Abstract
In Escherichia coli, the CpxA-CpxR two-component signal transduction system and the ςE and ς32 response pathways jointly regulate gene expression in adaptation to adverse conditions. These include envelope protein distress, heat shock, oxidative stress, high pH, and entry into stationary phase. Certain mutant versions of the CpxA sensor protein (CpxA* proteins) exhibit an elevated ratio of kinase to phosphatase activity on CpxR, the cognate response regulator. As a result, CpxA* strains display numerous phenotypes, many of which cannot be easily related to currently known functions of the CpxA-CpxR pathway. It is unclear whether CpxA* phenotypes are caused solely by hyperphosphorylation of CpxR. We here report that all of the tested CpxA* phenotypes depend on elevated levels of CpxR-P and not on cross-signalling of CpxA* to noncognate response regulators.
A typical sensor protein of a two-component signal transduction system catalyzes both the phosphorylation and dephosphorylation of the cognate response regulator. The ratio between the kinase and the phosphatase activities of the sensor determines the steady-state level of the phosphorylated or functional form of the response regulator (32). Thus, any mutation in the sensor protein that significantly affects its kinase/phosphatase activity ratio should have important physiological consequences. An example is provided by the Cpx system of Escherichia coli, comprising the CpxA sensor kinase/phosphatase and the CpxR response regulator. Certain mutant CpxA proteins, CpxA* proteins, appear to possess autokinase activity but are deficient in CpxR-P phosphatase activity, leading to an overaccumulation of CpxR-P (25). The elevated level of CpxR-P in turn hyperactivates the expression of the cpxRA operon (6, 27). Cells synthesizing CpxA* show numerous phenotypes, which include an impaired donor conjugative ability (11, 12, 28, 29); a deficiency in murein lipoprotein and OmpF in the cell envelope (14, 15); an anomalous positioning of the FtsZ ring during cell division (22); a decreased swarming ability (6); impaired abilities to grow on succinate (24), l-lactose (21), and l-proline (20); an acquired ability to utilize l-serine as the sole carbon source (17, 18, 33); partial auxotrophies for isoleucine and valine (13, 34); a growth sensitivity to high temperature (11) and sodium dodecyl sulfate (SDS) (1); and an enhanced tolerance to high pH (3), CuCl2 (6), colicins A and K (19), amikacin, and kanamycin (24, 35).
It is known that the Cpx system and the ςE and ς32 response pathways cooperatively manage envelope protein distress by activating the expression of ppiA and ppiD (encoding periplasmic peptidyl-prolyl cis-trans isomerases), dsbA (encoding a periplasmic disulfide oxidoreductase), degP (encoding a periplasmic protease), and cpxP (encoding a periplasmic protein) (2–5, 23, 26). The Cpx system has also been implicated in conjugation (11, 12, 28, 29), invasion of host cells, and virulence (8, 9). An even broader role for this two-component system is indicated by recent findings that the expression of the cpxRA operon increases at the onset of stationary growth in an RpoS-dependent manner and that operons involved in motility and chemotaxis (motAB cheAW and tsr) are under direct negative control of CpxR-P (6). In view of such an extensive role of the Cpx system, it is not surprising that a multitude of physiological anomalies are exhibited by CpxA* strains.
