Abstract
Several nalB-type multidrug-resistant mutants of Pseudomonas aeruginosa overexpressed MexAB-OprM and carried mutations in the local regulatory gene, mexR. Others, dubbed nalC types, carried mutations elsewhere and overexpressed MexAB-OprM less extensively than the nalB strains. Available evidence showed that MexR acted solely as repressor. Disruption of the mexR gene at various places suggested that the 5′ end of mexR may be a part of the mexAB-oprM promoter.
The intrinsic antibiotic resistance of Pseudomonas aeruginosa is attributable both to the limited permeability of the organism's outer membrane (12) and to the activity of broadly specific antibiotic efflux systems such as MexAB-OprM (3, 14, 15). MexAB-OprM is a member of a family of multidrug efflux systems of which there are several examples in P. aeruginosa including MexCD-OprJ (13), MexEF-OprN (6), and MexXY-OprM (1, 10). Mutants hyperexpressing MexAB-OprM and exhibiting an elevated multidrug-resistant (MDR) phenotype have been described (9, 22). Isolated in the laboratory (9, 22) and from patients after antibiotic therapy (4, 24), these so-called nalB mutants often carry mutations in a gene, mexR, which occurs immediately upstream of the efflux genes (4, 17, 24) and encodes a repressor of mexAB-oprM expression (17). Still, a mexR null mutant constructed in vitro, though MDR and expressing elevated levels of MexAB-OprM, was more antibiotic susceptible and exhibited reduced expression of mexAB-oprM compared with previously described nalB strains (17). One hypothesis stated that MexR functions as both repressor and activator, and those mutations in mexR which yield a nalB phenotype render MexR in an activator form. To assess, then, the role(s) of MexR in regulating mexAB-oprM expression, several nalB and mexR mutants were isolated, and their influence on MexAB-OprM was examined. We report here that MexR functions solely as a repressor in controlling mexAB-oprM expression and that nalB strains are simply derepressed for mexAB-oprM expression.
Methods.
Strains and plasmids used in this study are described in Table 1. Mutants hyperexpressing MexAB-OprM were selected on Luria broth (L-broth; Miller's Luria broth base [Difco] and 2 g of NaCl per liter of H2O) plates containing 0.2 μg of ciprofloxacin and 12 μg of cefoperazone per ml. MexAB-OprM hyperexpression was confirmed by Western immunoblotting with antibodies to MexB and OprM. Antibiotics were included in growth media as required at the following concentrations: tetracycline, 10 μg/ml (Escherichia coli) or 100 μg/ml (P. aeruginosa); chloramphenicol, 50 μg/ml (E. coli) or 200 μg/ml (P. aeruginosa); and mercuric chloride, 15 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| E. coli | ||
| 5K | thr lacZ rpsL thi ser hsdR hsdM | 16 |
| S17-1 | thi pro hsdR recA Tra+ | 19 |
| P. aeruginosa | ||
| K767 | PAO1 prototroph | 8 |
| OCR1 | nalB-type MDR mutant of K767 | 8 |
| K1454 to K1461 | MexAB-OprM hyperexpressing MDR mutants of K767 | This study |
| H103 | PAO1 prototroph | R. E. W. Hancock, University of British Columbia, Canada |
| K1462 to K1468 | MexAB-OprM hyperexpressing MDR mutants of H103 | This study |
| K1482 | K767 carrying a chromosomal ΩHg insertion in the SstI site of mexR | This study |
| K1483 | K1454 (nalC) carrying a chromosomal ΩHg insertion in the SstI site of mexR | This study |
| K1485 | K767 carrying a chromosomal ΩHg insertion in the MluI site of mexR | This study |
| K1486 | K1454 (nalC) carrying a chromosomal ΩHg insertion in the MluI site of mexR | This study |
| K1488 | K767 carrying a chromosomal ΩHg insertion in the Tth111I site of ORF2 | This study |
