Abstract
Background
In ST-elevation myocardial infarction (STEMI), transradial access (TRA) for percutaneous coronary intervention (PCI) is associated with less bleeding and mortality than transfemoral access (TFA). However, patients in cardiogenic shock (CS) are more often treated via TFA. The aim of this meta-analysis is to compare the safety and efficacy of TRA vs. TFA in CS.
Methods
Systematic review was performed querying PubMed, Google Scholar, Cochrane, and clinicaltrials.gov for studies comparing TRA to TFA in PCI for CS. Outcomes included in-hospital, 30-day and ≥1-year mortality, major and access site bleeding, TIMI3 (thrombolytics in myocardial infarction) flow, procedural success, fluoroscopy time, and contrast volume. Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using random effects models.
Results
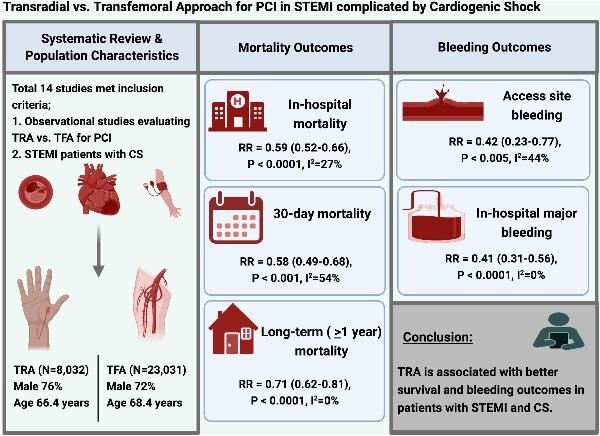
Six prospective and eight retrospective studies (TRA, n = 8032; TFA, n = 23 031) were identified. TRA was associated with lower in-hospital (RR 0.59, 95% CI 0.52–0.66, P < 0.0001), 30-day and ≥1-year mortality, as well as less in-hospital major (RR 0.41, 0.31–0.56, P < 0.001) and access site bleeding (RR 0.42, 0.23–0.77, P = 0.005). There were no statistically significant differences in post-PCI coronary flow grade, procedural success, fluoroscopy time, and contrast volume between TRA vs. TFA.
Conclusions
In PCI for STEMI with CS, TRA is associated with significantly lower mortality and bleeding complications than TFA while achieving similar TIMI3 flow and procedural success rates.
Graphical Abstract
Graphical Abstract.
Transradial vs. transfemoral approach for PCI in STEMI complicated by cardiogenic shock.
Introduction
Cardiogenic shock (CS) affects 4–12% of patients with acute ST-elevation myocardial infarction (STEMI) and is associated with increased mortality and morbidity.1,2 Urgent percutaneous coronary intervention (PCI) remains the gold standard treatment for STEMI patients with CS. The concomitant use of antiplatelet and antithrombotic agents during the management of STEMI increases the risks of bleeding and PCI access site complications.3
Transradial access (TRA) for PCI has been shown to have lower rates of bleeding and mortality than transfemoral access (TFA) in emergent PCI in the setting of STEMI.4,5 However, TFA has historically been preferred over TRA for patients with STEMI complicated by CS. This may be partially due to perceptions of achieving faster revascularization with TFA, using TFA access for mechanical circulatory support (MCS) device placement, and concerns about arterial vasoconstriction limiting TRA.6 Whether TRA or TFA results in lower access-site complications has been minimally studied. The aim of this systematic review and meta-analysis is to compare outcomes in STEMI patients with CS undergoing PCI via TRA vs. TFA.
Methods
Data sources and search strategy
Systematic review and meta-analysis were performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) methodology.7 A systematic search, without language restriction was performed in PubMed, EMBASE, Cochrane Library database, Google Scholar, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and ClinicalTrials.gov from inception to 9 October 2021 for studies comparing TRA vs. TFA in STEMI and CS. Conference proceedings of American College of Cardiology, American Heart Association, European Society of Cardiology (ESC) and Transcatheter Therapeutics (TCT) were also searched. The reference lists of original studies, conference abstracts, and relevant review articles were reviewed. We used combinations of the following keywords in our search strategy: radial access, TRA, femoral access, TFA, STEMI, acute myocardial infarction, acute coronary syndrome (ACS), PCI, coronary intervention, CS, randomized trial, and clinical trial. The search strategy was verified and independently validated by an experienced medical librarian.
Study selection, data extraction, and quality assessment
Studies that met the following criteria were included: (i) randomized trials or observational studies that included adults aged ≥18 years, (ii) studies evaluating the efficacy and safety of TRA vs. TFA, (iii) PCI (primary or rescue) in STEMI patients with CS. Case reports and editorials were excluded. Two investigators (SA and AL) independently performed the literature search, screened studies for eligibility, and extracted data using a standardized data collection form. Any differences in the included studies and collected data were resolved through consensus among the authors. The data for CS patients from the RIFLE-STEACS trial were abstracted from the meta-analysis by Pancholy et al., who reported that they had acquired the data from the authors of the paper by contacting them directly. The Newcastle and Ottawa Scale was used to assess the quality of observational studies (See supplementary material online, Table S3). The protocol for this meta-analysis was registered at PROSPERO, the international prospective register of systematic reviews.
Outcomes
The following clinical and procedural outcomes were extracted from individual studies: (i) all-cause mortality (cardiovascular and non-cardiovascular causes), (ii) study-defined major bleeding, (iii) access site bleeding, (iv) 30-day stroke, (v) 30-day major adverse cardiac and cerebrovascular events (MACCE), (vi) MCS utilization, (vii) post-PCI TIMI (Thrombolytics in Myocardial Infarction) flow grade, (viii) procedural success, (ix) procedure duration, (x) fluoroscopy time, (xi) contrast volume, and (xii) length of stay. Additionally, the definitions of MACCE and major bleeding were consistent across included studies. MACCE included mortality, myocardial reinfarction, target vessel revascularization, and cerebrovascular accident. Major bleeding used the TIMI definition of major bleeding.
