Abstract
Objective
The nine-item Shared Decision-Making Questionnaire (SDM-Q-9) is one of the most frequently used tools for assessing patients’ involvement in medical decision-making, but so far, it not been validated in Italian. We aimed to validate the Italian version of the SDM-Q-9 in a clinical sample of patients suffering from major psychiatric disorders.
Method
We involved 307 consecutive patients affected by major psychiatric disorders (including schizophrenia spectrum disorders, affective disorders and eating disorders) in a real-world outpatient clinical setting. Confirmatory Factor Analysis (CFA) was conducted to examine the latent structure of the SDM-Q-9. Cronbach’s alpha and correlations between the SDM-Q-9 and the Observing Patient Involvement (OPTION) scale were calculated to measure internal consistency and convergent validity respectively.
Results
The final sample was made up of 289 participants (response rate 94.1%) who completed the assessment. CFA confirmed the unidimensional structure as in the original version (χ2/df= 1.69; CFI= 0.98; TLI= 0.97; RMSEA= 0.05; SRMR= 0.08). Internal consistency of the total scale was Cronbach’s α = .86. Regarding construct validity, we found several correlations between the SDM-Q-9 and OPTION scale.
Conclusions
Our findings suggest that the Italian version of SDM-Q-9 performs well if compared to other languages validated versions, so it is a useful patient-centred measure to assess the involvement in medical decision-making (SDM) of patients from clinical samples from the Italian-speaking population.
Keywords: patient participation, psychometrics, shared decision making, survey and questionnaires, validation study
Introduction
In recent years, many studies place patients at the center of health care research and evaluate clinical care in order to improve their experience. Patients’ involvement in clinical research led to the use of patient-reported outcome measures (PROMs). These instruments describe a person’s perception of their own health through test and enable patients to report on their quality of life, symptoms, daily functioning, and other aspects of their health and well-being (Weldring & Smith, 2013).
In this theoretical framework, the Shared Decision Making (SDM) stands out. The SDM strategy is a clinical decision making model that ensures health care professionals (HCPs) not to make decisions solely on the basis of their own knowledge, experience, and the latest scientific evidence (namely paternalistic approach), but it also promotes HCPs to inform patients broadly, letting them take part in all important aspects of the medical decision (Charles et al., 1997; Kriston et al., 2010). Among several definitions of SDM (Makoul & Clayman, 2006; Moumjid et al., 2007), one of the most common descriptions is an interactive process in which both parties (patient and physician) are equally and actively involved and share information in order to reach an agreement, for which they are jointly responsible (Härter, 2004).
SDM is promoted in many health care systems and several medical and surgery specialties, and is gaining importance in many health contexts worldwide (Härter et al., 2011; France Légaré et al., 2008). There are numerous reasons for this shift in perspective: patients’ expanding knowledge of diseases and treatments through media, increasing numbers of available treatment options, and direct patients’ and physicians’ preferences for more active patient involvement (Adams et al., 2007; Chewning et al., 2012; Coulter & Jenkinson, 2005; Hamann et al., 2012; Say et al., 2006). SDM is therefore a kind of relationship that involves at least one patient and one HCP at the same time. Both parties take steps to actively participate in the process of decision making, share information and personal values, and together arrive at a treatment decision with shared responsibility. In fact, it stands in stark contrast to the old model of paternalistic medicine (Ankolekar et al., 2019).
SDM is indicated in most medical contexts, especially if multiple potential treatments and the alternatives are available with different and uncertain outcomes. This scenario is very common in most chronic diseases (Charles et al., 1997; Kriston et al., 2010; Makoul & Clayman, 2006; D Simon, Loh, & Härter, 2008), or when there is a medical decision-making process (F Légaré et al., 2012; Rockenbauch & Schildmann, 2011), and psychiatry often meets both criteria (Gunay Molu et al., 1969; Hays, 1995). SDM can help both patients and HCPs reach treatment agreement in long-term treatment decisions (de Filippis et al., 2021; Rockenbauch & Schildmann, 2011; D Simon, Loh, & Härter, 2008). Major patient involvement in treatment decision making leads to fewer decisional and clinical conflicts, which can be interpreted as a moderator of patient satisfaction (Joosten et al., 2008). SDM is associated with feelings of autonomy, control, and individual competence which have positive results in terms of compliance and therapeutic adherence, and follow-up (Hölzel et al., 2013). Interventions to facilitate SDM are becoming increasingly important, and their results need to be assessed and measured to be standardized. Up-to-date, more research is needed on the clinical implications of SDM (Glyn Elwyn et al., 2012).
Assessment tools to evaluate SDM can be classified by decision antecedents (e.g., role preference), the decision process (e.g., observed or perceived behavior of the clinician), or decision outcomes (e.g., decisional conflict, decisional regret, satisfaction) (Joosten et al., 2008). Thus, SDM can be assessed from different viewpoints: by an external observer, the patient, or the physician (I Scholl, Koelewijn-van Loon, et al., 2011). Although SDM is described as a relationship involving both patient and health care provider, only a few tools are available to assess SDM from both the patient’s and the physician’s points of view: the dyadic OPTION (observing patient involvement) scale (Melbourne et al., 2011), the MAPPIN'SDM measure (Kasper et al., 2012), and the 9-item Shared Decision Making Questionnaire (SDM-Q-9), published in 2010 (Kriston et al., 2010). Among them, the SDM-Q-9 appears to be the most used to assess interventions aiming to improve SDM. This is likely due to its features: psychometric testing, acceptance, and feasibility of administration with only nine items (I Scholl, Koelewijn-van Loon, et al., 2011). Indeed, the SDM-Q-9 is a patient-reported measure that focuses on the decisional process by rating physicians’ and patients’ behavior in medical encounters.
