Abstract
Background
Chronic neutrophilic inflammation, in both the presence and absence of infection, is a feature of bronchiectasis in adults and children. The anti‐inflammatory properties of non‐steroid anti‐inflammatory drugs (NSAIDs) may be beneficial in reducing airway inflammation, thus potentially improving lung function and quality of life in patients with bronchiectasis.
Objectives
To evaluate the efficacy of inhaled NSAIDs in the management of non‐cystic fibrosis bronchiectasis in children and adults:
• during stable bronchiectasis; and
• for reduction of:
∘ severity and frequency of acute respiratory exacerbations; and
∘ long‐term pulmonary decline.
Search methods
We searched the Cochrane Airways Group Trials Register, which includes reports identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). We also searched the trial registry ClinicalTrials.gov and the World Health Organization (WHO) trial portal. We carried out the latest searches on 22 September 2015.
Selection criteria
All randomised controlled trials comparing inhaled NSAIDs versus a control (placebo or usual treatment) in children or adults with bronchiectasis not related to cystic fibrosis.
Data collection and analysis
We reviewed the results of searches against predetermined criteria for inclusion.
Main results
One small, short‐term trial was eligible for inclusion. We included this study of 25 adults with chronic lung disease (only 32% of people included in the trial had bronchiectasis), as the other conditions were linked to development of bronchiectasis, and all were characterised by chronic sputum production. We were not able to obtain separate data for people with a diagnosis of bronchiectasis. We judged that the study was at a high risk of selection bias.
The primary outcome (mean difference in control of bronchiectasis severity, quality of life (Qol), cough scores) was not reported in the included study. The single trial in adults reported a significant reduction in sputum production over 14 days for the treatment group (inhaled indomethacin) compared with the placebo group (mean difference (MD) ‐75.00 g/day; 95% confidence interval (CI) ‐134.61 to ‐15.39) and a significant improvement in the Borg Dyspnoea Scale score (MD ‐1.90, 95% CI ‐3.15 to ‐0.65). We noted no significant differences between groups in lung function or blood indices and no reported adverse events.
Authors' conclusions
No new studies of adults or children have been conducted since the last version of this review was published. Therefore, final conclusions have not changed. Current evidence is insufficient to support or refute the use of inhaled NSAIDs for the management of bronchiectasis in adults or children. One small trial reported a reduction in sputum production and improved dyspnoea among adults with chronic lung disease who were treated with inhaled indomethacin, indicating that additional studies on the efficacy of NSAIDs for treatment of patients with bronchiectasis are warranted.
Keywords: Adult; Child; Humans; Administration, Inhalation; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/administration & dosage; Bronchiectasis; Bronchiectasis/drug therapy; Dyspnea; Dyspnea/drug therapy; Sputum; Sputum/metabolism
Plain language summary
Inhaled non‐steroid anti‐inflammatories (NSAIDs) for children and adults with bronchiectasis
People with bronchiectasis experience chronic inflammation of the lungs. Anti‐inflammatory effects of inhaled non‐steroid anti‐inflammatory drugs (NSAIDs) may be beneficial for patients with bronchiectasis. However, short‐term and long‐term benefits in adults and children must be investigated, in addition to potential side effects of NSAIDs used over the long term.
Results
We included one small study on 25 people with chronic lung disease. Of those 25, only eight people had bronchiectasis. Other individuals had chronic bronchitis of diffuse panbronchiolitis and were at risk for bronchiectasis. However, we must remember when interpreting the results that not all study participants had bronchiectasis.
Overall, the small study reported improvement in sputum production and dyspnoea (shortness of breath) in adults with chronic lung disease (chronic bronchitis, bronchiectasis or diffuse panbronchiolitis) who received inhaled indomethacin compared with placebo. Researchers observed no significant improvement in lung function (forced expiratory volume in one second (FEV1) and vital capacity (VC)) and reported no adverse events.
Conclusions
The small scale of this study and collective analysis of data from the three disease states made it difficult for review authors to draw solid conclusions on the benefit of using NSAIDs to treat adults with bronchiectasis. Review authors identified no studies examining the use of NSAIDs in children with bronchiectasis.
Background
Description of the condition
Bronchiectasis is increasingly recognised as a major cause of respiratory morbidity, especially in developing countries (Karadag 2005; Karakoc 2001) and in pockets of affluent countries (Chang 2008). The underlying aetiology of bronchiectasis varies; it may follow recurrent respiratory infection or may occur secondary to rare immune deficiencies. However, bronchiectasis is also a common pathway for a variety of diseases. Thus, the presence of bronchiectasis is increasingly recognised in common (e.g. chronic obstructive pulmonary disease (COPD) (O'Brien 2000)) and uncommon respiratory diseases (e.g. bronchiolitis obliterans, sarcoidosis (Lewis 2002)) as well as in non‐primary respiratory (e.g. autoimmune) diseases. When bronchiectasis is present along with another underlying disorder, morbidity and mortality of the underlying disease are increased (Keistinen 1997; Lewis 2002). For example, bronchiectasis has been reported in 29% to 50% (O'Brien 2000) of cohorts with COPD and when present increases the severity and frequency (Gursel 2006) of respiratory exacerbations.