| K1489 | K1454 (nalC) carrying a chromosomal ΩHg insertion in the Tth111I site of ORF2 | This study |
| K1491 | K767 ΔmexR; in-frame deletion | This study |
| K1494 | K767 carrying a chromosomal mexA-phoA fusion | This study |
| K1495 | K1454 (nalC) carrying a chromosomal a mexA-phoA fusion | This study |
| K1496 | K1455 (nalB) carrying a chromosomal a mexA-phoA fusion | This study |
| K1497 | K1482 (mexR::ΩHg [SstI]) carrying a chromosomal a mexA-phoA fusion | This study |
| K1498 | K1483 (nalC + mexR::ΩHg [SstI]) carrying a chromosomal a mexA-phoA fusion | This study |
| K1500 | K1485 (mexR::ΩHg [MluI]) carrying a chromosomal a mexA-phoA fusion | This study |
| K1501 | K1486 (nalC + mexR::ΩHg [MluI]) carrying a chromosomal a mexA-phoA fusion | This study |
| Plasmids | ||
| pRK415 | Broad-host-range cloning vector; plac MCS, Tcr | 5 |
| pMMB206 | Broad-host-range cloning vector; lacI plac MCS, Cmr | 11 |
| pEX18Tc | Gene replacement vector; Mob+sacB Tcr | H. Schweizer, Colorado State University |
| pMP190 | Broad-host-range, low-copy-number lacZ fusion vector; Cmr Smr | 20 |
| pHP45ΩHg | pHP45Ω derivative carrying the HgCl2 resistance operon of Tn501 | 2 |
| pMXR5 | pMP190 derivative carrying the mexR-mexA intergenic region, with the mexA promoter oriented toward the promoterless lacZ gene | 17 |
| pPV2 | pAK1900 derivative carrying mexAB, mexR and ORF2 on a 7.7-kb XhoI fragment, Apr Cbr | 17 |
| pMXA1 | pSUP202 derivative carrying a mexA-phoA fusion, Tcr | 17 |
| pRSP55 | pRK415::mexRK767 (i.e., mexR from K767) | This study |
| pRSP56 | pRK415::mexRK1454 | This study |
| pRSP58 | pRK415::mexRK1455 | This study |
| pRSP60 | pRK415::mexRK1456 | This study |
| pRSP64 | pEX18Tc derivative carrying mexR on a 3.4-kb EcoRI-SmaI fragment | This study |
| pRSP65 | pRSP64::ΩHg; ΩHg insertion in the unique SstI site of mexR | This study |
| pRSP67 | pMMB206::ORF2; ORF2 in the same orientation as plac | This study |
| pRSP70 | pRSP64::ΩHg; ΩHg insertion in the unique MluI site of mexR | This study |
| pRSP72 | pRSP64::ΩHg; ΩHg insertion in the unique Tth111I site of ORF2 | This study |
| pRSP75 | pEX18Tc::ΔmexR; carries a 261-bp SstI-MluI deletion | This study |
| pRSP83 | pRK415 derivative carrying the mexR::ΩHg (SstI) gene of pRSP65 on a 5.5-kb PstI fragment | This study |
MCS, multiple cloning site; Tcr, tetracycline resistant; Cmr, chloramphenicol resistant; Smr, streptomycin resistant; Apr, ampicillin resistant; Cbr, carbenicillin resistant; ΩHg, mercury resistant Ω interposon.
The mexR gene was amplified from P. aeruginosa strains by PCR by using chromosomal DNA as template and primers MEXRF1 (5′-GCGAGAATTCCGTTCGTTGCATAGCGTTGTC-3′) and MEXRB1 (5′-GCGAGAATTCCGAAGGCATTCGCCAGTAAGC-3′). The sequences of mexR and the mexR-mexA intergenic region were determined by sequencing of the PCR products directly and after cloning them into pRK415. The open reading frame (ORF) downstream of mexR (ORF2, Fig. 1) was also amplified by using primers K3 (5′-TACGGGATCCCGCGCAACCGCTTGAGATA-3′) and K4 (5′-GCATGCGCATGCCCTGTGGATGCGCGAACTGAG- 3′) and then sequenced. ORF2 from P. aeruginosa K767 was cloned into pMMB206 (yielding pRSP67) after digestion of the PCR product with BamHI and HindIII (site present downstream of ORF2) and sequenced. PCR reaction mixtures (100 μl), including 2.5 U of Taq DNA polymerase (Life Technologies), 1× PCR buffer (Life Technologies), 0.3 μM concentrations of each primer, 0.2 mM concentrations of deoxynucleoside triphosphates, 2 mM MgCl2, 10% (vol/vol) dimethyl sulfoxide, and 10 ng of template DNA, were heated for 1 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at 56°C, and 1 min at 72°C, before finishing with 10 min at 72°C.
