Abstract
Multisystem inflammatory syndrome in children (MIS-C) is an emerging phenomenon associated with SARS-COV-2 infection (COVID-19) occurring in < 1 % of infected children. MIS-C is characterized by a hyperinflammatory state with excessive cytokine release (‘storm’) leading to hemodynamic compromise and multiorgan failure, with a death rate of ∼2 %. Autopsy examination can play a particularly important role in helping to understand the pathogenesis of MIS-C. Yet, only five autopsy studies have been reported to date. We report a fatal case of MIS-C involving a previously healthy, 5-year-old Thai boy admitted with MIS-C, one month after exposure to SARS-COV-2. While in intensive care, he was found to have a hypertrophic cardiomyopathy, and despite immunosuppressive treatment for MIS-C, developed shock and died. Multiorgan inflammation was not found at autopsy, implying that the MIS-C had responded to treatment. However, there was disseminated aspergillosis and cytomegalovirus reactivation, attributed to the immunosuppression. SARS-COV-2 virus was also found in multiple organs. To the best of our knowledge, this is the first reported autopsy of an MIS-C patient from Asia, and the first report of aspergillosis in MIS-C. This case underscores that the risks of immunosuppression are also a concern in MIS-C. Although MIS-C is generally considered to be a post-infectious hyperimmune reaction, persistence of SARS-COV-2 is a feature in all autopsies of MIS-C patients reported to date, suggesting a possible role in the pathogenesis, at least in fatal cases.
Keywords: COVID-19, SARS-COV-2, MIS-C, Aspergillosis, Cytomegalovirus, Cardiomyopathy
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). In contrast to adults, most children with COVID-19 are either asymptomatic or have mild to moderate symptoms [1]. However, in this age group, SARS-CoV-2 infection may progress to a hyperinflammatory state with excessive cytokine release (‘storm’) leading to hemodynamic compromise and multiorgan failure, a condition referred to as multisystem inflammatory syndrome in children (MIS-C) [1], [2], [3], [4], [5], [6]. The incidence of MIS-C has been reported as 0.03 % of SARS-CoV-2 infections [7]. The clinical criteria for making such a diagnosis includes: fever of at least 24 h duration, severe illness requiring hospitalization, involvement of two or more organ systems, laboratory evidence of inflammation, laboratory or epidemiologic evidence of SARS-CoV-2 infection, and no alternative diagnosis, in particular Kawasaki disease, toxic shock syndrome, and macrophage activation syndrome, all which can resemble MIS-C.
The clinical picture of MIS-C has been the subject of several excellent reports [2], [3], [4], [5], [6], [8], [9]. There is a male predominance in MIS-C (55–69 %), with a median age of 8.3–8.6 years (range 3 months–20 years). Most patients are previously healthy, and the median interval from COVID-19 symptom onset to MIS-C symptom onset is 25 days (4–6 weeks). The most frequent organ system involved in gastrointestinal (>90 % of patients), with abdominal pain, diarrhea and vomiting. Second most common is cardiovascular, including myocarditis, depressed ventricular function, hypotension, arrhythmia, and less commonly coronary aneurysm. Abnormal echocardiogram with depressed ejection fraction is seen in 45 % of patients. The severity of illness is such that 68–80 % require intensive care admission and 48–63 %, inotropic support. Hematologic involvement is common (>80 %), typically with neutrophilia, but lymphopenia and thrombocytopenia. In contrast to adults with SARS-CoV-2 who often manifest lung involvement, in children with MIS-C, respiratory involvement less common, although 40 % show changes on imaging and 20–28 % need respiratory support (mechanically ventilation or rarely extra-corporeal membrane oxygenation (ECMO)). Dermatologic manifestations include rash, erythema, peeling, stomatitis, and conjunctivitis; neurologic can include headache, lethargy, and meningismus. Laboratory evidence of inflammation is evident by elevation in one or more of: C- reactive protein (CRP) (94 % of cases), erythrocyte sedimentation rate (ESR), fibrinogen, procalcitonin, D-dimer, ferritin, lactate dehydrogenase (LDH), interleukin − 6 (IL-6), procalcitonin, and creatine phosphokinase (CPK). Elevations in troponin T and pro B natriuretic peptide are indicative of cardiac damage. Laboratory confirmation of SARS-CoV-2 infection can be by RT-PCR or serology (70 % patients positive); otherwise, epidemiologic evidence requires known COVID-19 exposure within 4 weeks prior to onset of MIS-C.
Treatment usually consists of intravenous immune globulin (IVIG) and high-dose steroids; secondary options may include an IL-6 inhibitor (tocilizumab) and/or IL-1 receptor antagonist (anakinra) [3], [4], [6], [9]. With treatment, the majority of patients survive [3], [4], [6], [9]. Depending on the study, the mortality rate is low, in the range of 1.5–2.2 %, but rates of 15–25 % has been reported by some [2], [10], [11]. Most of the larger studies have not reported the causes of death. Autopsy examination can play a particularly important role in helping to define and understand MIS-C. Yet, very few autopsy studies have been reported to date, comprising five cases to date, originating from Brazil, Columbia and Spain [12], [13], [14], [15].
