Abstract
Stress-induced activation of the Bacillus subtilis transcription factor ςB is transitory. To determine whether the process that limits ςB activation is itself triggered by stress, B. subtilis strains in which the stress pathway was artificially activated by the induced expression of a positive regulatory protein (RsbT) were exposed to ethanol stress and were monitored for the persistence of ςB activity. Without ethanol treatment, the induced cultures displayed continuously high ςB activity. Ethanol treatment restricted ongoing ςB activity, but only in strains with intact rsbX and -S genes. The loss of other gene products (RsbR and Obg) known to participate in the stress activation pathway had little influence in blocking the ethanol effect. The data argue that stress upregulates the activity of the RsbX-S regulatory pair to restrict ςB induction following stress.
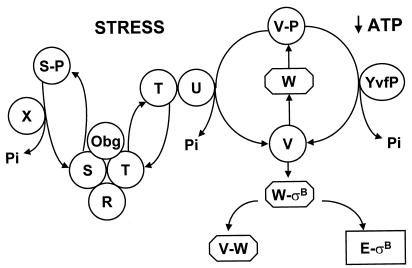
ςB is a transcription factor that controls the general stress regulon of Bacillus subtilis, a collection of genes whose products aid the bacterium in surviving any of a number of environmental traumas (10, 21, 23). Induction of the ςB regulon occurs by the activation of ςB itself, a process that is triggered by entry of B. subtilis into the stationary phase of growth or by the onset of environmental stress (e.g., heat, salt, acid, or ethanol) (12, 24, 25). A current model for ςB regulation is depicted in Fig. 1. ςB is present, but inactive, in the prestressed cell due to an association with the anti-ςB protein RsbW. ςB release from RsbW is effected by an additional protein (RsbV) which binds to RsbW in lieu of ςB (4, 5, 7). The abundance of active RsbV determines the level of free ςB (24). In unstressed cells, RsbV is largely inactive due to RsbW-catalyzed phosphorylation (7, 25). When B. subtilis enters stationary phase, unphosphorylated RsbV accumulates, likely due to the effects of an RsbV-P phosphatase (YvfP) as well as inefficient phosphorylation under the stationary-phase condition of low ATP (2, 24; K. Vijay, M. S. Brody, E. Fredlund, and C. W. Price, submitted for publication). As a result, RsbV is available to displace ςB from the RsbW-ςB complex and to induce the ςB regulon. Environmental stress also activates rsbV, but does so using a separate collection of Rsb proteins (1, 6, 8, 12, 25, 27, 28). RsbT is the most upstream effector in this pathway (28). Following exposure to stress, RsbT, normally inactive and complexed to RsbS, phosphorylates RsbS and becomes free to activate the stress-specific RsbV-P phosphatase, RsbU (28). RsbU can then activate RsbV. Obg, a GTP binding protein (14, 19, 26), is also needed for stress triggering of ςB activity; however, its explicit role in this process is unknown (17). Negative regulation is reestablished when RsbX, a RsbS-P phosphatase, dephosphorylates RsbS-P, thereby enabling RsbS to again inactivate RsbT (28).
FIG. 1.
Model of ςB regulation. Active ςB holoenzyme (E-ςB) forms when the RsbV protein (V) binds to the anti-ςB protein RsbW (W) to free ςB (2, 7). RsbV is normally inactive (V-P) due to phosphorylation by RsbW but is reactivated by stationary-phase or stress-activated phosphatases, YvfP and RsbU (U), respectively (7, 20, 24, 28; Vijay et al., submitted). The stress phosphatase RsbU is activated by RsbT (T) (28). RsbT is normally inactive due to an association with its negative regulator RsbS (S). Upon exposure to stress, RsbT phosphorylates and inactivates RsbS (S-P) and activates RsbU (28). RsbR (R) is believed to facilitate the RsbT-RsbS interaction (1, 9). Obg, a GTP binding protein, is necessary for stress activation of RsbT, but its role is unknown (17). Negative control is resumed when RsbX (X), a phosphatase, dephosphorylates and reactivates the RsbS phosphate (28).