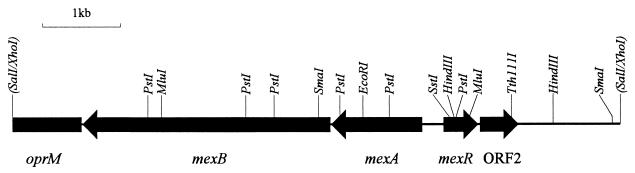
FIG. 1.
Restriction map of the mexRAB-oprM locus in plasmid pPV2. The SalI-XhoI junction that is not digested by either enzyme is within parenthesis.
To construct mexR::ΩHg insertion mutations, a 3.4-kb mexR-containing EcoRI-SmaI fragment of pPV2 was first cloned into pEX18Tc, yielding pRSP64. mexR was disrupted at its SstI site by digesting pRSP64 with SstI, followed by treatment with T4 DNA polymerase (New England Biolabs) and ligation with the SmaI-restricted ΩHg fragment of pHP45ΩHg, yielding pRSP65. Similarly, mexR and ORF2 were disrupted at their MluI and Tth111I sites, respectively, following digestion of pRSP64 with either MluI or Tth111I, treatment with the Klenow fragment (New England Biolabs), and ligation with the ΩHg SmaI fragment, creating pRSP70 and pRSP72. An internal deletion of mexR was constructed by digesting pRSP64 with SstI, treatment with T4 DNA polymerase, and digestion with MluI. The pEX18Tc-containing DNA was purified free of the SstI-MluI fragment, treated with Klenow fragment and ligated to yield pRSP75. These pEX18Tc-derived plasmids were mobilized from E. coli S17-1 into P. aeruginosa (14), and transconjugants carrying the plasmids in the chromosome were selected on L-agar containing tetracycline. mexR::ΩHg mutants were then selected on L-agar containing 10% (wt/vol) sucrose and HgCl2, while strains harboring the mexR deletion were selected on 10% (wt/vol) sucrose and screened for the mexR deletion by PCR.
Restriction digests, ligations, and transformations were carried out as described previously (18). Plasmid DNA was isolated with the aid of a plasmid Maxi Kit (Qiagen). DNA fragments used in cloning were purified from agarose gels with Prep-A-Gene (Bio-Rad). pRK415-, pMMB206-, and pMP190-derived vectors were introduced into P. aeruginosa from E. coli by triparental mating (22, 23). Cell envelopes were isolated as described previously (21) and resolved on sodium dodecyl sulfate-polyacrylamide gels (10% [wt/vol]). Gels were Coomassie blue stained or else electroblotted and developed with anti-MexB (21) or anti-OprM (23) antibodies. The antibiotic susceptibility of P. aeruginosa strains was assessed by using the broth dilution assay (7). β-Galactosidase assays were carried out as described elsewhere (17). The mexA-phoA fusion plasmid, pMXA1, was introduced into the chromosome of P. aeruginosa strains via conjugation from E. coli S17-1 as described previously (17). Fusion-containing strains were grown to an A600 of 1 in L-broth, concentrated twofold in 0.1 M Tris-HCl (pH 8.0) and assayed for alkaline phosphatase activity as described earlier (17).
Differential MexAB-OprM hyperexpression in nalB and nalC MDR strains.
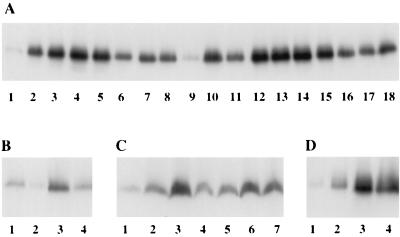
Many P. aeruginosa strains selected on ciprofloxacin and cefoperazone exhibited a MDR profile characteristic of the MexAB-OprM hyperexpressing nalB strain OCR1 (17) (Table 2). Western immunoblotting with anti-MexB (Fig. 2A) and anti-OprM (data not shown) demonstrated that the MDR mutants (eight derived from PAO1 strain K767 and seven derived from PAO1 strain H103) hyperexpressed the MexAB-OprM efflux components. MexB production in many of the mutants (Fig. 2A) was comparable to that of OCR1 (Fig. 2A, lane 10). In some, however, MexB production, though elevated, was reduced relative to this nalB strain (Fig. 2A). The antibiotic resistance of the MDR strains reflected these differences in MexB levels, with those strains producing less MexB consistently twofold more susceptible to several antibiotics (Table 2).
TABLE 2.
