Abstract
Purpose
To evaluate 3-year safety and efficacy of two second-generation trabecular micro-bypass stents. (iStent inject®) with phacoemulsification.
Materials and Methods
This multicenter retrospective study of iStent inject implantation with phacoemulsification included data from eight surgeons across Australia. Eyes with cataract and mild to advanced glaucoma [predominantly primary open-angle (POAG), primary angle closure (PAC), or normal-tension (NTG) glaucoma] or ocular hypertension (OHT) were included. Study assessments included intraocular pressure (IOP); number of ocular hypotensive medications; proportions of eyes with 0, 1, 2, or ≥3 IOP-lowering medications; IOP ≤15 mmHg or ≤18 mmHg; visual fields (VF); retinal nerve fiber layer thickness (RNFL); central corneal thickness (CCT); intraoperative complications; adverse events; and secondary surgeries.
Results
A total of 273 eyes underwent surgery and had 36-month follow-up. At 36 months versus preoperative, mean IOP decreased by 15.5% (16.4±4.6 mmHg to 13.9±3.5 mmHg; p<0.001), and 70.3% of eyes achieved IOP of ≤15 mmHg (versus 49.1% preoperatively; p<0.001). The mean medication burden decreased by 68.5% (from 1.51±1.17 to 0.48±0.89 medications; p<0.001); 71.4% of eyes were medication-free (versus 21.6% preoperatively; p<0.001), while 6.2% of eyes were on ≥3 medications (versus 22.3% preoperatively; p<0.001); 96.3% of eyes maintained or reduced medications vs preoperative. Significant IOP and medication reductions occurred across glaucoma subtypes (POAG, PAC, NTG, OHT): 13–22% for IOP (p<0.05 for all) and 42–94% for medication (p<0.05 for all). Favorable safety included few adverse events; stable VF, RNFL, and CCT; and filtering surgery in only 8 eyes (2.9%) over 3 years.
Conclusion
In this multicenter cohort from 8 surgeons across Australia, significant IOP and medication reductions were sustained through 3 years after iStent inject implantation with phacoemulsification. Results were favorable across different glaucoma subtypes (including POAG, PAC, NTG, OHT), severities, and surgeons, thereby underscoring the real-world relevance and efficacy of iStent inject implantation for glaucoma treatment.
Keywords: microinvasive glaucoma surgery, MIGS, glaucoma, iStent inject, intraocular pressure, second generation, multicenter
Introduction
Primary open-angle glaucoma (POAG) is the leading cause of irreversible blindness worldwide.1,2 A recent meta-analysis highlighted the importance of the disease: encompassing 50 publications and nearly 200,000 subjects, the study reported a worldwide prevalence of POAG in the adult general population of 2.4% over the last 20 years,3 with an estimated global population of POAG of approximately 69 million.
The primary goal of glaucoma treatment is to lower intraocular pressure (IOP) to a desired patient-specific goal, with the intent of stabilizing optic nerve/retinal nerve fiber layer (RNFL) status and visual fields.4 IOP lowering is achieved through a variety of interventions such as medical therapy, laser treatment such as selective laser trabeculoplasty (SLT), or incisional surgery.4 Minimally invasive glaucoma surgery, or MIGS, involves an ab interno surgical approach with minimal trauma to the surrounding ocular tissues.5 MIGS procedures have gained popularity in recent years and occupy a unique niche in the glaucoma treatment paradigm. In addition to reducing IOP, many MIGS options reduce patients’ dependence on ocular hypotensive medications, with a favorable safety profile.5
The iStent® trabecular micro-bypass stent (Glaukos Corporation, San Clemente, CA, USA) was the first MIGS device to gain approval by the US Food and Drug Administration (FDA).6,7 A recent study of data from the IRIS® registry by Yang et al8 showed that iStent procedures nearly tripled from 2013 to 2018, accounting for 43.7% of all US glaucoma surgeries (MIGS or otherwise) by 2017. Another study by Yang et al9 from over 200,000 eyes in the IRIS® registry from 2013 to 2018 found iStent was the most commonly performed MIGS procedure, with usage primarily in patients with normal tension glaucoma (NTG) or POAG. The iStent has been one of the most thoroughly studied of the MIGS devices, with outcomes through up to eight years in both standalone usage or combined with cataract surgery across a wide variety of glaucoma subtypes.10–35
More recently, a second-generation iStent device (iStent inject) has been developed, which comprises two conical-shaped heparin-coated titanium devices designed for ab interno injection directly into Schlemm’s canal through the trabecular meshwork.5 To-date, over 200 publications have provided an evidence base demonstrating favorable safety and effectiveness of both iStent and iStent inject through 8 years and 5 years, respectively.7,10–60
Two previous publications from our group reported 1-year and 2-year real-world multicenter results of iStent inject implantation with cataract surgery in 165 eyes (of 5 surgeons) and 340 eyes (of 9 surgeons), respectively.7,38 In both reports, iStent inject implantation with cataract surgery resulted in significant reductions in medications and IOP in eyes with various types and severities of glaucoma. Through two years of follow-up, a favorable safety profile was observed with no stent-related intraoperative complications; limited and transient postoperative adverse events; and stable cup-to-disk ratio, visual acuity (VA), and visual fields (VF). The current report examines 3-year effectiveness and safety of iStent inject implantation with cataract surgery in 273 eyes of 8 surgeons across Australia, including subgroup analyses of different glaucoma types.
Methods
Study Participants
This retrospective multicenter study involved pooled data from eight surgeons across Australia. Records were included from eyes receiving iStent® inject between January 2016 and February 2018. All patients signed informed consent forms, which permitted the retrospective evaluation of their de-identified clinical data. Data were collected in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Royal Australian and New Zealand College of Ophthalmology Human Research Ethics Committee.
Patients with the following glaucoma diagnoses were included, as these subtypes consistently have been shown to have favorable effectiveness and safety with trabecular microbypass implantation: POAG, pseudoexfoliative glaucoma (PXG), normal-tension glaucoma (NTG), pigmentary glaucoma (PG), combined-mechanism glaucoma (CMG), ocular hypertension/glaucoma suspect (OHT/GS), and primary angle closure (PAC).10–60 In addition, one case each of uveitic glaucoma, neovascular glaucoma, and angle recession glaucoma were included at the surgeons’ discretion. In accordance with clinical and regulatory guidelines, the enrolled eyes were eligible for cataract surgery and required additional glaucoma intervention due to inadequate IOP control, heavy ocular hypotensive medication burden, visual field progression, and/or nonadherence with topical medical therapy. Eyes were excluded from the study for having active ocular inflammation, significant ocular comorbidities confounding stent implantation, congenital glaucoma, or synechial angle closure. The two prior publications were each based on a consistent cohort (165 eyes from 5 surgeons at one year, 340 eyes from 9 surgeons at two years) with data at those respective time points. Given the different numbers of eyes and surgeons involved, the three cohorts (at one, two, and three years) are treated as distinct (albeit related) datasets.
