Abstract
Background
Over the past years, polysaccharide-based scaffolds have emerged as the most promising material for tissue engineering. In the present study, carrageenan, an injectable scaffold has been used owing to its advantage and superior property. Cissus quadrangularis, a natural agent was incorporated into the carrageenan scaffold. Therefore, the present study aimed to assess the antioxidant activity and biocompatibility of this novel material.
Methods
The present in vitro study comprised of four study groups each constituting a sample of 15 with a total sample size of sixty (n = 60). The carrageenan hydrogel devoid of Cissus quadrangularis acted as the control group (Group-I). Based on the concentration of aqueous extract of Cissus quadrangularis (10% w/v, 20% w/v and 30% w/v) in carrageenan hydrogel, respective study groups namely II, III and IV were considered. Antioxidant activity was assessed using a 1,1-diphenyl-2-picrylhydrazyl radical scavenging assay, whereas the biocompatibility test was performed using a brine shrimp lethality assay. The microstructure and surface morphology of the hydrogel samples containing different concentrations of Cissus quadrangularis aqueous extract was investigated using SEM. One-way ANOVA with the post hoc tukey test was performed using SPSS software v22.
Results
A significant difference (P < 0.05) in the antioxidant activity was observed among the study groups. Group III reported the highest activity, whereas the control group showed the least antioxidant activity. Additionally, a significant (P < 0.01) drop in the antioxidant activity was observed in group IV when compared with group III. While assessing the biocompatibility, a significant (P < 0.001) dose-dependent increase in biocompatibility was observed with the increasing concentration of aqueous extract of Cissus quadrangularis. SEM analysis in group III showed even distribution throughout the hydrogel although the particles are close and densely arranged. Reduced antioxidant activity in group IV was probably due to clumping of the particles, thus reducing the active surface area.
Conclusion
Keeping the limitations of in vitro study, it can be assumed that a carrageenan based injectable hydrogel scaffold incorporated with 20% w/v Cissus quadrangularis can provide a favourable micro-environment as it is biocompatible and possess better antioxidant property.
Keywords: Scaffold, Hydrogel, Carrageenan, Cissus quadrangularis, Regeneration, Dentin–pulp complex, Antioxidant activity, Tissue engineering
Background
In recent days, there is an immense focus on recreating the lost architecture of human tissue or defective tissues; however, it’s quite challenging [1]. Procedures such as harvesting a graft for regenerative purposes could lead to serious complications including pain, morbidity and a higher risk of infection [1]. Hence, tissue engineering principles are employed for repair, regeneration and enhancing the function of defective tissues.
The success of tissue regeneration depends on the material of choice. In this context, natural scaffold such as hydrogel claims to be a valid treatment option [2] as they carry a lower risk for cytotoxicity [3, 4]. They have a unique three-dimensional polymeric network wherein water is the main liquid component. The hydrophilic nature of these hydrogels helps in retaining the higher fluid content and thus allowing the diffusion of nutrients through their structure [5]. They are biocompatible with adjustable mechanical properties and their cross-linking structure renders them less soluble despite high-water concentrations [6]. They have a gelatinous structure, which provides essential cell support, along with their ability to get loaded with various drugs, making them a good drug delivery system [5, 7, 8]. Despite being biodegradable, hydrogels also release the bioactive molecules influencing the surrounding environment [9, 10]. In addition to being a carrier for the cells or bioactive molecules, they are mainly applied as a space-filling material for tissue engineering [11]. Among various hydrogel formulations [12], polysaccharide-based hydrogels have shown promising results in tissue engineering [13]. Being thixotropic, they can be injected into the targeted space without altering their physical, mechanical or biological properties [13].
Carrageenan-based hydrogel formulation is one such naturally occurring sulphate polysaccharide-based formulation having versatile properties [14]. Carrageenan is a natural compound obtained from red seaweed, which is a marine red alga [15]. Structurally, carrageenan has a resemblance to the glycosaminoglycans which form the extracellular matrix (ECM) of tissue, hence in physiological conditions, injecting this scaffold into the tissue defect will offer added benefits [16]. Studies have found that carrageenan-based extract is useful in the controlled delivery of drugs [17], bone tissue engineering purposes [18] as well as in wound healing [19].
Promising results have been documented with plant-derived bioactive compounds and their secondary metabolites in regenerative and therapeutic tissue engineering applications [20]. Cissus quadrangularis is a vitaceae plant that has been used as a medicinal herb in India and Africa for ages. In traditional medicine, it is used for its antibacterial, analgesic, anti-inflammatory and antioxidant properties. Along with this, it is employed for bone fracture healing and the prevention of osteoporosis [21]. Additionally, Calcium (about 4% by weight) and phosphorus ions are abundant in the Cissus quadrangularis stem extract [22].
In tissue engineering, as we are focused on achieving a favourable environment that could induce the claimed odontogenesis and osteogenesis, currently studied extract, being anti-inflammatory along with being antioxidant, could favour the environment for biomineralization. For a material to be used in tissue engineering, it needs to be biocompatible and antioxidant to prevent oxidative stress thereby inhibiting the damage caused by free radicals. Thus, the current study aimed at evaluating the activity of the Cissus quadrangularis incorporated in carrageenan-based injectable hydrogel as a natural scaffold. The present study is a preliminary one, which mainly focused on assessing the biocompatibility and antioxidant activity of a novel formulation of hydrogel in the in vitro models. The stated null hypothesis of the study was there is no statistically significant difference in the biocompatibility and antioxidant activity on using carrageenan-based injectable hydrogel incorporated with different concentrations of Cissus quadrangularis extract.
Methods
Study and sample characteristics
The present in vitro study was conducted after obtaining ethical approval from the institutional ethical committee (SRB/SDC/ENDO-2105/21/034). Depending on the con-centration of the Cissus quadrangularis aqueous extract, the current study had four study groups as mentioned below:
Group I: Carrageenan hydrogel (without any addition of Cissus quadrangularis, considered as control group (n=15);
Group II: Carrageenan hydrogel with 10% w/v of Cissus quadrangularis aqueous extracts (n=15)
Group III: Carrageenan hydrogel with 20% w/v of Cissus quadrangularis aqueous extracts (n=15),
Group IV: Carrageenan hydrogel with 30% w/v of Cissus quadrangularis aqueous extracts (n=15).