Antibiotic susceptibilities of P. aeruginosa strains carrying mutations in mexR
| Strain | Relevant genotype | MICa (μg/ml) of:
|
|||
|---|---|---|---|---|---|
| CAR | CEF | CAM | TET | ||
| K767 | Wild type | 128 | 2 | 64 | 16 |
| OCR1 | nalB | 1,024 | 8 | 512 | 64 |
| K1454b | nalC | 512 | 4 | 256 | 32 |
| K1455c | nalB | 1,024 | 8 | 512 | 64 |
| H103 | Wild type | 64 | 2 | 32 | 16 |
| K1464d | nalB | 512 | 8 | 256 | 64 |
| K1466e | nalC | 256 | 4 | 128 | 32 |
| K1482 | K767 mexR::ΩHg (SstI)f | 256 | 4 | 128 | 32 |
| K1483 | K1454 mexR::ΩHg (SstI) | 256 | 4 | 128 | 32 |
| K1485 | K767 mexR::ΩHg (MluI) | 1,024 | 8 | 512 | 64 |
| K1486 | K1454 mexR::ΩHg (MluI) | 1,024 | 8 | 512 | 64 |
| K1488 | K767 ORF2::ΩHg (Tth111I) | 128 | 2 | 64 | 16 |
| K1491 | K767 ΔmexR (SstI-MluI) | 1,024 | 8 | 512 | 64 |
CAR, carbenicillin; CEF, cefepime; CAM, chloramphenicol; TET, tetracycline.
K1459, K1460 and K1489 displayed the same MIC values as K1454.
K1456, K1457, K1458, and K1461 displayed the same MIC values as K1455.
K1462, K1463, and K1465 displayed the same MIC values as K1464.
K1467 and K1468 displayed the same MIC values as K1466.
Restriction sites in parenthesis denote either the position of insertion of the Ω interposon (mercury resistant) or the restriction sites that were used to construct defined deletions.
FIG. 2.
Expression of MexB in MexAB-OprM hyperexpressing strains of P. aeruginosa (A), strains carrying the cloned mexR gene (B), mexR::ΩHg insertion mutants (C), and mexR deletion strains (D). Cell envelopes (10 μg of protein) were subjected to Western immunoblotting with antibodies raised against MexB. (A) Lane 1, H103 (wild type); lane 2, K1462 (nalB); lane 3, K1463 (nalB); lane 4, K1464 (nalB); lane 5, K1465 (nalB); lane 6, K1466 (nalC); lane 7, K1467 (nalC); lane 8, K1468 (nalC); lane 9, K767 (wild type); lane 10, OCR1 (nalB); lane 11, K1454 (nalC); lane 12, K1455 (nalB); lane 13, K1456 (nalB); lane 14, K1457 (nalB); lane 15, K1458 (nalB); lane 16, K1459 (nalC); lane 17, K1460 (nalC); and lane 18, K1461 (nalB). (B) Lane 1, K767 carrying pRK415; lane 2, K767 carrying the mexR plasmid pRSP55; lane 3, K1482 (mexR::ΩHg [SstI]) carrying pRK415; and lane 4, K1482 carrying pRSP55. (C) Lane 1, K767; lane 2, K1454 (nalC); lane 3, K1455 (nalB); lane 4, K1482 (mexR::ΩHg [SstI]); lane 5, K1483 (nalC + mexR::ΩHg [SstI]); lane 6, K1485 (mexR::ΩHg [MluI]); and lane 7, K1486 (nalC + mexR::ΩHg [MluI]). (D) Lane 1, K767 (wild type); lane 2, K904 (mexR::ΩHg [SstI]); lane 3, K1455 (nalB); and lane 4, K1491 (ΔmexR).
MexAB-OprM hyperexpression in a variety of nalB strains correlates with a mutation in mexR (4, 17, 24). The nucleotide sequences of the mexR genes from K767 and H103 were identical to the previously published mexR sequence (17), and while several of the MDR mutants did carry mutations in mexR, others did not (Table 3). These latter mutants, which also lacked mutations in the mexR-mexA intergenic region, expressed reduced levels of MexAB-OprM and were less resistant than those MDR strains with mexR mutations (Fig. 2A; Table 2). Ziha-Zarifi et al. (24) described a single example of a clinical strain of P. aeruginosa hyperexpressing MexAB-OprM also lacking a mutation in mexR. To distinguish these from the MexAB-OprM hyperexpressing mutants that carry mexR mutations (nalB-type), we propose the term nalC for these mutants.