The same core research team created the physician version of the SDM-Q-9, the Shared Decision Making Questionnaire – Physician Version (SDM-Q-Doc), which measures the same aspects of SDM, but from the physician’s perspective (Isabelle Scholl et al., 2012).
Currently, the SMD-Q-9 is available in several languages (www.sdmq9.org,n.d.), including the original German version (I Scholl, Kriston, et al., 2011), as well as Arabic (Alzubaidi et al., 2019), Dutch (Rodenburg-Vandenbussche et al., 2015), Hungarian (Rencz et al., 2019), and Spanish (De las Cuevas et al., 2015), but, to the best of the authors’ knowledge, no validation of the SDM-Q-9 has been conducted in Italian, thus severely limiting the analysis of SDM in this language (www. sdmq9. org, n.d.).
Therefore, with this study, we aimed to assess the psychometric properties of the Italian version of SDM-Q-9 (i.e. factor structure, internal consistency, and construct validity) in a real-world outpatient clinical sample of patients suffering from major psychiatric disorders. We expected to find an adequate model fit as measured by the confirmatory factor analysis (CFA), a good internal consistency (α ≥ 70), and positive correlations between the SDM-Q-9 and the OPTION scale.
Materials and Methods
Participants and procedure
Participants have been consecutively recruited at the psychiatric outpatient services of the University Hospital ‘Mater Domini’, Catanzaro (Italy) for 12 months (October 2020 – September 2021) and have been evaluated by experienced clinicians according to the Mini International Neuropsychiatric Interview (MINI) with a minimum of 6 months follow-up time of patients. The interviewers were clinicians who worked in this area and were trained in the administration of specific neuropsychiatric and neuropsychological tests. We recruited subjects in a naturalistic setting with any kind of psychiatric disease according to DSM-5 diagnostic criteria, including major depressive disorder (MDD), bipolar disorder (BD), schizophrenia spectrum disorders (SSD), anxiety disorders, obsessive-compulsive disorder (OCD), personality disorders, and eating disorders (EDs).
All participants were considered eligible if they were: (1) free of diagnoses of substance abuse or dependence for ≥6 months; (2) free of any serious neurological or medical condition; (3) aged between 18 and 65 years and able to read and understand the informed consent form; (4) chart diagnosis of BD type I, BD type II, cyclothymia, MDD, dysthymia, SSD, OCD, anxiety disorders, personality disorders or EDs according to the DSM-5 (American Psychiatric Association, 2013); and (5) in remission at the time of the assessment according to a Clinical Global Impression (CGI) score ≤2 (Guy, 1976).
All participants who met the following criteria were excluded: (1) clinical diagnosis of pervasive developmental disorder or autism spectrum disorder according to the DSM-5; (2) difficulty in understanding the proposed assessment; (3) diagnosis of intellectual disability from mild to severe according to DSM-5 (corresponding to IQ < 70) (Gluck, 2014); (4) history of a medical or neurological disease that could affect cognitive function; and (5) medical and psychiatric history that was implausible or undocumented.
All eligible candidates were informed about the aims and procedures of the study, the voluntariness of participation, anonymity, and safety of data, and that no clinical or economic benefits would be given for the participation. A written informed consent was signed before any further step took place. The study protocol was submitted and approved by the local Ethical Committee of University Hospital Mater Domini at Catanzaro (Italy) ‘Regione Calabria, sezione Area Centro’ before collecting any data. The study protocol and procedures complied with the ethical principles set out in the revised version of Helsinki Declaration (World Medical Association, 2013).
Measures
All participants underwent a comprehensive assessment and clinical evaluation at their baseline or follow-up in person visits by means of the following tests: (1) the 9-item Shared Decision Making Questionnaire (SDM-Q-9) (Kriston et al., 2010), and (2) the OPTION Scale (Goss et al., 2007).
Consultations were evaluated using the OPTION Scale, already validated in Italian (Goss et al., 2007). Patients completed by themselves the SDM-Q-9 assessment, after the consultation.
The following assessment tools were administrated to every participant, without distinction from diagnosis:
The nine-item Shared Decision-Making Questionnaire (SDM-Q-9) is a self-reported questionnaire designed to assess patients’ views on SDM occurred in a consultation with a healthcare provider (Kriston et al., 2010). It contains two open-ended questions [‘Please indicate which health complaint/ problem/illness the consultation was about’ and ‘Please indicate which decision was made’] followed by nine closed questions.
Each closed question is represented by a statement featuring various aspects of SDM, rated on a 6-point balanced scale ranging from 0 (= ‘completely disagree’) to 5 (= ‘completely agree’). The total score, calculated by summing the score of the nine items, is expressed on a scale ranging between 0 and 45, where a higher score represents a greater level of perceived SDM. Following earlier studies, we will rescale the raw total scores to a 0–100 range (Kriston et al., 2010). The SDM-Q-9 was translated into English and Italian, allowing for use in international research (Kriston et al., 2010).
The OPTION (observing patient involvement) Scale (G Elwyn et al., 2005): this is a 12 item five-point scale (from 0 (behavior not observed) to 4 (high standard)). The raw total score ranges from 0 (0 level in all items) to 48 (four level in all items). Scores are normally transformed into a 0–100 score (Goss et al., 2007).
In addition, socio-demographic and clinical data were collected.
Statistical analysis
Confirmatory Factor Analysis (CFA) was run through the open-source software JASP (JASP, Version 0.15, University of Amsterdam, The Netherlands) to better examine the structure of the Italian version of SDM-Q-9. The Diagonal Weighted Least Squares (DWLS) estimator was used to estimate the parameters since it provides the best option for categorical or ordered data (Buck et al., 2020).