Sample size calculation
Considering the invitro nature of the study, the post-hoc power of the study was calculated using G Power software. The effect size of 1.70 was calculated based on the mean recorded in the current study for the variables tested in the study. The alpha error was kept at 0.05 for a study with four groups. Each group had a sample size of 15, making the total sample size of sixty. With the above input values the power of the study was 1.
Preparation of hydrogel
Commercially available Carrageenan powder (Tokyo Chemical Indrustries (TCI), CASReg no: 11114-20-8, MeronTM, India), Cissus quadrangularis powdered extract (Annai Aravindh Herbals®, ISO 9001:2015 Certified SKU-AAH_PH_S_PROI, Chennai, India) and distilled water were used to prepare the hydrogel. A 100 mL of distilled water was heated at 60 °C for 30 min and 0.5 g of commercially available carrageenan powder was then dissolved in it by continuous stirring to make the carrageenan hydrogel. It served as the control agent in the study. A 10 g, 20 g, and 30 g of commercially available Cissus quadrangularis powdered extract were then dissolved in 100 mL of distilled water to produce 10% w/v, 20% w/v and 30% w/v aqueous extract of Cissus quadrangularis respectively. A 100 mL of this aqueous extract was then added to 0.5 g commercially available carrageenan powder and continuously stirred at 60 °C to form 10%, 20%, 30% w/v Cissus quadrangularis hydrogel [23]. The prepared hydrogel was then poured into standard moulds of dimension 6 × 2 × 2 mm3 and stored at 4 °C Before testing, the prepared hydrogel was sterilized using autoclaving for 15 min at 121 °C at 15 psi (Fig. 1A–E).
Fig. 1.
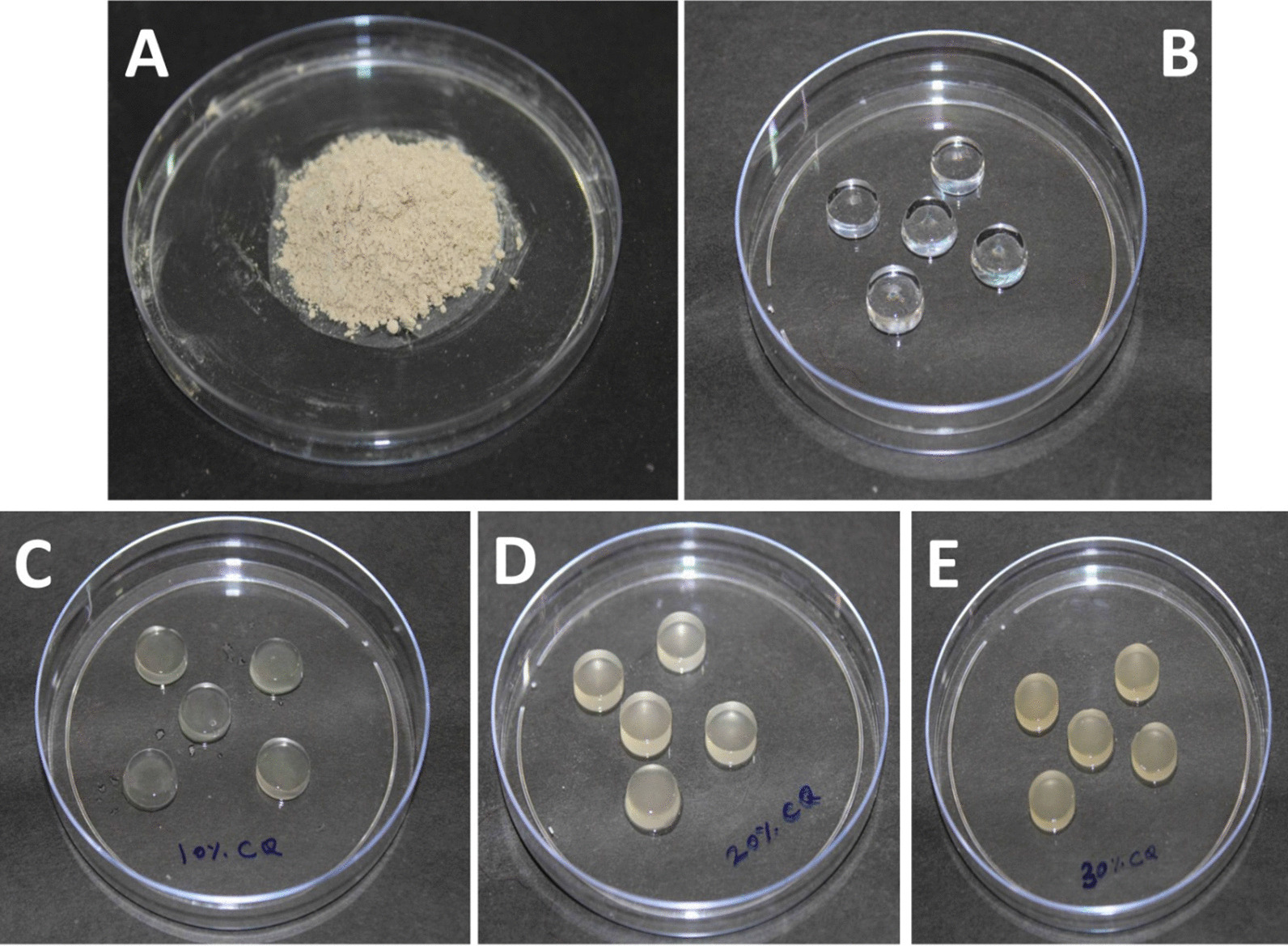
A Commercially available Cissus quadrangularis powder; B Carrageenan hydrogel (control); C 10% w/v Cissus quadrangularis hydrogel; D 20% w/v Cissus quadrangularis hydrogel; E 30% w/v Cissus quadrangularis hydrogel
Testing for antioxidant activity: DPPH radical scavenging assay [24]
The prepared samples of the hydrogels were then subjected to antioxidant assay using a 1,1-diphenyl-2-picrylhydrazyl (DPPH) model system. A 50 µL of control hydrogel and different concentrations (10%, 20%, 30% w/v) of Cissus quadrangularis hydrogel were taken in test tubes. Later, 1 mL of a 0.1 mM methanolic solution of DPPH and 450 µL of 50 mM of TrisHCl buffer (pH 7.4) were added and incubated for 30 min. Later, the reduction in the quantity of DPPH free radicals was assessed depending on the absorbance at 517 nm. The butylatedhydroxytoluene (BHT) was employed as a control. The percentage of inhibition was determined from the following equation
Testing for biocompatibility: brine shrimp lethality assay [25]
A 2 g of iodine-free salt was weighed and dissolved in 200 mL of distilled water. Fifteen, 6 well enzyme-linked immunoassay (ELISA) plates were taken and 10–12 mL of saline water was filled. Ten live nauplii were slowly added to each well. The wells were labelled as control, 10%, 20% and 30% according to the concentrations Cissus quadrangularis aqueous extract. A 50 µL of each concentration of hydrogel were added to each well as per their respective concentration and plates were incubated for 24 h. Later, the ELISA plates were observed and noted for the number of live nauplii present and calculated by using the following formula
Microstructure and surface morphology analysis using scanning electron microscopy
The microstructure and surface morphology of the hydrogel samples containing different concentrations of Cissus quadrangularis aqueous extract was investigated using SEM analysis. The hydrogel samples were stored at − 50 °C for 48 h and dried in a lyophilizer (Virtis Benchtop 4k freeze dryer). The cross-sectional surfaces of the sample in powder form were coated with a thin layer of platinum sputter and then SEM analysis was performed using field emission scanning electron microscopy (FESEM IT800).