Statistical analysis
The meta-analysis was performed using Review Manager (RevMan), Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Due to heterogeneity in the methodologies of the included studies, risk ratios (RRs) and 95% confidence intervals (CI) were calculated using the random effects Mantel–Haenszel method for dichotomous variables. Heterogeneity was assessed using Higgins’ and Thompson's I2 statistic, with I2 values of <25%, 25–75%, and >75% corresponding to low, moderate, and high levels of heterogeneity, respectively. Since the duration of follow-up was variable among the included studies, we performed a sub-group analysis for mortality based on the duration of follow-up (in-hospital vs. 30 day or longer). We performed meta-regression analyses using STATA 17.0 (STATA CORP, College Station, TX, USA) to measure the influence of cardiac arrest (CA) prior to PCI, intra-aortic balloon pump (IABP) use, and access site bleeding on 30-day all-cause mortality. Sensitivity analysis was performed by the study exclusion method. Publication bias was estimated using Egger's test and using funnel plots for meta-analyses involving 10 or more studies.8 The trim-and-fill method of Duval and Tweedie was employed to detect and adjust for any additional small study effects using JASP Version 0.15 (Amsterdam, The Netherlands).9 A 2-tailed P < 0.05 was considered statistically significant for all analyses. The graphical abstract was created with BioRender.com (2022).
Results
Systematic review and study population
A total of 789 articles were identified through database search. After excluding duplicates and studies that did not meet inclusion criteria, a total of 14 studies (6 prospective and 8 retrospective) comparing TRA and TFA in STEMI PCI for CS were selected for this meta-analysis (Figure 1). Characteristics of included studies are listed in Table 1. The aggregate study population included 8032 TRA patients and 2303 TFA patients.4,10–22 The TRA group had a mean age of 66.4 years comprised of 76% males; the TFA group had a mean age of 68.4 comprised of 72% males. The mean follow-up duration was 1.3 years. Baseline population characteristics are listed in Table 2. Utilization of MCS and procedural duration are summarized in Supplementary material online, Table S1. Target vessel and type of stent used are summarized in Supplementary material online, Table S2.
Figure 1.
Flow diagram of systematic review and meta-analysis using the PRISMA methodology.
Table 1.
Characteristics of included studies
| First author | Year | Nation | Design | Total cohort | TRA | TFA | Study quality |
|---|---|---|---|---|---|---|---|
| RIFLE-STEACS (Romagnoli) 4 | 2012 | Italy | Post-hoc analysis of RCT | 61 | 26 | 35 | 7 |
| Rodriguez-Leor 16 | 2013 | Spain | Retrospective cohort | 122 | 80 | 42 | 5 |
| Radial Pump UP (Romagnoli) 10 | 2013 | Italy | Retrospective cohort | 221 | 71 | 150 | 7 |
| Bernat 11 | 2013 | Czech Republic | Prospective cohort | 197 | 108 | 89 | 7 |
| Mamas 15 | 2014 | United Kingdom | Retrospective cohort | 7231 | 1877 | 5354 | 7 |
| Fuji 12 | 2014 | Japan | Retrospective cohort | 81 | 38 | 43 | 5 |
| Iga 13 | 2014 | Japan | Retrospective cohort | 85 | 60 | 25 | 5 |
| Kedev 14 | 2014 | Macedonia | Prospective cohort | 33 | 20 | 13 | 6 |
| Roule 17 | 2015 | France | Prospective cohort | 101 | 74 | 27 | 6 |
| Kubo 20 | 2019 | Japan | Prospective cohort | 16 740 | 4367 | 12 373 | 6 |
| CULPRIT-SHOCK TRIAL (Guedeney) 18 | 2020 | Germany | Post-hoc analysis | 673 | 118 | 555 | 7 |
| Tehrani 19 | 2020 | USA | Retrospective cohort | 153 | 82 | 71 | 7 |