The Tucker-Lewis Index (TLI), the Comparative Fit Index (CFI), the Root Mean Square Error of Approximation (RMSEA), Standardized Root Mean Squared Residual (SRMR) and its chi-square (χ2/df) were used to evaluate the goodness of adaptation of the data to the proposed model.
For TLI and CFI, values of .90 and above are considered adequate, while values of .95 or above are considered excellent; for RMSEA values of .08 and below are considered adequate and values of .05 or below are considered excellent; for SRMR a cut-off values close to .08 are considered adequate. Values of χ2/df <3.0 are considered good, while values <2.0 are excellent. The levels of these indices were assessed according to the recommendations of Hu and Bentler (Hu & Bentler, 1999).
We also calculated the internal consistency and the ideal Cronbach’s alpha was settled at >.70.
Finally, we ran the Spearman correlations between the SDM-Q-9 and the OPTION in order to measure the convergent validity. A p < 0.05 was considered statistically significant.
Results
We approached a total of 307 consecutive patients fulfilling the inclusion/exclusion criteria. Of these, 18 patients refused to participate in the study because they did not have the time to complete the assessment (n=11), did not sign the informed consent (n=5), or for other reasons (n=2). Therefore, we reached a 94.1% participation rate and the final sample was made up of 289 patients, with an average age of 42.4 ± 17.5 years; the majority of subjects were female (n=170; 58.8%), non-smoker (n=199; 68.9%), single (n=147; 50.9%), office workers or unemployed (n=65; 22.5%, each), mostly suffering from MDD (n=62; 21.5%) and SSD (n=60; 20.8%) (table 1).
Table 1.
Socio-demographics characteristics of the sample
| Total Sample N = 289 | |||
|---|---|---|---|
| Agea | 42.4 | (17.5) | |
| Genderb | Men | 119 | (41.2) |
| Women | 170 | (58.8) | |
| Smokerb | No | 199 | (68.9) |
| Yes | 90 | (31.1) | |
| Civil Statusb | Single | 147 | (50.9) |
| Married | 127 | (43.9) | |
| Divorced | 11 | (3.8) | |
| Widow | 4 | (1.4) | |
| Occupationb | Office worker | 65 | (22.5) |
| Unemployed | 65 | (22.5) | |
| Student | 63 | (21.8) | |
| Housewife | 33 | (11.4) | |
| Self-employer | 27 | (9.3) | |
| Retired | 26 | (9.0) | |
| Disable | 10 | (3.5) | |
| Diagnosisb | Schizophrenia Spectrum Disorder | 60 | (20.8) |
| Major Depressive Disorder | 62 | (21.5) | |
| Anxiety Disorder | 43 | (14.9) | |
| Bipolar Disorder | 32 | (11.1) | |
| Anorexia Nervosa | 24 | (8.3) | |
| Bulimia Nervosa | 20 | (6.9) | |
| Binge Eating Disorder | 15 | (5.2) | |
| Obsessive-Compulsive Disorder | 11 | (3.8) | |
| Personality Disorder | 13 | (4.5) | |
| Eating Disorders Not Otherwise Specified | 9 | (3.1) | |
a Data are presented as means (SD)
b Data are presented as frequencies (%)
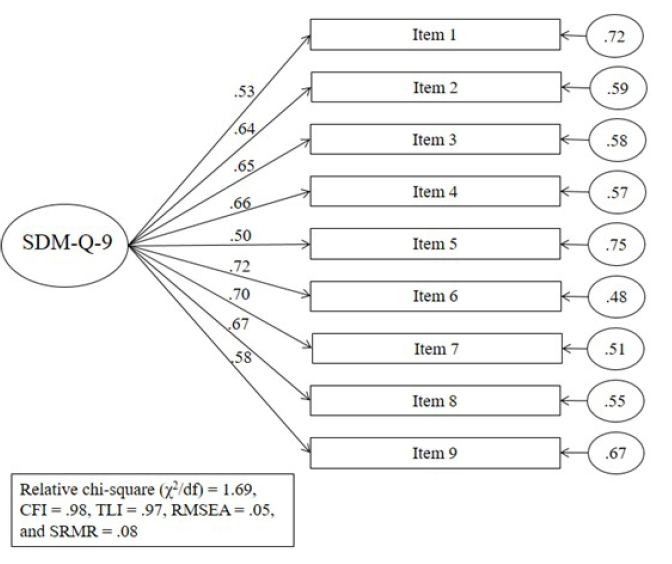
The CFA showed an excellent fit: relative chi-square (χ2/df) = 1.69, CFI = .98, TLI = .97, RMSEA = .05, and SRMR = .08, suggesting the suitability of the Italian version of SDM-Q-9 model (figure 1).
Figure 1.
Confirmatory Factor Analysis of the Italian version of the SDM-Q-9
The Cronbach’s alpha coefficient was .86 for the total score indicating very good reliability of the questionnaire. Regarding convergent validity, significant although weak correlations emerged between the items of OPTION and SDM-Q-9 (table 2).
Table 2.