Dominant symptoms and signs of bronchiectasis include productive or wet cough, dyspnoea on exertion and other respiratory signs (e.g. clubbing, chest wall deformity, respiratory noises such as wheeze or crepitations on auscultation). Pulmonary decline may occur over the long‐term (Keistinen 1997). Also, as with COPD, children and adults with bronchiectasis suffer from recurrent acute exacerbations, some of which require treatment in the hospital (Chang 2008). Effective management regimens for bronchiectasis improve quality of life (Courtney 2008; Martinez‐Gracia 2005; Muthalithas 2008) and could reduce the frequency or severity of respiratory exacerbations (Cymbala 2005) and/or long‐term pulmonary decline (Chang 2008). Thus, management of the symptoms and severity of bronchiectasis is important.
Description of the intervention
Non‐steroid anti‐inflammatory drugs (NSAIDs) are a class of medications that act as non‐selective inhibitors of the enzyme cyclo‐oxygenase, inhibiting both cyclo‐oxygenase‐1 (COX‐1) and cyclo‐oxygenase‐2 (COX‐2) isoenzymes. NSAIDs have analgesic, antipyretic and anti‐inflammatory effects and reduce pain, fever and inflammation. NSAIDs are usually given orally, but the inhaled formulation has been used in people with bronchorrhoea, a feature present in many patients with bronchiectasis (Tamaoki 1992).
How the intervention might work
Cole's 'vicious circle hypothesis' indicates that microbial colonisation/infection is important in the pathophysiology of bronchiectasis, as it leads to bronchial obstruction and an abnormal or exaggerated inflammatory response (Cole 1986). Anti‐inflammatory drugs may reduce the inflammatory cascade, thus ameliorating symptoms and reducing long‐term pulmonary decline.
As the airways of patients with bronchiectasis show intense neutrophilic inflammation (Cole 1986), the anti‐inflammatory effects of NSAIDs may have a beneficial effect for patients with bronchiectasis. "Blockade of cyclooxygenase pathway with indomethacin could decrease respiratory tract fluid and mucus by inhibiting chloride secretion and glandular secretion and by enhancing Na (sodium) absorption across airway mucosa" (Tamaoki 1992). Animal studies have shown that pretreatment with inhaled indomethacin protects the airway from distilled water and ozone, and this increases lung resistance through swelling of airway epithelial cells (Mochizuki 2002).
Why it is important to do this review
Although NSAIDs may have potential benefits for those with bronchiectasis, oral NSAIDs are associated with several adverse events, particularly of the gastrointestinal tract (Behrman 2003). NSAIDs may be better tolerated when inhaled; however, transient upper airway irritation has been reported (Ong 2004; Sestini 1999). It is therefore important to assess additional side effects associated with inhalation of NSAIDs.
Evidence suggests that NSAIDs may prevent pulmonary deterioration in people with mild lung disease due to cystic fibrosis (CF) (Lands 2013). However, extrapolation of treatment for individuals with CF to those with non‐CF bronchiectases may be harmful (e.g. recombinant human DNase efficacious in CF causes harm in non‐CF bronchiectasis (Crockett 2014)). Thus, a systematic review on the efficacy of inhaled NSAIDs in the treatment of children and adults with bronchiectasis could guide clinical practice.
Objectives
To evaluate the efficacy of inhaled NSAIDs for the management of non‐cystic fibrosis bronchiectasis in children and adults:
during stable bronchiectasis; and
for reduction of:
severity and frequency of acute respiratory exacerbations; and
long‐term pulmonary decline.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) comparing inhaled NSAIDs versus a control (placebo or usual treatment) in people with bronchiectasis. We planned to include studies reported as full text, those published as abstract only and unpublished data.
Types of participants
We included studies involving children or adults with a diagnosis of bronchiectasis (defined clinically or radiologically). We excluded studies of people with cystic fibrosis and COPD.
Types of interventions
We included studies comparing all types of inhaled NSAIDs (study group) versus usual care or placebo (control group). We included studies with co‐interventions provided they were not part of the randomly assigned treatment and both groups had equal access to the co‐interventions.
Types of outcome measures
Primary outcomes
For short‐term effectiveness (≤ 12 months): mean difference in control of bronchiectasis severity (quality of life (QOL), cough scores).
For medium‐ to long‐term outcomes (> 1 year): difference in lung function data (forced expiratory volume in one second (FEV1) % predicted).
Secondary outcomes
Short‐term effectiveness (≤ 12 months)
Total number of days with respiratory symptoms.
Mean difference in lung function indices (spirometry, other lung volumes, airway hyper‐responsiveness).
Proportions of participants who had respiratory exacerbations and/or hospitalisations.
Total number of hospitalised days.
Mean difference in other objective indices (e.g. airway markers of inflammation, exhaled nitric oxide).
