as the benchmark. Lastly, a national registry can assist heart centres in collecting high-quality data, for instance by performing audits and applying a data-quality control system with data-quality checks in the process of submitting data from the heart centres to the registry. High-quality data is of utmost importance when using real-world data to improve quality in healthcare.
The NHR is primarily set up to provide good data to support quality improvement by healthcare professionals. Mandated physicians from the participating hospitals are united in registration committees. They construct standard sets of patient-relevant outcome measures, process variables, and patient characteristics. Also, an environment to learn and share is created as in these committees physicians monitor outcomes and share hypotheses and good practices. However, the strong efforts in combination with the diversity in how outcomes are used within heart centres suggest that identifying and sharing best practices in the implementation of VBHC and specifically the implementation of outcomes within hospital quality management requires attention as well.
In the national benchmarks in the Netherlands, for several outcomes little variance is observed between heart centres. Although little variance is observed, measuring these outcomes is still relevant from a perspective of quality and safety monitoring. Given the criteria used by the heart centres to initiate quality improvement projects, little variation in outcomes will result in few improvement projects. Over time, it is to be expected that the quality registries will be expanded with new, additional indicators. As variance between heart centres might be shown in these indicators, the search for a solid organization and infrastructure for quality improvement remains relevant.
It is not possible to quantitatively compare the results on each of the questions in the questionnaire of the current study with the results of the 2016–17 study. The number and type of hospitals are different and not all hospitals from the previous study participated in the current study. However, an indication can be given of the overall difference. The 6 hospitals that participated in the 2016–17 study were all heart centres with a thoracic surgery department and a comparison can best be made with the 12 heart centres with thoracic surgery in the current study. Overall, the answers given in 2016–17 for the 11 elements of the HOME are similar or point in the direction of more use of outcome measures and more activities and investments related to outcome-based organization for 10 out of the 11 elements of the HOME model. This is not unexpected given the fact that within the Netherlands, outcome measurement is strongly stimulated in recent years.19 It should be stressed that the questions in the HOME model are not a measure of success of the heart centres, but a measure of the use of outcomes and outcome measures; it evaluates the extent to which outcomes are embedded in quality measurement and organization.
The results of the heart centres with and without thoracic surgery have been separately presented in the results of this study. This was done, because the type of care provided and therefore the type of organizations are different. Also, VBHC implementation in heart care in the Netherlands started with an initiative in which mostly heart centres with thoracic surgery participated. Outcome measurement for specific medical conditions therefore, on average, started sooner in heart centres with thoracic surgery. However, the results are very similar. Overall, the heart centres with thoracic surgery seem to be slightly more active in the use of outcome measures. Almost all heart centres with thoracic surgery have physicians that are ambassadors of measuring and using outcome measures, for heart centres without thoracic surgery this is half. In addition, the heart centres with thoracic surgery seem to use a wider range of learning strategies to initiate improvement initiatives.
The study has a simple setup and its strength is the number of participating hospitals. However, the study suffers from limitations. First, differences might exist in the involvement of different specialties in filling out the questionnaire. Clear instructions were given, however in 30% the involvement of physicians was not confirmed or it was not confirmed how many/or from what specialism physicians were involved. Secondly, the interpretation of questions might differ per heart centre. For instance the definition of an ‘additional analysis’ may differ from a simple data-analysis up to extensive research based on patient files. Thirdly, in contrast to the previously performed study no extensive interviews were performed. It was therefore not possible to elaborate on to the provided answers. However, in the earlier study little difference was observed between the results of the survey and the interviews. Finally, the self-reporting of hospitals might have introduced a bias, as self-reporting might lead to socially desirable answers and reporting that gives a more positive reflection of reality. This can for instance occur with the questions regarding the level of trust to openly discuss outcomes within and between specialties.