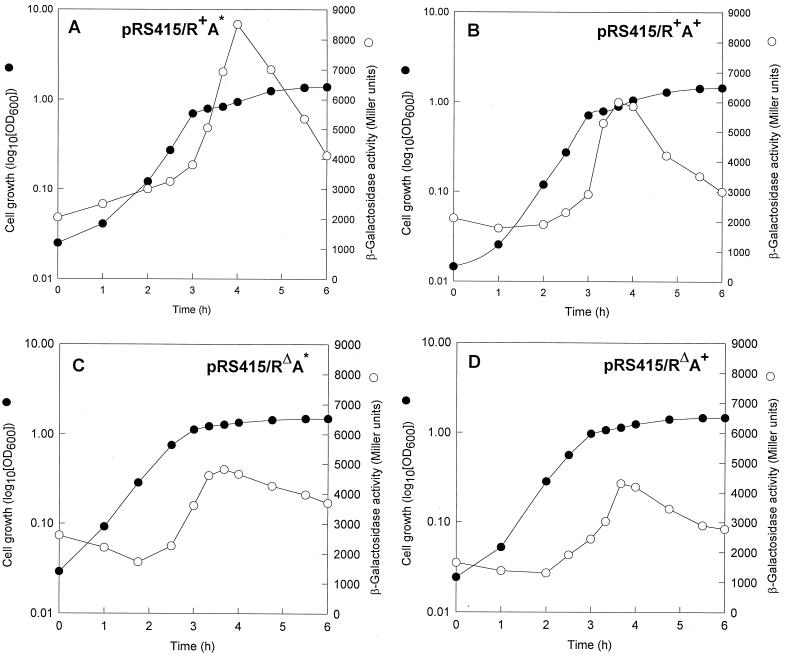
To test whether the CpxA* phenotypes are exclusively attributable to exaggerated levels of CpxR-P, we compared the phenotypes of strain ECL3501 (ΔcpxRA) (Table 1) (6) expressing four different cpxRA operons: (i) wild-type cpxR+A+, (ii) cpxR+A*, (iii) cpxRΔA+; and (iv) cpxRΔA*. The low-copy-number plasmid pRS415 (30) was used to express the various cpx operons, since cpxRA is autogenously activated by CpxR-P (6, 27). Consequently, in the absence of CpxR, a single copy of the operon may not supply an adequate amount of CpxA* for phenotypic manifestation. The missense mutation in CpxA* consists of a Leu38-to-Phe substitution (22). The deletion in CpxRΔ extends from amino acid residues 28 to 166 (6), a region that includes the conserved phosphoryl acceptor Asp51 (25). The plasmidborne operons carried the cpx promoter region that stretches 309 bp upstream of the cpxR start codon and includes the CpxR-P boxes (6). To monitor the transcription of the cpx constructs, a promoterless lacZYA operon was placed downstream of cpxA or cpxA* (Fig. 1). The synthesis of CpxA, CpxA*, CpxR, and CpxRΔ by the transformants was confirmed by SDS-polyacrylamide (12.5 and 15%) gel electrophoresis. No Cpx proteins were synthesized by ECL3501 bearing vector pRS415 without a cpx insert (data not shown). The levels of expression of the various cpx operons were quantified by β-galactosidase activities (Miller units) during growth of the transformants in glucose (0.2%) minimal medium. The β-galactosidase activity levels obtained from pRS415/R+A* (Fig. 2A) exceeded those obtained from pRS415/R+A+ (Fig. 2B). This result was expected because the increased kinase/phosphatase ratio of CpxA* should cause elevated CpxR-P levels, which in turn should enhance cpxR+A* expression by autoactivation. Likewise, the β-galactosidase activity levels obtained from pRS415/RΔA* (Fig. 2C) and pRS415/RΔA+ (Fig. 2D) were lower than those obtained from pRS415/R+A+, due to the absence of autoactivation. It should be mentioned, however, that even in the absence of CpxR, the expression of cpxA and cpxA* (from 1,500 to 4,500 Miller units) (Fig. 2B and C) exceeded that from single-copy cpxR+A* (from 900 to 1,800 Miller units [6]). The β-galactosidase activity levels in the control transformant, bearing vector pRS415, which contains a promoterless lacZYA, varied from 15 to 35 Miller units (data not shown). Thus, the levels of plasmid-specified CpxA and CpxA* should be sufficient to give the appropriate phenotypes in the absence of CpxR-P.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristic | Reference |
|---|---|---|
| Strains | ||
| BW21355 | K-12 F−rph-1 Δ(lac)X74 | 6 |
| ECL3501 | BW21355 Δ(cpxRA)2 | 6 |
| Plasmids | ||
| pRS415 | lacYZA-based promoter-fusion vector | 30 |
| pRS415/R+A+ | pRS415 containing the cpxR+A+ operon | 6 |
| pRS415/RΔA+ | pRS415 containing the cpxRΔA+ operon | 6 |
| pRS415/R+A* | pRS415 containing the cpxR+A* operon | 6 |
| pRS415/RΔA* | pRS415 containing the cpxRΔA* operon | 6 |
| pEXT20 | Expression vector | 7 |
| pEXTA* | pEXT20 containing cpxA* | This study |
FIG. 1.