The genes for ςB and seven of its regulators (RsbR, -S, -T, -U, -V, -W, and -X) are cotranscribed as an eight-gene operon from a promoter (PA) that is recognized by the B. subtilis housekeeping ς factor (ςA) (11, 27). An internal ςB-dependent promoter (PB) enhances the expression of the four downstream genes when ςB is active (11). Thus, the levels of ςB, its principal regulators (RsbV and -W), and the RsbX phosphatase are elevated following activation of ςB.
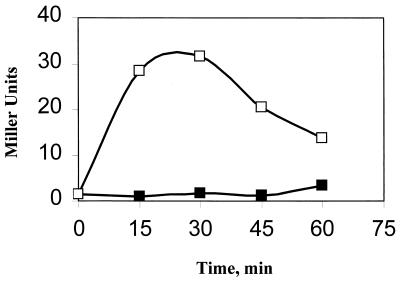
ςB-dependent transcription is only transiently activated by stress (18, 25), declining by 20 to 30 min after its initial induction (Fig. 2). The observation that RsbX, the most upstream negative regulator of the stress pathway, is expressed at higher levels when ςB becomes active suggested that the transience of the ςB stress response could be attributed to an effect of elevated RsbX protein levels on the phosphorylation state of RsbS (28). Although the persistence of ςB activity following stress induction of mutant strains lacking RsbX indicated that RsbX had a role in this process, manipulating RsbX levels by its expression from inducible promoters failed to show a credible correlation between the absolute levels of RsbX and the degree of ςB activation in stressed cells (22). These results suggested that the RsbX protein was necessary, but not sufficient, to limit the induction of ςB following stress.
FIG. 2.
Ethanol induction of ςB in wild-type B. subtilis. BSA46 (ctc::lacZ) was grown in Luria broth (LB) (16) at 37°C. At an optical density at 540 nm (OD540) of 0.15, ethanol (4% vol/vol) was added to half of the culture (0 time). Samples were taken at 15-min intervals and were analyzed for β-galactosidase (13). The data is given in Miller units (15).
To further investigate the mechanism responsible for the transience of the stress induction of ςB, we sought to separate the effect of stress in triggering the pathway from its possible effect in limiting the duration of the response. To accomplish this, we took advantage of the finding that the enhanced synthesis of RsbT, relative to its negative regulator, RsbS, is sufficient to induce the ςB stress pathway in the absence of stress (17, 28). This allowed us to artificially activate the pathway and then test the effects of stress and the need for particular rsb gene products on the duration of the response.
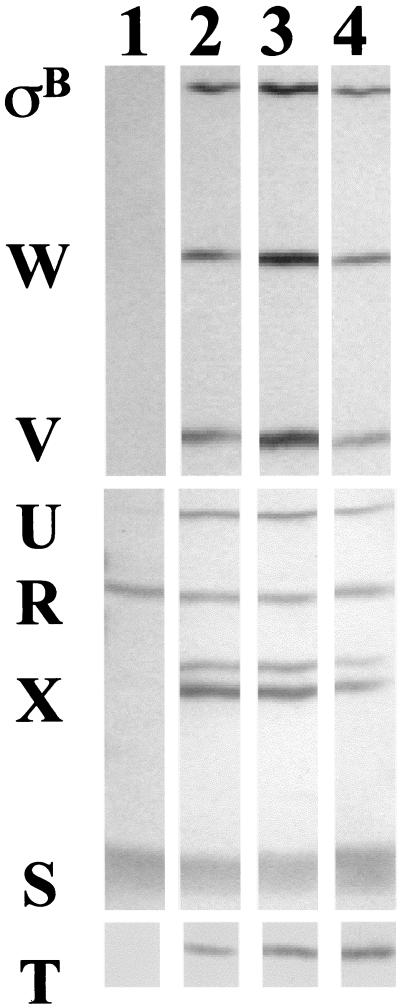
We used a B. subtilis strain (BSA419), in which a Pspac::rsbT fusion plasmid (pHV501T) had entered the chromosome by a single-site recombination event at rsbT (Table 1). BSA419 contains a sigB operon in which rsbR, -S, and -T are expressed from the PA promoter and a second copy of rsbT and the remaining downstream sigB genes, separated from PA by the plasmid sequences, are expressed under the control of the inducible spac promoter (17). When Pspac is not induced, only RsbR and -S are evident in Western blots (Fig. 3, lane 1). rsbT is also expressed, but is difficult to detect in unstressed cells by Western blotting (8). Induction of Pspac with isopropyl-β-d-thiogalactopyranoside (IPTG) yields the anticipated increase in the products of the six genes that are downstream of Pspac (Fig. 3, lane 2).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Constructiona or source |
|---|---|---|
| Bacillus strains | ||