Several studies have documented a preferential occurrence of MIS-C in black and Hispanic populations compared to white populations [2], [3], [5], [6], [8], [16]. A similar higher occurrence in Asian patients living in western countries such as the USA and UK was based on studies that focused on South Asians, rather than those of the Asian race [17], [18], [19]. When Asian patients were specifically separated out, there was a relative sparing of this population, accounting for only 2–3 % of MIS-C cases [8], [16]. Attention was initially drawn to that fact that MIS-C was not seen in Asian countries [3], [5]. This has proven to be incorrect, as there are now reports, albeit very few, documenting MIS-C in patients in Asia, including Japan, South Korea, Indonesia, VietNam and India [10], [11], [20], [21], [22], [23].
We report herein a fatal case of MIS-C in a Thai patient. To the best of our knowledge, this is the first reported autopsy of an MIS-C patient from Asia.
2. Clinical presentation
The patient was male, 5 years 7 months old, who was hospitalized because of frequent vomiting for 2 days prior to admission. He had been exposed to SARS-CoV-2 from his grandmother, who had died from the infection, 1 month earlier. At presentation, his temperature was 38.8 °C, BP 83/62 mmHg and pulse rate 170 beats/min. The patient was in fluid-refractory shock and was transferred to the intensive care unit. Increased serum biomarker levels were detected for inflammation (CRP, ESR, ferritin, pro-calcitonin, D-dimer, LDH, and IL-6) ( Table 1). There was lymphopenia and platelets were low normal. A cardiac murmur was auscultated and chest x-ray showed cardiomegaly. An echocardiogram revealed a reduced left ventricular ejection fraction of 22 %, right and left atrial enlargement, atrial and ventricular septum deviation to the right, and marked left ventricular dilation, with a normal pattern of coronary arteries. There was serologic evidence of cardiac injury with elevated troponin T and pro B natriuretic peptide (Table 1). Testing for SARS-CoV-2 was negative by RT-PCR but serum was positive for IgG antibodies.
Table 1.
Laboratory test results on admission.
| Laboratory value | Result | Reference range |
|---|---|---|
| Complete blood count | ||
| Hemoglobin | 12.1 | 13–17 g/dL |
| Hematocrit | 35.1 | 39–51 % |
| White blood cell count (%neutrophils; %lymphocytes) | 11,730 (79.1;13.7) | 45,000–110,000/mm3 |
| Platelets | 150,000 | 150,000–450,000/mm3 |
| Coagulation profile | ||
| Prothrombin time | 34.7 | 9.9–12.7 s |
| International normalized ratio | 3.21 | 0.86–1.22 s |
| Activated partial thromboplastin time | 80 | 19.9–28.9 s |
| Cardiac biomarkers | ||
| High-sensitivity troponin T | 221.4 | 0–13.99 ng/L |
| N-terminal pro-brain natriuretic peptide | > 35,000 | 5–390 pg/mL |
| Inflammatory markers | ||
| Erythrocyte sedimentation rate | 2 | 0–10 mm/hr |
| High-sensitivity C-reactive protein | 6.44 | 0.1–2.8 mg/L |
| Ferritin | 2702 | 30–400 ng/mL |
| Procalcitonin | 21.65 | 0.1–0.49 µg/L |
| D-dimer | 8873.33 | 0–229 ng/mL |
| Lactate dehydrogenase | 7308 | 125–220 U/L |
| Interleukin-6 | 61.42 | 0–7 pg/mL |
| Liver function tests | ||
| Albumin | 2.9 | 3.5–5.0 g/dL |
| Alkaline phosphatase | 84 | 40–120 U/L |
| Aspartate aminotransferase | 4718 | 14–59 U/L |
| Alanine aminotransferase | 2250 | 10–55 U/L |
| Bilirubin, total | 1.23 | 0.2–1.2 mg/dL |
| Bilirubin, direct | 0.81 | 0.0–0.5 mg/dL |
The patient met the six WHO criteria for a diagnosis of MIS-C including (1) age 0–19 years; (2) Fever for ≥ 3 days; (3) clinical signs of multisystem involvement, which included hypotension/shock, cardiac dysfunction, evidence of coagulopathy, acute gastrointestinal symptoms, (4) elevated markers of inflammation, (5) no other obvious microbial cause of inflammation; and (6) evidence of SARS-CoV-2 infection. Endotracheal intubation was established and ECMO begun. Inotropic drugs, IVIG and high dose methylprednisolone were started. IVIG was given for two days and steroids were continued throughout the admission.