During the last decade, the Netherlands has become one of the leading clusters internationally on VBHC implementation. Reports on VBHC provide a relatively large amount of Dutch examples.1 Within the Netherlands, heart care has been at the forefront of VBHC implementation. Therefore, our hypothesis is that on average hospitals have a more basic level of outcome-based quality improvement compared to the hospitals in this study. This is relevant, because even within Dutch hospitals a standard and systematic approach for outcome-based quality improvement is still not in place. The results of the current study mainly show that most of these hospitals invest in the implementation of outcome measurement. Given the fact that measuring patient-relevant outcomes for medical conditions has become a joint global effort, relatively little effort as yet is put in transforming hospital quality management and hospital planning and control cycles to systematically embed outcomes. For VBHC to become a success creating standard models for outcome-based or value-based quality improvement within hospitals should become a joint effort as well.16 This topic is missing in Michael Porter's strategic agenda for value transformation.22
Acknowledgements
We thank the participating heart centres: Amsterdam UMC (location AMC), Albert Schweitzer, Catharina Hospital, Elisabeth-TweeSteden Hospital, Haaglanden Medical Centre, Isala, Jeroen Bosch Hospital, Maasstad Hospital, Maastricht UMC+, Meander Medical Centre, Medical Centre Leeuwarden, Medisch Spectrum Twente, Noordwest Ziekenhuisgroep, OLVG, Radboudumc, St. Antonius Hospital, Tergooi, UMC Utrecht, and Zuyderland Medical Centre for their time and contribution.
Contributor Information
Paul B van der Nat, Department of Value-Based Healthcare, St. Antonius Hospital, Nieuwegein, the Netherlands; Radboud University Medical Center, Radboud Institute for Health Sciences, Scientific Center for Quality of Healthcare (IQ healthcare), Nijmegen, the Netherlands.
Lineke Derks, Netherlands Heart Registration, Utrecht, the Netherlands.
Dennis van Veghel, Netherlands Heart Registration, Utrecht, the Netherlands.
Funding
None.
Conflict of interest
None declared.
Data availability statement
The data underlying this article are available in the article.
References
- 1. EIT Health . Implementing Value-Based Health Care in Europe: Handbook for Pioneers (Director: Gregory Katz) [Internet]; 2020[cited 2021 May 14]. Available from: https://eithealth.eu/wp-content/uploads/2020/06/Implementing-Value-Based-Healthcare-In-Europe.pdf
- 2. Porter ME, Teisberg EO.. Redefining Health Care: Creating Value-Based Competition on Results. Boston: Harvard Business School Press; 2006. [Google Scholar]
- 3. Porter ME. What is value in health care? N Engl J Med 2010;363:2477–2481. [DOI] [PubMed] [Google Scholar]
- 4. Mjåset C, Ikram U, Nagra NS, Feeley TW.. Value-based health care in four different health care systems. NEJM Catalyst 2010;1,DOI:10.1056/CAT.20.0530. [Google Scholar]
- 5. Steinmann G, van de Bovenkamp H, de Bont A, Delnoij D.. Redefining value: a discourse analysis on value-based health care. BMC Health Serv Res 2020;20, DOI:10.1186/s12913-020-05614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Porter ME, Larsson S, Lee TH.. Standardizing patient outcomes measurements. N Engl J Med 2016;374:504–506. [DOI] [PubMed] [Google Scholar]
- 7. Daeter EJ, Timmermans MJC, Hirsch A, Lipsic E, Houterman S, board Meetbaar Beter advisory, van Veghel D, van der Nat PB. Defining and measuring a standard set of patient-relevant outcomes in coronary artery disease. Am J Cardiol 2018;121:1477–1488. [DOI] [PubMed] [Google Scholar]