Study Outcomes and Statistical Analysis
Effectiveness outcomes included mean IOP; percent IOP reductions; eyes with IOP ≤15 mmHg or ≤18 mmHg; number of ocular hypotensive medications; and proportional analyses of eyes on 0, 1, 2, or ≥3 medications. These outcomes were calculated for the overall cohort as well as for the four most prevalent glaucoma subgroups (POAG, PAC, OHT, NTG). Safety outcomes included visual field mean deviation (VF MD), retinal Nerve Fiber Layer thickness (RNFL by optical coherence tomography), central corneal thickness (CCT), intraoperative complications, postoperative adverse events, and secondary surgical interventions.
Pre- and postoperative data were summarized using descriptive statistics, including means (± standard deviation) and proportional analyses. Pre- and postoperative mean IOP and medication values were compared using paired t-tests. The proportions of eyes with IOP ≤15 mmHg or ≤18 mmHg, and the proportions of eyes on 0, 1, 2, or ≥3 medications, were compared using the McNemar test. Qualified and complete success rates were calculated for attaining IOP ≤15 mmHg or ≤18 mmHg either with or without medication, respectively. Since some patients achieved IOP ≤15 mmHg or ≤18 mmHg at both preoperative and postoperative visits, we completed a separate analysis of those eyes, with efficacy quantified by medication reduction. Results were considered statistically significant for p-values <0.05. Patients have been followed for 36 months, and follow-up continues.
Surgical Technique and Postoperative Care
The implantation technique has been detailed previously elsewhere.7,36,38 Under gonioscopic visualization, the injector was advanced ab internally through the phacoemulsification incision to the nasal angle. The stents were then implanted approximately two clock hours apart into two separate regions of the trabecular meshwork and Schlemm’s canal. This placement is designed to access up to six clock hours of collector channels for aqueous outflow through a single intraocular entry point.51 The viscoelastic was then removed, the eye was irrigated with balanced salt solution, and incision patency was confirmed. After surgery, patients were prescribed the surgeon’s standard postoperative medication regimen that typically included a topical antibiotic for 1–2 weeks and a topical steroid (eg prednisolone) tapered over 4 weeks.
Results
Demographic and Preoperative Characteristics
A total of 273 eyes underwent cataract surgery with iStent inject trabecular micro-bypass stent implantation and reached 36 months of follow-up by the time of data collection.
Preoperative demographic and ocular characteristics are shown in Table 1. The mean age of this cohort was 72.4 years. The most common diagnosis was POAG (67.0%). Approximately 30% of eyes (83/273) had a history of prior glaucoma procedure(s). Mean preoperative IOP was 16.4 mmHg with 75.8% of eyes having baseline IOP of ≤18 mmHg and 49.1% of eyes having baseline IOP of ≤15 mmHg. Patients were on an average of 1.51 medications before surgery, with similar proportions of eyes on 0 medications (n=59 or 21.6%) or ≥3 medications (n=61 or
Table 1.
Demographic and Preoperative Characteristics
| Demographics | n | % | |
|---|---|---|---|
| Total | 273 | 100 | |
| Age in years | Mean | SD | |
| 72.4 | 8.3 | ||
| Type of Glaucoma (n = 273 eyes) | POAG | 183 | 67.0 |
| PAC | 28 | 10.3 | |
| OHT/GS | 23 | 8.4 | |
| NTG | 17 | 6.2 | |
| PXG | 7 | 2.6 | |
| CMG | 6 | 2.2 | |
| PG | 6 | 2.2 | |
| Uveitic | 1 | 0.4 | |
| Neovascular | 1 | 0.4 | |
| Angle recession | 1 | 0.4 | |
| Eyes with prior glaucoma surgical or laser procedures | No prior surgery | 190 | 69.6% |
| Prior surgery/laser | 83 | 30.4% | |
| Preoperatve IOP | Mean | SD | |
| Mean ± SD | 16.4 | 4.6 | |
| Proportional analysis | n | % | |
| ≤15 mmHg | 134 | 49.1% | |
| ≤18 mmHg | 207 | 75.8% | |
| Preoperative medications | Mean | SD | |
| Mean ± SD | 1.51 | 1.17 | |
| Proportional analysis | n | % | |
| 0 medications | 59 | 21.6% | |
| 1 medication | 91 | 33.3% | |
| 2 medications | 62 | 22.7% | |
| 3 to 5 medications | 61 | 22.3% | |
Abbreviations: POAG, primary open-angle glaucoma; PAC, primary angle closure; OHT/GS, ocular hypertension/glaucoma suspect; NTG, normal-tension glaucoma; PXG, pseudoexfoliative glaucoma; CMG, combined-mechanism glaucoma; PG, pigmentary glaucoma; IOP, intraocular pressure; SD, standard deviation.