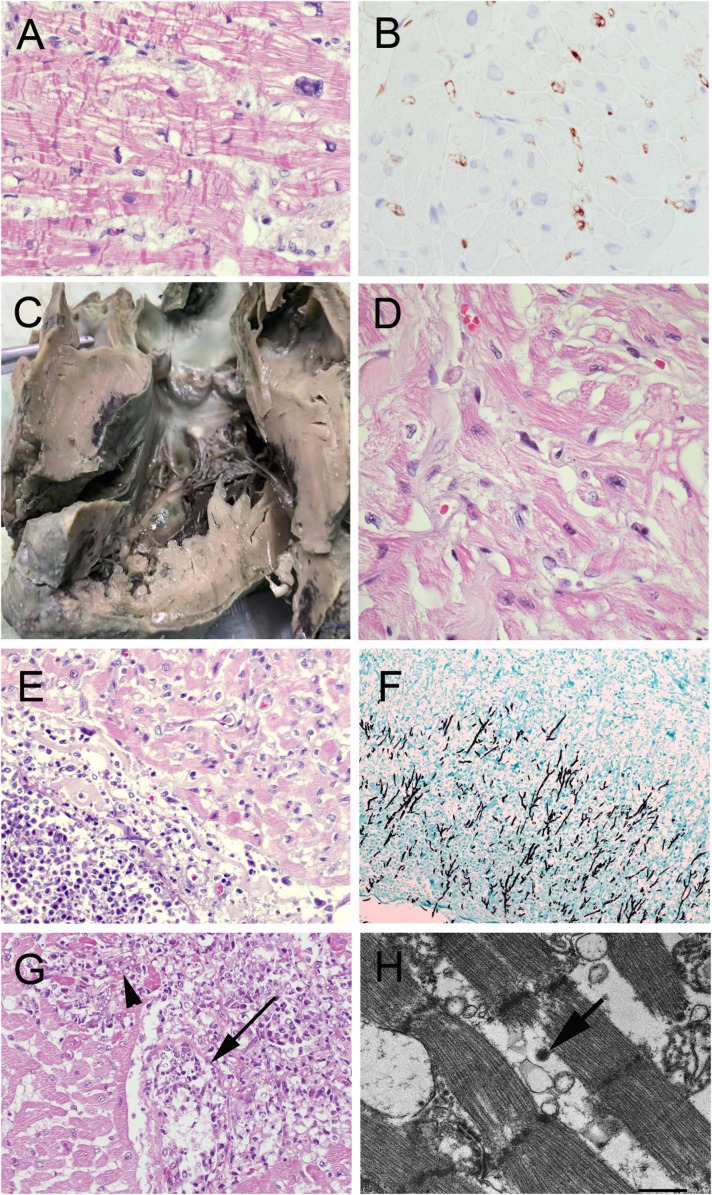
By day 7, inflammatory markers were improving (IL-6 had dropped from 61.42 to 3.68 pg/mL; ferritin had dropped from 2702 to 354.40 ng/mL). However, cardiac function did not recover and the patient could not be weaned from ECMO. Based on the echocardiogram, hypertrophic cardiomyopathy was considered. The circulatory support was changed from ECMO to a biventricular assist device. A myocardial biopsy was done showing hypertrophic myocardium, myocyte disarray and branching, consistent with hypertrophic cardiomyopathy. There was contraction band necrosis, and positive C4d immunostaining of cardiac vessels consistent with an immunologic reaction ( Fig. 1A and 1B), but no evidence of myocarditis. Whole exome sequencing revealed a MYLK2 gene mutation, confirming the diagnosis of hypertrophic cardiomyopathy. The patient remained on the biventricular assist device for the remainder of the hospital stay.
Fig. 1.
Cardiac pathology. The myocardial biopsy showed (A) contraction band necrosis with (B) complement C4d deposits in heart vessels. Post-mortem examination showed (C) hypertrophic cardiomyopathy, with the ventricular septum thicker than left ventricular free wall, and (D) myofiber hypertrophy and disarray on microscopy. (E-F) Fungal hyphae invaded the pericardium along with (G) angioinvasion leading to fungal myocarditis (necrotic vessel with fungal thrombus marked by arrow; fungal hyphae in myocardium marked by arrowhead) (H) Electron micrograph of the myocardium shows sarcomeres with a virus particle in the adjacent cytoplasm (arrow). Scale bar = 500 nm. (A,D,E,G: H&E; B: immunohistochemistry; F: Gomori methenamine silver) (Original magnifications A,B,D, x600, E,F,G x100).
Two weeks after admission, the patient developed a fever of 37.8 oC with dyspnea and a productive cough, associated with a leukocytosis (leukocytes 25,130/mm3; neutrophils 89.3 %) and thrombocytopenia (platelets 73,000/mm3). There was elevated high-sensitivity troponin T (87.7 ng/L) and interleukin-6 (29.7 pg/mL), although these values were lower than those on admission. Chest x-ray showed pneumonia with patchy infiltration in both upper lobes of the lungs. Alveolar fluid culture detected Pseudomonas aeruginosa. Hemoculture from an arterial line showed P. aeruginosa and Acinetobacter buamanii. Increased galactomannan was detected in bronchoalveolar lavage fluid (1.35; cutoff 0.488), but not in serum. This was interpreted as contamination because there was no other evidence to support aspergillus infection. Serology for respiratory pathogens was positive for adenovirus and negative for bocavirus, coronavirus229E, coronavirus HKU1, influenza A (H1), influenza (H3), influenza B, parainfluenza 1, parainfluenza 2, parainfluenza 3, parainfluenza 4, respiratory syncytial virus A, respiratory syncytial virus B, enterovirus/rhinovirus, Chamyphilia pneumoniae, Legionella pneumophilia, Mycoplasma pneumoniae. Serology for herpesvirus (HSV) 1–8 was positive for Epstein Barr virus (EBV; HSV 4), but negative for the others. EBV IgG was positive and IgM negative with a low viral load < 316. Mean arterial pressure dropped and septic shock was diagnosed. Empirical antibiotics were given, but his status did not improve, and the patient died 36 days after admission.
Permission for a full autopsy and publication of the case was obtained from the parents.