| PY22 | trpC2 | P. Youngman |
| BSA46 | trpC2 SPβ ctc::lacZ | 3 |
| XS352 | trpC2 aph3′5"/sigB rsbST | 18 |
| BSA419 | trpC2 Pspac::rsbT SPβ ctc::lacZ | 17 |
| BSJ13 | trpC2 Pspac::rsbT Pxyl::obg SPβ ctc::lacZ | 17 |
| BSJ38 | trpC2 Pspac::rsbT rsbX::spec SPβ ctc::lacZ | BSA625 → BSA419 |
| BSJ39 | trpC2 aph3′5"/sigB ΔrsbST | XS352 → PY22 |
| BSJ40 | trpC2 aph3′5"/sigB ΔrsbST Pspac::rsbT SPβ ctc::lacZ | pHV501T → BSJ39 |
| BSJ41 | trpC2 aph3′5"/sigB ΔrsbST Pspac::rsbT SPβ ctc::lacZ | BSA46 → BSJ40 |
| BSJ42 | trpC2 aph3′5"/sigB rsbRΔ5 Pspac::rsbT SPβ ctc::lacZ | pJM49 → BSA419 |
| Plasmids | ||
| pHV501T | Apr Emr Pspac::rsbT | 17 |
| pRS11 | Apr KanrPA::rsbR rsbS | 18 |
| pJM49 | Apr KanrPA::rsbRΔ5 rsbS | This study |
| pML7/X::spec | Apr Cmr SprPBrsbVW sigB rsbX::spec | 4 |
Transformations were performed as described by Yasbin et al. (29).
FIG. 3.
Western blot analysis of BSA419 after treatment with ethanol. Cells were grown as described in the legend to Fig. 4, with samples harvested 30 min after induction by pouring over ice chips. Following centrifugation, the cells were resuspended in buffer (50 mM Tris-HCl [pH 8.0], 0.1 mM ETDA, 0.03% phenylmethylsulfonyl fluoride) and were disrupted by passage through a French press. The extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, were transferred to nitrocellulose, and were probed by Western blotting by using monoclonal antibodies raised against RsbV, -W, -X, -R, -S, -T, and -U and ςB (8). The anti-RsbX antibody detects doublet bands of unknown significance (24). Lane 1, cells immediately before addition of IPTG; lane 2, 30 min after IPTG induction; lane 3, 90 min after addition of IPTG, without ethanol treatment; lane 4, 90 min after addition of IPTG, with ethanol treatment (60 min).
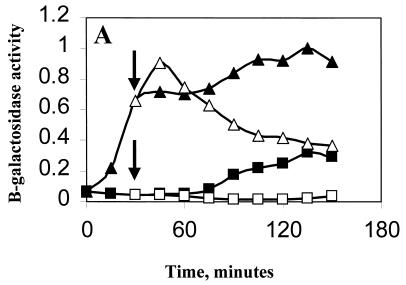
BSA419 contains a lacZ reporter gene fused to a ςB-dependent promoter (ctc::lacZ). Concomittant with induction of Pspac, there was a rapid rise in ςB-dependent transcription, which remained high throughout the duration of the experiment (Fig. 4A). When the induced culture was exposed to ethanol stress 30 min after IPTG induction (Fig. 4A), reporter gene activity showed a small increase, followed by a decline in β-galactosidase activity that resembled the decline seen when ςB is induced by stress in wild-type B. subtilis (Fig. 2). This difference in the activity of ςB in stressed and unstressed cultures was also evident in the accumulation of the sigB operon products (Fig. 3). The sigB genes (rsbV, rsbW, sigB, and rsbX), controlled from PB, continued to generate products in the absence, but not in the presence, of ethanol stress (Fig. 3, lane 3 versus lane 4). Ethanol treatment thus curtails the activity of ςB, even when the activation of ςB is independent of stress. The culture which was not IPTG treated did not show ethanol induction. This is likely due to the restricted expression of rsbU, which is downstream of the integrated plasmid. The uninduced culture did, however, display a modest increase in ςB activity upon entry into stationary phase. Presumably, this occurred when ςB, present at low levels in this strain, became active and triggered its further expression from PB. The IPTG- and ethanol-treated cultures were growth impaired and did not enter stationary phase during the course of the experiment.