22.3%). In medication-free eyes at baseline, stent implantation was chosen in place of initiating topical treatment in patients with medication hypersensitivities or noncompliance, since stent implantation has been shown to be a viable alternative to medication through at least 5 years postoperative in newly-diagnosed glaucoma.12
IOP and Medications, Overall Cohort
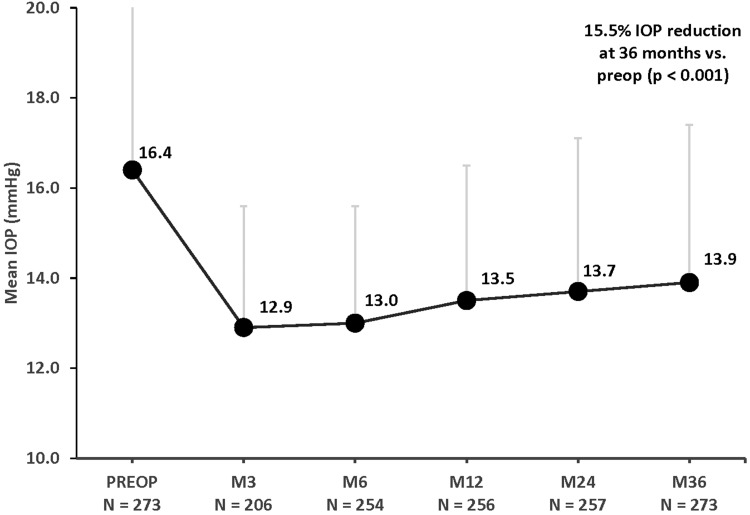
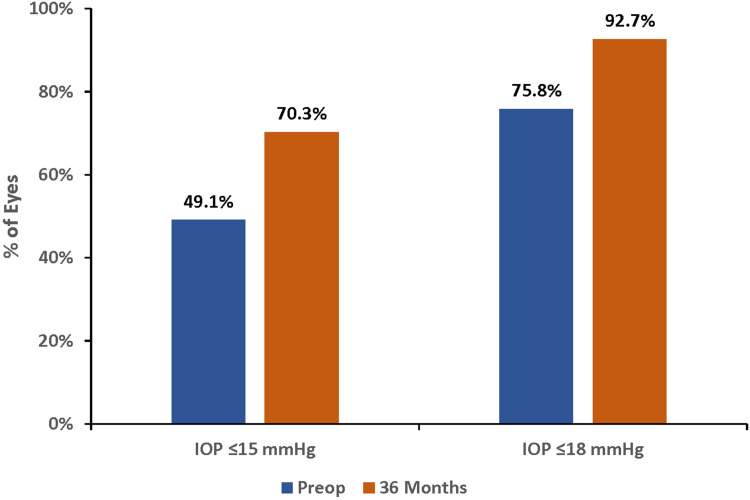
Following cataract surgery combined with iStent inject implantation, significant reductions in IOP were maintained for 3 years (p<0.001 at all time points). At 3 years, mean IOP decreased from 16.4 mmHg preoperatively to 13.9 mmHg, a 15.5% reduction (p<0.001) (Figure 1). The proportions of eyes with target IOP of ≤15 mmHg and ≤18 mmHg were substantially higher at 3 years compared with baseline. More than two-thirds of eyes (70.3%) had IOP of ≤15 mmHg at 3 years (vs 49.1% preoperatively), and 92.7% of eyes achieved IOP of ≤18 mmHg (vs 75.8% preoperatively) (Figure 2) (p<0.001 for both). Qualified success rates for attaining the ≤15 mmHg and ≤18 mmHg cutoffs were 70.0% and 92.3%, respectively. Complete success rates for attaining the 15 mmHg and ≤18 mmHg cutoffs without medication were 50.9% and 66.7%, respectively. In eyes that achieved IOP ≤15 mmHg or ≤18 mmHg at both the preoperative and postoperative visits (n=114 and n=202, respectively), effectiveness was additionally characterized by mean medication reduction: 64.9% and 71.8%, respectively.
Figure 1.
Mean IOP through 36 months postoperative. P < 0.001 for all postoperative time points vs preoperative IOP.
Abbreviations: IOP, intraocular pressure; M, month; Preop, preoperative. Vertical bars represent standard deviation.
Figure 2.
Proportional analysis of IOP at 36 months vs preoperative, all eyes (n=273). P<0.001 for both IOP cutoffs.
Abbreviations: IOP, intraocular pressure; Preop, preoperative.
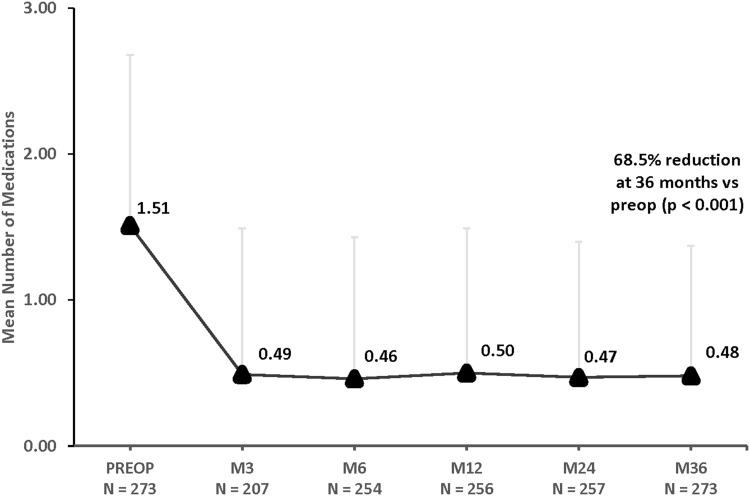
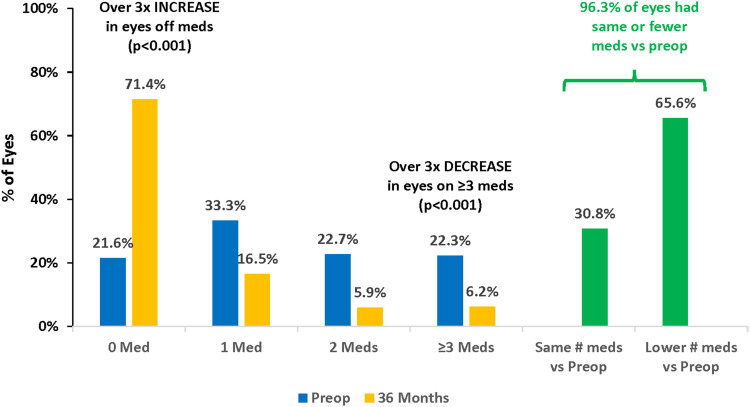
The changes in medication burden over 3 years are shown in Figures 3 and 4. Three years after iStent inject implantation with cataract surgery, the mean number of ocular hypotensive medications had decreased to 0.48 compared to 1.51 preoperatively, a 68.5% reduction (p<0.001). At 3 years, 71.4% of eyes needed no medications (versus 21.6% preoperatively) (p<0.001), while 6.2% of eyes required 3 medications (versus 22.3% preoperatively)(p<0.001). Furthermore, nearly all eyes (263/273 or 96.3%) maintained or decreased their medication burden compared with their preoperative regimen.
Figure 3.
Mean number of medications through 36 months postoperative, all eyes (n=273). p < 0.001 for all postoperative time points vs preoperative number of medications. Vertical bars represent standard deviation.
Abbreviations: M, month; Preop, preoperative.
Figure 4.
Proportional analysis of medication burden at 36 months vs preoperative, all eyes (n=273).
Abbreviations: Preop, preoperative; Meds, medications.