| Zahn 21 | 2020 | Germany | Retrospective cohort | 1700 | 111 | 1589 | 5 |
| Tokarek 22 | 2021 | Poland | Prospective cohort | 3565 | 959 | 2606 | 7 |
RCT, randomized controlled trial; TRA, transradial access; TFA, transfemoral access
Table 2.
Baseline characteristics of study participants
| First author | Year | Access | Age (y) | Male (%) | DM (%) | HTN (%) | HLD (%) | eGFR | Smoking (%) | CAD | Prior MI (%) | CA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RIFLE-STEACS (Romagnoli) 4 | 2012 | TRA | 64 | 69 | NR | NR | 44 | NR | 35 | NR | 14 | NR |
| TFA | 70 | 74 | NR | NR | 40 | NR | 34 | NR | 14 | NR | ||
| Rodriguez-Leor 16 | 2013 | TRA | 65 | 89 | 30 | 58 | 51 | 66 | 36 | 28 | 28 | 33 |
| TFA | 68 | 74 | 19 | 57 | 62 | 53 | 26 | 45 | 45 | 38 | ||
| Radial Pump UP (Romagnoli) 10 | 2013 | TRA | 66 | 72 | 23 | 62 | 50 | 75 | 25 | 20 | 24 | NR |
| TFA | 69 | 70 | 25 | 71 | 44 | 63 | 23 | 19 | 22 | NR | ||
| Bernat 11 | 2013 | TRA | 69 | 71 | 13 | 47 | 38 | 73 | 38 | 14 | 14 | 16 |
| TFA | 64 | 71 | 26 | 54 | 46 | 72 | 44 | 18 | 18 | 15 | ||
| Mamas 15 | 2014 | TRA | 67 | 74 | 17 | 44 | 42 | NR | 30 | 21 | 21 | NR |
| TFA | 67 | 69 | 21 | 47 | 42 | NR | 31 | 25 | 25 | NR | ||
| Fuji 12 | 2014 | TRA | 71 | 82 | 63 | 84 | 53 | 54 | 66 | 8 | 8 | NR |
| TFA | 73 | 70 | 49 | 91 | 56 | 49 | 51 | 19 | 19 | NR | ||
| Iga 13 | 2014 | TRA | 68 | 83 | 32 | 53 | 52 | NR | 58 | 14 | 10 | 32 |
| TFA | 70 | 72 | 44 | 64 | 32 | NR | 40 | 17 | 8 | 28 | ||
| Kedev 14 | 2014 | TRA | 57 | 60 | 35 | 45 | 37 | NR | 55 | 5 | NR | NR |
| TFA | 63 | 46 | 31 | 54 | 31 | NR | 46 | 15 | NR | NR | ||
| Roule 17 | 2015 | TRA | 67 | 77 | 18 | 53 | 31 | 58 | 31 | NR | 12 | 19 |
| TFA | 73 | 44 | 11 | 59 | 11 | 49 | 19 | NR | 19 | 44 | ||
| Kubo 20 | 2019 | TRA | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| TFA | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| CULPRIT-SHOCK TRIAL (Guedeney) 18 | 2020 | TRA | 68 | 80 | 27 | 50 | 34 | NR | 33 | NR | 18 | 46 |
| TFA | 69 | 76 | 34 | 62 | 35 | NR | 25 | NR | 17 | 55 | ||
| Tehrani 19 | 2020 | TRA | 64 | 75 | 43 | NR | NR | NR | 57 | NR | 16 | 23 |
| TFA | 67 | 67 | 52 | NR | NR | NR | 35 | NR | 23 | 45 | ||
| Zahn 21 | 2020 | TRA | 69 | 78 | 30 | NR | NR | NR | 52 | NR | 19 | NR |
| TFA | 68 | 70 | 31 | NR | NR | NR | 45 | NR | 27 | NR | ||
| Tokarek 22 | 2021 | TRA | 68 | 65 | 24 | 56 | NR | NR | 24 | NR | 17 | 30 |
| TFA | 69 | 62 | 23 | 52 | NR | NR | 20 | NR | 20 | 47 |
TRA, transradial access; TFA, transfemoral access, DM, diabetes mellitus; HLD, hyperlipidaemia; HTN, hypertension; M, male; CAD, coronary artery disease; MI, myocardial infraction.
Mortality
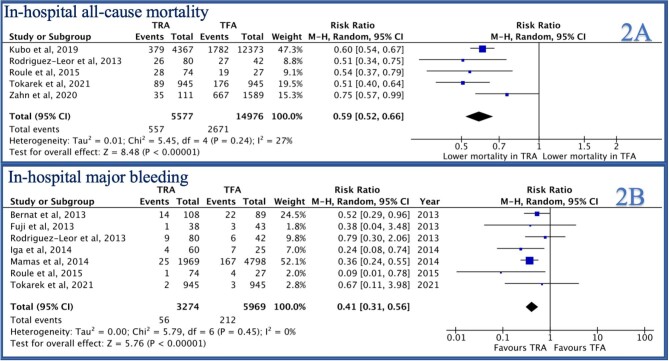
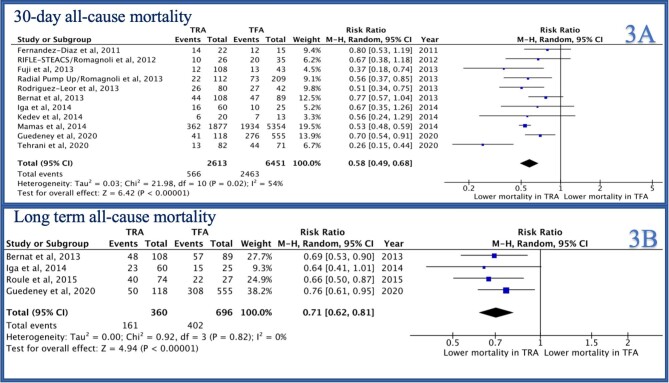
In-hospital all-cause mortality was reported by 5/14 studies and was significantly lower in the TRA group as compared to the TFA group [RR 0.59 (95% CI 0.52–0.66), P < 0.0001, I2 = 27%, Figure 2A]. Similarly, 30-day mortality was reported by 11/14 studies and was lower in the TRA group as compared to the TFA group [RR 0.58 (0.49–0.68), P < 0.001, I2 = 54%, Figure 3A]. Mortality at the longest follow-up (>1 year) was reported by 4/14 studies and was similarly lower in the TRA group as compared to the TFA group [RR 0.71 (0.62–0.81), P < 0.0001, I2 = 0%, Figure 3B].
Figure 2.
Forest plot comparing TRA vs. TFA for PCI in patients with STEMI complicated by cardiogenic shock (A: in-hospital all-cause mortality, B: in-hospital major bleeding).
Figure 3.
Forest plot comparing TRA vs. TFA for PCI in patients with STEMI complicated by cardiogenic shock (A: 30-day all-cause mortality, B: long-term all-cause mortality).