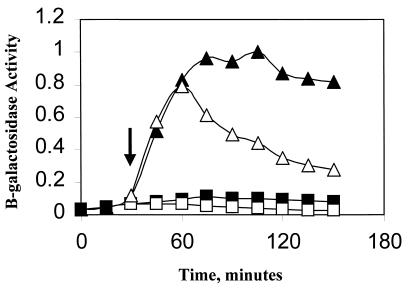
FIG. 4.
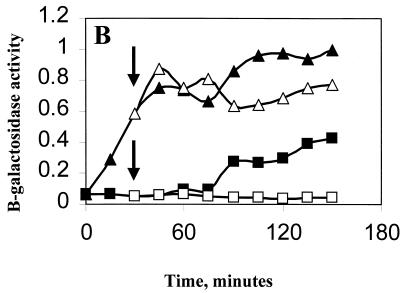
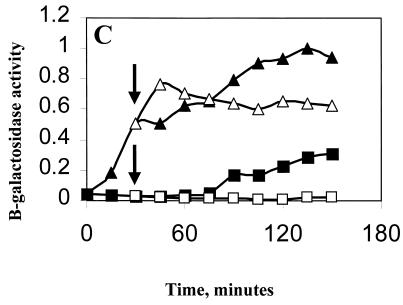
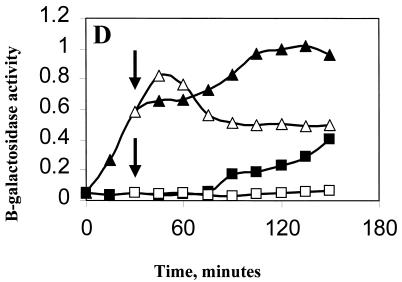
Effect of ethanol on ςB induction in Pspac::rsbT strains. B. subtilis strains carrying Spβ ctc::lacZ were grown at 37°C in LB to an OD540 of 0.1. The cultures were diluted 1:10 into fresh LB and were incubated further. When growth had recovered (OD540 of 0.05), portions of the culture were either left untreated (■) or were treated with 1 mM IPTG (▴). Thirty minutes later, as indicated by the arrows, ethanol (4%, vol/vol) was added to a portion of each of the cultures (open symbols). Samples from each of the cultures were taken every 15 min and were analyzed for β-galactosidase. Results are the averages of two experiments. The Miller unit values (15) were normalized to 1 by using the highest respective value of each strain. (A) BSA419 (Pspac::rsbT), 1 = 113 Miller units; (B) BSJ38 (Pspac::rsbT rsbX::spec), 1 = 128 Miller units; (C) BSJ41 (Pspac::rsbT ΔrsbST), 1 = 151 Miller units; (D) BSJ42 (Pspac::rsbT rsbRTΔ5), 1 = 163 Miller units.