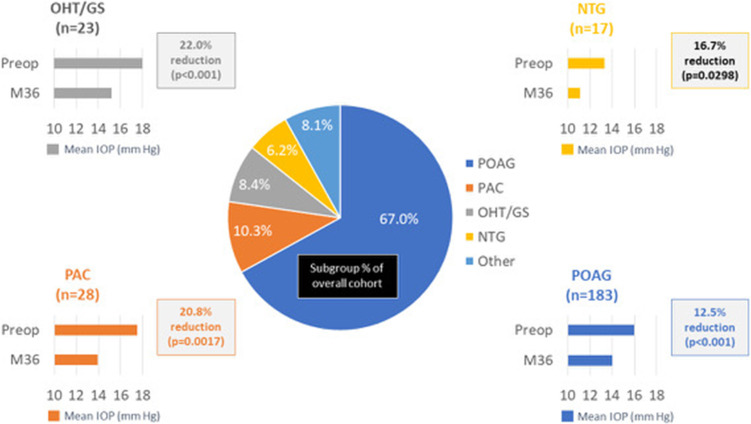
IOP and Medications, Subgroup Analysis
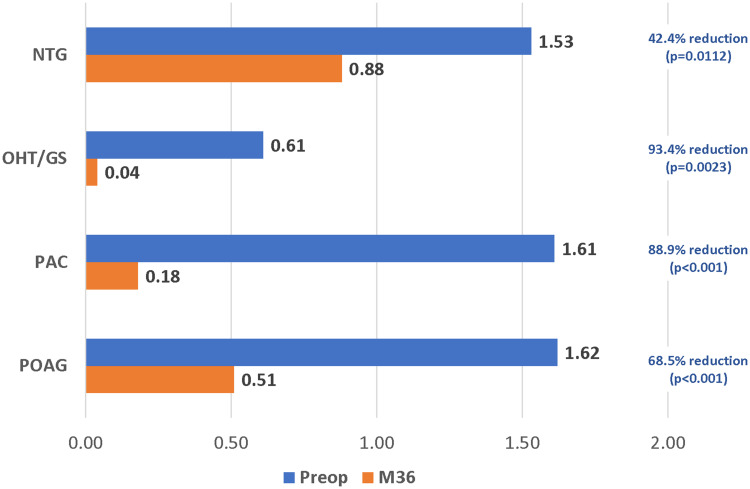
Results were stratified by glaucoma subtype for the 4 most prevalent diagnoses in the cohort (POAG n=183, PAC n=28, OHT/GS n=23, NTG n=17). Significant reductions in IOP and ocular hypotensive medications were achieved across all glaucoma subtypes. At 36 months compared with baseline, mean IOP decreased by 12.5% in POAG eyes (16.0 mmHg to 14.0 mmHg, p<0.001), 20.8% in PAC eyes (17.5 mmHg to 13.9 mmHg, p=0.0017), 22.0% in OHT/ GS eyes (19.5 mmHg to 15.2 mmHg, p<0.001), and 16.7% in NTG eyes (13.3 mmHg to 11.1 mmHg, p=0.0298) (Figure 5). At 36 months, medication reductions compared to baseline were 68.5% in POAG eyes (1.62 to 0.51, p<0.001), 88.9% in PAC eyes (1.61 to 0.18, p<0.001), 93.4% in OHT/GS eyes (p=0.0023), and 42.4% in NTG eyes (p=0.0112) (Figure 6).
Figure 5.
IOP reduction from preoperative, by glaucoma subtype (p<0.05 for all).
Abbreviations: IOP, intraocular pressure; POAG, primary open-angle glaucoma; PAC, primary angle closure; OHT/GS, ocular hypertension/glaucoma suspect; NTG, normal-tension glaucoma; M, month.
Figure 6.
Mean medication reduction by glaucoma subtype (p<0.05 for all).
Abbreviations: POAG, primary open-angle glaucoma; PAC, primary angle closure; OHT/GS, ocular hypertension/glaucoma suspect; NTG, normal-tension glaucoma; M, month.
Safety: General Parameters
Safety outcomes are detailed in Table 2. Two stents were successfully implanted in all but 2 eyes in this study (those 2 eyes each received 1 stent and experienced no associated sequelae). Over 3 years of follow-up, no adverse event occurred at a rate above 2%, a threshold often used in clinical studies involving MIGS.36,61 The adverse events that occurred were generally mild, transient, resolved with minimal to no intervention, and did not result in sequelae. Most events occurred within the immediate postoperative period; only 3 events occurred after 1 month postoperative (Table 2).
Table 2.
Adverse Events and Secondary Glaucoma Procedures
| Number of Eyes (%) | |
|---|---|
| Intraoperative adverse events | |
| Only 1 stent implanted | 2 (<1%) |
| Postoperative adverse events occurring after 1 month postoperative | |
| Stent-iris touch without blockage (noted at 3 months, transient, self-resolved without intervention or sequelae) | 1 (<1%) |
| Stent-iris touch with blockage (noted at 6 months, transient, self-resolved without intervention or sequelae) | 1 (<1%) |
| Peripheral anterior synechiae with stent obstruction (noted at 12 months; treated with Baerveldt tube implantation and SLT)* | 1 (<1%) |
| Secondary glaucoma procedures | |
| SLT | 12 (4.4%) |
| Filtering surgery | 5 (1.8%) |
| Both filtering surgery and SLT | 3 (1.1%) |
| Second iStent inject (standalone procedure) | 2 (<1%) |
| Non-filtering procedure (deep sclerectomy + mitomycin C) | 1 (<1%) |
Note: *This eye is also included in the “both filtering surgery and SLT” category of “Secondary glaucoma procedures” in the table.
Over the three years of follow-up, 20 eyes underwent secondary glaucoma procedures, most commonly SLT (12 eyes or 4.4%). Five eyes (1.8%) underwent a filtering surgery such as trabeculectomy or tube implantation, and 3 eyes (1.1%) underwent both SLT and a filtering surgery (Table 2). In these 8 eyes, there appeared to be no specific preoperative characteristic predisposing to ultimate filtering surgery. For example, the eyes had different diagnoses (5 POAG, 1 PXG, 1 PAC, 1 NVG); preoperative IOP ranging 13 to 28 mmHg (mean 20.4 mmHg); medication burden ranging from 0 to 3 glaucoma medications (mean 1.88 medications); and VF MD ranging from −1.79 to −28.59 dB (average −13.11 dB). Three of the 8 eyes had undergone prior SLT; no eyes had a history of prior filtering surgery. In 7 of the 8 eyes, the filtering surgery was unrelated to the iStent inject device, and was undertaken due to VF and/or optic nerve progression despite an otherwise favorable postoperative course: post-stent IOP had been controlled (between 8–20 mmHg) prior to filtering surgery; and post-stent IOP was reduced compared to patients’ preoperative IOP. However, given that IOP targets are individualized, even though the IOP may have been “normal”, it would have been too high for these specific patients. In the one remaining eye, post-stent IOP had ranged from 18 to 25 mmHg (vs 22 mmHg preoperatively), and peripheral anterior synechiae (PAS) were noted to obstruct the stents at 12 months postoperative; SLT and Baerveldt tube implantation were then completed.
Safety: Long-Term Disease Stability
From baseline to 3 years postoperative, there was no appreciable change in the average VF MD (from −5.09 dB to −5.14 dB); mean RNFL thickness (from 72.85 µm to 72.06 µm); average cup-to-disc ratio (from 0.703 to 0.715); nor mean central corneal thickness (from 530.6 µm to 529.6 µm) (Table 3).
Table 3.