3. Pathology findings
A complete autopsy was performed. The heart was hypertrophic and weighed 183.5 g (reference 85 g) with the ventricular septum thicker than the left ventricular free wall. By microscopy, there was hypertrophic cardiomyopathy; interstitial edema and multiple areas of contraction band necrosis (Fig. 1C and D). C4d immunostaining was negative. In addition, there were scattered foci of acute fungal pericarditis and myocarditis, with angioinvasion by fungus (Fig. 1E-G). Virus particles in the range of 100 nm diameter were identified in cardiac myocytes by electron microscopy (Fig. 1H). There was an outer membrane surrounding electron-dense small granules. Similar virus particles were also visible in proximal tubular epithelial cells of the kidney and enterocytes of the small bowel (results not shown). The morphology of the virus particles did not match adenovirus nor any herpes type virus (including CMV), but were in keeping with the morphology described for SARS-Co-V2 [24], [25], [26]. Heart and bowel were tested for adenovirus by immunostaining and EBV by in situ hybridization and both tests were negative.
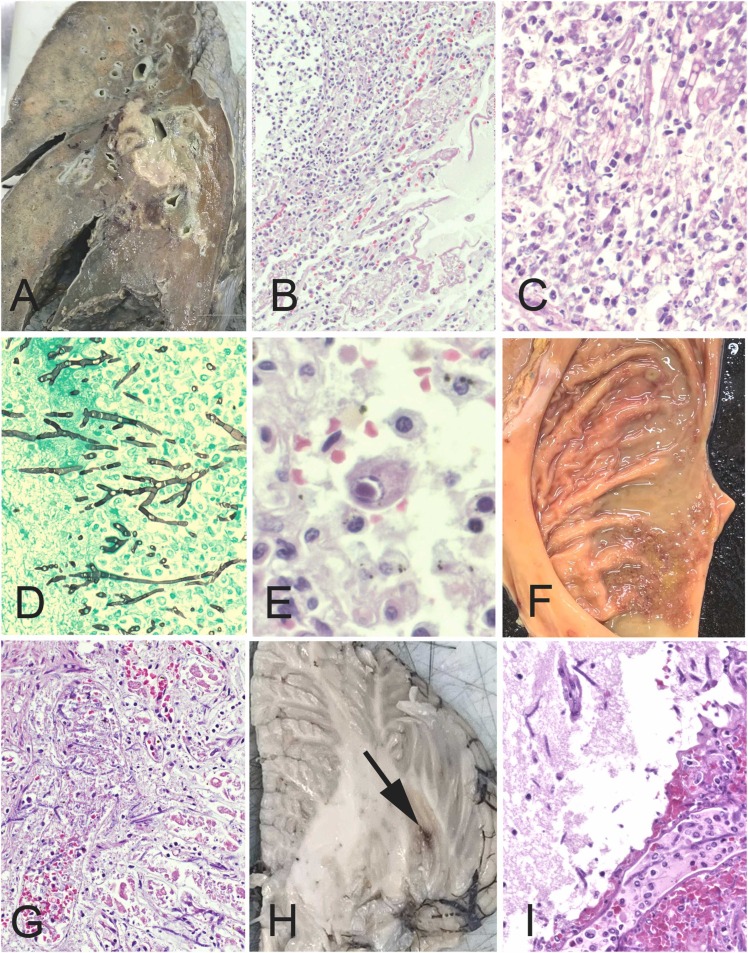
The combined weight of the lungs was 436.9 g (reference 211 g) ( Fig. 2A). There was diffuse consolidation of both lungs with abscesses in the right middle and lower lobes around the hilar region. These abscesses contained angioinvasive fungal hyphae associated with a neutrophilic infiltrate (Fig. 2B-2D) and vascular thrombosis. The fungal hyphae were septate with acute-angle branching, consistent with Aspergillus sp. In addition, both lungs revealed occasional cytomegalovirus-infected cells (Fig. 2E) confirmed by immunohistochemistry (not shown). Notably, there was no evidence of diffuse alveolar damage. There were multiple ulcers in the gastric body and antrum, which showed angioinvasive fungal hyphae (Fig. 2F). The brain (1060 g; reference 1237 g) showed red foci in the left cerebral white matter and left cerebellar hemisphere, which were the result of angioinvasive fungal infection (Fig. 2G and H). The liver (750 g; reference 596 g) showed macrovesicular steatosis; with bile pigment in of hepatocytes and bile ductules, but no hepatitis. No microthrombi were identified except those associated with fungal hyphae. Death was attributed to disseminated aspergillus infection.
Fig. 2.
Additional autopsy findings.(A) Cut surface of right lung showed abscesses in the middle and lower lobes. (B) The abscess consisted of a dense neutrophilic infiltrate, associated with (C) angioinvasive fungal hyphae that have the morphology Aspergillus sp, with septate hyphae and acute angle branching (D). (E) In addition, the lung showed cytomegalovirus-infected cells. (F) There were multiple gastric antral ulcers with angioinvasive fungal hyphae extending into the gastric mucosa. The left cerebellum (G) and left cerebral white matter showed soft red foci (arrow), which were the result of angioinvasive fungal hyphae (H). (B,C,E,G,I: H&E; D: Gomori methenamine silver) (Original magnifications B x100, C,D,G I x400, E x600).
4. Discussion
Our patient fulfilled the criteria for MIS-C as defined in previous publications [3], [5], [6] and summarized in the Clinical Presentation. Most patients (∼75 %) with MIS-C are previously healthy, but comorbidities are reported in up to 20 % of cases, the most common of which is obesity, followed by asthma [2], [3], [6], [14], [27]. Our patient did not have these conditions, but instead had an underlying cardiomyopathy. Of note, 5 % of children with MIS-C are reported to have cardiac conditions, but these were not detailed [3]. Given that one of the life-threatening events in MIS-C is compromise of cardiac function on one basis or another, it seems reasonable that the underlying cardiomyopathy in our patient led to the patient’s demise, whereas the vast majority of children with MIS-C (>97 %) survive [3], [4], [6], [9]. Thus, the clinical course of our patient is not typical of most MIS-C patients.