Plasmid constructs for expressing cpxR+A+, cpxRΔA+, cpxR+A*, and cpxRΔA* in strain ECL3501 (ΔcpxRA). The dark boxes depict the deleted regions within the chromosomal cpxRA and lacZYA operons. The hatched boxes depict the in-frame deletion (418 bp) within cpxR. The cpx operon constructs contain 309 bp upstream from the cpxR start codon, including the cpx promoter region (PcpxRA).
FIG. 2.
Growth and specific β-galactosidase activity profiles of E. coli ECL3501 expressing various cpx operon constructs. The cells were grown in 20 ml of glucose (0.2%)-containing minimal medium containing 50 μg of ampicillin per ml (300 rpm, 37°C) and assayed for their β-galactosidase activity as described by Miller (16). OD600, optical density at 600 nm.
ECL3501 strains bearing pRS415 expressing different cpx alleles were then scored for CpxA* anomalies: temperature sensitivity (11); inability to grow on succinate (24); resistance to amikacin (24, 35), high pH (3), CuCl2 (6), the redox dye toluidine blue (J. M. Dong, unpublished data), H2O2 (P. De Wulf, unpublished data), and SDS (1); sensitivity to EDTA (P. De Wulf, unpublished data); diminished swarming ability (6); and aberrant positioning of the division septum (22). The conditions and results of the analyses are shown in Table 2. None of the 11 CpxA* phenotypes was expressed in the absence of CpxR, indicating that the anomalies result exclusively from excessive levels of CpxR-P caused by CpxA*. To confirm this conclusion, a 1.65-kb segment containing cpxA* was excised from pRS415/RΔA* with SmaI and BamHI. The fragment was then treated with DraI (a site is present 43 bp upstream of the CpxR start codon), to remove the cpx promoter region, and cloned into SmaI/BamHI-restricted single-copy plasmid pEXT20 (7), yielding pEXTA*. The expression of cpxA* from the tac promoter in pEXTA* is IPTG (isopropyl-β-d-thiogalactopyranoside) inducible. Because cpxA* is dominant over the wild-type allele (23), we transformed pEXTA* into strains ECL3501 (ΔcpxRA) and BW21355 (cpxR+A+ parent of ECL3501) (Table 1) to test whether cpxA* phenotypes strictly depend on the presence of CpxR. The resistance of the transformants to amikacin, CuCl2, and pH 9 in the presence of 1 mM IPTG was scored under conditions described in Table 2. All three CpxA* phenotypes were observed only in BW21355 (data not shown).
TABLE 2.