Correlation of single items and total scores of the SDM-Q-9 and OPTION Scale
| SDM-Q-9 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | TOTAL | ||
| OPTION | Item 1 | .109 | .100 | .064 | .075 | .094 | .058 | .118* | .019 | .001 | .096 |
| Item 2 | .159** | .129* | .055 | .064 | .094 | .011 | .115 | .097 | .093 | .113 | |
| Item 3 | .118* | .090 | .084 | -.010 | .042 | -.004 | .021 | .063 | .047 | .099 | |
| Item 4 | .071 | .095 | .107 | .175** | .169** | .126* | .178** | .157** | .135* | .184** | |
| Item 5 | -.026 | .034 | .072 | .055 | .122* | .107 | .125* | .066 | .064 | .107 | |
| Item 6 | .087 | .032 | .132* | .034 | .142* | .057 | .085 | .083 | .078 | .131* | |
| Item 7 | .007 | .088 | .076 | .065 | .111 | .024 | -.004 | -.058 | .035 | .074 | |
| Item 8 | .087 | .096 | .160** | .140* | .046 | .121* | .001 | .007 | .090 | .146* | |
| Item 9 | .116* | .007 | .058 | .004 | .083 | .124* | .034 | .020 | -.004 | .077 | |
| Item 10 | .047 | .017 | .050 | .044 | .025 | .056 | .028 | -.034 | -.027 | .062 | |
| Item 11 | .094 | .043 | -.011 | -.005 | .049 | .021 | .105 | -.003 | -.019 | .062 | |
| Item 12 | .107 | .035 | -.063 | -.011 | .036 | -.011 | .036 | .015 | -.030 | .023 | |
| TOTAL | .171** | .133* | .132* | .111 | .129* | .113 | .141* | .117* | .088 | .200** | |
OPTION: observing patient involvement scale; SDM-Q-9: The nine-item Shared Decision-Making Questionnaire.
* p-value < .05; ** p-value < .01.
Discussion
The SDM collaborative process by which HCPs support patients in decision-making process about their treatment has gained fundamental importance in today’s health practice. Interventions to improve SDM have become relevant, and routine and their results have to be measured. In this cross-sectional study we evaluated the psychometric features of the Italian version of the SDM-Q-9 in a real-world clinical sample of patients affected by several major psychiatric disorders. The aim was to validate the Italian translated version of the scale in an outpatient clinical setting. The Italian version of the SMD-Q-9 showed excellent performance and the overall quality data was adequate, thus demonstrating to be a reliable, brief and well accepted instrument even in a clinical sample.
In our study, we excluded patients affected by comorbid substance and alcohol dependence during the previous 6 months. This is because patients with alcohol or drug abuse more frequently report an impaired SDM ability and high levels of impulsivity, thus resulting into a biased outcome which could affect scores interpretation (Carrà et al., 2016; Luciano et al., 2020).
Our results show up a high internal consistency (Cronbach’s alpha >0.9) of the Italian version of the SDM-Q-9 as well as satisfactory item discriminations, both implying a good reliability of the instrument. This result stands in line with similar acceptable reliability of the scale in other languages (Alzubaidi et al., 2019; Rodenburg-Vandenbussche et al., 2015) and analogous results to those observed for English-speaking primary care patients (Glass et al., 2012).
Additionally, the low patients drop-out rate, with complete response rate over 94.1% denotes a high acceptance of the assessment by the patients. This result confirms and improves what is already known about SDM-Q-9 good acceptability, reliability and validity as indicated by good response rates (≥80%) (De las Cuevas et al., 2015), and appears to be very high even when compared to the response rate to online surveys (Burns et al., 2008). Therefore, we can summarize that the adequate sample size together with a high response rate constitute a strength of our work that reinforces its validity (Nulty, 2008; Sihoe, 2015).
The internal consistency results was high and comparable to those reported for the German (Kriston et al., 2010) (0.94), Spanish (De las Cuevas et al., 2015) (0.89) and Arabic (Alzubaidi et al., 2019) (0.929) versions, thus demonstrating a similar scale reliability in different languages.
In our study, there was no need to test different single-factor models excluding specific SDM-Q-9 items, as we tested all its items as expected in the first original study version with excellent results (Kriston et al., 2010; I Scholl, Kriston, et al., 2011). Therefore, these findings are superior and in contrast to what reported in some other validation versions in different languages, where it was necessary to test four single-factor models, finally excluding SDM-Q-9 item 1 and 9, to obtain a significative statistical model (Alvarez et al., 2016; Rodenburg-Vandenbussche et al., 2015).
Thus, our psychometric results for the Italian version of SDM-Q-9 are consistent with what has been previously reported in the literature for the English, Dutch, German, and Spanish versions (De las Cuevas et al., 2015; Glass et al., 2012; Kriston et al., 2010; Rodenburg-Vandenbussche et al., 2015).
Based on these results, we can therefore speculate that although some peculiar dimensions of SDM (e.g., clarity on the need to make a medical decision, or mutual agreement on how to proceed) might be culturally influenced, and experienced and rated differently depending the cultural values and expectations for empowerment of diverse patients (Cortes et al., 2009), the different translations of SDM-Q-9 seem to be a valid tool to assess SDM in already validated languages, including Italian (Alvarez et al., 2016).
Among a huge range of assessment tools measuring different aspects of SDM (Austin et al., 2015; Daniela Simon et al., 2007), only a small number have been widely validated and psychometrically tested (H et al., 2017; Kriston et al., 2010). In this scenario, the SDM-Q-9 represents a quick, feasible and consistent self-assessment tool measuring the soundness of SDM (Alzubaidi et al., 2019; Rencz et al., 2019). Since the psychometric properties of instruments and the clinical validity of assessments cannot a priori be transferred to its translations (D Simon, Loh, & Harter, 2008), and considering that the validation of the Italian version of SDM-Q-9 was still missing (www.sdmq9.org, n.d.), its evaluation was long time overdue.
However, it should not be underestimated that the SDM-Q-9 focuses on the progression rather than on the outcome of SDM (Kriston et al., 2010). Therefore, we might speculate that informing about different treatment options, explaining their advantages and disadvantages, exploration of treatment preference and weighing options build a core set that patients and physicians share, before or even without achieving full recovery (Kriston et al., 2012).