Statistical analysis
The gathered data was entered into the MS excel sheet. The normality of the data was assessed using the Shapiro–Wilk test. Data was presented in mean ± standard deviation and considering the parametric nature of data, One-way ANOVA with Post Hoc Tukey test was performed to derive inferential statistics. The data was analysed using IBM (IBM Corporation Business Analytics) SPSS software 23.0 version.
Results
Comparative analysis of antioxidant activity among study groups
A statistically significant (P < 0.001) difference in the antioxidant activity was observed among the study groups with control (group I) and group III reported with the least and highest antioxidant activity (Fig. 2). On post-hoc evaluation, the group I exhibited significantly (P < 0.001) least antioxidant activity compared with other study groups. There was a non-significant (P˃0.05) gradual increase in the antioxidant activity between group II and III. On the other hand, a significant (P < 0.01) drop in the activity was recorded with increased concentration of aqueous extract of Cissus quadrangularis hydrogel from 20% w/v (group III) to 30% w/v (group IV) (Table 1).
Fig. 2.
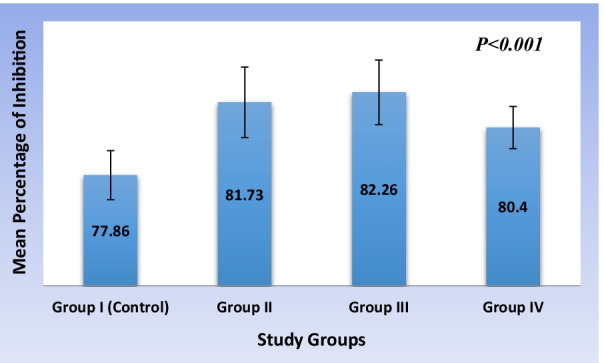
Comparative analysis of antioxidant activity among the study groups
Table 1.
Post-hoc evaluation of antioxidant activity among study groups
| Pair-wise comparison groups | Mean difference | P value | 95% confidence interval | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Group I | ||||
| Group II | − 3.867 | .000¶ | − 5.35 | − 2.39 |
| Group III | − 4.400 | .000¶ | − 5.88 | − 2.92 |
| Group IV | − 2.533 | .000¶ | − 4.01 | − 1.05 |
| Group II | ||||
| Group I | 3.867 | .000¶ | 2.39 | 5.35 |
| Group III | − .533 | .776 | − 2.01 | .95 |
| Group IV | 1.333 | .092 | − .15 | 2.81 |
| Group III | ||||
| Group I | 4.400 | .000¶ | 2.92 | 5.88 |
| Group II | .533 | .776 | − .95 | 2.01 |
| Group IV | 1.867 | .008€ | .39 | 3.35 |
| Group IV | ||||
| Group I | 2.533 | .000¶ | 1.05 | 4.01 |
| Group II | − 1.333 | .092 | − 2.81 | .15 |
| Group III | − 1.867 | .008€ | − 3.35 | − .39 |
Group I: Carrageenan hydrogel (without any addition of cissus quadran-gularis; Group II: Carrageenan hydrogel with 10% w/v of Cissus quadrangularis aqueous extracts; Group III: Carrageenan hydrogel with 20% w/v of Cissus quadrangularis aqueous extracts; Group IV: Carrageenan hydrogel with 30% w/v of Cissus quadrangularis aqueous extracts; Intergroup comparison was carried out with one-way ANOVA, which showed a statistically significant difference in antioxidant activity
¶p < 0.001; €P < 0.01
Comparative analysis of biocompatibility among study groups
A statistically significant (P < 0.001) increase in the biocompatibility was observed among the study groups with group I and group IV reported with the least and highest level of biocompatibility (Fig. 3). On post-hoc evaluation, a significant (P < 0.001) increase in the biocompatibility was observed with each increment increase in concentration of aqueous extract of Cissus quadrangularis hydrogel (Table 2).
Fig. 3.
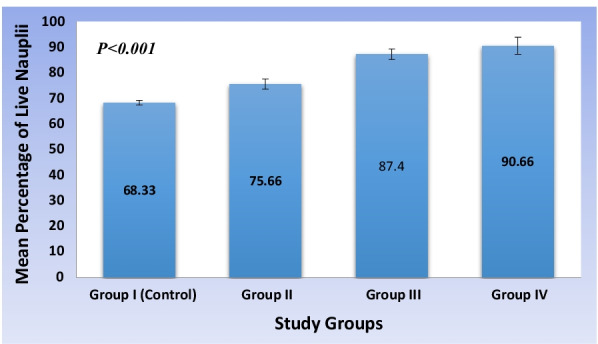
Comparative analysis of biocompatibility among the study groups
Table 2.