Proportions experiencing adverse effects of the intervention (e.g. gastritis, haematemesis, ecchymoses).
Serious adverse events (e.g. haemoptysis, bronchospasm).
Medium‐ to long‐term outcomes (> 1 year)
Radiology scores (high‐resolution computed tomography scans or chest radiograph).
Clinical indices of control of bronchiectasis severity (e.g. QOL, cough diary, Likert scale, visual analogue scale, level of interference of cough).
Mortality.
Proportions experiencing adverse effects of the intervention (e.g. gastric bleeding, gastritis, haematemesis, cardiac events).
Serious adverse events (e.g. haemoptysis, bronchospasm).
Search methods for identification of studies
Electronic searches
Search methods used in the previous version of this review are detailed in Appendix 1. The previously published version included searches up to October 2009. The search period for this update is October 2009 through September 2015.
For this update, we identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 2 for details). We searched all records in the CAGR using the search strategy provided in Appendix 3.
We also searched ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/). We searched all databases from their inception to the present, and we imposed no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information. We planned to communicate with the authors of trials included in the review, when necessary.
We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
From the title, abstract or descriptors, two review authors (SP, AC) independently reviewed the literature searches to identify potentially relevant trials for full review. We conducted searches of bibliographies and texts to identify additional studies. From the full text and on the basis of specified criteria, the same two review authors independently selected trials for inclusion. The review authors (SP, AC) reported no disagreement on the selection of trials. We documented ineligible studies in the table, Characteristics of excluded studies.
Data extraction and management
We reviewed trials that satisfied the inclusion criteria for the following information: study setting; year of study; source of funding; patient recruitment details (including number of eligible participants); inclusion and exclusion criteria; other symptoms; randomisation and allocation concealment methods; numbers of participants randomly assigned; blinding (masking) of participants, care providers and outcome assessors; dose and type of intervention; duration of therapy; co‐interventions; numbers of participants not followed up; reasons for withdrawal from study protocol (clinical events, side effects, refusal and other); details on side effects of therapy; and whether intention‐to‐treat analyses were used when possible. We would have extracted data on the outcomes described previously. When required, we planned to obtain further information from the study authors.
Assessment of risk of bias in included studies
To assess risk of bias, two review authors (SP, AC) independently assessed the quality of the studies on the basis of the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Review authors assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
This is briefly described in the following sections.
Allocation concealment
Adequate: if allocation of participants involved a central independent unit, an on‐site locked computer, identical appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator or sealed opaque envelopes.
Unclear: if the method used to conceal allocation was not described.
Inadequate: if the allocation sequence was known to investigators who assigned participants, or if the study was quasi‐randomised.
Random sequence generation
Adequate: if methods of randomisation included using a random number table, computer‐generated lists or similar methods.
Unclear: if the trial was described as randomised, but the methods used to allocate participants to treatment groups were not described.
Inadequate: if methods of randomisation included alternation, use of case record numbers, date of birth or day of the week and any procedure that was entirely transparent before allocation.
Blinding of participants and personnel
Blinding of clinician (person delivering treatment) to treatment allocation.
Blinding of participant to treatment allocation.
Blinding of outcome assessor to treatment allocation.
Follow‐up
We planned to grade numbers and reasons for drop‐outs and withdrawals in each intervention group.
Measures of treatment effect
We analysed data using RevMan 2014. We analysed continuous data as mean differences (MDs) with 95% confidence intervals (CIs). If studies reported outcomes by using different measurement scales, we planned to use standardised mean differences (SMDs).
For dichotomous variables, we planned to analyse data as odds ratios (ORs) with 95% CIs.
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
We planned to request further information from primary investigators when required, but as the only included study was published in 1992, we did not contact study authors (Tamaoki 1992).
Assessment of heterogeneity
We planned to describe heterogeneity between study results and to use the Chi2 test to see if it reached statistical significance. We would have considered heterogeneity as significant if the P value was less than 0.10 (Higgins 2011). We also planned to use the I2 statistic when heterogeneity was categorised in such a way that a value less than 25% was considered low, around 50% was considered moderate and over 75% was considered high for heterogeneity (Higgins 2003). As only one eligible study was identified, assessment of heterogeneity was not applicable.
Assessment of reporting biases
If more than 10 eligible studies were found, we would have assessed publication bias by using a funnel plot. We intended to investigate and report on any cases of selective reporting.
Data synthesis
For the dichotomous outcome variables of each individual study, we had planned to calculate odds ratios (ORs) using a modified intention‐to‐treat (ITT) analysis. This analysis assumes that children not available for outcome assessment have not improved (and probably represents a conservative estimate of effect). An initial qualitative comparison of all individually analysed studies examines whether pooling of results (meta‐analysis) is reasonable. This would take into account differences in study populations, inclusion/exclusion criteria, interventions, outcome assessments and estimated effect size.
Results from studies that met the inclusion criteria and reported any of the outcomes of interest were to be included in subsequent meta‐analyses. We planned to calculate the summary OR and 95% CI (fixed‐effect model) (RevMan 2014).