- 8. Kampstra NA, Grutters JC, van Beek FT, Culver DA, Baughman RP, Renzoni EA, Wuyts W, Wijsenbeek MS, Biesma DH, van der Wees PJ, van der Nat PB.. A first patient-centred set of outcomes for pulmonary sarcoidosis: a multicentre initiative. BMJ Open Respir Res 2019;6:e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reesink DJ, van de Garde EWM, Somford DM, Meijer RP, Los M, Biesma DH, Horenblas S, van Melick HHE, van der Nat PB, for the Santeon MIBC Study Group . Development of the first patient-centred set of outcomes for muscle-invasive and metastatic bladder cancer: a multicentre initiative. Eur Urol Open Sci 2021;26:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mulder JWCM, Galema-Boers AMH, de Jong-Verweij LM, Hazelzet JA, Roeters van Lennep JE. The development and first results of a health-related outcomes set in familial hypercholesterolemia (FH) patients: knowledge is health. Atherosclerosis 2020;293:11–17. [DOI] [PubMed] [Google Scholar]
- 11. Oerlemans S, Bennink MC, Levin MD, Broijl A, Van der Klift M, Van Deursen J, Vogels D, Van de Poll-Franse LV, Sonneveld P, Hazelzet JA, Tick LW.. Development of a Patient Centered Outcome Set for Patients With Multiple Myeloma to be Used in Clinical Practice. Hemasphere 2020;4:e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Veghel D, Marteijn M, de Mol BA, Measurably better study group (The Netherlands) and advisory board. First results of a national initiative to enable quality improvement of cardiovascular care by transparently reporting on patient-relevant outcomes. Eur J Cardiothorac Surg 2016;49:1660–1669. [DOI] [PubMed] [Google Scholar]
- 13. Nederlandse Hart Registratie . NHR Rapportage 2020 [Internet]; 2020[cited 2021 Jan 22]. Available at: https://nederlandsehartregistratie.nl/publicaties/
- 14. van der Nat PB, van Veghel D, Daeter E, Crijns HJ, Koolen J, Houterman S, Soliman MA, de Mol BA, Measurably better study group. Insights on value-based healthcare implementation from Dutch heart care. Int J Healthc Manag 2020;13:189–192. [Google Scholar]
- 15. Spanjersberg AJ, Ottervanger JP, Nierich AP, Speekenbrink RGH, Stooker W, Hoogendoorn M, van Veghel D, Houterman S, Brandon Bravo Bruinsma GJ. Implementation of a specific safety check is associated with lower postoperative mortality in cardiac surgery. J Thorac Cardiovasc Surg 2020;159:1882–1890.e2e2. [DOI] [PubMed] [Google Scholar]
- 16. van der Nat PB. The new strategic agenda for value transformation. Health Serv Manage Res 2021;26:9514848211011739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kampstra NA, Zipfel N, van der Nat PB, Westert GP, van der Wees PJ, Groenewoud AS.. Health outcomes measurement and organizational readiness support quality improvement: a systematic review. BMC Health Serv Res 2018;18:1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Veghel D, Daeter EJ, Bax M, Amoroso G, Blaauw Y, Camaro C, Cummins P, Halfwerk FR, Wijdh-den Hamer IJ, de Jong JSSG, Stooker W, van der Wees PJ, van der Nat PB. Organization of outcome-based quality improvement in Dutch heart centres. Eur Heart J Qual Care Clin Outcomes 2020;6:49–54. [DOI] [PubMed] [Google Scholar]
- 19. Convenant . Bestuurlijk akkoord medisch-specialistische zorg 2019 t/m 2022 [in Dutch]. 2018 Jun. Available from: https://www.rijksoverheid.nl/documenten/convenanten/2019/06/04/bestuurlijk-akkoord-medisch-specialistische-zorg-2019-t-m-2022.
- 20. Porter ME, Lee TH.. Integrated practice units: a playbook for health care leaders. NEJM Catal 2021;2. DOI: 10.1056/CAT.20.0237. [Google Scholar]
- 21. Mercuri M, Gafni A.. Examining the role of the physician as a source of variation: are physician-related variations necessarily unwarranted? J Eval Clin Pract 2018;24:145–151. [DOI] [PubMed] [Google Scholar]
- 22. Porter ME, Lee TH.. The Strategy That Will Fix Healthcare. Boston: Harvard Business Review; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.