Phenotypes of ECL3501 expressing mutant cpx operons from plasmid pRS415a
| Phenotype and conditionsb | pRS415/R+A* | pRS415/R+A+ | pRS415/RΔA* | pRS415/RΔA+ | pRS415 |
|---|---|---|---|---|---|
| Growth (colony diameter, cm) | |||||
| MM-glucose (0.2%) (30°C) | 0.16 ± 0.02 | 0.18 ± 0.03 | 0.15 ± 0.04 | 0.16 ± 0.01 | 0.18 ± 0.02 |
| MM-glucose (0.2%) (42°C) | 0.10 ± 0.02 | 0.21 ± 0.03 | 0.23 ± 0.02 | 0.23 ± 0.05 | 0.25 ± 0.03 |
| MM-succinate (0.1%) | 0c | 0.01 ± 0.02 | 0.09 ± 0.01 | 0.10 ± 0.03 | 0.11 ± 0.02 |
| LB-glucose (0.2%)-amikacin (3 μg/ml) | 0.18 ± 0.04 | 0 | 0 | 0 | 0 |
| LB (pH 9)d | 0.20 ± 0.02 | 0 | 0 | 0 | 0 |
| LB-CuCl2 (4 mM) | 0.21 ± 0.02 | 0 | 0 | 0 | 0 |
| NT-toluidene blue (1.8 mg/ml) | 0.12 ± 0.02 | 0 | 0 | 0 | 0 |
| Growth inhibition (diameter, cm)e | |||||
| LB-H2O2 (30%) | 4.60 ± 0.2 | 3.68 ± 0.12 | 3.63 ± 0.10 | 3.60 ± 0.10 | 3.53 ± 0.07 |
| LB-EDTA (500 mM, pH 8) | 2.00 ± 0.15 | 2.70 ± 0.10 | 2.90 ± 0.10 | 2.80 ± 0.05 | 2.80 ± 0.20 |
| LB-SDS (10%) | 3.1 ± 0.10 | 0 | 0 | 0 | 0 |
| Swarming zone (diameter, cm)f | 2.25 ± 0.05 | 3.20 ± 0.11 | 3.50 ± 0.15 | 3.55 ± 0.15 | 3.52 ± 0.12 |
| Part of population (%) with randomly positioned septumg | 82 ± 4 | 3.00 ± 0.09 | 0 | 2.00 ± 0.02 | 0 |
Ampicillin was used at 100 μg/ml, except in MM-glucose-amikacin and swarming medium (both 35 μg/ml) and MM-succinate (150 μg/ml).
MM, minimal agar medium (pH 7.0) comprising 34 mM NaH2PO4, 66 mM K2HPO4, 20 mM (NH4)2SO4, 1 μM FeSO4, 30 mM MgSO4, 1 mM ZnCl2, 10 μM CaCl2, 0.3 mM isoleucine, 0.3 mM valine, 2 mM thiamine, and ultrapure agarose (17 mg/ml; Seakem LA); LB, Luria-Bertani broth containing Bacto Agar (17 mg/ml; Difco); NT, NaCl (8 mg/ml)-tryptone (10 mg/ml) broth containing Bacto Agar (17 mg/ml; Difco). Growth or growth inhibition was scored after overnight incubation, with the exception of growth on MM-glucose at 30°C (4 days) and MM-succinate (8 days). Growth was scored at 37°C unless otherwise specified.
A zero indicates no growth or no growth inhibition even upon prolonged incubation.
Contains 100 mM CHES (Sigma).
100 μl of a mid-exponential-phase culture was spread on LB in the center of which a paper disk (1.2-cm diameter; Schleicher & Schuell) containing 30 μl of H2O2 or 50 μl of EDTA) or a 5-μl drop of SDS was placed.
Motility was analyzed (overnight) after spotting of 5 μl of a mid-exponential-phase culture at the center of a Difco nutrient (8-mg/ml) soft agar (4-mg/ml) plate supplied with 0.2% glucose and ampicillin.
Cells were grown overnight in 5 ml of LB-ampicillin medium, and growth was scored using phase-contrast microscopy. The percentage of aberrantly dividing cells was calculated from at least 300 cells per culture.
The pleiotropy of cpxA* mutations is reminiscent of the envZ* mutations, whose manifestations are also dependent on the cognate response regulator (31). In principle, a structural alteration of CpxA may entail a specificity change that leads to an aberrant phenotype by phosphorylating or dephosphorylating a noncognate response regulator. Cross-phosphorylation between different two-component systems has been observed and was postulated to integrate adaptive responses by separate control networks. For instance, PhoB, the cognate response regulator of the Pi-sensing PhoR, can also be phosphorylated by the catabolite sensor CreC (36). OmpR, the cognate response regulator of the osmo-sensing EnvZ, can be cross-phosphorylated by an as-yet-unidentified histidine sensor kinase (10). However, it should be emphasized that in both cases the cross-phosphorylation of the noncognate response regulators was observed in mutant backgrounds that lack the cognate sensor kinase. In a wild-type situation, such cross-phosphorylation may not have a chance to occur to a significant extent. Theoretically, cross-talk at the level of dephosphorylation of response regulators by noncognate sensor kinases may also occur, but to the best of our knowledge, no such phenomenon has yet been reported. In view of the above discussion, it is not surprising that the CpxA* phenotypes did not apparently involve cross-phosphorylation of noncognate response regulators.