Bleeding, stroke, and MACCE
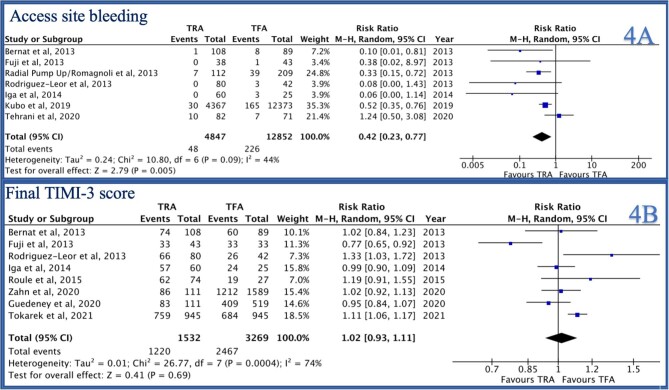
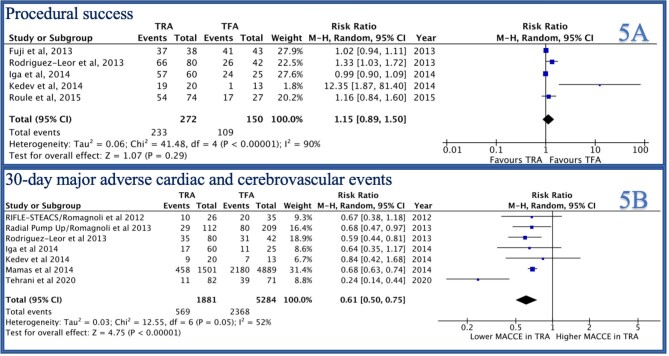
In-hospital major bleeding was reported by 7/14 studies and was lower in the TRA group compared to TFA group [RR 0.41 (0.31–0.56), P < 0.0001, I2 = 0, Figure 2B]. Similarly, access site bleeding was reported by 7/14 studies and was lower in the TRA group as compared to the TFA group [RR 0.42 (0.23–0.77), P = 0.005, I2 = 44%, Figure 4A]. Thirty-day stroke was reported by 3/14 studies and was not statistically different between the two groups [RR 1.29 (0.37–4.47), P = 0.69, I2 = 53%, Supplementary material online, Figure S2B]. Thirty-day MACCE was reported by 7/14 studies and was lower in the TRA group as compared to the TFA group [RR 0.61 (0.50–0.75), P < 0.001, I2 = 52%, Figure 5B).
Figure 4.
Forest plot comparing TRA vs. TFA for PCI in patients with STEMI complicated by cardiogenic shock (A: access site bleeding, B: final TIMI-3 score).
Figure 5.
Forest plot comparing TRA vs. TFA for PCI in patients with STEMI complicated by cardiogenic shock (A: procedural success, B: 30-day MACCE).
Procedural outcomes
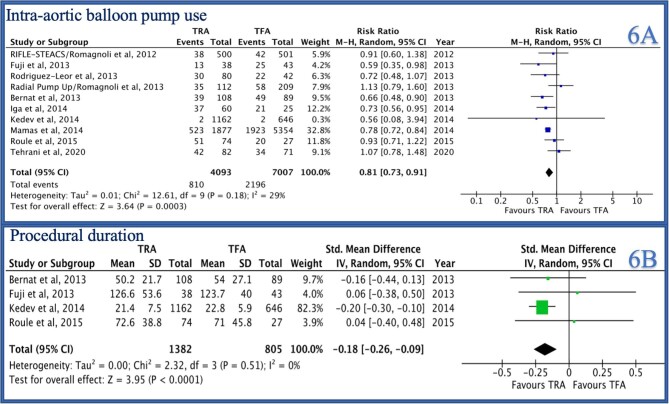
Post-PCI TIMI-3 flow was reported by 8/14 studies and was not significantly different between the two groups [RR 1.02 (0.93–1.11), P = 0.69, I2 = 74%, Figure 4B]. Procedure success was reported by 5/14 studies and was not statistically different between the two groups [RR 1.15 (0.89–1.50), P = 0.29, I2 = 90%, Figure 5A]. IABP use was reported by 10/14 studies and was significantly higher in the TFA group as compared to the TRA group [RR 0.81 (0.73–0.91), P = 0.0003, I2 = 29%, Figure 6A]. Procedure duration was reported by 4/14 studies and was significantly lower in the TRA as compared to TFA group [mean difference (MD) −0.18 [−0.26–0.09], P < 0.0001, I2 = 0, Figure 6B]. Fluoroscopy time was reported by 6/14 studies and was similar between the two groups [MD 0.30 (−0.25–0.85), P = 0.28, I2 = 0, Supplementary material online, Figure S1A]. Contrast volume was reported by 9/14 studies and was similar between the two groups [MD 14.14 (−2.02–30.30), P = 0.09, I2 = 0, Supplementary material online, Figure S1B]. Hospital length of stay was reported by 4/14 studies and was not statistically different between the two groups [MD −0.95 (−1.19 to −0.70), P < 0.0001, I2 = 0, Supplementary material online, Figure S2A].
Figure 6.
Forest plot comparing TRA vs. TFA for PCI in patients with STEMI complicated by cardiogenic shock (A: IABP, B: procedure duration).
Sensitivity analyses
Sensitivity analysis of matched/randomized studies showed that use of TRA in CS patients was associated with lower in-hospital mortality [2/6, RR = 0.51 (0.42–0.63), P < 0.0001, I2 = 0], 30-day mortality [3/6, RR = 0.60 (0.49—0.74), P < 0.0001, I2 = 51%], and mortality at long term [2/6, RR 0.72 (0.61–0.86), P = 0.002, I2 = 0], respectively, when compared to TFA (Supplementary material online, Figure S3A, B, C). Similarly, TRA use in CS patients was associated with lower in-hospital major bleeding [3/6, RR 0.36 (0.23–0.55), P < 0.00001, I2 = 2%] and IABP use [3/6, RR 0.79 (0.73–0.86), P < 0.00001, I2 = 2%] (Supplementary material online, Figure S4A, B). Sensitivity analysis by the study exclusion method was performed to assess the effects of the largest study on the mortality outcomes. There was no significant change in the results for in-hospital all-cause mortality, 30-day all-cause mortality, and long-term all-cause mortality after the exclusion of studies by Kubo et al. and Guedeney et al., respectively (Supplementary material online, Figure S5A, B, C). Adjusted summary estimates with inverse variance analysis were calculated for in-hospital mortality, major bleeding, and 30-day mortality. The results of adjusted analyses were consistent with the main analyses (Supplementary material online, Figure S6A, B, C). Funnel plot distributions of RRs for 30-day all-cause mortality and IABP use showed a small degree of asymmetry. However, Egger's regression test and trim-and-fill models excluded the presence of significant publication bias (Supplementary material online, Figures S9A and S9B).
Meta-regression for mortality and IABP use
Random effects meta-regression analysis was performed to estimate the influence of IABP use on study outcomes. Our analysis showed that the difference in IABP use between TRA and TFA groups was not significantly associated with 30-day mortality (P = 0.281), access site bleeding (P = 0.195), or in-hospital major bleeding (P = 0.130) (Supplementary material online, Figure S7A, B, C). However, with a decrease in access site bleeding, the risk of all-cause mortality was significantly reduced as defined by the adjusted R2 statistics with up to 90% of mortality explained by access site bleeding [R2 = 89.7%, P = 0.003) (Supplementary material online, Figure S8A).