For the small number of patients who do not survive, in most studies, the causes of death are either not provided [3], [5], [6], [10] or based on clinical impressions [11]. Given the relative novelty of MIS-C, post mortem examination can provide valuable information about this incompletely understood condition. Commonalities in the pathology of MIS-C can come from comparison of our case to published autopsies. Unfortunately, there are very few autopsy studies on MIS-C reported to date, to which we can compare our finding. Four cases are from South America and a fifth from Spain [12], [13], [14], [15]. Ages ranged from 8 to 12 years except one case at 10 months. Three were of African race and the racial origins other two were not specified. Three were obese and one also had asthma, and no comorbidities were mentioned for the other two. Causes of death for the five cases were myocarditis, colits, acute encephalopathy, septic shock, and hepatic failure, respectively. Summarizing the findings for the four cases in older children, the lungs showed only focal diffuse alveolar damage, but with microthrombi in arterioles and capillaries. Microthrombi were variably seen in the kidneys, colon and liver accompanied by C4d deposition. The hearts showed foci of contraction band necrosis with C4d deposition, as well as extensive inflammation with lymphocytes and macrophages in two cases, accompanied by a right ventricle thrombus in one. Similar inflammation was noted in the colon of one case. Changes attributed to hypotension included centrilobular necrosis of the liver, acute tubular necrosis, and ischemic damage to the brain. All cases identified continued presence of SARS-CoV-2 virus, by one or more of RT-PCR, electron microscopy or immunostaining. Affected organs included heart, lung, colon and brain, and involved cell types including cardiac myocytes, endothelial cells, macrophages, neutrophils, renal tubular epithelial cells, colonic glands, and fibroblasts. The fifth case, the one of hepatic failure [12] is unusual for MIS-C in many ways. The patient was only 10 months, which is younger than the median age of 8 years for MIS-C. The patient presented with hepatic failure, which is unusual in MIS-C, and the patient developed acute respiratory distress syndrome (ARDS), which is also unusual in MIS-C. At autopsy, the liver showed massive necrosis and the lungs showed diffuse alveolar damage, and both findings are atypical for MIS-C.
Comparing these results to our case, the lungs also showed only focal inflammation, further supporting the idea that diffuse alveolar damage is not part of the pathologic spectrum of MIS-C. We were also able to detect virus in cardiac myocytes, renal tubular epithelial cells, and small bowel enterocytes, supporting the above reports that the virus can continue to reside in organs weeks after the initial infection in MIS-C. In contrast to the above cases, microthrombi were not found in any organs, and C4d deposition was only detected at the time of myocardial biopsy, but not post mortem. If microthrombi are part of the pathologic spectrum of MIS-C, it is possible the treatment in our patient was successful in the resolution of MIS-C and that is why these were not found in our case. However, since the treatment involves immunosuppression, that carries risks of complications, and in our case, those manifested as disseminated aspergillosis and cytomegalovirus infection, neither of which has received attention in cases of MIS-C.
The incidence of pulmonary aspergillosis as a complication of SARS-CoV-2 infection in adults is believed to be underappreciated because of the overlapping clinical and radiological findings between aspergillosis and SARS-CoV-2 pneumonia, particularly when associated with ARDS [28]. Most affected patients are critically-ill with a mean age of 66 years, and are receiving corticosteroid and anti-IL-6 therapy. Such patients are generally immunocompetent and have no other risk factor except SARS-CoV-2 associated ARDS [28], [29]. Our patient was also immunosuppressed by similar therapy, but did not have any lung changes to support a diagnosis of ARDS, a condition which is unusual in MIS-C [2], [3], [4], [5], [6], [8], [9]. The diagnosis of aspergillosis is challenging because of the low sensitivity of blood tests [28] and specimen collection from the primary site of infection may be delayed due to concern about the risk of SARS-CoV-2 transmission. After death, lung necropsy may be preferred over a full autopsy, but this more limited sampling may be insufficient to detect early fungal infection [29]. It is of interest to note that IL-6, which is generally increased in MIS-C, also increases after aspergillus infection and is believed to play a role in protective immunity against this fungus [30]. Nevertheless, pulmonary aspergillosis as a complication of SARS-CoV-2 in adults has a high mortality rate, and was also the cause of death in our patient.
We believe the cytomegalovirus detected in the lungs of our patient may reflect reactivation of latent virus. It has been reported that immune activation caused by SARS-CoV-2 infection can trigger cytomegalovirus reactivation [31]. In adults, such reactivation is usually detected around one month following COVID-19 diagnosis, and is seen in critically-ill patients with persistent hyperinflammation and who continue to receive corticosteroids beyond the time used for initial COVID-19 treatment [31]. Cytomegalovirus can suppress or kill T cells and natural killer cells as well as trigger neutrophil activation. Thus, cytomegalovirus reactivation in severe SARS-CoV-2 infection might be an independent factor triggering hyperinflammation, which could play a role in MIS-C. Alternatively, reactivations of cytomegalovirus may simply be an epiphenomenon in a critically-ill patient. In our case, there was no associated inflammatory reaction seen, which favors the latter.