We planned to use data from parallel studies only (not from cross‐overs). We planned to calculate number needed to treat for an additional beneficial outcome (NNTB) from the pooled OR and its 95% CI applied to a specified baseline risk by using an online calculator (Cates 2003). If studies reported outcomes based on different measurement scales, we would have estimated standardised mean differences (SMDs).
Subgroup analysis and investigation of heterogeneity
We planned the following a priori subgroup analyses.
Children (≤ 18 years of age) and adults (> 18 years of age).
Severity of bronchiectasis (based on FEV1: > 80% classified as mild, 50% to 79% classified as moderate, 30% to 49% classified as severe, < 30% classified as very severe).
We would have described and explored heterogeneity between study results. We had planned to include the 95% CI estimated by using a random‐effects model when we had concerns about statistical heterogeneity.
Sensitivity analysis
We also planned sensitivity analyses to assess the impact of potentially important factors on overall outcomes.
Variation in inclusion criteria.
Differences in medications used in intervention and comparison groups.
Differences in outcome measures.
Analysis using a random‐effects model.
Analysis by treatment received.
Analysis by ITT.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The Airways Group specialised register/literature search performed in October 2008 and October 2009 yielded 173 (153 and 20 respectively) references. We found no RCTs that focused specifically on adults or children with bronchiectasis. We identified two publications that were considered for inclusion in this review. We included one study (Tamaoki 1992); and we excluded the second study (Llewellyn‐Jones 1995), which did not meet eligibility criteria.
The latest search for this update was performed in September 2015 and yielded 104 additional potential studies, but none met our eligibility criteria (Figure 1); therefore, the results of this review remain unchanged.
1.
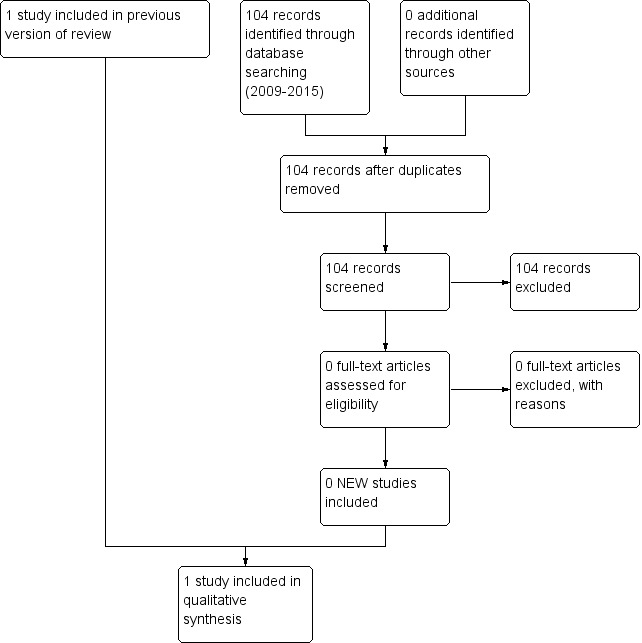
Study flow diagram: review update.
Included studies
We identified no studies that focused solely on bronchiectasis in adults or children. However, we included a single, small study on 25 adults with chronic lung disease, including eight with bronchiectasis (32%), as the two additional chronic lung disease conditions in the study led to bronchiectasis, and bronchorrhoea is a key clinical feature of bronchiectasis. Tamaoki and colleagues (Tamaoki 1992) examined the short‐term effects (14 days) of inhaled indomethacin compared with placebo on sputum and blood indices, dyspnoea scale and lung function in 25 adults with chronic lung disease (eight with bronchiectasis (32%), 12 with chronic bronchitis and five with diffuse panbronchiolitis). We have provided details of this study in the Characteristics of included studies table.
Excluded studies
We excluded one study (Llewellyn‐Jones 1995), as it was not a randomised controlled trial.
Risk of bias in included studies
Overall we assessed the sole included study (Tamaoki 1992) to be at high risk of bias, as detailed in the 'Risk of bias' table and summarised below.
Allocation
The doctor responsible for allocating treatment groups was not blinded (but was not involved in follow‐up or data analysis). This approach does not fulfill the criteria for adequate allocation concealment and is considered to present high risk of selection bias.
Blinding
Participants and investigators responsible for disease follow‐up and data analysis were blinded, representing low risk of performance and detection bias.
Incomplete outcome data
Data were complete for all participants.
Selective reporting
We identified no selective reporting bias in the study and consider it to present low risk.
Other potential sources of bias
We identified no other potential sources of bias. However, data analysis did not distinguish participants with bronchiectasis from other respiratory groups, other than for sputum production. We consider this to represent an unclear risk of bias.
Effects of interventions
Short‐term effects
The primary outcome (MD in bronchiectasis severity control, QOL, cough scores) was not reported in the included study.
Respiratory symptoms
The only clinical data reported consisted of the Borg score, which showed significant differences between groups (MD ‐1.90, 95% CI ‐3.15 to ‐0.65; Analysis 1.1).