Cardiac arrest as covariate for 30-day mortality
Six studies reported the incidence of CA prior to PCI. Patients undergoing PCI via TRA had a lower incidence of CA as compared to the TFA group [RR 0.72 (0.59–0.87), P = 0.001, I2 = 52%]. Random effects meta-regression showed that the difference in the incidence of CA prior to PCI was not significantly associated with access site bleeding (P = 0.13) or in-hospital major bleeding (P = 0.37). However, CA had a significant effect on 30-day mortality (R2 = 79.08%, P = 0.006) (Supplementary material online, Figure S8B). Multivariate analysis showed that the combined difference in CA and access site bleeding could fully account for the observed variability in 30-day mortality (R2 = 100%, P = 0.0008).
Discussion
We performed a systematic review and 14-study meta-analysis to compare periprocedural and clinical outcomes in patients with STEMI and CS undergoing PCI via TRA vs. TFA. We found that TRA was associated with lower all-cause mortality in the hospital, at 30-days and at long-term follow-up. Furthermore, TRA was associated with lower major bleeding, access site bleeding, MACCE, IABP utilization, procedure duration, and length of stay. There was no significant difference in post-PCI TIMI flow grade, procedural success, contrast volume, and fluoroscopy time between TRA and TFA.
Mortality
In the three decades since its first description, TRA has been increasingly used in clinical practice due to lower mortality compared with TFA for PCI in general. Specifically in STEMI with CS, our analysis showed TRA was associated with lower mortality than TFA at every time point. These findings are consistent with overall TRA vs. TFA use in STEMI PCI meta-analyses not specific to CS performed by Karrowni et al.23 and Singh et al.,24 respectively, but contradict the findings of the Minimizing Adverse Haemorrhagic Events by MATRIX (Transradial Access Site and Systemic Implementation of AngioX) trial25 and SAFARI-STEMI (Safety and Efficacy of Femoral Access vs. Radial Access in STEMI),26 which showed all-cause mortality was non-significantly different with TRA vs. TFA PCI in ACS patients. The benefit of TRA over TFA may be less pronounced in non-STEMI and unstable angina patients so that smaller trials with mixed ACS populations did not detect a difference.
Despite its mortality benefit, TRA is underutilized in STEMI patients with CS with significant operator and institutional variation.27 Valle et al.27 showed significant geographic, operator, and institutional variation in the use of TRA for STEMI PCI, but TRA use among all participating institutions was associated with mortality benefit. Reluctance to TRA adoption may also be due to the initial observational data, which showed lower procedural success and longer reperfusion time with TRA. These findings were also observed in analysis of the National Cardiovascular Disease Registry (NCDR) by Baklanov et al.,28 which showed slightly increased door-to-balloon time (DTB) with TRA, but slightly increased DTB was balanced by the more favourable risk-adjusted mortality rates. Finally, not only short-term mortality, but even 1-year mortality may be influenced by the choice of access site. Non-fatal femoral site complications may leave patients with significant morbidity and deconditioning, which may not be fatal immediately but still potentiate 1-year mortality.
Bleeding, stroke, and MACCE
Patients with STEMI and CS represent a high-risk population often treated with aggressive antithrombotic pharmacological and vascular interventions that convey benefits against ischaemia, albeit with higher vascular and bleeding complications. Our analysis showed TRA in STEMI with CS was associated with lower periprocedural bleeding than TFA, similar to the findings of the RIFLE-STEACS (Radial vs. Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) trial.4 A prospective analysis by Nishihira et al.29 demonstrated that periprocedural bleeding is associated with higher mortality in patients with STEMI and CS. Similarly, around 14% of noncardiac deaths reported in the Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock (SHOCK) trial were attributed to periprocedural bleeding.18,30,31 To further this observation, our analysis showed a 30-day all-cause mortality benefit among TRA patients driven by the significant difference in access site bleeding between the groups. To help with access site bleeding, ultrasound guidance may be beneficial in patients with a weak pulse and hypotension such as STEMI patients with CS. The randomized RAUST trial (Radial Artery Access with Ultrasound Trial) demonstrated reduced time and number of attempts to achieve arterial access with ultrasound guidance.32 Thus, TRA for PCI in STEMI patients with CS not only decreased periprocedural bleeding and thereby mortality, but also permitted safer utilization of robust antithrombotic therapies to improve overall ischemic outcomes in this high-risk patient population.31
There was no difference in stroke between TRA and TFA groups, which was consistent with the findings reported by Sirker et al.,33 who concluded that radial access for cardiac catheterization was not associated with an increased risk of stroke. Although stroke risk was similar among the two groups, 30-day MACCE was lower in the TRA group, driven by the difference in mortality.
IABP Utilization
Patients with STEMI and CS are frequently treated with MCS devices, for which evidence is still accumulating. Our study found that IABP use was lower in the TRA group possibly suggesting preferential selection of TFA for patients who will receive post-PCI IABP, which may be inserted transfemorally through the access site used for PCI.34 This could introduce selection bias, and the benefit of TRA on mortality and access site bleeding in our study could have been attributed to difference in the use of MCS. Therefore, we performed a meta-regression analysis based on the use of IABP and its association with 30-day mortality and access site bleeding. On meta-regression analysis, IABP use was not statistically associated with 30-day mortality or access site bleeding. Similar to IABP, newer left ventricular MCS devices including the Impella system (Abiomed, Danvers, MA, USA) also typically require femoral access for implantation,34,35 further highlighting the importance of the radial-first approach in patients for whom post-PCI transfemoral MCS devices are planned to preserve the femoral site for that purpose.