Although there is a growing body of literature about the positive influence of high SDM level in all medicine fields, including psychiatry, with positive impact on adherence and clinical outcomes, the real application of SDM techniques in clinical practice is still modest (Chong et al., 2013; Clarke et al., 2015). This could be explained both from the poor health care providers attention to this aspect, and to the lack of adequate and reliable tools to measure and evaluate SDM in the everyday clinical practice (Brinkman, 2011; Luciano et al., 2020). Our study stands in line with this idea and aims to increase clinicians’ awareness about the clinical utility of SDM as well as to provide a solid and feasible assessment tool translated and validated in Italian, with the goal to measure and increase the SDM and its clinical applications.
Strengths and limitations
The results of our study should be interpretated in the light of some limitations. First, the main limitation of the study is the nature itself of the SDM-Q-9 as a self-report questionnaire that is potentially biased by the risk of inaccurate recall of respondents’ experiences, hiding, social desirability, and misunderstanding. For this reason, further studies are needed to evaluate the clinical properties of the SDM-Q-9 using clinimetrics, an innovative approach, which has been refined as the science of clinical measurements. Clinimetrics has seen important developments with the introduction of Clinimetric Patient-Reported Outcome Measures (CLIPROM) criteria that represent a step forward to the development and validation process of rating scales (Carrozzino et al., 2021). Second, in the study protocol we did not include a test-retest reliability. Nevertheless, the reproducibility over time, otherwise known as test-retest reliability, is just one of various methods to evaluate and measure reliability, which include also internal consistency, inter-rater reliability and convergent validity compared to the gold-standard tool (i.e., the OPTION scale). Indeed, internal consistency estimates how the individual scores of the items correlate with each other (Cook & Beckman, 2006), and in the current study, it was demonstrated to be satisfactory. Third, the enrolled sample presented a broad clinical variability in terms of diagnosis, severity, and duration of disease, which makes the final sample very heterogeneous. Such a varied sample composition has long been aimed at by previous SDM-Q-9 validation studies, in order to increase the environmental complexity and shared clinical decision making and thus make the study more reliable and reproducible in the real-world (De las Cuevas et al., 2015; Isabelle Scholl et al., 2015).
On the other hand, our study presents some important strengths. First, although a comparable gold-standard self-administrated instrument does not exist, we used the OPTION scale as a proper assessment of the convergent validity. Indeed, these two tools represent the most validated assessments in literature to evaluate SDM from the patient (Kriston et al., 2010), physician (Isabelle Scholl et al., 2012), or an external observer point of view (G Elwyn, 2003). Moreover, the validation was performed in a real-world clinical setting, with a sample made up of patients suffering from several different major psychiatric disorders, and with a large and adequate sample size. Indeed, recommendations for the sample size used to validate an assessment tool suggest ranging from 2 to 20 subjects per tool item (Hair et al., 1995), with a total minimum number of 100 to 250 subjects anyway (Anthoine et al., 2014). Moreover, Comrey and Lee (1992) provided the following guidance: 100 subjects=poor, 200=fair, 300=good, 500=very good, ≥1000=excellent (Comrey & Lee, 1992). Therefore, with a total of 231 participants with a 9-items tool, according to all these recommendations, our sample can be considered more than fair to validate the scale.
Conclusion
In conclusion, the psychometric properties of the Italian version of SDM-Q-9 are largely equivalent to the original and other language validation versions with a high internal consistency, even when compared to OPTION scale. Therefore, the Italian version of the SDM-Q-9 has been demonstrated in a sample of patients affected by major psychiatric disorders to be a useful tool to investigate shared decision making in the clinical setting.
References
- Adams, J. R., Drake, R. E., & Wolford, G. L. (2007). Shared decision-making preferences of people with severe mental illness. Psychiatric Services (Washington, D.C.), 58(9), 1219–1221. 10.1176/ps.2007.58.9.1219 [DOI] [PubMed] [Google Scholar]
- Alvarez, K., Wang, Y., Alegria, M., Ault-Brutus, A., Ramanayake, N., Yeh, Y.-H., Jeffries, J. R., & Shrout, P. E. (2016). Psychometrics of shared decision making and communication as patient centered measures for two language groups. Psychological Assessment, 28(9), 1074– 1086. 10.1037/pas0000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzubaidi, H., Hussein, A., McNamara, K., & Scholl, I. (2019). Psychometric properties of the Arabic version of the 9-item Shared Decision-Making Questionnaire: the entire process from translation to validation. BMJ Open, 9(4), e026672. 10.1136/bmjopen-2018-026672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.).