It should be recognized, however, that the excessively high levels of CpxR-P in CpxA* mutants might recruit nonphysiological target operons whose promoter regions possess a sequence(s) that resembles the recognition consensus for CpxR-P. The occurrence of such “cross-regulation” may contribute to the complexity of CpxA* phenotypes and cannot be excluded by the approach of this study.
Acknowledgments
P.D.W. is a postdoctoral D. Collen Fellow of the Belgian American Educational Foundation. This work was financed by Public Health Service grants GM40993 and GM39693 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Cosma C L, Danese P N, Carlson J H, Silhavy T J, Snyder W B. Mutational activation of the Cpx signal transduction pathway of Escherichia coli suppresses the toxicity conferred by certain envelope-associated stresses. Mol Microbiol. 1995;18:491–505. doi: 10.1111/j.1365-2958.1995.mmi_18030491.x. [DOI] [PubMed] [Google Scholar]
- 2.Danese P N, Silhavy T J. The ςE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- 3.Danese P N, Silhavy T J. Cpx-P, a stress-combative member of the Cpx regulon. J Bacteriol. 1998;180:831–839. doi: 10.1128/jb.180.4.831-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danese P N, Snyder W B, Cosma C L, Davis L J B, Silhavy T J. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 1995;9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- 5.Dartigalongue C, Raina S. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 1998;14:3968–3980. doi: 10.1093/emboj/17.14.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Wulf P, Kwon O, Lin E C C. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J Bacteriol. 1999;181:6772–6778. doi: 10.1128/jb.181.21.6772-6778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykxhoorn D M, St. Pierre R, Linn T. A set of compatible tac promoter expression vectors. Gene. 1996;177:133–136. doi: 10.1016/0378-1119(96)00289-2. [DOI] [PubMed] [Google Scholar]
- 8.Jacob-Dubuisson F, Pinkner J, Xu Z, Striker R, Padmanhaban A, Hultgren S J. PapD chaperone function in pilus biosynthesis depends on oxidant and chaperone-like activities of DsbA. Proc Natl Acad Sci USA. 1994;91:11552–11556. doi: 10.1073/pnas.91.24.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones C H, Danese P N, Pinkner J S, Silhavy T J, Hultgren S J. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 1997;16:6394–6406. doi: 10.1093/emboj/16.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsubara M, Mizuno T. EnvZ-independent phosphotransfer signaling pathway of the OmpR-mediated osmoregulatory expression of OmpC and OmpF in Escherichia coli. Biosci Biotechnol Biochem. 1999;63:408–414. doi: 10.1271/bbb.63.408. [DOI] [PubMed] [Google Scholar]
- 11.McEwen J, Silverman P. Chromosomal mutations of Escherichia coli that alter expression of conjugative plasmid functions. Proc Natl Acad Sci USA. 1980;77:513–517. doi: 10.1073/pnas.77.1.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen J, Silverman P. Genetic analysis of Escherichia coli K-12 chromosomal mutants defective in expression of F-plasmid functions: identification of genes cpxA and cpxB. J Bacteriol. 1980;144:60–67. doi: 10.1128/jb.144.1.60-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen J, Silverman P. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in isoleucine and valine synthesis. J Bacteriol. 1980;144:68–73. doi: 10.1128/jb.144.1.68-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen J, Silverman P M. Mutations in genes cpxA and cpxB alter the protein composition of Escherichia coli inner and outer membranes. J Bacteriol. 1982;151:1553–1559. doi: 10.1128/jb.151.3.1553-1559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen J, Sambucetti L, Silverman P M. Synthesis of outer membrane proteins in cpxA cpxB mutants of Escherichia coli K-12. J Bacteriol. 1983;154:375–382. doi: 10.1128/jb.154.1.375-382.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 17.Morris J M, Newman E B. Map location of the ssd mutation in Escherichia coli K-12. J Bacteriol. 