1.1. Analysis.
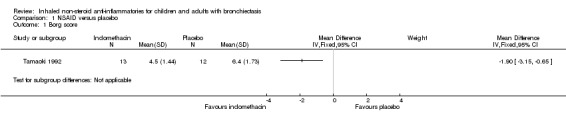
Comparison 1 NSAID versus placebo, Outcome 1 Borg score.
Lung function
We noted no significant differences between groups for FEV1 % predicted at the end of the study (MD ‐2.90%, 95% CI ‐13.30 to 7.50; Analysis 1.2) nor for vital capacity (VC) % predicted (MD ‐2.90%, 95% CI ‐10.58 to 4.78; Analysis 1.3).
1.2. Analysis.
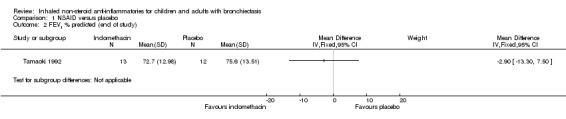
Comparison 1 NSAID versus placebo, Outcome 2 FEV1 % predicted (end of study).
1.3. Analysis.
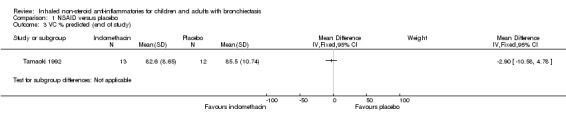
Comparison 1 NSAID versus placebo, Outcome 3 VC % predicted (end of study).
Other indices
For sputum indices, we found a significant decrease in wet weight of sputum at the end of the study in the indomethacin group compared with the placebo group (MD ‐75.00 g/day; 95% CI ‐134.61 to ‐15.39; Analysis 1.4) but no difference in bacterial load per gram of sputum (MD ‐0.30, 95% CI ‐1.71 to 1.11; Analysis 1.5).
1.4. Analysis.
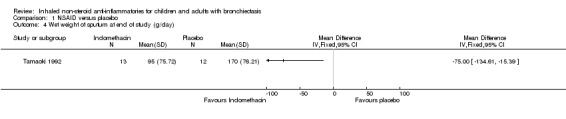
Comparison 1 NSAID versus placebo, Outcome 4 Wet weight of sputum at end of study (g/day).
1.5. Analysis.
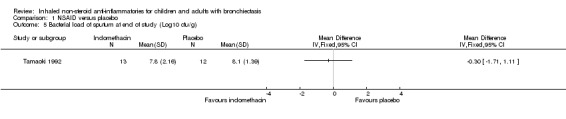
Comparison 1 NSAID versus placebo, Outcome 5 Bacterial load of sputum at end of study (Log10 cfu/g).
For blood indices, we found no significant differences between groups for erythrocyte sedimentation rate (ESR) (MD ‐2.00 mm/h, 95% CI ‐13.42 to 9.42; Analysis 1.6) nor for total white cell count (MD ‐400.00 cells/mL, 95% CI ‐1654.94 to 854.94; Analysis 1.7).
1.6. Analysis.
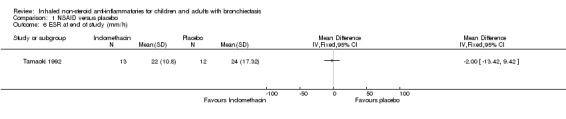
Comparison 1 NSAID versus placebo, Outcome 6 ESR at end of study (mm/h).
1.7. Analysis.
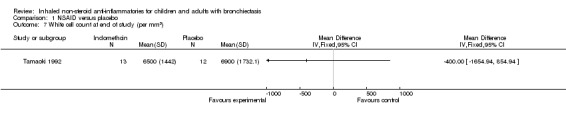
Comparison 1 NSAID versus placebo, Outcome 7 White cell count at end of study (per mm3).
Adverse events
Investigators reported no adverse events.
Discussion
Summary of main results
Data from one small, short‐term (14‐day) study of 25 adults with chronic lung disease (12 with chronic bronchitis, eight with bronchiectasis and five with panbronchiolitis) suggest that inhaled indomethacin (a type of non‐steroid anti‐inflammatory drug (NSAID)) was significantly beneficial in reducing sputum production compared with placebo. The clinically important difference for bronchiectasis on the Borg scale is unknown, but that for chronic obstructive pulmonary disease (COPD) is 1 unit (Ries 2005); thus the difference between groups for dyspnoea (mean difference (MD) ‐1.90, 95% confidence interval (CI) ‐3.15 to ‐0.65) is likely to be clinically important.
Investigators reported no differences between groups for lung function nor for blood indices.
Overall completeness and applicability of evidence
The sole small study included with its limited number of participants with bronchiectasis (n = 8; 32%) limits definitive conclusions.
We identified no randomised controlled trials of inhaled NSAIDs in children with bronchiectasis.
Quality of the evidence
The sole included study in this review (Tamaoki 1992) was double‐blind and randomised, although allocation of concealment remains unknown. Overall, we judged the quality of the evidence to be low. We downgraded the score two‐fold on the basis of (1) imprecision of results, caused by the small sample size, and (2) indirect evidence, based on collective analysis of data from three disease states; hence bronchiectasis‐specific data are unknown.