Post-hoc evaluation of biocompatibility among study groups
| Pair-wise comparison groups | Mean difference | P value | 95% confidence interval | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Group I | ||||
| Group II | − 7.333 | .000¶ | − 9.52 | − 5.15 |
| Group III | − 19.067 | .000¶ | − 21.25 | − 16.88 |
| Group IV | − 22.333 | .000¶ | − 24.52 | − 20.15 |
| Group II | ||||
| Group I | 7.333 | .000¶ | 5.15 | 9.52 |
| Group III | − 11.733 | .000¶ | − 13.92 | − 9.55 |
| Group IV | − 15.000 | .000¶ | − 17.18 | − 12.82 |
| Group III | ||||
| Group I | 19.067 | .000¶ | 16.88 | 21.25 |
| Group II | 11.733 | .000¶ | 9.55 | 13.92 |
| Group IV | − 3.267 | .001€ | − 5.45 | − 1.08 |
| Group IV | ||||
| Group I | 22.333 | .000¶ | 20.15 | 24.52 |
| Group III | 15.000 | .000¶ | 12.82 | 17.18 |
| Group IV | 3.267 | .001€ | 1.08 | 5.45 |
Group I: Carrageenan hydrogel (without any addition of Cissus quadrangularis; Group II: Carrageenan hydrogel with 10% w/v of Cissus quadrangularis aqueous extracts; Group III: Carrageenan hydrogel with 20% w/v of Cissus quadrangularis aqueous extracts; Group IV: Carrageenan hydrogel with 30% w/v of Cissus quadrangularis aqueous extracts; Intergroup comparison was carried out with one-way ANOVA, which showed a statistically significant difference in biocompatibility
¶p < 0.001; €P < 0.01
Microstructure and surface morphology analysis using scanning electron microscopy
The SEM analysis of the control group revealed a porous structure of the hydrogel. It is essential for the regeneration as it provides a matrix for the stem cells of the apical papilla to embed and differentiate further into odontoblasts to lay down the dentin. In group II (10% w/v aqueous extract of Cissus quadrangularis hydrogel), an even distribution of Cissus quadrangularis was seen throughout the hydrogel, whereas in group III, in addition to even distribution Cissus quadrangularis, the particles were shown to be close and densely arranged. The group IV, showed an uneven distribution of Cissus quadrangularis particles with the particles being clumped together (Figs. 4, 5).
Fig. 4.
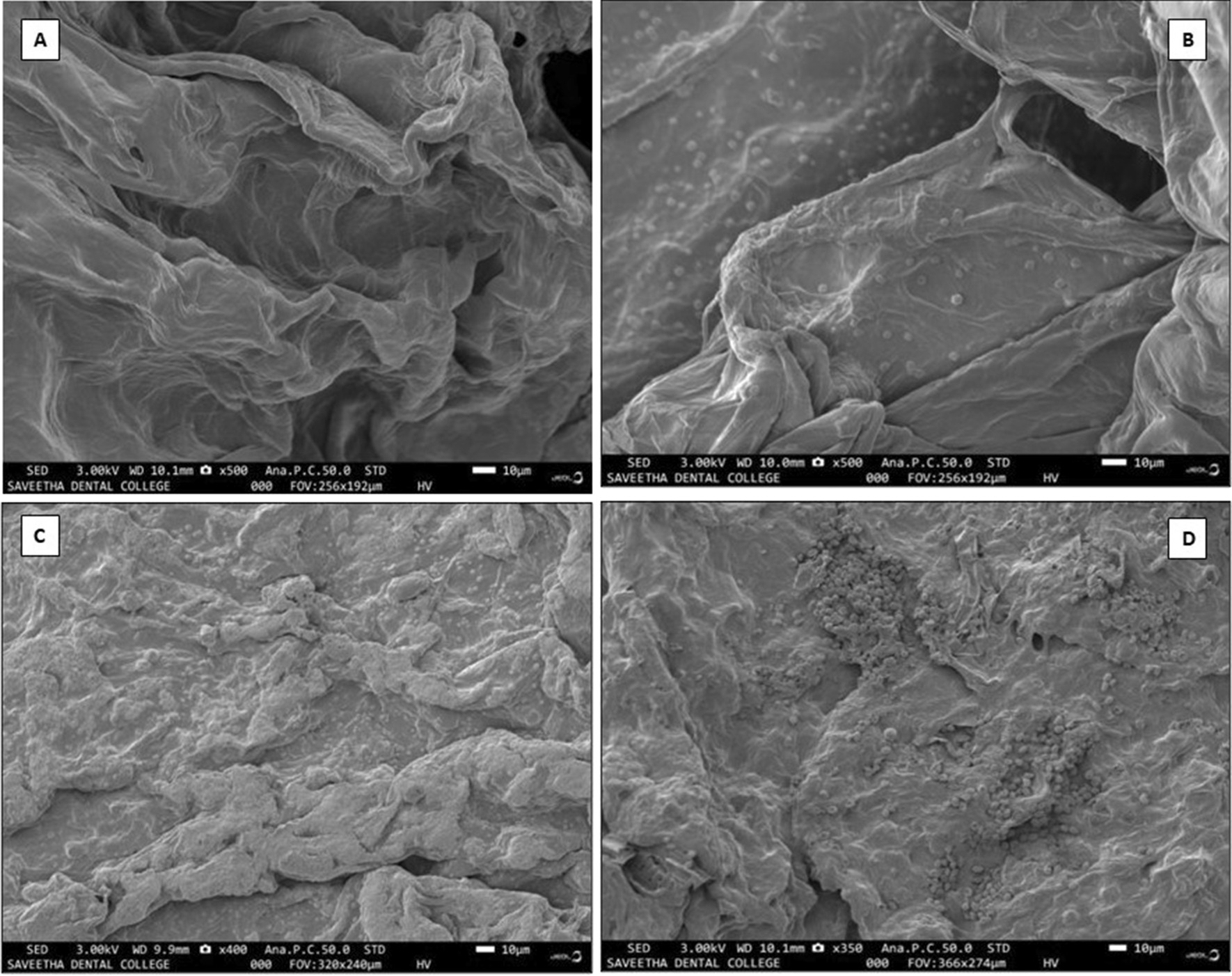
A Porous microstructure of carrageenan hydrogel. B 10% w/v aqueous extract of Cissus quadrangularis hydrogel showing evenly dispersed particles of Cissus quadrangularis C 20% w/v aqueous extract of Cissus quadrangularis hydrogel showing evenly dispersed dense arrangement of Cissus quadrangularis particles. D 30% w/v aqueous extract of Cissus quadrangularis hydrogel showing clumping of Cissus quadrangularis particles
Fig. 5.