TABLE 3.
Characteristics of MexAB-OprM hyperexpressing multidrug resistant mutants
| Strain | mexR mutationa | MexR aab change | MexB expressionc |
|---|---|---|---|
| K767 | Wild type | + | |
| OCR1 | C208 to T | Arg70 to Trp | ++++ |
| K1454 | Wild type | +++ | |
| K1455 | A388 to C | Thr130 to Pro | ++++ |
| K1456 | Deletion from C294 to G300 | –d | ++++ |
| K1457 | Insertion of T after T203 | – | ++++ |
| K1458 | Deletion from C261 to G272 | Deletion from Ser88 to Arg91 | ++++ |
| K1459 | Wild type | +++ | |
| K1460 | Wild type | +++ | |
| K1461 | A281 to C | Gln94 to Pro | ++++ |
| H103 | Wild type | + | |
| K1462 | T170 to G | Leu57 to Arg | ++++ |
| K1463 | A388 to C | Thr130 to Pro | ++++ |
| K1464 | Insertion of T after A80 | – | ++++ |
| K1465 | Deletion from T71 to A425 | – | ++++ |
| K1466 | Wild type | +++ | |
| K1467 | Wild type | +++ | |
| K1468 | Wild type | +++ |
Changes to the nucleotide (A, G, C, or T) sequence of mexR are shown. The numbers in subscript denote the positions of the nucleotides with reference to the A1TG start codon of mexR.
Changes to the amino acid (aa; three-letter code) sequence of MexR are shown. The numbers in subscript denote the positions of the amino acids with reference to the f-Met1 of MexR.
Levels of MexB expression are reported qualitatively as determined by visual assessment of Western immunoblots of cell envelopes developed with anti-MexB antibodies.
–, Sequence out of frame; full-length MexR not made.
The observation that nalC strains express reduced levels of MexAB-OprM relative to nalB strains contrasts with a previous report (24), although OprM, and not MexB, levels were assessed as a marker of MexAB-OprM expression. Indeed, we also observed no differences in OprM levels between nalC and nalB strains (data not shown). Since OprM can be expressed and function independently of MexAB (23), a finding consistent with its role as the outer membrane component of additional MDR efflux systems in P. aeruginosa (e.g., MexXY [1, 10]), MexB may be a more accurate marker of MexAB-OprM production. Certainly, the antibiotic susceptibility data is consistent with nalC strains expressing less MexAB-OprM than the nalB strains.
MexR functions solely as a repressor of the mexAB-oprM operon.
The mexR mutations identified in the aforementioned nalB strains included base substitutions, insertions and deletions, although none of these were described previously (see OCR1, [Table 3] and references 4 and 24), and only one mutation was recovered more than once (i.e., T130P) (Table 3). One mutant, K1465, had 80% of its mexR sequence deleted while another, K1464, had a frameshift early in the mexR sequence (Table 3). That these expressed levels of MexAB-OprM indistinguishable from that of mexR point mutants (Fig. 2A, lanes 4 and 5, compare lanes 2 and 3) strongly suggested that mexAB-oprM hyperexpression in nalB strains, including those with base substitutions in mexR, results from loss of MexR (and its repressor activity). Thus, our previous suggestion that MexR is converted to an activator in nalB strains (17) seems unlikely. Consistent with this, the wild-type mexR genes of the prototrophic strain K767 (on plasmid pRSP55) and the nalC strain K1454 (on plasmid pRSP56; data not shown) reduced expression of a plasmid-borne mexA-lacZ fusion (pMXR5) in E. coli (from 782 ± 28 Miller units in the absence of the mexR plasmid to 108 ± 9 Miller units in the presence of pRSP55), while the mutated mexR genes of nalB strains K1455 (on plasmid pRSP58) and K1456 (on plasmid pRSP60) had no effect on the mexA-lacZ expression (pRSP58, 794 ± 66 Miller units; pRSP60, 609 ± 22 Miller units). Similarly, the wild-type mexR gene repressed mexA-lacZ expression (data not shown) and MexB production (Fig. 2B, lanes 2 and 4, compare lanes 1 and 3) in P. aeruginosa, while the mutated mexR genes had no effect on mexA-lacZ expression (data not shown). The mexR mutations, thus, obviated the repressor activity of MexR without converting it into an activator of mexAB-oprM expression.
mexR cis effects on mexAB-oprM expression.