Potential biases in the review process
This review has been conducted in accordance with the published protocol. We did not contact the authors (for bronchiectasis‐specific data) of the one included study, Tamaoki 1992, due to the age of the study and the small number of participants with bronchiectasis (n = 8). Such data would be unlikely to change the final conclusions of this review.
Agreements and disagreements with other studies or reviews
The Cochrane review of oral NSAIDs for cystic fibrosis concluded that NSAIDs are likely to slow the progression of lung disease (Lands 2013). Review authors reported no data on sputum production or dyspnoea. The Cochrane review of oral NSAIDs for bronchiectasis (Kapur 2007) identified no relevant studies.
Authors' conclusions
Implications for practice.
Although a single study has shown some benefit for short‐term use of inhaled indomethacin in adults with chronic lung disease (including participants with bronchiectasis and those at risk of bronchiectasis), evidence is currently insufficient to support or refute the use of inhaled NSAIDs in children or adults with bronchiectasis. NSAIDs may be beneficial in the immediate term for reducing sputum production. However, there were too few participants with bronchiectasis in the included study, and the duration of treatment was too short, to provide adequate information on beneficial or adverse effects of inhaled NSAIDs in adults with bronchiectasis. No data are currently available on the effectiveness of inhaled NSAIDs in children with bronchiectasis.
Implications for research.
Data presented in the only study included in this review indicate that a double‐blind, randomised, placebo‐controlled trial is warranted to investigate short‐term (≤ 12 months) and long‐term (> one year) beneficial and adverse effects of inhaled NSAIDs for both adults and children with bronchiectasis. Randomised controlled trials should investigate children and adults separately and should include data as highlighted in the Types of outcome measures section of this review.
What's new
| Date | Event | Description |
|---|---|---|
| 28 September 2015 | New search has been performed | The latest search was conducted on 28 September 2015. Minor amendments have been made to the review for consistency with updated guidelines of the Cochrane Airways Group. |
| 28 September 2015 | New citation required but conclusions have not changed | No new studies were identified for this update. The final conclusion remains unchanged. |
Acknowledgements
We thank Emma Welsh, Emma Jackson, Dr Chris Cates and Elizabeth Stovold from the Cochrane Airways Group for advice, support and comments provided on this updated review.
Chris Cates was the Editor for this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods used up to 2010
Electronic searches
We used the following topic search strategy to identify the relevant randomised controlled trials listed in the electronic databases:
("bronchiectasis" OR "suppurative lung disease" as (textword) or (MeSH )) AND ("inhaled" OR "nebulise" OR "nebulised" as (textword) or (MeSH )) AND ("anti‐inflammatory" OR "diclofenac" OR "etodolac" OR "ketorolac" OR "sulindac" OR "tolmentin" OR "diflunisal" OR "salsalate" OR "meloxicam" OR "piroxicam" OR "flurbiprofen" OR "Ibupropen" OR "ketoprofen" OR "naproxen" OR "oxaprozin" OR "indomethacin" OR "COX2 inhibitors" OR "celecoxib" OR "rofecoxib" OR "valdecoxib") as (textword) or (MeSH)
We identified trials from the following sources.
Cochrane Airways Group Trials Register.
Cochrane Central Register of Controlled Trials (CENTRAL) (2009, Issue 3).
MEDLINE (1966 to present).
OLDMEDLINE (1950 to 1965).
EMBASE (1980 to present).
For MEDLINE, OLDMEDLINE and EMBASE, we combined the topic search strategy with the RCT search filter as outlined in the Airways Group module.
Searching other resources
We also searched the references in relevant publications. We planned to communicate with the authors of trials included in the review, when necessary.
Appendix 2. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Bronchiectasis search
1. exp Bronchiectasis/
2. bronchiect$.mp.
3. bronchoect$.mp.
4. kartagener$.mp.
5. (ciliary adj3 dyskinesia).mp.
6. (bronchial$ adj3 dilat$).mp.
7. or/1‐6
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and the RCT filter are adapted to identify trials in other electronic databases.
Appendix 3. Search strategy to identify relevant trial reports from the CAGR
#1 BRONCH:MISC1 #2 MeSH DESCRIPTOR Bronchiectasis Explode All #3 bronchiect* #4 #1 or #2 or #3 #5 MeSH DESCRIPTOR Anti‐Inflammatory Agents, Non‐Steroidal #6 anti NEXT inflammat* #7 NSAID* #8 diclofenac #9 etodolac #10 ketorolac #11 sulindac #12 diflunisal #13 salsalate #14 meloxicam #15 piroxicam #16 flurbiprofen #17 Ibuprofen #18 ketoprofen #19 naproxen #20 oxaprozin #21 indometacin #22 tolmetin #23 MeSH DESCRIPTOR Cyclooxygenase 2 Inhibitors #24 cyclooxygenase* #25 cox NEXT 2 #26 celecoxib #27 rofecoxib #28 valdecoxib #29 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 #30 #4 AND #29
[Note: in search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, bronchiectasis.]