Higher magnification view of 30% w/v Cissus quadrangularis hydrogel (Group IV) with evident clumping of Cissus quadrangularis particles
Discussion
In recent years, research is ongoing to determine the use of hydrogel as a scaffold for regenerative procedures. Although many studies have focused on the use of scaffolds in tissue engineering, reports are scarce on the use of injectable carrageenan hydrogel scaffolds infused with Cissus quadrangularis. Hence, as a preliminary study, we focused on assessing the biocompatibility and antioxidant property of the Cissus quadrangularis incorporated carrageenan-based hydrogel scaffold. A patent under the World intellectual property organization, with international publication no—WO 2008/081233 A2, with the inventor/applicant AVESTHA, GENGRAINE TECHNOLOGIES PVT LTD states that the percentage yield of whole plant extracts with active ingredients was the highest in water which was about 17.31%. The rest of the solvents used for extraction were hexane, 80% ethanol and acetone whose yields were much lesser than that of water. In the current study, this was the rationale for using aqueous extract of Cissus quadrangularis for the preparation of hydrogel and for further tests conducted [26].
In the present study, the rationale for selecting the carrageenan hydrogel lies in the fact that it enhances favourable results in the tissue regeneration process. This hydrogel has superior mechanical properties that depend on its molecular weight, source, concentration, type and degree of cross-linkage [27]. The molecular weight of the hydrogel influences its degradation property. It has been found that as the molecular weight increases the degradation rate decreases [28]. Studies showed that the degradation rate was higher when a lower-weight scaffold such as chitosan-based hydrogels was used [29]. Furthermore, the three-dimensional structure of carrageenan has shown osteoblastic proliferation and adhesion [30]. A combination of carrageenan hydrogels with different delivery systems has shown successful outcomes [31]. The prerequisites for an injectable hydrogel include flowability under low pressure, rapid setting at the target site and preserving the appropriate integrity and strength [32]. Due to its emulsifying and thixotropic property, it could be used as an injectable scaffold [33].
In the present study, we have used a carrageenan-based hydrogel incorporated with various concentrations of Cissus quadrangularis extracts which is a bioactive compound. To the best of our knowledge, the latter has not been explored for its therapeutic potential; however, its potential for osteogenesis has been extensively studied. The osteogenesis potential of Cissus quadrangularis extract has been explored in various dental clinical situations such as periodontal bone regeneration [34], mandibular alveolar ridge distractions [35] and in maxillofacial [36] and mandibular fractures [37]. In particular, the Cissus quadrangularis extracts have been shown to contain calcium, along with other compounds [38] and thus probably have shown to regulate osteoblastic activity [39] by enhancing osteoblastogenesis [39], mineralization [40] and eventually induce bone formation for faster bone healing [41]. Literature also showed that Cissus quadrangularis extract stimulated the mineralised nodules in dental pulp cells [42].
Recent literature found the stage-specific and tissue expression of BSP [43], and OPN [43] in reparative dentinogenesis. On the other hand, studies are showing the efficacy of Cissus quadrangularis extract on OPN [44], bone morphogenetic protein (BMP) [45] and BSP [46] activation, which in turn gives us an idea of the usage of this current material as a scaffold for tissue engineering, as the material has immense potentiality for osteogenesis and mineralisation.
In the present study, Cissus quadrangularis and carrageenan hydrogels are combined to assess antioxidant activity and biocompatibility. To date, there are no reports on the usage of combinations of these injectable hydrogels for assessing these properties. The reason for choosing only 10–30 weight/volume incorporations of Cissus quadrangularis extracts was based on the results from the previous study [39], which showed maximum bone healing at 10gm/100 ml. Additionally, multiple observations in the prior experiments of SEM of the present lab showed maximum antioxidant activity with evenly distributed extract throughout the hydrogel on 20% w/v Cissus quadrangularis. Observations above concentration 30% w/v showed clumping, hence, in the present study, it was restricted to 30 w/v concentration extract. Considering the above observations, the above-mentioned incorporations of cissus extracts in the hydrogel were considered in the present study.
Reactive oxygen species are well recognised for their dual role play. Although they might play constructive in cell physiology, they may also cause the destruction of cell membranes and DNA [47, 48], which could be deleterious to the regenerative process. They cause significant damage to the cell structures during oxidative stress. They are cytotoxic and implicated in the aetiology of various pathological conditions [49]. Hence, antioxidants counteract these reactive oxygen species and thereby reducing the harmful effects induced by them. Especially the antioxidants play an immense role during regeneration by promoting the environment favourable. Hence, before usage of any biomaterial, it’s important also to assess its antioxidant activity. For the assessment of the antioxidant activity, the DPPH test was performed in the present study as it has been popularly used to test the antioxidant properties of the plant extracts. In the DPPH assay, the addition of the extract to a violet-coloured DPPH solution reduces it to a yellow-coloured product, diphenylpicryl hydrazine in a concentration-dependent manner. The convenience offered by the short duration of the assay, allows its wide applications to predict antioxidant activity [50]. Dhanasekaran S et al. (2020) evaluated the antioxidant property of Cissus quadrangularis with different concentrations ranging from 25 to 400 μg/mL in ethanolic, and methanolic extracts. In this in vitro model, the free radical scavenging activity was more in methanolic extract compared to ethanolic extract in a dose-dependent manner [24]. In the present study, 10 and 20% w/v Cissus quadrangularis showed a significant gradual increase in the antioxidant activity as compared to the control group, however, a significant drop in the activity was observed with a further increase in the concentration of the agent to 30% w/v. On SEM analysis it was observed that 10% and 20% w/v Cissus quadrangularis hydrogel showed an even distribution of the incorporated agent throughout the hydrogel but when the concentration is increased to 30% w/v, an uneven distribution with clumping of the Cissus quadrangularis particles was noted. This could be a probable reason for the reduced antioxidant activity seen with the increasing concentration.