Long-Term Disease Stability: Preoperative and Year 3 Safety Parameters, Eyes with Data at Both Time Points
| Preoperative | Year 3 | |
|---|---|---|
| Visual Field MD (db) | ||
| # eyes with data at both time points | 180 | 180 |
| Mean ± SD | −5.09 ± 5.62 | −5.14 ± 6.61 |
| p-value | 0.8584 | |
| Retinal Nerve Fiber Layer Thickness (µm) | ||
| # eyes with data at both time points | 106 | 106 |
| Mean ± SD | 72.85 ± 13.29 | 72.06 ± 16.43 |
| p-value | 0.4030 | |
| Central Corneal Thickness (µm) | ||
| # eyes with data at both time points | 44 | 44 |
| Mean ± SD | 530.6 ± 40.5 | 529.6 ± 39.4 |
| p-value | 0.6535 |
Abbreviations: VF, visual field; MD, mean deviation; SD, standard deviation.
Discussion
The present multicenter, multi-surgeon study is one of the largest and longest-term realworld cohorts yet reported on iStent inject implantation with cataract surgery, providing useful longitudinal data on the performance of this stent in an Asia-Pacific population in a routine clinical setting. The outcomes add to our previous results at 1 and 2 years and support the efficacy and safety of this treatment modality for various types of mild to advanced glaucoma and OHT. Both IOP and topical ocular hypotensive medication use decreased significantly from baseline, and were maintained consistently across time points through 3 years postoperative. Safety parameters such as visual fields, retinal nerve fiber layer thickness, and central corneal thickness remained stable, suggesting long-term preservation of structure and function.
Additionally, subgroup analyses showed similarly favorable effectiveness regardless of glaucoma subtype (including POAG, PAC, NTG, OHT/GS).
This study’s real-world patient population was heterogeneous in disease severity, preoperative medication burden, and glaucoma subtype, especially when compared to randomized controlled trials with pre-defined narrow inclusion/exclusion criteria. Therefore, the outcomes in this cohort may be more generalizable and relevant to current ophthalmologists in routine practice who manage diverse patient populations. In this study, patients ranged from those who had no previous glaucoma procedures or ocular hypotensive medications, to those who had undergone prior glaucoma surgery, had uncontrolled IOP, and/or required up to five IOP-lowering medications. Because of the range of disease burden, outcomes should be assessed in the context of a patient’s entire clinical situation, considering both IOP and medications as well as patients’ preoperative risk factors and disease severity. For example, in eyes with relatively well-controlled IOP on topical medications, a key goal for surgery may be to reduce the reliance on medications, while lowering or maintaining IOP. Conversely, in eyes with uncontrolled IOP despite numerous medications, a realistic goal for surgery may be to reduce IOP, while also reducing or maintaining the number of medications. In the current cohort, the vast majority (96.3%) of eyes maintained or decreased their medication burden at 3 years compared to their preoperative regimen.
The favorable outcomes from the present study corroborate those of prior studies of iStent inject with cataract surgery. For example, Hengerer et al49 conducted a 5-year prospective, nonrandomized, interventional case series in patients with mild to severe OAG who underwent iStent inject implantation either with or without cataract surgery. At 5 years postoperative, the mean IOP decreased to 14.1 mmHg (40% reduction vs 23.5 mmHg preoperatively, p<0.001), and mean medication burden decreased to 0.77 (71% reduction vs 2.68 medications preoperatively, p<0.001). The magnitude of the reductions was similar in both stent-cataract and stentstandalone cases, corroborating that the stents (rather than the phacoemulsification) were producing the change. In comparison, our study cohort had lower baseline IOP (16.4 mmHg) and included eyes with NTG, limiting the amount of postoperative reduction that can be achieved. Despite this, the present cohort achieved significant IOP and medication reductions, and maintained them consistently through 3 years. The reductions were significant across all glaucoma subtypes including in NTG eyes, which are well-known to be challenging in terms of IOP reduction.
Another notable long-term real-world case series was conducted by Salimi et al, who published 3-year outcomes of iStent inject with concomitant cataract surgery in 124 eyes with various glaucoma subtypes and severities.48 At 3 years versus baseline, the mean IOP decreased from 16.90 mmHg to 13.17 mmHg (a 22% reduction, p<0.001) and the mean topical ocular hypotensive medication burden decreased from 2.38 medications to 1.16 medications (a 51% reduction, p<0.001). Additionally, at 3 years, nearly all (96%) eyes achieved IOP ≤18 mmHg (vs 69% at baseline) and 80% of eyes achieved IOP ≤15 mmHg (vs 40% at baseline). Medication use was reduced by 1 medication in 76% of eyes and by 2 medications in 37% of eyes versus the preoperative medication burden. Following the combined procedure, BCVA improvement was preserved, and VF mean deviation and RNFL and GCIPL thickness remained stable Similar to the current study, significant and sustained IOP and medication reductions, with an excellent safety profile, were achieved through 3 years following iStent inject implantation with cataract surgery in a real-world clinical setting of patients with mild to severe glaucoma.
Fea et al50 conducted a randomized study to compare outcomes of patients with OAG not controlled on one medication who underwent either implantation of two iStent inject stents or received medical therapy with a fixed combination of latanoprost/timolol. Of the 192 patients enrolled, 94 underwent implantation of two iStent inject stents in the treated eye and 98 received ocular hypotensive medical therapy. At 1 year, an IOP ≤18 mmHg was reported in 92.6% of iStent inject eyes (87/94) and 89.8% of medical therapy eyes (88/98). The iStent inject and medical therapy groups reported mean IOP decreases from baseline of 8.1 mmHg and 7.3 mmHg, respectively. These data demonstrated that iStent inject implantation was at least as effective as a fixed combination of two ocular hypotensive medications, while not having the downsides of topical ocular hypotensive medication use.
Indeed, the reductions in medication use sustained over this 3-year study are particularly valuable. Medication therapy is known to be effective and safe, but its utility is often limited by
adherence issues.62,63 In a cross-sectional survey of 190 participants, Newman-Casey et al64 found that the most prevalent barriers to adherence were low self-efficacy, forgetfulness, and difficulty with both drop administration and complex medication schedules. Furthermore, treatment adherence tends to decrease when regimens increase from single to multiple topical eye drops.65 Topical drops also can carry caveats such as deleterious effects on the ocular surface (especially with chronic dosing of preservative-containing formulations), financial and logistic burdens for the patient and caregiver, and diminished patient quality of life.62,65–69 Local adverse effects of topical drops may include conjunctival hyperemia, corneal and conjunctival cell toxicity, loss of orbital fat (ie, prostaglandin-associated periorbitopathy) iris darkening, periocular skin pigmentation, and greater risk of surgical failure.70 Thus the reductions in IOP and medication use observed in the current study equate to tangible benefits for glaucoma patients, whose ultimate goal is to achieve target IOP with the fewest medications and minimum adverse effects.70
As with any clinical study, this retrospective real-world study carried some limitations. In this patient cohort, a medication washout was not performed, as this was not part of routine clinical practice. Medications were prescribed according to the routine clinical practice of each surgeon rather than being dictated by a formal protocol. However, medication recommendations were generally observed to be consistent across the eight surgeons. In addition, any differences probably had a little impact, since the same effectiveness outcomes were evaluated in the same patients by the same clinicians throughout follow-up, so changes over time should have been detected. Diurnal IOP measurements were not taken and thus regression to the mean could have occurred. However, this was likely to have had minimal to no impact due to the robust sample size of the study. There was no phacoemulsification control group, and thus the effects of the stents compared with cataract surgery could not be separated. However, patients could be considered their own controls, as is commonly done in retrospective real-world case series. Additionally, numerous other comparative trials of iStent-phaco or iStent inject-phaco versus phacoemulsification alone10,11,16,21,32,36 have established that iStent-phaco has greater efficacy for IOP lowering and medication reduction than phacoemulsification alone. Furthermore, the IOP-lowering effect of cataract surgery would not be expected to persist to the same degree over 3 years following surgery.71
Conclusions
This study represents one of the largest long-term multicenter real-world datasets amassed to-date of iStent inject with cataract surgery. This report provides 3-year safety and efficacy results for patients with a variety of glaucoma subtypes and severities, including those that are less often addressed in MIGS research studies (such as NTG and PAC). Subgroup analysis of the 4 most prevalent diagnoses in the cohort (POAG, PAC, OHT/GS, NTG) revealed clinically and statistically significant IOP and medication reductions (13–22% for IOP and 42–94% for medication).