The pathogenesis of MIS-C is not completely understood. The preferred mechanism involves dysregulation of the immune system occurring several weeks after a SARS-COV-2 infection. Testing for SARS-COV-2 in MIS-C patients typically shows positive serology for IgG rather than virus by PCR, accompanied by the presence of complement C4d in microvessels, suggesting MIS-C is a post-infectious immune-mediated event [1], [3], [5], [6]. As well, MIS-C patients typically respond to immunosuppressive therapy, which should worsen the disease if it is virus-mediated [32]. In this proposed mechanism, SARS-COV-2 infection triggers macrophage activation and stimulation of T-helper cells, leading to widespread cytokine release ((IL-18 and IL-6 and others), followed by heightened stimulation of macrophages, neutrophils and production of antibodies leading to a hyperinflammatory and hyperimmune response [27], [32]. Tissue damage by virus may lead to antibody production, followed by antibody-mediated inflammatory reactions [8]. MIS-C plasma has been shown to contain autoantibodies that can target endothelial, gastrointestinal, and immune-cell antigens, which is in keeping with the multi-organ involvement in patients with MIS-C [32].
If MIS-C is immune-mediated, however, one might predict worsening of illness in patients treated with convalescent plasma, which does not occur [33]. There is, then, the alternate hypothesis that MIS-C is the direct result of a residual or second hit SARS-COV-2 infection. Coronaviruses are known to be able to block interferon responses and it has been proposed that MIS-C might then result from delayed cytokine storm in patients who do not control viral replication well or suffered an initially high SARS-CoV-2 viral load [33]. Of note, virus has been demonstrated in 100 % of autopsies reported to date (including our case) and involving organs related to the cause of death [13], [15]. This finding suggests that the pathogenesis of MIS-C may not be so straightforward as a post-infectious event. Perhaps fatal cases are the ones in which there is persistence of virus. If this is true, then continued detection of SARS-CoV-2 in cases of MIS-C might even serve as a prognostic marker; further studies are needed to evaluate this possibility.
Even less well understood is why there is an apparent preferential occurrence of MIS-C in black and Hispanic populations [2], [3], [5], [6], [8], [16] and why Asian populations seem relatively spared, especially in Asian countries, where there are so few reports of MIS-C cases [11], [20], [21], [22], [23]. These racial differences may be related to reporting procedures, health care disparities, environmental factors or possibly genetic differences that influence inflammatory and immune responses [16], [17]. Our report provides the first autopsy results on a patient with MIS-C from Asia. The patient was unusual in several aspects: (1) having an underlying cardiomyopathy, (2) showing persistent SARS-CoV-2 in multiple organs, and (3) dying from the complications of immune suppression, cytomegalovirus reactivation and aspergillosis, while the MIS-C was seemingly responding to immunodulatory treatment. Cytomegalovirus and aspergillus infections have not received attention to date in MIS-C, and should be kept in mind when treating such patients.
Funding
There is no funding to report for this project.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Frenkel L.D., Gomez F., Bellanti J.A. COVID-19 in children: pathogenesis and current status. Allergy Asthma Proc. 2021;42:8–15. doi: 10.2500/aap.2021.42.200104. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed M., Advani S., Moreira A., Zoretic S., Martinez J., Chorath K., Acosta S., Naqvi R., Burmeister-Morton F., Burmeister F., Tarriela A., Petershack M., Evans M., Hoang A., Rajasekaran K., Ahuja S., Moreira A. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., Newburger J.W., Kleinman L.C., Heidemann S.M., Martin A.A., Singh A.R., Li S., Tarquinio K.M., Jaggi P., Oster M.E., Zackai S.P., Gillen J., Ratner A.J., Walsh R.F., Fitzgerald J.C., Keenaghan M.A., Alharash H., Doymaz S., Clouser K.N., Giuliano J.S., Jr., Gupta A., Parker R.M., Maddux A.B., Havalad V., Ramsingh S., Bukulmez H., Bradford T.T., Smith L.S., Tenforde M.W., Carroll C.L., Riggs B.J., Gertz S.J., Daube A., Lansell A., Coronado Munoz A., Hobbs C.V., Marohn K.L., Halasa N.B., Patel M.M., Randolph A.G. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lima-Setta F., Magalhaes-Barbosa M.C., Rodrigues-Santos G., Figueiredo E., Jacques M.L., Zeitel R.S., Sapolnik R., Borges C., Lanziotti V.S., Castro R.E.V., Bellinat A.P.N., Silva T.P.D., Oliveira F.R.C., Reis B., Castro N., Macedo J., Scarlato A., Riveiro P.M., Mota I., Lorenzo V.B., Lucena N.M.L., Azevedo Z.M.A., Cunha A., Prata-Barbosa A. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J. Pedia. 2021;97:354–361. doi: 10.1016/j.jped.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakra N.A., Blumberg D.A., Herrera-Guerra A., Lakshminrusimha S. Multi-System inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. 