Data and analyses
Comparison 1. NSAID versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Borg score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 FEV1 % predicted (end of study) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 VC % predicted (end of study) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Wet weight of sputum at end of study (g/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Bacterial load of sputum at end of study (Log10 cfu/g) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 ESR at end of study (mm/h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 White cell count at end of study (per mm3) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Tamaoki 1992.
| Methods | Double‐blind, randomised, placebo‐controlled trial Pulmonary function was assessed by a change in VC and FEV1 before treatment (day 0) and on day 14. Quality of life was assessed by the Borg ratio scale for questions related to breathlessness and dyspnoea Sputum was analysed for change in production (g/day), cyclo‐oxygenase products (PGE2, PGF2a, 6‐oxo‐PGF1a, TxB2) and microbiological culture Statistical analysis: data were expressed as means ± SEM. Two‐way analysis of variance and Student's paired t test were used for normally distributed variables. The Newman‐Keuls test was used for multiple comparisons. A P value less than 0.05 was considered statistically significant |
|
| Participants | 25 adults (age 29 to 78 years) with a diagnosis of chronic lung disease (chronic bronchitis (N = 12), diffuse panbronchiolitis (N = 5) or bronchiectasis (N = 8)) and bronchorrhoea of at least 4 weeks. Eight of the 25 participants had bronchiectasis, but all had symptoms of bronchiectasis and 21 had chronic colonisation with respiratory pathogens present in the adults with bronchiectasis ‐ 17 had Pseudomonas aeruginosa, 3 Haemophilus influenzae and 1 Staphylococcus aureus. Of the 8 participants with bronchiectasis, 4 were allocated to the indomethacin group and 4 to the placebo group. All had no history of respiratory allergy | |
| Interventions | Treatment group 1: inhaled indomethacin, 2 mL aerosol preparation of 1.2 μg/mL in saline 3 times daily for 14 days Treatment group 2: inhaled placebo, 2 mL aerosolised saline alone 3 times daily for 14 days Method of delivery: nebuliser delivering aerosolised particles with a median particle diameter of 4.5 to 5 μm |
|
| Outcomes | Data for all 3 disease states were analysed collectively. Outcomes were sputum indices (% solid composition, sputum bacterial density and inflammatory markers ‐ prostaglandin E2, PGF2a, 6‐oxo‐PGF1a, TxB2), Borg score ratio scale for breathlessness and dyspnoea, WCC, ESR and spirometry The only outcome for which results were reported separately for participants with bronchiectasis was effect on sputum production |
|
| Notes | We elected to include all outcomes, as although not all participants had the diagnosis of bronchiectasis, the additional 2 diseases (chronic bronchitis and panbronchiolitis) overlap with bronchiectasis and eventually can lead to bronchiectasis. Furthermore, the large number colonised with bacteria, especially with Pseudomonas, indicates that bronchiectasis would have been likely to be identified if a multi‐detector high‐resolution CT scan had been performed on all participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | The doctor responsible for allocating treatment groups was not blinded but was not involved in follow‐up or data analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators responsible for disease follow‐up and data analysis were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were complete for all outcomes |
| Selective reporting (reporting bias) | Low risk | We identified no selective reporting |
| Other bias | Unclear risk | We identified no other potential sources of bias. However, data analysis did not distinguish individuals with bronchiectasis from people with other respiratory disease |
CT: computed tomography. ESR: erythrocyte sedimentation rate. FEV1: forced expiratory volume in one second. 6‐oxo‐PGF1a: 6‐oxo‐prostaglandin F1 alpha. PGE2: prostaglandin E2. PGF2a: prostaglandin F2 alpha. SEM: standard error of the mean. TxB2: thromboxane B2. VC: vital capacity. WCC: white cell count.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Llewellyn‐Jones 1995 | Study using oral indomethacin |
Contributions of authors
SP and AC updated the review. JU and SY contributed to editing of the update.
Sources of support
Internal sources
-
Royal Children's Hospital Foundation, Australia.
Salary support for AC
External sources
-
NHMRC, Australia.
AC and JU are supported by the NHMRC
-
Australian Cochrane Airways Group, Australia.
Support for SP to complete this review
Declarations of interest
None of the review authors have reported any conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Tamaoki 1992 {published data only}
- Tamaoki J, Chiyotani A, Kobayashi K, Sakai N, Kanemura T, Takizawa T. Effect of indomethacin on bronchorrhea in patients with chronic bronchitis, diffuse panbronchiolitis, or bronchiectasis. American Review of Respiratory Disease 1992;145(3):548‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Llewellyn‐Jones 1995 {published data only}
- Llewellyn‐Jones CG, Johnson MM, Mitchell JL, Pye A, Okafor VC, Hill SL, et al. In vivo study of indomethacin in bronchiectasis: effect on neutrophil function and lung secretion. European Respiratory Journal 1995;8(9):1479‐87. [PubMed] [Google Scholar]
Additional references
Behrman 2003
- Behrman RE, Kliegman RM, Jenson HB. Nelson Textbook of Pediatrics. 17th Edition. Philadelphia: Saunders, 2003. [Google Scholar]
Cates 2003 [Computer program]
- [Author Cates C]. Visual Rx. Online NNT Calculator. www.nntonlinenet, 2003.