Procedural parameters
Post-PCI TIMI flow grade, procedural duration and fluoroscopy time were similar with TRA and TFA in our analysis. This finding differed from the all-STEMI meta-analysis by Singh et al.36 and reported increased fluoroscopy and DTB with TRA use in STEMI PCI, although these findings were associated with significant heterogeneity in their analysis. Also, contrast volume and procedural success were similar in our study that was contrary to the results of Sciahbasi et al.,37 who reported TRA PCI to be associated with higher contrast volume and difficulty in obtaining radial access successfully in patients with CS. These findings may reflect increasing proficiency in the TRA approach with growing adoption of TRA as a primary PCI approach. Consistent with this reasoning, Liam et al.38 reported contrast volume to be lower in procedures performed by high-volume TRA operators than low-volume TRA operators. Finally, although TRA was not associated with difference in length of stay in this high-risk population, use of TRA has been established to be associated with improved patient comfort, early ambulation, and lower healthcare cost in broad PCI populations.39–41
Clinical implications
Although TRA PCI has been associated with lower vascular complications and better mortality, this finding is linked to both procedural volume and operator expertise.42 Consequently, given better outcomes with increased operator proficiency, ESC has proposed >50 TRA cases to achieve TRA proficiency, and the Society for Cardiovascular Angiography and Interventions (SCAI) transradial working group has proposed >80 TRA cases to achieve proficiency.43,44 Additionally, given the complexity of CS PCI and decision-making for MCS with the use of TFA and TRA, a randomized controlled trial with randomization to TRA vs. TFA following SCAI shock staging at time of index CS diagnosis could delineate more definitively the optimal access approach for these high-risk STEMI patients.45 However, performing a randomized controlled comparison may itself present challenges of difficulty obtaining consent, operator proficiency and preference, and use of MCS.
Meta-analyses by Pancholy et al.,6 Gandhi et al.,46 and Del Rio-Pertuz et al.47 evaluating TRA vs. TFA PCI in STEMI-CS were published previously. The analysis by Gandhi et al. was small, including only six studies, and reported only in-hospital outcomes. Del Rio-Perutz’s work was a brief communication including only mortality as its outcome. In contrast to the analysis of eight studies by Pancholy et al., our contemporary meta-analysis adds to previous findings by including six additional studies. The previous meta-analyses differed substantially from ours by focusing only on 30-day all-cause mortality and 30-day MACCE and did not include details about periprocedural outcomes or long-term all-cause mortality. Additionally, our analysis reported details on procedural success, post-PCI coronary flow grade, procedural duration and use of IABP, which were not previously studied and are important considerations when choosing a PCI access site for STEMI patients with CS. As a result, the present study adds substantially to the literature.
Limitations
There are several important limitations of our meta-analysis. First, TRA use was highly operator-dependent with no specific selection criteria for PCI access site, leading to potential selection bias. Only one study reported when unsuccessful attempted TRA resulted in TFA use. Second, only four studies reported patient outcomes data at ≥1 year, leading to limited applicability of our results over a longer follow-up period. However, a sensitivity analysis based on matched/randomized studies and study exclusion method was reported to further minimize the unmeasured confounding in the results. Third, data about ischemic outcomes such as recurrent MI, repeat revascularization, and crossover between access sites were not available. Finally, IABP was the most commonly reported MCS device in our analysis with limited information about the use of newer MCS devices such as Impella. Impella use was only reported explicitly by one study with less than 15 patients receiving the device. All studies either reported IABP as the only MCS device or reported MCS in aggregated, so specific data about Impella use were not available. However, since 9 of the 14 studies comprising this meta-analysis were conducted before the 2015 commercial release of Impella, Impella use was likely minimal. The on-going RECOVER-IV trial will report how Impella use affects mortality in patients with STEMI and CS.
Conclusions
In PCI for STEMI with CS, TRA is associated with significantly lower mortality and bleeding complications than TFA while achieving similar TIMI3 coronary flow and procedural success rates. A randomized controlled trial evaluating the optimal access for STEMI-CS should be pursued in accordance with SCAI shock staging to evaluate the role of a ‘radial-first’ approach in this high-risk population.
Supplementary Material
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Muhammad Junaid Ahsan, Division of Cardiovascular Medicine, Iowa Heart Center, Des Moines, IA, USA.
Soban Ahmad, Department of Internal Medicine, East Carolina University, Greenville, NC, USA.
Azka Latif, Division of Cardiovascular Medicine, Creighton University, Omaha, NE, USA.
Noman Lateef, Division of Cardiovascular Medicine, University of Nebraska Medical Center, Omaha, NE, USA.
Mohammad Zoraiz Ahsan, Department of Internal Medicine, Fatima Memorial Hospital, Pakistan.
Waiel Abusnina, Division of Cardiovascular Medicine, Creighton University, Omaha, NE, USA.
Sandeep Nathan, Division of Cardiovascular Medicine, University of Chicago, Chicago, IL, USA .
S Elissa Altin, Division of Cardiovascular Medicine, Yale University, New Haven, CT, USA.
Dhaval S Kolte, Division of Cardiovascular Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
John C Messenger, Division of Cardiology Medicine, University of Colorado, Aurora, CO, USA.
Mark Tannenbaum, Division of Cardiovascular Medicine, Iowa Heart Center, Des Moines, IA, USA.
Andrew M Goldsweig, Division of Cardiovascular Medicine, University of Nebraska Medical Center, Omaha, NE, USA.
Funding
Dr Goldsweig reports receiving consulting fees from Inari Medical and grant support from the National Institute of General Medical Sciences, 1U54GM115458, and the UNMC Center for Heart and Vascular Research.
Conflict of interest
No authors report conflicts of interest to disclose.
Data availability
Data underlying this article are derived from a source in the public domain.