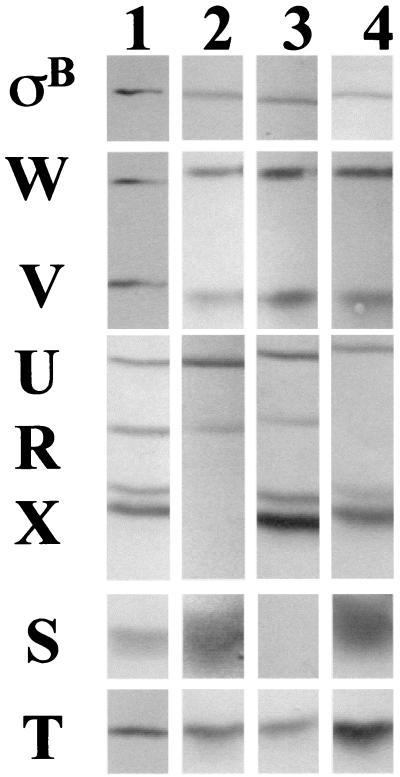
In previous studies, we noted that stress-induced ςB activity did not decline in B. subtilis strains lacking RsbX (18, 22). We therefore tested whether the fall in ςB activity, which occurred when the IPTG-induced culture was ethanol treated, also required RsbX. BSJ38 (Table 1) is a strain containing the Pspac::rsbT integration present in BSA419 plus a disruption of rsbX (rsbX::spec) (Fig. 5, lane 2). A culture of BSJ38 was induced with IPTG and a portion was exposed to ethanol stress. As was the case with the RsbX+ strain, IPTG induction resulted in ςB activation; however, unlike the RsbX+ strain, ethanol treatment did not lead to a decline in ςB reporter gene activity (Fig. 4B). Thus, the ethanol-dependent drop in ςB activity requires functional RsbX.
FIG. 5.
Rsb profiles of BSA419 and mutant strains. B. subtilis strains were grown at 37°C in LB to an OD540 of 0.1, were treated with IPTG (1 mM) to induce Pspac upstream of rsbT, and were harvested 30 min after induction and processed as described in the legend to Fig. 3. Lane 1, BSA419 (Pspac::rsbT); lane 2, BSJ38 (Pspac::rsbT rsbX::spec); lane 3, BSJ41 (Pspac::rsbT ΔrsbST); lane 4, BSJ42 (Pspac::rsbT rsbRΔ5).
The role of RsbX in the stress-induction pathway is thought to involve reactivation of RsbS, a negative regulator of RsbT (28). Given that the activation of ςB in our artificial system was caused by the induced expression of RsbT rather than by a putative stress-triggered inactivation of RsbS by RsbT, we asked whether the fall in ςB activity following ethanol treatment required RsbS. The RsbS− strain was constructed by transforming the Pspac::rsbT plasmid into BSJ39, a strain containing a deletion in the rsbS and -T region of the sigB operon (Table 1). The resulting strain (BSJ41) has an inducible source of RsbT but lacks RsbS (Fig. 5, lane 3). As was also observed with the RsbX− strain, the strain lacking RsbS failed to restrict ςB activity after stress (Fig. 4C). This result is consistent with the notion that gratuitous expression of rsbT results in an inactivation of RsbS, which can be at least partially reactivated by RsbX in stressed B. subtilis but not in unstressed cells.
Recently, Gaidenko et al. found that RsbR could influence the ability of RsbT to phosphorylate RsbS (9). They proposed that RsbR modulated the inactivation of RsbS by RsbT, either in response to environmental signals or as part of a feedback mechanism to prevent continued stress signaling (9). This result prompted us to ask whether RsbR played a role in the stress-dependent restriction of ςB activity which we observed in our present experiments. A RsbR− mutation was constructed by deleting a 500-bp EcoRI fragment from the interior of rsbR on the plasmid pRS11 (18). The resulting plasmid (pUM49) was then linearized with ScaI and was transformed into BSA419 to generate BSA42 (rsbRΔ5 Pspac::rsbT) (Fig. 5, lane 4). When BSA42 was induced with IPTG and treated with ethanol, its ςB activity profile (Fig. 4D) resembled that of the parent strain (Fig. 4A). There was a small reproducible difference (15% lower) in the degree to which ςB activity fell in the RsbR− strain compared to the decline in the RsbR+ strain; however, given that the principal pattern of decline was still evident, we conclude that RsbR is not an important component of this process. Thus, RsbX and -S, but not RsbR, are essential for the stress-activated drop in ςB activity. Presumably, stress influences the activation state of the RsbX phosphatase and its ability to reactivate RsbS-P.