Outcomes were captured in patients within real-world settings, and thus may be more directly relevant to practicing clinicians. The data showed that iStent inject plus phacoemulsification provides safe, significant, and sustained reductions in IOP and topical ocular hypotensive medication burden through 3 years following surgery. The majority of eyes achieved both qualified and complete success in attaining IOP ≤15 mmHg and ≤18 mmHg at 3 years postoperatively. These beneficial effects were seen across a wide variety of glaucoma subtypes, supporting the long-term durability and versatility of this treatment regimen for managing glaucoma.
Acknowledgments
No financial support was provided for the work in this study. The authors would like to thank Julie Crider, PhD, and Dana Hornbeak, MD, MPH, for medical writing and editing contributions.
Editorial assistance and publication processing charges were provided by Glaukos Corporation.
Abbreviations
POAG, primary open-angle glaucoma; PAC, primary angle closure; OHT, ocular hypertension; GS, glaucoma suspect; NTG, normal-tension glaucoma; PXG, pseudoexfoliative glaucoma; CMG, combined-mechanism glaucoma; PG, pigmentary glaucoma; IOP, intraocular pressure; SD, standard deviation; MD, mean deviation; VF, visual field.
Disclosure
CC reports personal fees from Allergan, Glaukos, Alcon, Merck, Sharp, Dohme. FH reports personal fees from Glaukos. ASI is a consultant for Glaukos. DM received personal fees from Glaukos, Allergan, and Alcon including being a consultant for Alcon. JL reports research grants from Sydney Eye Hospital Foundation, Glaukos, and Zeiss. SS reports honoraria as a consultant from Glaukos and Allergan. TG reports personal fees from and serves in the advisory board for Glaukos. The authors report no other conflicts of interest in this work.
References
- 1.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S. Glaucoma. Lancet. 2017;390(10108):2183–2193. doi: 10.1016/S0140-6736(17)31469-1 [DOI] [PubMed] [Google Scholar]
- 3.Zhang N, Wang J, Li Y, Jiang B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Sci Rep. 2021;11(1):13762. doi: 10.1038/s41598-021-92971-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The American Academy of Ophthalmology. Primary open-angle glaucoma preferred practice pattern. San Francisco, CA; 2020. [Google Scholar]
- 5.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. doi: 10.1097/ICU.0b013e32834ff1e7 [DOI] [PubMed] [Google Scholar]
- 6.Glaukos® Corporation iStent inject® Trabecular Micro-Bypass System. [Directions for use]. San Clemente, CA; Glaukos Corp; 2020. [Google Scholar]
- 7.Clement C, Howes F, Ioannidis AS, et al. Two-year multicenter outcomes of iStent inject trabecular micro-bypass stents combined with phacoemulsification in various types of glaucoma and ocular hypertension. Clin Ophthalmol. 2020;14:3507–3517. doi: 10.2147/OPTH.S271646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang SA, Mitchell W, Hall N, et al. Trends and usage patterns of minimally invasive glaucoma surgery in the United States: IRIS(R) Registry analysis 2013–2018. Ophthalmol Glaucoma; 2021;4(6):558–568. [DOI] [PubMed] [Google Scholar]
- 9.Yang SA, Mitchell WG, Hall N, et al. Usage patterns of Minimally Invasive Glaucoma Surgery (MIGS) differ by glaucoma type: IRIS registry analysis 2013–2018. Ophthalmic Epidemiol. 2021:2021:1–9. [DOI] [PubMed] [Google Scholar]
- 10.Craven ER, Katz LJ, Wells JM, Giamporcaro JE; iStent Study G. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–1345. doi: 10.1016/j.jcrs.2012.03.025 [DOI] [PubMed] [Google Scholar]
- 11.El Wardani M, Bergin C, Achache F, Sharkawi E. Evaluating the trabecular micro-bypass stent combined with phacoemulsification compared to phacoemulsification alone. Klin Monbl Augenheilkd. 2015;232(4):442–445. doi: 10.1055/s-0035-1545798 [DOI] [PubMed] [Google Scholar]
- 12.Fechtner RD, Voskanyan L, Vold SD, et al. Five-year, prospective, randomized, multi-surgeon trial of two trabecular bypass stents versus prostaglandin for newly diagnosed open-angle glaucoma. Ophthalmol Glaucoma. 2019;2(3):156–166. doi: 10.1016/j.ogla.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 13.Ferguson T, Swan R, Ibach M, Schweitzer J, Sudhagoni R, Berdahl JP. Evaluation of a trabecular microbypass stent with cataract extraction in severe primary open-angle glaucoma. J Glaucoma. 2018;27(1):71–76. doi: 10.1097/IJG.0000000000000825 [DOI] [PubMed] [Google Scholar]
- 14.Ferguson TJ, Swan RJ, Bleeker A, et al. Trabecular microbypass stent implantation in pseudoexfoliative glaucoma: long-term results. J Cataract Refract Surg. 2020;46(9):1284–1289. doi: 10.1097/j.jcrs.0000000000000243 [DOI] [PubMed] [Google Scholar]
- 15.Ferguson TJ, Ibach M, Schweitzer J, Karpuk KL, Stephens JD, Berdahl JP. Trabecular micro-bypass stent implantation with cataract extraction in pigmentary glaucoma. Clin Exp Ophthalmol. 2020;48(1):37–43. doi: 10.1111/ceo.13638 [DOI] [PubMed] [Google Scholar]
- 16.Scott RA, Ferguson TJ, Stephens JD, Berdahl JP. Refractive outcomes after trabecular microbypass stent with cataract extraction in open-angle glaucoma. Clin Ophthalmol. 2019;13:1331–1340. doi: 10.2147/OPTH.S206619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al Habash A, Khan O. Outcomes of combined iStent trabecular micro-bypass and cataract surgery for the treatment of open-angle glaucoma in a Saudi population. Clin Ophthalmol. 2020;14:1573–1580. doi: 10.2147/OPTH.S249261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular microbypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911–1917. doi: 10.1016/j.jcrs.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 19.Berdahl JP, Dockter Z, Schweitzer J, Ibach M, Ferguson T Long-term evaluation of a trabecular microbypass stent with cataract surgery in open-angle glaucoma: 8-year results. Presentation at the Annual Meeting of the American Society of Cataract and Refractive Surgery (ASCRS); July 23–27, 2021; Las Vegas, NV. [Google Scholar]
- 20.Chang DF, Donnenfeld ED, Katz LJ, et al. Efficacy of two trabecular micro-bypass stents combined with topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 3-year follow-up. Clin Ophthalmol. 2017;11:523–528. doi: 10.2147/OPTH.S121041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen DZ, Sng CCA, Sangtam T, et al. Phacoemulsification vs phacoemulsification with micro-bypass stent implantation in primary angle closure and primary angle closure glaucoma: a randomized single-masked clinical study. Clin Exp Ophthalmol. 2020;48(4):450–461. doi: 10.1111/ceo.13721 [DOI] [PubMed] [Google Scholar]