Any biomaterial used for regeneration, should not elicit any undesirable effects and should perform its desired function with appropriate beneficial cellular or tissue response [51–53]. The success of any biomaterial inevitably depends on its acceptability by the native tissue [54]. Hence, it’s important to evaluate the biocompatibility of the material used for regeneration. To evaluate the same, carrageenan-based hydrogel infused with Cissus quadrangularis was subjected to brine shrimp lethality assay. The current study showed a significant increase in biocompatibility with the increasing concentration of the additive agent in the scaffold. Cissus quadrangularis extract has been extensively explored for its antioxidant, anti-inflammatory and bone tissue regeneration in various in vitro studies [20]. Previous reports have shown better cytocompatibility of Cissus quadrangularis when performed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay for bone tissue engineering [20]. In another study, the least cytotoxicity of carrageenan hydrogels was seen when performed on L929 fibroblast cells [16]. In the present study, Cissus quadrangularis and carrageenan hydrogels are combined to assess antioxidant activity and biocompatibility. To date, there are no reports on the usage of combinations of these injectable hydrogels.
Limitations and future directions
This is a preliminary in vitro study to assess the antioxidant and biocompatible property of carrageenan injectable hydrogel infused with Cissus quadrangularis. It’s important to assess the setting time and degradation rate of the material which is intended to be used for regeneration. The current in-vitro study is a preliminary one where we have not focussed on assessing the properties of the prepared hydrogel. The interaction between Cissus quadrangularis extract and carrageenan needs to be studied further. Whether the sulphated groups and slightly acidic nature of the carrageenan inhibit the bio-active compounds from Cissus quadrangularis is unknown. Further studies need to be done using stem cells from the apical papilla (SCAP) cell line to evaluate the biocompatibility of the hydrogel prepared. Studies need to be performed using organic and polar solvents to see if more active ingredients are released which can be used to synthesise a hydrogel with better antioxidant potential.
Conclusions
Within the limitation of the study, it can be concluded that 20% w/v Cissus quadrangularis infused carrageenan based injectable hydrogel showed enhanced antioxidant activity whereas when the concentration of Cissus quadrangularis was increased to 30% w/v, has shown diminished response. However, there was a significant increase in the biocompatibility in a dose-dependent manner.
Acknowledgements
None.
Author contributions
All authors contributed to this article. “Conceptualization, S.S., N.M.S., K.V.T., and M.K.A; methodology, S.S., N.M.S., R.E, and S.M.P.; formal analysis, S.S. and K.C.S.; investigation, S.S., N.M.S., K.V.T., K.J., R.E, S.M.P.; resources, N.M.S., K.V.T., K.J., R.E, S.M.P., K.C.S.; data curation, D.S., M.A.O., H.A.A., M.M., A.R.Q., N.Q., K.C.S.; writing—original draft preparation, S.S., N.M.S., D.S., K.V.T., K.J., K.C.S.; writing—review and editing, S.S., N.M.S., D.S., M.A.O., H.A.A., M.M., A.R.Q., N.Q., K.V.T., K.J., R.E, S.M.P., M.K.A, K.C.S.; visualization, M.A.O., H.A.A., M.M., A.R.Q., N.Q., M.K.A,; supervision, N.M.S.; project administration, N.M.S., K.V.T. and K.C.S.; funding acquisition, M.M., A.R.Q., N.Q., K.V.T., K.J., R.E, S.M.P., M.K.A, K.C.S. All authors read and approved the final manuscript.
Funding
The current study is Self-funded.
Availability of data and materials
The datasets generated and/or analyzed during the present study are not publicly available as ethics approval was granted on the basis that only the researchers involved in the study could access the identified data but are available and accessible from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The present in vitro study was conducted according to the guidelines of the Declaration of Helsinki and approved by the institutional Ethics Committee (SRB/SDC/ENDO-2105/21/034) of Saveetha Institute of Medical and Technical Sciences (SIMATS), Chennai, Tamil Nadu, India. Informed consent was waived by the institutional Ethics Committee of Saveetha Institute of Medical and Technical Sciences (SIMATS) due to in vitro nature of the study.
Consent for publication
Due to the in vitro nature of the study, it is not applicable.
Competing interests
We declare no potential conflicts of interest with respect to the authorship or publication of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Deepti Shrivastava, Email: sdeepti20@gmail.com.
Kumar Chandan Srivastava, Email: drkcs.omr@gmail.com.