Disruption of the 5′ end of mexR (at the SstI site [27th codon]) (Fig. 1) with a ΩHg cartridge did not enhance mexAB-oprM expression and antibiotic resistance to the extent seen in nalB strains (17), a result reproduced here (see K1482) (Fig. 2C, lane 4 [see lane 3]; Table 2). One explanation is that the ΩHg insertion exerted a polar effect on a downstream gene(s) required for mexAB-oprM hyperexpression and, perhaps, the target of mutation in nalC mutants. Although an ORF was identified downstream of mexR in strain K767 (ORF2, Fig. 1), no sequence changes were observed in ORF2 of the nalC strain K1454. Moreover, the cloned ORF2 failed to restore MexAB-OprM expression in the mexR::ΩHg (SstI) derivative of a nalC strain (e.g., K1483) to levels seen for the original nalB strains (data not shown), and disruption of ORF2 by insertion of the ΩHg cartridge at the Tth111I site (Fig. 1) in wild-type strain K767 (yielding K1488; Table 2) or nalC strain K1454 (yielding K1489; data not shown) did not alter their drug resistance properties. Finally, disruption of mexR with an ΩHg at the MluI site (codon 114; Fig. 1) in strain K767 (yielding K1485) produced an MDR phenotype reminiscent of nalB strains (e.g. OCR1; Table 2) and levels of MexB (as a marker of MexAB-OprM expression) which were higher, like other nalB strains, than that seen in the mexR::ΩHg (SstI) mutant K1482 (Fig. 2C, compare lanes 4 and 6). Thus, ORF2 is not involved in mexAB-oprM expression, and disruption of mexR alone is sufficient to produce a nalB phenotype.
The differential effect of ΩHg insertions at the SstI and MluI on MexAB-OprM production was reflected in the expression of the efflux genes, as assessed by using chromosomal mexA-phoA fusions. Disruption of mexR at the SstI site (see K1497) yielded a modest ca. twofold increase in expression from the mexA promoter, while disruption of this gene at the MluI site (K1500) produced a fourfold increase in efflux gene expression (Table 4). This suggested a possible cis effect of the SstI::ΩHg insertion on mexAB-oprM expression, perhaps because sequences beyond the SstI site in mexR were needed for full mexAB-oprM promoter activity. This was consistent with an earlier observation that a mexR::ΩHg (SstI) mutant and a nalB strain showed comparably elevated expression of a plasmid-borne mexA-lacZ fusion (data not shown), while expression of a chromosomal mexA-phoA fusion was reduced in the SstI insertion mutant relative to the nalB strain (17). Still, deletion of the SstI-MluI fragment from mexR (in strain K1491) produced a phenotype indistinguishable from that of a nalB strain (Fig. 2D, compare lanes 3 and 4) or the mexR::ΩHg (MluI) mutant (Table 3). Although it was possible that a MexR peptide resulting from the ΩHg insertion at the SstI site had partial repressor activity, the cloned mexR (SstI)::ΩHg fragment from K1482 (pRSP83) did not repress MexAB-OprM expression in trans (data not shown). Thus, while the increase in MexAB-OprM and antibiotic resistance seen in strain K1482 (K767 mexR::ΩHg [SstI]) likely results from loss of MexR and subsequent derepression of mexAB-oprM, failure to see expression at the level typical of nalB or mexR::ΩHg (MluI) insertion mutants apparently results from a negative impact of the SstI insertion on mexA(B-oprM) promoter activity. Perhaps the ΩHg element altered the topology of the DNA at the 5′ end of mexR and this region is important for mexAB-oprM expression. It is unclear, however, whether this reflects involvement of additional regulator(s) or the need for a specific DNA conformation (for full promoter activity) which requires sequences in the vicinity of mexR. The ΩHg insertion at the SstI site of mexR in a nalC strain (K1483) increased its susceptibility to antibiotics (Table 2) and reduced expression of MexB (Fig. 2C, lane 5) and a mexA-phoA fusion (see K1498, Table 4), suggesting that the 5′ region of mexR is important for mexAB-oprM hyperexpression associated with the nalC mutation. We are currently delineating the region required for optimal mexAB-oprM expression.
TABLE 4.