- 22.Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary openangle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol. 2015;2015:795357. doi: 10.1155/2015/795357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallardo MJ, Supnet RA. Three-year outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic population with primary open-angle glaucoma. Clin Ophthalmol. 2019;13:869–879. doi: 10.2147/OPTH.S189071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuhann TH, Hornbeak DM, Neuhann RT, Giamporcaro JE. Long-term effectiveness and safety of trabecular microbypass stent implantation with cataract surgery in patients with glaucoma or ocular hypertension: five-year outcomes. J Cataract Refract Surg. 2019;45(3):312–320. doi: 10.1016/j.jcrs.2018.10.029 [DOI] [PubMed] [Google Scholar]
- 25.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE; Group USiS. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi: 10.1016/j.ophtha.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 26.Ansari E. 5-year outcomes of single iStent (G1) trabecular microbypass implantation with phacoemulsification in moderately advanced primary open angle glaucoma. PLoS One. 2021;16(9):e0257015. doi: 10.1371/journal.pone.0257015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bargoud AR, Lira J, An S, Walsman SM, Herndon LW, Khouri AS. Trabecular microbypass stent and phacoemulsification in african american patients with openangle glaucoma: outcomes and effect of prior laser trabeculoplasty. J Glaucoma. 2021;30(1):89–93. doi: 10.1097/IJG.0000000000001692 [DOI] [PubMed] [Google Scholar]
- 28.Kozera M, Konopinska J, Mariak Z, Rekas M. Effectiveness of iStent trabecular microbypass system combined with phacoemulsification versus phacoemulsification alone in patients with glaucoma and cataract depending on the initial intraocular pressure. Ophthalmic Res. 2021;64(2):327–336. doi: 10.1159/000511456 [DOI] [PubMed] [Google Scholar]
- 29.Kozera M, Konopinska J, Mariak Z, Rekas M. Treatment of open-angle glaucoma with iStent implantation combined with phacoemulsification in Polish Caucasian population. Clin Ophthalmol. 2021;15:473–480. doi: 10.2147/OPTH.S293637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozera M, Konopinska J, Rekas M. Mid-term evaluation of the safety and efficacy of the iStent trabecular micro-bypass system combined with phacoemulsification. Adv Clin Exp Med. 2021;30(1):49–54. doi: 10.17219/acem/129576 [DOI] [PubMed] [Google Scholar]
- 31.Paletta Guedes RA, Gravina DM, Paletta Guedes VM, Chaoubah A. Two-year comparative outcomes of first- and second-generation trabecular micro-bypass Stents with cataract surgery. Clin Ophthalmol. 2021;15:1861–1873. doi: 10.2147/OPTH.S302684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salimi A, Abu-Nada M, Harasymowycz P. Matched cohort study of cataract surgery with and without trabecular microbypass Stent implantation in primary angle-closure glaucoma. Am J Ophthalmol. 2021;224:310–320. doi: 10.1016/j.ajo.2020.12.032 [DOI] [PubMed] [Google Scholar]
- 33.Shalaby WS, Jia J, Katz LJ, Lee D. iStent inject: comprehensive review. J Cataract Refract Surg. 2021;47(3):385–399. doi: 10.1097/j.jcrs.0000000000000325 [DOI] [PubMed] [Google Scholar]
- 34.Shalaby WS, Lam SS, Arbabi A, et al. iStent versus iStent inject implantation combined with phacoemulsification in open angle glaucoma. Indian J Ophthalmol. 2021;69(9):2488–2495. doi: 10.4103/ijo.IJO_308_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziaei H, Au L. Manchester iStent study: long-term 7-year outcomes. Eye. 2021;35(8):2277–2282. doi: 10.1038/s41433-020-01255-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuelson TW, Sarkisian SR Jr., Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811–821. doi: 10.1016/j.ophtha.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 37.Berdahl J, Voskanyan L, Myers JS, Katz LJ, Samuelson TW. iStent inject trabecular micro-bypass stents with topical prostaglandin as standalone treatment for open-angle glaucoma: 4-year outcomes. Clin Exp Ophthalmol. 2020;48(6):767–774. doi: 10.1111/ceo.13763 [DOI] [PubMed] [Google Scholar]
- 38.Clement CI, Howes F, Ioannidis AS, Shiu M, Manning D. One-year outcomes following implantation of second-generation trabecular micro-bypass stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: multicenter, multisurgeon study. Clin Ophthalmol. 2019;13:491–499. doi: 10.2147/OPTH.S187272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson TJ, Dockter Z, Bleeker A, et al. iStent inject trabecular microbypass stent implantation with cataract extraction in open-angle glaucoma: early clinical experience. Eye Vis. 2020;7(1):28. doi: 10.1186/s40662-020-00194-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guedes RAP, Gravina DM, Lake JC, Guedes VMP, Chaoubah A. Intermediate results of iStent or iStent inject implantation combined with cataract surgery in a real-world setting: a longitudinal retrospective study. Ophthalmol Ther. 2019;8(1):87–100. doi: 10.1007/s40123-019-0166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hengerer FH. Four-year real-world outcomes of iStent Inject® Trabecular microbypass stents. E-Poster Discussion at the Annual Meeting of the American Academy of Ophthalmology (AAO); October 13, 2019; San Francisco, CA. [Google Scholar]