In earlier studies, we discovered that an essential GTP binding protein of B. subtilis, Obg, is needed for ςB activation by stress (17). Obg was also found to interact with RsbT, -W, and -X in the yeast dihybrid system (17). Given the possible interaction of Obg with RsbX, we tested whether the stress-dependent stimulation of RsbX is affected by Obg. B. subtilis BSJ13 (Table 1), which carries the Pspac::rsbT construction within rsbT, as well as a second inducible promoter (Pxyl) driving the expression of obg, was used for this experiment. By withholding xylose, we can deplete Obg from the culture. This depletion of Obg causes a slowing of growth and a failure of stress to induce ςB (17). After culturing BSJ13 in a medium without xylose to a point where growth had slowed and ςB could no longer be activated by stress, we induced the stress pathway with IPTG and examined the ability of ethanol to restrict ςB activity in these Obg-depleted cells. As is seen in Fig. 6, ethanol treatment could still curtail ςB activity in the absence of Obg. Thus, the putative stress activation of RsbX appears to be independent of Obg.
FIG. 6.
Effect of ethanol on ςB induction in Obg-depleted cells. BSJ-13 (Pspac::rsbT Pxyl::obg) was grown in LB without xylose in order to deplete Obg. When growth slowed (time 0), IPTG (1 mM) was added to a portion of the culture (triangles) to induce Pspac upstream of rsbT, while the remaining portion was left untreated (squares). Thirty minutes later, as indicated by the arrows, ethanol (4%, vol/vol) was added to a portion of each of the cultures (open symbols). Samples from each of the cultures were taken every 15 min and were analyzed for β-galactosidase (13). Results are the averages of two experiments. The Miller unit values were normalized to 1 by using the highest value for the strain (1 = 132 Miller units).
The data presented herein argue that, aside from inducing ςB activity, ethanol stress activates a process that limits this induction. Although ethanol treatment was the only stress examined in the present study, other stresses (e.g., acid shock and salt stress) also induce ςB transiently and likely engage in a similar process. The ethanol-responsive process requires RsbX and RsbS and presumably involves the ability of RsbX to dephosphorylate and reactivate RsbS-P. The limiting factor in this reaction is not the RsbX protein, but rather is its activation. RsbX was present at higher levels in the culture that was not ethanol treated than in the ethanol-treated culture (Fig. 3, lane 3 versus lane 4) and yet was relatively ineffective in curtailing ςB activity. We conclude that either stress activates RsbX directly or there are additional stress-responsive factors which modulate the activity of RsbX.
Acknowledgments
This work was supported by NIH grant GM-48220.
REFERENCES
- 1.Akbar S, Kang C M, Gaidenko T A, Price C W. Modulator protein RsbR regulates environmental signaling in the general stress pathway of Bacillus subtilis. Mol Microbiol. 1997;24:567–578. doi: 10.1046/j.1365-2958.1997.3631732.x. [DOI] [PubMed] [Google Scholar]
- 2.Alper S, Dufour A, Garsin D A, Duncan L, Losick R. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J Mol Biol. 1996;260:165–177. doi: 10.1006/jmbi.1996.0390. [DOI] [PubMed] [Google Scholar]
- 3.Benson A K, Haldenwang W G. Characterization of a regulatory network that controls ςB expression in Bacillus subtilis. J Bacteriol. 1992;174:749–757. doi: 10.1128/jb.174.3.749-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson A K, Haldenwang W G. Regulation of ςB levels and activity in Bacillus subtilis. J Bacteriol. 1993;175:2347–2356. doi: 10.1128/jb.175.8.2347-2356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson A K, Haldenwang W G. Bacillus subtilis ςB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc Natl Acad Sci USA. 1993;90:2330–2334. doi: 10.1073/pnas.90.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boylan S A, Redfield A R, Brody M S, Price C W. Stress-induced activation of the ςB transcription factor of Bacillus subtilis. J Bacteriol. 1993;175:7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufour A, Haldenwang W G. Interactions between a Bacillus subtilis anti-ς factor (RsbW) and its antagonist (RsbV) J Bacteriol. 1994;176:1813–1820. doi: 10.1128/jb.176.7.1813-1820.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufour A, Voelker U, Voelker A, Haldenwang W G. Relative levels and fractionation properties of Bacillus subtilis ςB and its regulators during balanced growth and stress. J Bacteriol. 1996;178:3701–3709. doi: 10.1128/jb.178.13.3701-9sigma.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaidenko T A, Yang X, Lee Y M, Price C W. Threonine phosphorylation of modulator protein RsbR governs its ability to regulate a serine kinase in the stress signaling pathway of Bacillus subtilis. J Mol Biol. 1999;288:29–39. doi: 10.1006/jmbi.1999.2665. [DOI] [PubMed] [Google Scholar]