- Ankolekar, A., Vanneste, B. G. L., Bloemen-van Gurp, E., van Roermund, J. G., van Limbergen, E. J., van de Beek, K., Marcelissen, T., Zambon, V., Oelke, M., Dekker, A., Roumen, C., Lambin, P., Berlanga, A., & Fijten, R. (2019). Development and validation of a patient decision aid for prostate Cancer therapy: from paternalistic towards participative shared decision making. BMC Medical Informatics and Decision Making, 19(1), 130. 10.1186/s12911-019-0862-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthoine, E., Moret, L., Regnault, A., Sébille, V., & Hardouin, J.-B. (2014). Sample size used to validate a scale: a review of publications on newly-developed patient reported outcomes measures. Health and Quality of Life Outcomes, 12(1), 2. 10.1186/s12955-014-0176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin, C. A., Mohottige, D., Sudore, R. L., Smith, A. K., & Hanson, L. C. (2015). Tools to Promote Shared Decision Making in Serious Illness. JAMA Internal Medicine, 175(7), 1213. 10.1001/jamainternmed.2015.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman, W. B. (2011). Physicians’ Shared Decision-Making Behaviors in Attention-Deficit/Hyperactivity Disorder Care. Archives of Pediatrics & Adolescent Medicine, 165(11), 1013. 10.1001/archpediatrics.2011.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, B., Browne, J., Gagen, E. C., & Penn, D. L. (2020). Hostile attribution bias in schizophrenia-spectrum disorders: narrative review of the literature and persisting questions. Journal of Mental Health, 1–18. 10.1080/09638237.2020.1739240 [DOI] [PubMed]
- Burns, K. E. A., Duffett, M., Kho, M. E., Meade, M. O., Adhikari, N. K. J., Sinuff, T., & Cook, D. J. (2008). A guide for the design and conduct of self-administered surveys of clinicians. Canadian Medical Association Journal, 179(3), 245–252. 10.1503/cmaj.080372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrà, G., Johnson, S., Crocamo, C., Angermeyer, M. C., Brugha, T., Azorin, J.-M., Toumi, M., & Bebbington, P. E. (2016). Psychosocial functioning, quality of life and clinical correlates of comorbid alcohol and drug dependence syndromes in people with schizophrenia across Europe. Psychiatry Research, 239, 301–307. 10.1016/j.psychres.2016.03.038 [DOI] [PubMed] [Google Scholar]
- Carrozzino, D., Patierno, C., Guidi, J., Berrocal Montiel, C., Cao, J., Charlson, M. E., Christensen, K. S., Concato, J., De las Cuevas, C., de Leon, J., Eöry, A., Fleck, M. P., Furukawa, T. A., Horwitz, R. I., Nierenberg, A. A., Rafanelli, C., Wang, H., Wise, T. N., Sonino, N., & Fava, G. A. (2021). Clinimetric Criteria for Patient-Reported Outcome Measures. Psychotherapy and Psychosomatics, 90(4), 222–232. 10.1159/000516599 [DOI] [PubMed] [Google Scholar]
- Charles, C., Gafni, A., & Whelan, T. (1997). Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Social Science & Medicine (1982), 44(5), 681–692. 10.1016/s0277-9536(96)00221-3 [DOI] [PubMed] [Google Scholar]
- Chewning, B., Bylund, C. L., Shah, B., Arora, N. K., Gueguen, J. A., & Makoul, G. (2012). Patient preferences for shared decisions: a systematic review. Patient Education and Counseling, 86(1), 9–18. 10.1016/j.pec.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, W. W., Aslani, P., & Chen, T. F. (2013). Shared decision-making and interprofessional collaboration in mental healthcare: a qualitative study exploring perceptions of barriers and facilitators. Journal of Interprofessional Care, 27(5), 373–379. 10.3109/13561820.2013.785503 [DOI] [PubMed] [Google Scholar]
- Clarke, E., Puschner, B., Jordan, H., Williams, P., Konrad, J., Kawohl, W., Bär, A., Rössler, W., Del Vecchio, V., Sampogna, G., Nagy, M., Süveges, A., Krogsgaard Bording, M., & Slade, M. (2015). Empowerment and satisfaction in a multinational study of routine clinical practice. Acta Psychiatrica Scandinavica, 131(5), 369–378. 10.1111/acps.12365 [DOI] [PubMed] [Google Scholar]
- Comrey, A. L., & Lee, H. B. (1992). A first course in factor analysis (2nd ed.). Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Cook, D. A., & Beckman, T. J. (2006). Current Concepts in Validity and Reliability for Psychometric Instruments: Theory and Application. The American Journal of Medicine, 119(2), 166.e7-166.e16. 10.1016/j.amjmed.2005.10.036 [DOI] [PubMed] [Google Scholar]
- Cortes, D. E., Mulvaney-Day, N., Fortuna, L., Reinfeld, S., & Alegría, M. (2009). Patient—Provider Communication. Health Education & Behavior, 36(1), 138–154. 10.1177/1090198108314618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter, A., & Jenkinson, C. (2005). European patients’ views on the responsiveness of health systems and healthcare providers. European Journal of Public Health, 15(4), 355– 360. 10.1093/eurpub/cki004 [DOI] [PubMed] [Google Scholar]
- de Filippis, R., De Fazio, P., Gaetano, R., Steardo, L., Cedro, C., Bruno, A., Zoccali, R. A., & Muscatello, M. R. A. (2021). Current and emerging long-acting antipsychotics for the treatment of schizophrenia. Expert Opinion on Drug Safety, 20(7), 771–790. 10.1080/14740338.2021.1910674 [DOI] [PubMed] [Google Scholar]
- De las Cuevas, C., Perestelo-Perez, L., Rivero-Santana, A., Cebolla-Martí, A., Scholl, I., & Härter, M. (2015). Validation of the Spanish version of the 9-item Shared Decision-Making Questionnaire. Health Expectations, 18(6), 2143–2153. 10.1111/hex.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn, G. (2003). Shared decision making: developing the OPTION scale for measuring patient involvement. Quality and Safety in Health Care, 12(2), 93–99. 10.1136/qhc.12.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn, G, Hutchings, H., Edwards, A., Rapport, F., Wensing, M., Cheung, W., & Grol, R. (2005). The OPTION Scale: Measuring the Extent That Clinicians Involve Patients in Decision-Making Tasks. Health Expectations : An International Journal of Public Participation in Health Care and Health Policy, 8(1). 10.1111/J.1369-7625.2004.00311.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn, Glyn, Frosch, D., Thomson, R., Joseph-Williams, N., Lloyd, A., Kinnersley, P., Cording, E., Tomson, D., Dodd, C., Rollnick, S., Edwards, A., & Barry, M. (2012). Shared decision making: a model for clinical practice. Journal of General Internal Medicine, 27(10), 1361–1367. 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, K. E., Wills, C. E., Holloman, C., Olson, J., Hechmer, C., Miller, C. K., & Duchemin, A.-M. (2012). Shared decision making and other variables as correlates of satisfaction with health care decisions in a United States national survey. Patient Education and Counseling, 88(1), 100–105. 10.1016/j.pec.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck, S. (2014). Mild, Moderate, Severe Intellectual Disability Differences. HealthyPlace.