- 42.Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Prospective, non-randomized, 36-month study of second-generation trabecular micro-bypass stents with phacoemulsification in eyes with various types of glaucoma. Ophthalmol Ther. 2018;7(2):405–415. doi: 10.1007/s40123-018-0152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Second-generation trabecular micro-bypass Stents as standalone treatment for glaucoma: a 36-month prospective study. Adv Ther. 2019;36(7):1606–1617. doi: 10.1007/s12325-019-00984-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manning D. Real-world case series of iStent or iStent inject trabecular micro-bypassstents combined with cataract surgery. Ophthalmol Ther. 2019;8(4):549–561. doi: 10.1007/s40123-019-00208-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuhann R, Neuhann T. Second-generation trabecular micro-bypass stent implantation: retrospective analysis after 12- and 24-month follow-up. Eye Vis. 2020;7(1):1. doi: 10.1186/s40662-019-0169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salimi A, Clement C, Shiu M, Harasymowycz P. Second-generation trabecular micro- bypass (iStent inject) with cataract surgery in eyes with normal-tension glaucoma: one-year outcomes of a multi-centre study. Ophthalmol Ther. 2020;9(3):585–596. doi: 10.1007/s40123-020-00266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salimi A, Lapointe J, Harasymowycz P. One-year outcomes of second-generation trabecular micro-bypass Stents (iStent Inject) Implantation with cataract surgery in different glaucoma subtypes and severities. Ophthalmol Ther. 2019;8(4):563–575. doi: 10.1007/s40123-019-00214-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salimi A, Watt H, Harasymowycz P. Three-Year outcomes of second-generation trabecular micro-bypass Stents (iStent inject) With phacoemulsification in various glaucoma subtypes and severities. J Glaucoma. 2021;30(3):266–275. doi: 10.1097/IJG.0000000000001716 [DOI] [PubMed] [Google Scholar]
- 49.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Morales-Fernandez L, Fernandez-Perez C, Garcia-Feijoo J. Glaukos iStent inject® trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1056573. doi: 10.1155/2016/1056573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject ((R)) versus two ocular hypotensive agents in patients with primary openangle glaucoma. Clin Ophthalmol. 2014;8:875–882. doi: 10.2147/OPTH.S59932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang AS, Penteado RC, Papoyan V, Voskanyan L, Weinreb RN. Aqueous angiographic outflow improvement after trabecular microbypass in glaucoma patients. Ophthalmol Glaucoma. 2019;2(1):11–21. doi: 10.1016/j.ogla.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klamann MK, Gonnermann J, Pahlitzsch M, et al. iStent inject in phakic open angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253(6):941–947. doi: 10.1007/s00417-015-3014-2 [DOI] [PubMed] [Google Scholar]
- 53.Lindstrom R, Sarkisian SR, Lewis R, Hovanesian J, Voskanyan L. Four-year outcomes of two second-generation trabecular micro-bypass stents in patients with open-angle glaucoma on one medication. Clin Ophthalmol. 2020;14:71–80. doi: 10.2147/OPTH.S235293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voskanyan L, Garcia-Feijoo J, Belda JI, et al. Prospective, unmasked evaluation of the iStent(R) inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014;31(2):189–201. doi: 10.1007/s12325-014-0095-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al Habash A, Otaif W. Surgical outcomes of combined 2nd-generation trabecular microbypass (iStent Inject) and cataract surgery for the treatment of primary open-angle glaucoma in the Saudi population. Ophthalmol Ther. 2021;10(4):923–933. doi: 10.1007/s40123-021-00380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu YJ, Ang CB, Tecson IOC, Kan Tsia-Chuen J, Yip WL. Combined phacoemulsification and iStent inject implantation in Asian eyes. Eur J Ophthalmol. 2021;32:288–295. [DOI] [PubMed] [Google Scholar]
- 57.Paletta Guedes RA, Gravina DM, Paletta Guedes VM, Chaoubah A. iStent inject (Second-generation Trabecular Microbypass) versus nonpenetrating deep Sclerectomy in association with phacoemulsification for the surgical treatment of open-angle glaucoma and cataracts: 1-year results. J Glaucoma. 2020;29(10):905–911. doi: 10.1097/IJG.0000000000001576 [DOI] [PubMed] [Google Scholar]
- 58.Singh IP, Sarkisian S, Hornbeak D, Katz LJ, Samuelson T; iStent inject Study G. Treatment success across different levels of preoperative disease burden: stratified two-year outcomes from the pivotal trial of iStent inject ((R)) Trabecular micro-bypass in primary open-angle glaucoma and cataract. Clin Ophthalmol. 2021;15:3231–3240. doi: 10.2147/OPTH.S316270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hengerer FH, Auffarth GU, Conrad-Hengerer I. iStent inject trabecular micro-bypass with or without cataract surgery yields sustained 5-year glaucoma control. Adv Ther. 2021;39(3):1417–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Businesswire.com Press Release August 23, 2021. Glaukos announces market-leading clinical milestone of 200 peer-reviewed publications on iStent Technologies. Available from: https://www.businesswire.com/news/home/20210823005049/en/Glaukos-Announces-Market-Leading-Clinical-Milestone-of-200-Peer-Reviewed-Publications-on-iStent%C2%AE-Technologies. Accessed August 26, 2022.
- 61.Samuelson TW, Chang DF, Marquis R, et al. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126(1):29–37. doi: 10.1016/j.ophtha.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 62.Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116(11 Suppl):S30–36. doi: 10.1016/j.ophtha.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 63.Tsai JC, McClure CA, Ramos SE, Schlundt DG, Pichert JW. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12(5):393–398. doi: 10.1097/00061198-200310000-00001 [DOI] [PubMed] [Google Scholar]
- 64.Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316. doi: 10.1016/j.ophtha.2015.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533–540. doi: 10.1016/j.ajo.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 66.Baudouin C, Liang H, Hamard P, et al. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and Thelper 2 pathways. Ophthalmology. 2008;115(1):109–115. doi: 10.1016/j.ophtha.2007.01.036 [DOI] [PubMed] [Google Scholar]
- 67.Samuelson TW, Singh IP, Williamson BK, et al. Quality of Life in Primary Open-Angle Glaucoma and Cataract: an Analysis of VFQ-25 and OSDI from the iStent inject® Pivotal Trial. Am J Ophthalmol. 2021;229:220–229. doi: 10.1016/j.ajo.2021.03.007 [DOI] [PubMed] [Google Scholar]
- 68.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f [DOI] [PubMed] [Google Scholar]
- 69.Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606. doi: 10.1016/j.ajo.2005.04.051 [DOI] [PubMed] [Google Scholar]
- 70.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vizzeri G, Weinreb RN. Cataract surgery and glaucoma. Curr Opin Ophthalmol. 2010;21(1):20–24. doi: 10.1097/ICU.0b013e328332f562 [DOI] [PubMed] [Google Scholar]