- 10.Hecker M, Schumann W, Voelker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalman S, Duncan M L, Thomas S M, Price C W. Similar organization of the sigB and spoIIA operons encoding alternate sigma factors of Bacillus subtilis RNA polymerase. J Bacteriol. 1990;172:5575–5585. doi: 10.1128/jb.172.10.5575-5585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang C M, Brody M S, Akbar S, Yang X, Price C W. Homologous pairs of regulatory proteins control activity of Bacillus subtilis transcription factor ςB in response to environmental stress. J Bacteriol. 1996;178:3846–3853. doi: 10.1128/jb.178.13.3846-3853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenney T J, Moran C P., Jr Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J Bacteriol. 1987;169:3329–3339. doi: 10.1128/jb.169.7.3329-3339.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kok J, Trach K A, Hoch J A. Effects on Bacillus subtilis of a conditional lethal mutation in the essential GTP-binding protein Obg. J Bacteriol. 1994;176:7155–7160. doi: 10.1128/jb.176.23.7155-7160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Scott J M, Haldenwang W G. Obg, an essential GTP binding protein of Bacillus subtilis, is necessary for stress activation of transcription factor ςB. J Bacteriol. 1999;181:4653–4660. doi: 10.1128/jb.181.15.4653-4660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirnova N, Scott J, Voelker U, Haldenwang W G. Isolation and characterization of Bacillus subtilis sigB operon mutations that suppress the loss of the negative regulator RsbX. J Bacteriol. 1998;180:3671–3680. doi: 10.1128/jb.180.14.3671-3680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trach K, Hoch J A. The Bacillus subtilis spo0B stage 0 sporulation operon encodes an essential GTP-binding protein. J Bacteriol. 1989;171:1362–1371. doi: 10.1128/jb.171.3.1362-1371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voelker U, Dufour A, Haldenwang W G. The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of ςB. J Bacteriol. 1995;177:114–122. doi: 10.1128/jb.177.1.114-122.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voelker U, Engelmann S, Maul B, Riethdorf S, Voelker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 22.Voelker U, Luo T, Smirnova N, Haldenwang W G. Stress activation of Bacillus subtilis ςB can occur in the absence of the ςB negative regulator RsbX. J Bacteriol. 1997;179:1980–1984. doi: 10.1128/jb.179.6.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voelker U, Maul B, Hecker M. Expression of the ςB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J Bacteriol. 1999;181:3942–3948. doi: 10.1128/jb.181.13.3942-3948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voelker U, Voelker A, Haldenwang W G. Reactivation of the Bacillus subtilis anti-ςB antagonist, RsbV, by stress or starvation-induced phosphatase activities. J Bacteriol. 1996;178:5456–5463. doi: 10.1128/jb.178.18.5456-5463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voelker U, Voelker A, Maul B, Hecker M, Dufour A, Haldenwang W G. Separate mechanisms activate ςB of Bacillus subtilis in response to environmental and metabolic stresses. J Bacteriol. 1995;177:3771–3780. doi: 10.1128/jb.177.13.3771-3780.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh K, Trach K A, Folger C, Hoch J A. Biochemical characterization of the essential GTP-binding protein Obg of Bacillus subtilis. J Bacteriol. 1994;176:7161–7168. doi: 10.1128/jb.176.23.7161-7168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise A A, Price C W. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor ςB in response to environmental signals. J Bacteriol. 1995;177:123–133. doi: 10.1128/jb.177.1.123-133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Kang C M, Brody M S, Price C W. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 1996;10:2265–2275. doi: 10.1101/gad.10.18.2265. [DOI] [PubMed] [Google Scholar]
- 29.Yasbin R E, Wilson G A, Young F E. Transformation and transfection of lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973;113:540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]