- Goss, C., Fontanesi, S., Mazzi, M., Del Piccolo, L., Rimondini, M., Elwyn, G., & Zimmermann, C. (2007). Shared Decision Making: The Reliability of the OPTION Scale in Italy. Patient Education and Counseling, 66(3), 296–302. 10.1016/J.PEC.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Gunay Molu, N., ÖZKAN, B., & İçel, S. (1969). Quality of life for chronic psychiatric illnesses and home care. Pakistan Journal of Medical Sciences, 32(2). 10.12669/pjms.322.8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy, W. (1976). Clinical Global Impression (CGI). In W. Guy (Ed.), ECDEU Assessment Manual for Psychopharmacology. Department of Health, Education, and Welfare.
- H, D., E, C., L, K., M, H., & I, S. (2017). Use of the 9-item Shared Decision Making Questionnaire (SDM-Q-9 and SDM-Q-Doc) in Intervention studies-A Systematic Review. PloS One, 12(3). 10.1371/JOURNAL.PONE.0173904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair, J., Anderson, R., Tatham, R., & Black, W. (1995). Multivariate Data Analysis: With Readings (N. P.-H. Englewood Cliffs (ed.)).
- Hamann, J., Mendel, R., Bühner, M., Kissling, W., Cohen, R., Knipfer, E., & Eckstein, H. (2012). How Should Patients Behave to Facilitate Shared Decision Making--The Doctors’ View. Health Expectations : An International Journal of Public Participation in Health Care and Health Policy, 15(4). 10.1111/J.1369-7625.2011.00682.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härter, M. (2004). [Shared decision making--from the point of view of patients, physicians and health politics is set in place]. Zeitschrift Fur Arztliche Fortbildung Und Qualitatssicherung, 98(2), 89–92. http://www.ncbi.nlm.nih.gov/pubmed/15106486 [PubMed] [Google Scholar]
- Härter, M., van der Weijden, T., & Elwyn, G. (2011). Policy and practice developments in the implementation of shared decision making: an international perspective. Zeitschrift Fur Evidenz, Fortbildung Und Qualitat Im Gesundheitswesen, 105(4), 229–233. 10.1016/j.zefq.2011.04.018 [DOI] [PubMed] [Google Scholar]
- Hays, R. D. (1995). Functioning and Well-being Outcomes of Patients With Depression Compared With Chronic General Medical Illnesses. Archives of General Psychiatry, 52(1), 11. 10.1001/archpsyc.1995.03950130011002 [DOI] [PubMed] [Google Scholar]
- Hölzel, L., Kriston, L., & Härter, M. (2013). Patient Preference for Involvement, Experienced Involvement, Decisional Conflict, and Satisfaction With Physician: A Structural Equation Model Test. BMC Health Services Research, 13. 10.1186/1472-6963-13-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, L., & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Joosten, E. A. G., DeFuentes-Merillas, L., de Weert, G. H., Sensky, T., van der Staak, C. P. F., & de Jong, C. A. J. (2008). Systematic review of the efects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychotherapy and Psychosomatics, 77(4), 219–226. 10.1159/000126073 [DOI] [PubMed] [Google Scholar]
- Kasper, J., Hoffmann, F., Heesen, C., Köpke, S., & Geiger, F. (2012). MAPPIN’SDM--the Multifocal Approach to Sharing in Shared Decision Making. PloS One, 7(4). 10.1371/JOURNAL.PONE.0034849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriston, L., Härter, M., & Scholl, I. (2012). A latent variable framework for modeling dyadic measures in research on shared decision-making. Zeitschrift Für Evidenz, Fortbildung Und Qualität Im Gesundheitswesen, 106(4), 253–263. 10.1016/j.zefq.2012.03.021 [DOI] [PubMed] [Google Scholar]
- Kriston, L., Scholl, I., Hölzel, L., Simon, D., Loh, A., & Härter, M. (2010). The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Education and Counseling, 80(1), 94–99. 10.1016/j.pec.2009.09.034 [DOI] [PubMed] [Google Scholar]
- Légaré, F, Politi, M., Drolet, R., Desroches, S., Stacey, D., & Bekker, H. (2012). Training Health Professionals in Shared Decision-Making: An International Environmental Scan. Patient Education and Counseling, 88(2). 10.1016/J.PEC.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Légaré, France, Ratté, S., Gravel, K., & Graham, I. D. (2008). Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Education and Counseling, 73(3), 526–535. 10.1016/j.pec.2008.07.018 [DOI] [PubMed] [Google Scholar]
- Luciano, M., Sampogna, G., Del Vecchio, V., Loos, S., Slade, M., Clarke, E., Nagy, M., Kovacs, A., Munk-Jørgensen, P., Krogsgaard Bording, M., Kawohl, W., Rössler, W., Puschner, B., & Fiorillo, A. (2020). When does shared decision making is adopted in psychiatric clinical practice? Results from a European multicentric study. European Archives of Psychiatry and Clinical Neuroscience, 270(6), 645–653. 10.1007/s00406-019-01031-y [DOI] [PubMed] [Google Scholar]