Expression of chromosomal mexA-phoA fusion in P. aeruginosaa
| Strain | Relevant genotype | Alkaline phosphatase activity (A405/A600)b |
|---|---|---|
| K1494 | Wild type | 0.54 ± 0.06 |
| K1495 | nalC | 1.15 ± 0.05 |
| K1496 | nalB | 2.26 ± 0.09 |
| K1497 | mexR::ΩHg (SstI)c | 0.80 ± 0.05 |
| K1498 | nalC + mexR::ΩHg (SstI) | 0.81 ± 0.05 |
| K1500 | mexR::ΩHg (MluI) | 2.26 ± 0.08 |
| K1501 | nalC + mexR::ΩHg (MluI) | 1.59 ± 0.09 |
P. aeruginosa strains carrying a mexA-phoA chromosomal fusion were grown to A600 of 1 in L-broth and assayed for alkaline phosphatase activity.
Activity is reported as the amount of p-nitrophenyl released from p-nitrophenyl phosphate, measured at A405, as a function of the amount of cell material used in the assay, measured at A600. The data are reported as the means of three determinations ± the standard deviations and are representative of three repetitions.
As described in Table 3.
Interestingly, a ΩHg insertion at the MluI site of mexR in the nalC strain K1454 (yielding K1486) also caused an increase in antibiotic resistance (Table 2) and MexB expression (Fig. 2C, lane 7), though the latter was less than that seen for a nalB strain (K1455; Fig. 2C, lane 3) or a mexR::ΩHg (MluI) derivative of strain K767 (K1485; Fig. 2C, lane 6). This difference in expression was also observed when mexA-phoA fusions were employed (Table 4). Thus, while a nalC mutation afforded increased MexAB-OprM production and MDR, it also compromised full expression of this efflux system when mexR was inactivated. This suggests some interplay between mexR and nalC in the regulation of mexAB-oprM.
Acknowledgments
We thank Xian-Zhi Li and Nicole Barré for isolation of the P. aeruginosa strains K1462 to K1468.
We also gratefully acknowledge the financial support of the Canadian Cystic Fibrosis Foundation (CCFF). R.S. is a Medical Research Council of Canada and CCFF Postdoctoral Fellow. K.P. is a CCFF Martha Morton Scholar.
REFERENCES
- 1.Aires J R, Köhler T, Nikaido H, Plesiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–2628. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 3.Gotoh N, Tsujimoto H, Poole K, Yamagishi J-I, Nishino T. The outer membrane protein OprM of Pseudomonas aeruginosa is encoded by oprK of the mexA-mexB-oprK multidrug resistance operon. Antimicrob Agents Chemother. 1995;39:2567–2569. doi: 10.1128/aac.39.11.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalal S, Wretlind B. Mechanisms of quinolone resistance in clinical strains of Pseudomonas aeruginosa. Microbiol Drug Resist. 1998;4:257–261. doi: 10.1089/mdr.1998.4.257. [DOI] [PubMed] [Google Scholar]
- 5.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 6.Koehler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 7.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1847–1851. doi: 10.1128/aac.36.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mine T, Morita Y, Kataoka A, Mitzushima T, Tsuchiya T. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–417. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morales V M, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J-I, Li X-Z, Nishino T. Overexpression of the mexC-mex-oprJ efflux operon in nfxB multidrug resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 14.Poole K, Heinrichs D E, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 15.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole K, Schiebel E, Braun V. Molecular characterization of the hemolysin determinant of Serratia marcescens. J Bacteriol. 1988;170:3177–3188. doi: 10.1128/jb.170.7.3177-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole K, Tetro K, Zhao Q, Neshat S, Heinrichs D, Bianco N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob Agents Chemother. 1996;40:2021–2028. doi: 10.1128/aac.40.9.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Simon R, Priefer U, Puehler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 20.Spaink H P, Okker R J H, Wijffelman C A, Pees E, Lugtenberg B J J. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- 21.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srikumar R, Li X-Z, Gotoh N, Poole K. The inner membrane efflux components are responsible for the β-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol. 1997;179:7875–7881. doi: 10.1128/jb.179.24.7875-7881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Q, Li X-Z, Srikumar R, Poole K. Contribution of outer membrane efflux protein OprM to antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Antimicrob Agents Chemother. 1998;42:1682–1688. doi: 10.1128/aac.42.7.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziha-Zarifi I, Llanes C, Koehler T, Pechere J-C, Plesiat P. In vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob Agents Chemother. 1999;43:287–291. doi: 10.1128/aac.43.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]