References
- 1.Sevari SP, Ansari S, Moshaverinia A. A narrative overview of utilizing biomaterials to recapitulate the salient regenerative features of dental-derived mesenchymal stem cells. Int J Oral Sci. 2021;13(1):22. doi: 10.1038/s41368-021-00126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catoira MC, Fusaro L, Di Francesco D, Ramella M, Boccafoschi F. Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med. 2019;30(10):115. doi: 10.1007/s10856-019-6318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbass MMS, El-Rashidy AA, Sadek KM, Moshy SE, Radwan IA, Rady D, Dörfer CE, Fawzy El-Sayed KM. Hydrogels and dentin–pulp complex regeneration: from the benchtop to clinical translation. Polymers. 2020;12(12):2935. doi: 10.3390/polym12122935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai JY. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J Mater Sci Mater Med. 2010;21(6):1899–1911. doi: 10.1007/s10856-010-4035-3. [DOI] [PubMed] [Google Scholar]
- 5.Mantha S, Pillai S, Khayambashi P, Upadhyay A, Zhang Y, Tao O, Pham HM, Tran SD. Smart hydrogels in tissue engineering and regenerative medicine. Materials. 2019;12(20):3323. doi: 10.3390/ma12203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polym J. 2015;65:252–267. doi: 10.1016/j.eurpolymj.2014.11.024. [DOI] [Google Scholar]
- 7.Chang B, Ahuja N, Ma C, Liu X. Injectable scaffolds: preparation and application in dental and craniofacial regeneration. Mater Sci Eng R Rep. 2017;111:1–26. doi: 10.1016/j.mser.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan C, Wang DA. Macroporous hydrogel scaffolds for three-dimensional cell culture and tissue engineering. Tissue Eng Part B Rev. 2017;23(5):451–461. doi: 10.1089/ten.TEB.2016.0465. [DOI] [PubMed] [Google Scholar]
- 9.Tabata Y, Nagano A, Ikada Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng. 1999;5(2):127–138. doi: 10.1089/ten.1999.5.127. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara M, Obara K, Nakamura S, Fujita M, Masuoka K, Kanatani Y, Takase B, Hattori H, Morimoto Y, Ishihara M, Maehara T, Kikuchi M. Chitosan hydrogel as a drug delivery carrier to control angiogenesis. J Artif Organs. 2006;9(1):8–16. doi: 10.1007/s10047-005-0313-0. [DOI] [PubMed] [Google Scholar]
- 11.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 12.El-Sherbiny IM, Yacoub MH. Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract. 2013;2013(3):316–342. doi: 10.5339/gcsp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camponeschi F, Atrei A, Rocchigiani G, Mencuccini L, Uva M, Barbucci R. New formulations of polysaccharide-based hydrogels for drug release and tissue engineering. Gels. 2015;1(1):3–23. doi: 10.3390/gels1010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yegappan R, Selvaprithiviraj V, Amirthalingam S, Jayakumar R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr Polym. 2018;198:385–400. doi: 10.1016/j.carbpol.2018.06.086. [DOI] [PubMed] [Google Scholar]
- 15.Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, Neves NM, Reis RL. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface. 2007;4(17):999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popa EG, Carvalho PP, Dias AF, Santos TC, Santo VE, Marques AP, Viegas CA, Dias IR, Gomes ME, Reis RL. Evaluation of the in vitro and in vivo biocompatibility of carrageenan-based hydrogels. J Biomed Mater Res A. 2014;102(11):4087–4097. doi: 10.1002/jbm.a.35081. [DOI] [PubMed] [Google Scholar]
- 17.Rasool A, Ata S, Islam A, Khan RU. Fabrication of novel carrageenan based stimuli responsive injectable hydrogels for controlled release of cephradine. RSC Adv. 2019;9(22):12282–12290. doi: 10.1039/c9ra02130b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santo VE, Frias AM, Carida M, Cancedda R, Gomes ME, Mano JF, Reis RL. Carrageenan-based hydrogels for the controlled delivery of PDGF-BB in bone tissue engineering applications. Biomacromol. 2009;10(6):1392–1401. doi: 10.1021/bm8014973. [DOI] [PubMed] [Google Scholar]
- 19.Mokhtari H, Tavakoli S, Safarpour F, Kharaziha M, Bakhsheshi-Rad HR, Ramakrishna S, Berto F. Recent advances in chemically-modified and hybrid carrageenan-based platforms for drug delivery, wound healing, and tissue engineering. Polymers. 2021;13(11):1744. doi: 10.3390/polym13111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair PR, Sreeja S, Sailaja GS. In vitro biomineralization and osteogenesis of Cissus quadrangularis stem extracts: an osteogenic regulator for bone tissue engineering. J Biosci. 2021;46:88. doi: 10.1007/s12038-021-00206-x. [DOI] [PubMed] [Google Scholar]
- 21.Tamburaci S, Kimna C, Tihminlioglu F. Novel phytochemical Cissus quadrangularis extract–loaded chitosan/Na-carboxymethyl cellulose-based scaffolds for bone regeneration. J Bioact Compat Polym. 2018;33(6):629–646. doi: 10.1177/0883911518793913. [DOI] [Google Scholar]
- 22.Sanyal A, Ahmad A, Sastry M. Calcite growth in Cissus quadrangularis plant extract, a traditional Indian bone-healing aid. Curr Sci. 2005;89:1742–1745. [Google Scholar]
- 23.Rode MP, Batti Angulski AB, Gomes FA, da Silva MM, Jeremias TDS, de Carvalho RG, Iucif Vieira DG, Oliveira LFC, Fernandes Maia L, Trentin AG, Hayashi L, de Miranda KR, de Aguiar AK, Rosa RD, Calloni GW. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J Biomater Appl. 2018;33(3):422–434. doi: 10.1177/0885328218795569. [DOI] [PubMed] [Google Scholar]
- 24.Dhanasekaran S. Phytochemical characteristics of aerial part of Cissus quadrangularis (L) and its in-vitro inhibitory activity against leukemic cells and antioxidant properties. Saudi J Biol Sci. 2020;27(5):1302–1309. doi: 10.1016/j.sjbs.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ntungwe NE, Domínguez-Martín EM, Roberto A, Tavares J, Isca VMS, Pereira P, et al. Artemia species: an important tool to screen general toxicity samples. Curr Pharm. 2020;26(24):2892–2908. doi: 10.2174/1381612826666200406083035. [DOI] [PubMed] [Google Scholar]
- 26.Patell VM. Cissus quadrangularis plant extracts for treating osteoporosis and the extraction process thereof [Internet]. WO2008081233A2, 2008. https://patents.google.com/patent/WO2008081233A2/en.