- Makoul, G., & Clayman, M. L. (2006). An integrative model of shared decision making in medical encounters. Patient Education and Counseling, 60(3), 301–312. 10.1016/j.pec.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Melbourne, E., Roberts, S., Durand, M., Newcombe, R., Légaré, F., & Elwyn, G. (2011). Dyadic OPTION: Measuring Perceptions of Shared Decision-Making in Practice. Patient Education and Counseling, 83(1). 10.1016/J.PEC.2010.04.019 [DOI] [PubMed] [Google Scholar]
- Moumjid, N., Gafni, A., Brémond, A., & Carrère, M.-O. (2007). Shared decision making in the medical encounter: are we all talking about the same thing? Medical Decision Making : An International Journal of the Society for Medical Decision Making, 27(5), 539–546. 10.1177/0272989X07306779 [DOI] [PubMed] [Google Scholar]
- Nulty, D. D. (2008). The adequacy of response rates to online and paper surveys: what can be done? Assessment & Evaluation in Higher Education, 33(3), 301–314. 10.1080/02602930701293231 [DOI] [Google Scholar]
- Rencz, F., Tamási, B., Brodszky, V., Gulácsi, L., Weszl, M., & Péntek, M. (2019). Validity and reliability of the 9-item Shared Decision Making Questionnaire (SDM-Q-9) in a national survey in Hungary. The European Journal of Health Economics, 20(S1), 43–55. 10.1007/s10198-019-01061-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenbauch, K., & Schildmann, J. (2011). [Shared decision making (SDM): a systematic survey of terminology use and concepts]. Gesundheitswesen (Bundesverband Der Arzte Des Offentlichen Gesundheitsdienstes (Germany)), 73(7), 399– 408. 10.1055/s-0030-1262870 [DOI] [PubMed] [Google Scholar]
- Rodenburg-Vandenbussche, S., Pieterse, A. H., Kroonenberg, P. M., Scholl, I., van der Weijden, T., Luyten, G. P. M., Kruitwagen, R. F. P. M., den Ouden, H., Carlier, I. V. E., van Vliet, I. M., Zitman, F. G., & Stiggelbout, A. M. (2015). Dutch Translation and Psychometric Testing of the 9-Item Shared Decision Making Questionnaire (SDM-Q-9) and Shared Decision Making Questionnaire-Physician Version (SDM-Q-Doc) in Primary and Secondary Care. PLOS ONE, 10(7), e0132158. 10.1371/journal.pone.0132158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Say, R., Murtagh, M., & Thomson, R. (2006). Patients’ Preference for Involvement in Medical Decision Making: A Narrative Review. Patient Education and Counseling, 60(2). 10.1016/J.PEC.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Scholl, I, Koelewijn-van Loon, M., Sepucha, K., Elwyn, G., Légaré, F., Härter, M., & Dirmaier, J. (2011). Measurement of Shared Decision Making – A Review of Instruments. Zeitschrift Fur Evidenz, Fortbildung Und Qualitat Im Gesundheitswesen, 105(4). 10.1016/J.ZEFQ.2011.04.012 [DOI] [PubMed] [Google Scholar]
- Scholl, I, Kriston, L., & Härter, M. (2011). PEF-FB-9 – Fragebogen zur Partizipativen Entscheidungsfindung (revidierte 9-Item-Fassung). In Klin. Diagnostik und Evaluation (pp. 46–49). Vandenhoeck & Ruprecht GmbH & Co. KG. [Google Scholar]
- Scholl, Isabelle, Kriston, L., Dirmaier, J., Buchholz, A., & Härter, M. (2012). Development and psychometric properties of the Shared Decision Making Questionnaire--physician version (SDM-Q-Doc). Patient Education and Counseling, 88(2), 284–290. 10.1016/j.pec.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Scholl, Isabelle, Kriston, L., Dirmaier, J., & Härter, M. (2015). Comparing the nine-item Shared Decision-Making Questionnaire to the OPTION Scale – an attempt to establish convergent validity. Health Expectations, 18(1), 137–150. 10.1111/hex.12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihoe, A. D. L. (2015). Rationales for an accurate sample size evaluation. Journal of Thoracic Disease, 7(11), E531-6. 10.3978/j.issn.2072-1439.2015.10.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, D, Loh, A., & Harter, M. (2008). Development and evaluation of interventions to support shared decision making – framework and measuring instruments. Z Med Psychol, 28, 699–705. [Google Scholar]
- Simon, D, Loh, A., & Härter, M. (2008). [Foundations of shared decision making and examples of its application in rehabilitation]. Die Rehabilitation, 47(2), 84–89. 10.1055/s-2008-1042446 [DOI] [PubMed] [Google Scholar]
- Simon, Daniela, Loh, A., & Härter, M. (2007). Measuring (shared) decision-making – a review of psychometric instruments. Zeitschrift Für Ärztliche Fortbildung Und Qualität Im Gesundheitswesen – German Journal for Quality in Health Care, 101(4), 259–267. 10.1016/j.zgesun.2007.02.029 [DOI] [PubMed] [Google Scholar]
- Weldring, T., & Smith, S. M. S. (2013). Article Commentary: Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Services Insights, 6, HSI.S11093. 10.4137/HSI.S11093 [DOI] [PMC free article] [PubMed]
- World Medical Association. (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA, 310(20), 2191. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- www.sdmq9.org. (n.d.). SDM-Q-9 / SDM-Q-Doc The 9-item Shared Decision Making Questionnaire. http://www.patient-als-partner.de/index.php?article_id=20&clang=2/