References
- 1. Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JPet al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2020;323:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation 2009;119:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehran R, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BRet al. ; HORIZONS-AMI Trial Investigators . Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009;374:1149–1159. [DOI] [PubMed] [Google Scholar]
- 4. Romagnoli E, Biondi-Zoccai, G, Sciahbasi, A, Politi, L, Rigattieri, S, Pendenza, Get al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol 2012;60:2481–2489. [DOI] [PubMed] [Google Scholar]
- 5. Vorobcsuk A, Kónyi A, Aradi D, Horváth IG, Ungi I, Louvard Yet al. Transradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction Systematic overview and meta-analysis. Am Heart J 2009;158:814–821. [DOI] [PubMed] [Google Scholar]
- 6. Pancholy SB, Palamaner Subash Shantha G, Romagnoli E, Kedev S, Bernat I, Rao SVet al. Impact of access site choice on outcomes of patients with cardiogenic shock undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Am Heart J 2015;170:353–361.e6. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 8. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 10. Romagnoli E, De Vita M, Burzotta F, Cortese B, Biondi-Zoccai G, Summaria Fet al. Radial versus femoral approach comparison in percutaneous coronary intervention with intraaortic balloon pump support: the Radial Pump Up registry. Am Heart J 2013;166:1019–1026. [DOI] [PubMed] [Google Scholar]
- 11. Bernat I, Abdelaal E, Plourde G, Bataille Y, Cech J, Pesek Jet al. Early and late outcomes after primary percutaneous coronary intervention by radial or femoral approach in patients presenting in acute ST-elevation myocardial infarction and cardiogenic shock. Am Heart J 2013;165:338–343. [DOI] [PubMed] [Google Scholar]
- 12. Fujii T, Masuda N, Ijichi T, Kamiyama Y, Tanaka S, Nakazawa Get al. Transradial intervention for patients with ST elevation myocardial infarction with or without cardiogenic shock. Catheter Cardiovasc Interv 2014;83:E1–E7. [DOI] [PubMed] [Google Scholar]
- 13. Iga A, Wagatsuma K, Yamazaki J, Ikeda T. Transradial versus transfemoral coronary intervention for acute myocardial infarction complicated by cardiogenic shock: is transradial coronary intervention suitable for emergency PCI in high-risk acute myocardial infarction? J Invasive Cardiol 2014;26:196–202. [PubMed] [Google Scholar]
- 14. Kedev S, Kalpak O, Dharma S, Antov S, Kostov J, Pejkov Het al. Complete transitioning to the radial approach for primary percutaneous coronary intervention: a real-world single-center registry of 1808 consecutive patients with acute ST-elevation myocardial infarction. J Invasive Cardiol 2014;26:475–482. [PubMed] [Google Scholar]
- 15. Mamas MA, Anderson SG, Ratib K, Routledge H, Neyses L, Fraser DGet al. ; British Cardiovascular Intervention Society; National Institute for Cardiovascular Outcomes Research . Arterial access site utilization in cardiogenic shock in the United Kingdom: is radial access feasible? Am Heart J 2014;167:900–908.e1e1. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez-Leor O, Fernandez-Nofrerias E, Carrillo X, Mauri J, Oliete C, Rivas Mdel Cet al. Transradial percutaneous coronary intervention in cardiogenic shock: a single-center experience. Am Heart J 2013;165:280–285. [DOI] [PubMed] [Google Scholar]
- 17. Roule V, Lemaitre A, Sabatier R, Lognoné T, Dahdouh Z, Berger Let al. Transradial versus transfemoral approach for percutaneous coronary intervention in cardiogenic shock: a radial-first centre experience and meta-analysis of published studies. Arch Cardiovasc Dis 2015;108:563–575. [DOI] [PubMed] [Google Scholar]
- 18. Guedeney P, Thiele H, Kerneis M, Barthélémy O, Baumann S, Sandri Met al. ; CULPRIT-SHOCK Investigators . Radial versus femoral artery access for percutaneous coronary artery intervention in patients with acute myocardial infarction and multivessel disease complicated by cardiogenic shock: subanalysis from the CULPRIT-SHOCK trial. Am Heart J 2020;225:60–68. [DOI] [PubMed] [Google Scholar]
- 19. Tehrani BN, Damluji AA, Sherwood MW, Rosner C, Truesdell AG, Epps KCet al. Transradial access in acute myocardial infarction complicated by cardiogenic shock: stratified analysis by shock severity. Catheter Cardiovasc Interv 2021;97:1354–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kubo S, Yamaji K, Inohara T, Kohsaka S, Tanaka H, Ishii Het al. In-hospital outcomes after percutaneous coronary intervention for acute coronary syndrome with cardiogenic shock [from a Japanese nationwide registry (J-PCI Registry)]. Am J Cardiol 2019;123:1595–1601. [DOI] [PubMed] [Google Scholar]
- 21. Zahn R, Hochadel M, Schumacher B, Pauschinger M, Stellbrink C, Schaechinger Vet al. Cardiogenic shock and radial access in patients with an acute ST elevation myocardial infarction. Eur Heart J 2020;41. [Google Scholar]
- 22. Tokarek T, Dziewierz A, Plens K, Rakowski T, Dudek D, Siudak Z. Radial approach reduces mortality in patients with ST-segment elevation myocardial infarction and cardiogenic shock. Pol Arch Intern Med 2021;131:421–428. [DOI] [PubMed] [Google Scholar]
- 23. Karrowni W, Vyas A, Giacomino B, Schweizer M, Blevins A, Girotra Set al. Radial versus femoral access for primary percutaneous interventions in ST-segment elevation myocardial infarction patients: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv 2013;6:814–823. [DOI] [PubMed] [Google Scholar]
- 24. Singh S, Singh M, Grewal N, Khosla S. Transradial vs transfemoral percutaneous coronary intervention in ST-segment elevation myocardial infarction: a systemic review and meta-analysis. Can J Cardiol 2016;32:777–790. [DOI] [PubMed] [Google Scholar]
- 25. Valgimigli M, Frigoli E, Leonardi S, Vranckx P, Rothenbühler M, Tebaldi Met al. ; MATRIX Investigators . Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet 2018;392:835–848. [DOI] [PubMed] [Google Scholar]