2020;7 doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radia T., Williams N., Agrawal P., Harman K., Weale J., Cook J., Gupta A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021;38:51–57. doi: 10.1016/j.prrv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne A.B., Gilani Z., Godfred-Cato S., Belay E.D., Feldstein L.R., Patel M.M., Randolph A.G., Newhams M., Thomas D., Magleby R., Hsu K., Burns M., Dufort E., Maxted A., Pietrowski M., Longenberger A., Bidol S., Henderson J., Sosa L., Edmundson A., Tobin-D'Angelo M., Edison L., Heidemann S., Singh A.R., Giuliano J.S., Jr., Kleinman L.C., Tarquinio K.M., Walsh R.F., Fitzgerald J.C., Clouser K.N., Gertz S.J., Carroll R.W., Carroll C.L., Hoots B.E., Reed C., Dahlgren F.S., Oster M.E., Pierce T.J., Curns A.T., Langley G.E., Campbell A.P., Balachandran N., Murray T.S., Burkholder C., Brancard T., Lifshitz J., Leach D., Charpie I., Tice C., Coffin S.E., Perella D., Jones K., Marohn K.L., Yager P.H., Fernandes N.D., Flori H.R., Koncicki M.L., Walker K.S., Di Pentima M.C., Li S., Horwitz S.M., Gaur S., Coffey D.C., Harwayne-Gidansky I., Hymes S.R., Tobin-D'Angelo N.J., Ackerman K.G., Cholette J.M. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein S., Willis E., Lythgoe H., McCann L., Cleary A., Mahmood K., Porter D., Jones J., McDonagh J., Chieng A., Varnier G., Hughes S., Boullier M., Ryan F., Awogbemi O., Soda G., Duong P., Pain C., Riley P., Hedrich C.M. Presentation, treatment response and short-term outcomes in paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS) J. Clin. Med. 2020;9 doi: 10.3390/jcm9103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sood M., Sharma S., Sood I., Sharma K., Kaushik A. Emerging evidence on Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 infection: a systematic review with meta-analysis. SN Compr. Clin. Med. 2021:1–10. doi: 10.1007/s42399-020-00690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashyap H., Kumar R.N.S., Gautam S., Gupta A., Gupta S., Tiwari P.K. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19 infection. Indian J. Pedia. 2021;88:1053. doi: 10.1007/s12098-021-03832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putri N.D., Prawira Y., Tartila T., Jasin M.R., Puspitasari H.A., Puspaningtyas N.W., Indawati W., Karyanti M.R., Setyanto D.B., Prayitno A., Yuniar I., Alatas F.S., Hidayati E.L., Muhaimin R., Prawitasari T., Soebadi A., Muktiarti D., Primacakti F., Rahmadhany A., Octavius G.S., Djer M.M., Hendarto A., Dewi R., Kaswandani N., Pudjiadi A.H. Clinical features of Multisystem Inflammatory Syndrome in Children Associated with COVID-19 in Indonesia. J. Trop. Pedia. 2022;68 doi: 10.1093/tropej/fmac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonilla Gonzalez C., Hincapie Echeverria M., Plazas Pachon R., Mora Umana P., Diaz Gomez B.L., Gualdron Barreto N. Case report: fatal acute liver failure with giant cell transformation in a pediatric patient associated With MIS-C. Front. Pedia. 2022;9 doi: 10.3389/fped.2021.780258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolhnikoff M., Ferreira Ferranti J., de Almeida Monteiro R.A., Duarte-Neto A.N., Soares Gomes-Gouvea M., Viu Degaspare N., Figueiredo Delgado A., Montanari Fiorita C., Nunes Leal G., Rodrigues R.M., Taverna Chaim K., Rebello Pinho J.R., Carneiro-Sampaio M., Mauad T., Ferraz da Silva L.F., Brunow de Carvalho W., Saldiva P.H.N., Garcia Caldini E. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc. Health. 2020;4:790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duarte-Neto A.N., Caldini E.G., Gomes-Gouvea M.S., Kanamura C.T., de Almeida Monteiro R.A., Ferranti J.F., Ventura A.M.C., Regalio F.A., Fiorenzano D.M., Gibelli M., Carvalho W.B., Leal G.N., Pinho J.R.R., Delgado A.F., Carneiro-Sampaio M., Mauad T., Ferraz da Silva L.F., Saldiva P.H.N., Dolhnikoff M. An autopsy study of the spectrum of severe COVID-19 in children: from SARS to different phenotypes of MIS-C. EClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayordomo-Colunga J., Vivanco-Allende A., Lopez-Alonso I., Lopez-Martinez C., Fernandez-Vega I., Gil-Pena H., Rey C. SARS-CoV-2 spike protein in intestinal cells of a patient with coronavirus disease 2019 multisystem inflammatory syndrome. J. Pedia. 2022;243 doi: 10.1016/j.jpeds.2021.11.058. 214-218 e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stierman B., Abrams J.Y., Godfred-Cato S.E., Oster M.E., Meng L., Yip L., Patel P., Balachandran N., Prezzato E., Pierce T., Hsu K.K., Burns M., Peterson Pompa X., Lauro P., Hartley A., Jones C., Gretsch S., Reid H., Lim S., Campbell A.P., Belay E.D. Racial and ethnic disparities in Multisystem Inflammatory Syndrome in Children in the United States, March 2020 to February 2021. Pedia Infect. Dis. J. 2021;40:e400–e406. doi: 10.1097/INF.0000000000003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middelburg J.G., Crijnen T.E.M., D'Antiga L., Verdoni L., Chikermane A., Garg P., Acharyya B.C., Pruccoli G., Schnapp A., Rauf A., Middelburg R.A. Association of ethnicity with Multisystem Inflammatory Syndrome in Children related to SARS-CoV-2 infection: an international case-referent study. Front. Pedia. 