1980;143:1504–1505. doi: 10.1128/jb.143.3.1504-1505.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman E B, Malik N, Walker C. l-Serine degradation in Escherichia coli K-12: directly isolated ssd mutants and their intergenic revertants. J Bacteriol. 1982;150:710–715. doi: 10.1128/jb.150.2.710-715.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plate C A. Mutant of Escherichia coli defective in response to colicin K and in active transport. J Bacteriol. 1976;125:467–474. doi: 10.1128/jb.125.2.467-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plate C A, Suit J L. The eup genetic locus of Escherichia coli and its role in H+/solute symport. J Biol Chem. 1981;256:12974–12980. [PubMed] [Google Scholar]
- 21.Plate C A, Seely S A, Laffler T G. Evidence for a protonmotive force related regulatory system in Escherichia coli and its effects on lactose transport. Biochemistry. 1986;25:6127–6132. doi: 10.1021/bi00368a044. [DOI] [PubMed] [Google Scholar]
- 22.Pogliano J, Dong J M, De Wulf P, Furlong D, Boyd D, Losick R, Pogliano K, Lin E C C. Aberrant cell division and random FtsZ ring positioning in Escherichia coli cpxA* mutants. J Bacteriol. 1998;180:3486–3490. doi: 10.1128/jb.180.13.3486-3490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogliano J, Lynch A S, Belin D, Lin E C C, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 24.Rainwater S, Silverman P M. The Cpx proteins of Escherichia coli K-12: evidence that cpxA, ecfB, ssd, and eup mutations all identify the same gene. J Bacteriol. 1990;172:2456–2461. doi: 10.1128/jb.172.5.2456-2461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raivio T L, Silhavy T J. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol. 1997;179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raivio T L, Silhavy T J. The ςE and Cpx regulatory pathways: overlapping but distinct envelope stress responses. Curr Opin Microbiol. 1999;2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
- 27.Raivio T L, Popkin D L, Silhavy T J. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol. 1999;181:5263–5272. doi: 10.1128/jb.181.17.5263-5272.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambucetti L, Eoyang L, Silverman P M. Cellular control of conjugation in Escherichia coli K12. Effect of chromosomal cpx mutations on F-plasmid gene expression. J Mol Biol. 1982;161:13–31. doi: 10.1016/0022-2836(82)90275-3. [DOI] [PubMed] [Google Scholar]
- 29.Silverman P M, Tran L, Harris R, Gaudin H M. Accumulation of the F plasmid TraJ protein in cpx mutants of Escherichia coli. J Bacteriol. 1993;175:921–925. doi: 10.1128/jb.175.4.921-925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 31.Slauch J M, Garrett S, Jackson D E, Silhavy T J. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988;170:439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stock J B, Surette M G, Levit M, Park P. Two-component signal transduction systems: structure-function relationships and mechanisms of catalysis. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 25–51. [Google Scholar]
- 33.Su H S, Lang B F, Newman E B. l-Serine degradation in Escherichia coli K-12: cloning and sequencing of the sdaA gene. J Bacteriol. 1989;171:5095–5102. doi: 10.1128/jb.171.9.5095-5102.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutton A, Newman T, McEwen J, Silverman P M, Freundlich M. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in acetohydroxyacid synthase I function in vivo. J Bacteriol. 1982;151:976–982. doi: 10.1128/jb.151.2.976-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorbjarnardottir S H, Magnusdottir R A, Eggertsson G. Mutations determining generalized resistance to aminoglycoside antibiotics in Escherichia coli. Mol Gen Genet. 1978;161:89–98. doi: 10.1007/BF00266619. [DOI] [PubMed] [Google Scholar]
- 36.Wanner B L. Signal transduction and cross regulation in the Escherichia coli phosphate regulon by PhoR, CreC, and acetyl phosphate. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: ASM Press; 1995. pp. 203–221. [Google Scholar]