Chang 2008
- Chang AB, Bilton D. Non‐cystic fibrosis bronchiectasis exacerbations. Thorax 2008;63(3):269‐76. [DOI] [PubMed] [Google Scholar]
Cole 1986
- Cole PJ. Inflammation: a two edged sword. The model of bronchiectasis. European Journal of Respiratory Disease 1986;147(Suppl):6‐15. [PubMed] [Google Scholar]
Courtney 2008
- Courtney J, Kelly M, Watt A, Garske L, Bradley J, Ennis M, et al. Quality of life and inflammation in exacerbations of bronchiectasis. Chronic Respiratory Disease 2008;5(3):161‐8. [DOI] [PubMed] [Google Scholar]
Crockett 2014
- Crockett AJ, Cranston JM, Latimer KM, Alpers JH. Mucolytics for bronchiectasis. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001289.pub2] [DOI] [Google Scholar]
Cymbala 2005
- Cymbala AA, Edmonds LC, Bauer MA, Jederlinic PJ, May JJ, Victory JM, et al. The disease‐modifying effects of twice‐weekly oral azithromycin in patients with bronchiectasis. Treatments in Respiratory Medicine 2005;4(2):117‐22. [DOI] [PubMed] [Google Scholar]
Gursel 2006
- Gursel G. Does coexistence with bronchiectasis influence intensive care unit outcome in patients with chronic obstructive pulmonary disease?. Heart & Lung 2006;35(1):58‐65. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kapur 2007
- Kapur N, Chang AB. Oral non steroid anti‐inflammatories for children and adults with bronchiectasis. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006427.pub2] [DOI] [PubMed] [Google Scholar]
Karadag 2005
- Karadag B, Karakoc F, Ersu R, Kut A, Bakac S, Dagli E. Non‐cystic‐fibrosis bronchiectasis in children: a persisting problem in developing countries. Respiration 2005;72(3):233‐8. [DOI] [PubMed] [Google Scholar]
Karakoc 2001
- Karakoc GB, Yilmaz M, Altintas DU, Kendirli SG. Bronchiectasis: still a problem. Pediatric Pulmonology 2001;32(2):175‐8. [DOI] [PubMed] [Google Scholar]
Keistinen 1997
- Keistinen T, Saynajakangas O, Tuuponen T, Kivela SL. Bronchiectasis: an orphan disease with a poorly‐understood prognosis. European Respiratory Journal 1997;10(12):2784‐7. [DOI] [PubMed] [Google Scholar]
Lands 2013
- Lands C, Stanojevic S. Oral non‐steroidal anti‐inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001505.pub3] [DOI] [PubMed] [Google Scholar]
Lewis 2002
- Lewis MM, Mortelliti MP, Yeager H Jr, Tsou E. Clinical bronchiectasis complicating pulmonary sarcoidosis: case series of seven patients. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases 2002;19(2):154‐9. [PubMed] [Google Scholar]
Martinez‐Gracia 2005
- Martinez‐Garcia MA, Perpina‐Tordera M, Roman‐Sanchez P, Soler‐Cataluna JJ. Quality‐of‐life determinants in patients with clinically stable bronchiectasis. Chest 2005;128(2):739‐45. [DOI] [PubMed] [Google Scholar]
Mochizuki 2002
- Mochizuki H, Ohki Y, Arakawa H, Kato M, Tokuyama K, Morikawa A. Effect of inhaled indomethacin on distilled water‐induced airway epithelial cell swelling. Journal of Applied Physiology 2002;92:155‐61. [DOI] [PubMed] [Google Scholar]
Muthalithas 2008
- Mutalithas K, Watkin G, Willig B, Wardlaw A, Pavord I, Birring SS. Improvement in health status following bronchopulmonary hygiene physical therapy in patients with bronchiectasis. Respiratory Medicine 2008;102(8):1140‐4. [DOI] [PubMed] [Google Scholar]
O'Brien 2000
- O'Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax 2000;55(8):635‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ong 2004
- Ong KC, Kor AC, Earnest A, Wang YT. Effects of inhaled furosemide on exertional dyspnea in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;169(9):1028‐33. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ries 2005
- Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and visual analog scale. COPD: Journal of Chronic Obstructive Pulmonary Disease 2005;2:105‐10. [DOI] [PubMed] [Google Scholar]
Sestini 1999
- Sestini P, Refini RM, Pieroni MG, Vaghi A, Robuschi M, Bianco S. Different effects of inhaled asprinlike drugs on allergy‐induced early and late asthmatic responses. American Journal of Respiratory and Critical Care Medicine 1999;159(4 pt 1):1228‐33. [DOI] [PubMed] [Google Scholar]