2021;9 doi: 10.3389/fped.2021.707650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramcharan T., Nolan O., Lai C.Y., Prabhu N., Krishnamurthy R., Richter A.G., Jyothish D., Kanthimathinathan H.K., Welch S.B., Hackett S., Al-Abadi E., Scholefield B.R., Chikermane A. Paediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pedia Cardiol. 2020;41:1391–1401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swann O.V., Holden K.A., Turtle L., Pollock L., Fairfield C.J., Drake T.M., Seth S., Egan C., Hardwick H.E., Halpin S., Girvan M., Donohue C., Pritchard M., Patel L.B., Ladhani S., Sigfrid L., Sinha I.P., Olliaro P.L., Nguyen-Van-Tam J.S., Horby P.W., Merson L., Carson G., Dunning J., Openshaw P.J.M., Baillie J.K., Harrison E.M., Docherty A.B., Semple M.G. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. Bmj. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choe Y.J., Choi E.H., Choi J.W., Eun B.W., Eun L.Y., Kim Y.J., Kim Y.H., Kim Y.A., Kim Y.K., Kwak J.H., Lee H.M., Lee H., Lee J.K., Park J.D., Kim E.J., Park Y.J., Gwack J., Lee S.W. Surveillance of COVID-19-Associated Multisystem Inflammatory Syndrome in Children, South Korea. Emerg. Infect. Dis. 2021;27:1196–1200. doi: 10.3201/eid2704.210026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohri Y., Shimizu M., Fujimoto T., Nishikawa Y., Ikeda A., Matsuda Y., Wada T., Kawaguchi C. A young child with pediatric multisystem inflammatory syndrome successfully treated with high-dose immunoglobulin therapy. IDCases. 2022;28 doi: 10.1016/j.idcr.2022.e01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.N.T.N. Phung, T.T. Tran, T.H. Nguyen, T.H. Nguyen, T.M.T. Nguyen, Cardiovascular injury and clinical features of multisystem inflammatory syndrome in children (MIS-C) related to Covid-19 in Vietnam. Pediatr Neonatol (In press). [DOI] [PMC free article] [PubMed]
- 23.Takasago S., Sakai A., Sugiyama M., Mizokami M., Hamada H., Ishizaka Y., Miyoshi-Akiyama T., Matsunaga A., Ueno M., Shichino H., Mizukami A. Case report: Changes in cytokine kinetics during the course of disease in a Japanese Patient With Multisystem Inflammatory Syndrome in Children. Front Pedia. 2021;9 doi: 10.3389/fped.2021.702318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopfer H., Herzig M.C., Gosert R., Menter T., Hench J., Tzankov A., Hirsch H.H., Miller S.E. Hunting coronavirus by transmission electron microscopy - a guide to SARS-CoV-2-associated ultrastructural pathology in COVID-19 tissues. Histopathology. 2021;78:358–370. doi: 10.1111/his.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones M.S., 2nd, Harrach B., Ganac R.D., Gozum M.M., Dela Cruz W.P., Riedel B., Pan C., Delwart E.L., Schnurr D.P. New adenovirus species found in a patient presenting with gastroenteritis. J. Virol. 2007;81:5978–5984. doi: 10.1128/JVI.02650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Said J.W., Chien K., Tasaka T., Koeffler H.P. Ultrastructural characterization of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) in Kaposi's sarcoma lesions: electron microscopy permits distinction from cytomegalovirus (CMV) J. Pathol. 1997;182:273–281. doi: 10.1002/(SICI)1096-9896(199707)182:3<273::AID-PATH835>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Gruber C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., Wilson K.M., Onel K., Geanon D., Tuballes K., Patel M., Mouskas K., O'Donnell T., Merritt E., Simons N.W., Barcessat V., Del Valle D.M., Udondem S., Kang G., Gangadharan S., Ofori-Amanfo G., Laserson U., Rahman A., Kim-Schulze S., Charney A.W., Gnjatic S., Gelb B.D., Merad M., Bogunovic D. Mapping systemic inflammation and antibody responses in Multisystem Inflammatory Syndrome in Children (MIS-C) Cell. 2020;183 doi: 10.1016/j.cell.2020.09.034. 982-995 e914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoenigl M. Invasive fungal disease complicating coronavirus disease 2019: when it rains, it spores. Clin. Infect. Dis. 2021;73:e1645–e1648. doi: 10.1093/cid/ciaa1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koehler P., Cornely O.A., Bottiger B.W., Dusse F., Eichenauer D.A., Fuchs F., Hallek M., Jung N., Klein F., Persigehl T., Rybniker J., Kochanek M., Boll B., Shimabukuro-Vornhagen A. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai C., Yu W. COVID-19 associated with pulmonary aspergillosis: a literature review. J. Microbiol. Immunol. Infect. 2021;54:46–53. doi: 10.1016/j.jmii.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soderberg-Naucler C. Does reactivation of cytomegalovirus contribute to severe COVID-19 disease? Immun. Ageing. 2021;18:12. doi: 10.1186/s12979-021-00218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., Tan Z., Zicari S., Ruggiero A., Pascucci G.R., Santilli V., Campbell T., Bryceson Y., Eriksson D., Wang J., Marchesi A., Lakshmikanth T., Campana A., Villani A., Rossi P., Landegren N., Palma P., Brodin P. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183 doi: 10.1016/j.cell.2020.09.016. 968-981 e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowley A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 2020;20:453–454. doi: 10.1038/s41577-020-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]