- 26. Le May M, Wells G, So D, Chong AY, Dick A, Froeschl Met al. Safety and efficacy of femoral access vs radial access in ST-segment elevation myocardial infarction: The SAFARI-STEMI randomized clinical trial. JAMA Cardiol 2020;5:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valle JA, Kaltenbach LA, Bradley SM, Yeh RW, Rao SV, Gurm HSet al. Variation in the adoption of transradial access for ST-segment elevation myocardial infarction: insights from the NCDR CathPCI registry. JACC Cardiovasc Interv 2017;10:2242–2254. [DOI] [PubMed] [Google Scholar]
- 28. Baklanov DV, Kaltenbach LA, Marso SP, Subherwal SS, Feldman DN, Garratt KNet al. The prevalence and outcomes of transradial percutaneous coronary intervention for ST-segment elevation myocardial infarction: analysis from the National Cardiovascular Data Registry (2007 to 2011). J Am Coll Cardiol 2013;61:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishihira K, Honda S, Takegami M, Kojima S, Asaumi Y, Suzuki Met al. Impact of bleeding on mortality in patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2021;10:388–396. [DOI] [PubMed] [Google Scholar]
- 30. Jeger RV, Assmann SF, Yehudai L, Ramanathan K, Farkouh ME, Hochman JS. Causes of death and re-hospitalization in cardiogenic shock. Acute Card Care 2007;9:25–33. [DOI] [PubMed] [Google Scholar]
- 31. Schoenfeld MS, Kassas I, Shah B. Transradial artery access in percutaneous coronary intervention for ST-segment elevation myocardial infarction and cardiogenic shock. Curr Treat Options Cardiovasc Med 2018;20:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seto AH, Roberts JS, Abu-Fadel MS, Czak SJ, Latif F, Jain SPet al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv 2015;8:283–291. [DOI] [PubMed] [Google Scholar]
- 33. Sirker A, Kwok CS, Kotronias R, Bagur R, Bertrand O, Butler Ret al. Influence of access site choice for cardiac catheterization on risk of adverse neurological events: a systematic review and meta-analysis. Am Heart J 2016;181:107–119. [DOI] [PubMed] [Google Scholar]
- 34. Nwaejike N, Son AY, Patel CB, Schroder JN, Milano CA, Daneshmand MA. The axillary intra-aortic balloon pump as a bridge to recovery allows early ambulation in long-term use: case series and literature review. Innovations (Phila) 2017;12:472–478. [DOI] [PubMed] [Google Scholar]
- 35. Estep JD, Cordero-Reyes AM, Bhimaraj A, Trachtenberg B, Khalil N, Loebe Met al. Percutaneous placement of an intra-aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long-term support as bridge to heart transplantation. JACC Heart Fail 2013;1:382–388. [DOI] [PubMed] [Google Scholar]
- 36. Singh S, Singh M, Grewal N, Khosla S. The fluoroscopy time, door to balloon time, contrast volume use and prevalence of vascular access site failure with transradial versus transfemoral approach in ST segment elevation myocardial infarction: a systematic review & meta-analysis. Cardiovasc Revasc Med 2015;16:491–497. [DOI] [PubMed] [Google Scholar]
- 37. Sciahbasi A, Romagnoli E, Burzotta F, Trani C, Sarandrea A, Summaria Fet al. Transradial approach (left vs right) and procedural times during percutaneous coronary procedures: TALENT study. Am Heart J 2011;161:172–179. [DOI] [PubMed] [Google Scholar]
- 38. Lim YH, Lee Y, Shin J, Yoon J, Lee SH, Rha SWet al. Comparisons of clinical and procedural outcomes between transradial and transfemoral approaches in percutaneous coronary intervention (from the Korean Transradial Intervention Prospective Registry). Am J Cardiol 2016;117:1272–1281. [DOI] [PubMed] [Google Scholar]
- 39. Cooper CJ, El-Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu Aet al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J 1999;138:430–436. [DOI] [PubMed] [Google Scholar]
- 40. Mitchell MD, Hong JA, Lee BY, Umscheid CA, Bartsch SM, Don CW. Systematic review and cost–benefit analysis of radial artery access for coronary angiography and intervention. Circ Cardiovasc Qual Outcomes 2012;5:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Safley DM, Amin AP, House JA, Baklanov D, Mills R, Giersiefen Het al. Comparison of costs between transradial and transfemoral percutaneous coronary intervention: a cohort analysis from the Premier research database. Am Heart J 2013;165:303–309e2. [DOI] [PubMed] [Google Scholar]
- 42. Hulme W, Sperrin M, Rushton H, Ludman PF, De Belder M, Curzen Net al. Is there a relationship of operator and center volume with access site-related outcomes? An analysis from the British Cardiovascular Intervention Society. Circ Cardiovasc Interv 2016;9:e003333. [DOI] [PubMed] [Google Scholar]
- 43. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti Fet al. ; ESC Scientific Document Group . [2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC)]. G Ital Cardiol (Rome) 2016;17:831–872. [DOI] [PubMed] [Google Scholar]
- 44. Rao SV, Tremmel JA, Gilchrist IC, Shah PB, Gulati R, Shroff ARet al. ; Society for Cardiovascular Angiography and Intervention's Transradial Working Group . Best practices for transradial angiography and intervention: a consensus statement from the Society for Cardiovascular Angiography and Intervention's Transradial Working Group. Catheter Cardiovasc Interv 2014;83:228–236. [DOI] [PubMed] [Google Scholar]
- 45. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TDet al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29–37. [DOI] [PubMed] [Google Scholar]
- 46. Gandhi S, Kakar R, Overgaard CB, Comparison of radial to femoral PCI in acute myocardial infarction and cardiogenic shock: a systematic review. J Thromb Thrombolysis 2015;40:108–117. [DOI] [PubMed] [Google Scholar]
- 47. Del Rio-Pertuz G, Mekraksakit P, Ansari MM. Meta-analysis comparing vascular access site on mortality in patients undergoing primary percutaneous coronary intervention with ST-elevation myocardial infarction complicated by cardiogenic shock. Am J Cardiol 2022;168:173–174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying this article are derived from a source in the public domain.