- 27.Lee H, Noh K, Lee SC, Kwon IK, Han DW, Lee IS, et al. Human hair keratin and its-based biomaterials for biomedical applications. Tissue Eng Regen Med. 2014;11(4):255–265. doi: 10.1007/s13770-014-0029-4. [DOI] [Google Scholar]
- 28.Niloy KK, Gulfam M, Compton KB, Li D, Huang GTJ, Lowe TL. Methacrylated hyaluronic acid-based hydrogels maintain stemness in human dental pulp stem cells. Regen Eng Transl Med. 2020;6(3):262–272. doi: 10.1007/s40883-019-00115-4. [DOI] [Google Scholar]
- 29.Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R, Saldaña-Koppel DA, Quiñones-Olvera LF. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed Res Int. 2015;2015:821279. doi: 10.1155/2015/821279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mihaila SM, Popa EG, Reis RL, Marques AP, Gomes ME. Fabrication of endothelial cell-laden carrageenan microfibers for microvascularized bone tissue engineering applications. Biomacromol. 2014;15(8):2849–2860. doi: 10.1021/bm500036a. [DOI] [PubMed] [Google Scholar]
- 31.González Ocampo JI, Bassous N, Ossa Orozco CP, Webster TJ. Evaluation of cytotoxicity and antimicrobial activity of an injectable bone substitute of carrageenan and nano hydroxyapatite. J Biomed Mater Res A. 2018;106(11):2984–2993. doi: 10.1002/jbm.a.36488. [DOI] [PubMed] [Google Scholar]
- 32.Pettinelli N, Rodríguez-Llamazares S, Bouza R, Barral L, Feijoo-Bandín S, Lago F. Carrageenan-based physically crosslinked injectable hydrogel for wound healing and tissue repairing applications. Int J Pharm. 2020;589:119828. doi: 10.1016/j.ijpharm.2020.119828. [DOI] [PubMed] [Google Scholar]
- 33.Diekjürgen D, Grainger DW. Polysaccharide matrices used in 3D in vitro cell culture systems. Biomaterials. 2017;141:96–115. doi: 10.1016/j.biomaterials.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Jain A, Dixit J, Prakash D. Modulatory effects of Cissus quadrangularis on periodontal regeneration by bovine-derived hydroxyapatite in intrabony defects: exploratory clinical trial. J Int Acad Periodontol. 2008;10(2):59–65. [PubMed] [Google Scholar]
- 35.Altaweel AA, Baiomy AABA, Shoshan HS, Abbas H, Abdel-Hafiz AA, Gaber AE, Zewail AA, Elshiekh MAM. Evaluation of osteogenic potential of Cissus quadrangularis on mandibular alveolar ridge distraction. BMC Oral Health. 2021;21(1):491. doi: 10.1186/s12903-021-01847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brahmkshatriya HR, Shah KA, Ananthkumar GB, Brahmkshatriya MH. Clinical evaluation of Cissus quadrangularis as osteogenic agent in maxillofacial fracture: a pilot study. Ayu. 2015;36(2):169–173. doi: 10.4103/0974-8520.175542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nayak T. An assessment of the osteogenic potential of Cissus quadrangularis in mandibular fractures: a pilot study. J Maxillofac Oral Surg. 2020;19(1):106–112. doi: 10.1007/s12663-019-01230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udayakumar R, Sundaran M, Krishna R. Mineral and biochemical analysis of various parts of Cissus quadrangularis linn. Anc Sci Life. 2004;24(2):79–82. [PMC free article] [PubMed] [Google Scholar]
- 39.Potu BK, Rao MS, Nampurath GK, Chamallamudi MR, Nayak SR, Thomas H. Anti-osteoporotic activity of the petroleum ether extract of Cissus quadrangularis Linn in ovariectomized Wistar rats. Chang Gung Med J. 2010;33(3):252–7. [PubMed] [Google Scholar]
- 40.Aswar UM, Bhaskaran S, Mohan V, Bodhankar SL. Estrogenic activity of friedelin rich fraction (IND-HE) separated from Cissus quadrangularis and its effect on female sexual function. Pharmacogn Res. 2010;2(3):138–145. doi: 10.4103/0974-8490.65507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramachandran S, Fadhil L, Gopi C, Amala M, Dhanaraju MD. Evaluation of bone healing activity of Cissus quadrangularis (Linn), Cryptolepis buchanani, and Sardinella longiceps in Wistar rats. Beni-Suef Univ J Basic Appl Sci. 2021;10(1):30. doi: 10.1186/s43088-021-00120-z. [DOI] [Google Scholar]
- 42.Fuangtharnthip P, Chaitisanan A. Khovidhunkit siribang on. Cissus quadrangularis extract stimulated mineralized nodules of dental pulp cells; 2011.
- 43.Zhang Q, Fan M, Bian Z, Chen Z, Zhu Q. Immunohistochemistry of bone sialoprotein and osteopontin during reparative dentinogenesis in vivo. Chin J Dent Res. 2000;3(2):38–43. [PubMed] [Google Scholar]
- 44.Singh N, Singh V, Singh RK, Pant AB, Pal US, Malkunje LR, Mehta G. Osteogenic potential of cissus qudrangularis assessed with osteopontin expression. Natl J Maxillofac Surg. 2013;4(1):52–56. doi: 10.4103/0975-5950.117884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerra JM, Hanes MA, Rasa C, Loganathan N, Innis-Whitehouse W, Gutierrez E, Nair S, Banu J. Modulation of bone turnover by Cissus quadrangularis after ovariectomy in rats. J Bone Miner Metab. 2019;37(5):780–795. doi: 10.1007/s00774-018-0983-3. [DOI] [PubMed] [Google Scholar]
- 46.Tasadduq R, Gordon J, Al-Ghanim KA, Lian JB, Van Wijnen AJ, Stein JL, Stein GS, Shakoori AR. Ethanol extract of Cissus quadrangularis enhances osteoblast differentiation and mineralization of murine pre-osteoblastic MC3T3-E1 cells. J Cell Physiol. 2017;232(3):540–547. doi: 10.1002/jcp.25449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerutti PA. Oxidant stress and carcinogenesis. Eur J Clin Invest. 1991;21(1):1–5. doi: 10.1111/j.1365-2362.1991.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 48.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 49.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24(5):287–296. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 50.Rahman MM, Islam MB, Biswas M, Khurshid Alam AH. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015;8:621. doi: 10.1186/s13104-015-1618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 52.Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P. Biocompatibility of engineered nanoparticles for drug delivery. J Control Release. 2013;166(2):182–194. doi: 10.1016/j.jconrel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Ghasemi-Mobarakeh L, Kolahreez D, Ramakrishna S, Williams DF. Key terminology in biomaterials and biocompatibility. Curr Opin Biomed Eng. 2019;10:45–50. doi: 10.1016/j.cobme.2019.02.004. [DOI] [Google Scholar]
- 54.Raut HK, Das R, Liu Z, Liu X, Ramakrishna S. Biocompatibility of biomaterials for tissue regeneration or replacement. Biotechnol J. 2020;15(12):e2000160. doi: 10.1002/biot.202000160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are not publicly available as ethics approval was granted on the basis that only the researchers involved in the study could access the identified data but are available and accessible from the corresponding author on reasonable request.


