Summary
This study identified helminthic species among wild boars (Sus scrofa) in Primorsky Krai, Russia. In total, 66 fecal samples were taken from wild boars and examined using the floatation-sedimentation method to identify helminths eggs and protozoan cysts. Age and sex were estimated for each host animal investigated. The helminthic fauna of the wild boars examined involved six parasite genera, but 4 are helminths and 2 are protozoans: the nematodes Metastrongylus spp., Trichuris suis, Capillaria sp. and Ascaris suum; and the protozoan parasites Eimeria sp. and Cystoisospora suis.
The most prevalent parasite was Metastrongylus spp. (13.6 %) followed by Trichuris suis (7.6 %).
The other parasites found were Eimeria sp. (3 %), Ascaris suum (3 %), Capillaria sp. (1.5 %) and Cystoisospora suis (1.5 %).
Not found positive correlation between the host’s age and sex and the parasite prevalence. This was the first detailed study on helminths infections among wild boars in Primorsky Krai.
Keywords: helminths, protozoans, feces, prevalence, wild boars
Introduction
Wild boars (Sus scrofa) have worldwide distribution and are commonly encountered in Primorsky Krai (Primorye region), Russia. This area is located in the extreme southeastern part of Russia and have border with North Korea and the Heilongjiang province of China. Wild boars are living in the forests of southern, south-western, northern and northeastern parts of the Primorye region in dense populations (Ramayo et al., 2011). They form part of the diet of rare carnivores living in this region, such as the Amur tiger (Panthera tigris altaica) and Far Eastern leopard (Panthera pardus orientalis).
Wild boars have also an important role in the epizootiology and circulation of some major infections (African Swine Fever; Classical
Swine Fever) and some zoonotic parasites. They can also transmit zoonotic helminths and protozoa to humans living in rural communities where wild boars roam freely around the villages. There is a lack of data regarding helminths in the gastrointestinal and hepatic systems of wild boar populations in Primorsky Krai. We focused our work on this area of research through by microscopic fecal examination.
Materials and Methods
Between December 2017 and January 2018, fecal samples were taken from 66 wild boars in Primorsky Krai. Totally investigated of 34 males and 32 females. These samples were collected from 10 areas, in hunting areas including Anuchinskii, Mihailovskii, Ussuriiskii, Spasskii, Partizanskii, Pogranichnii, Yakovlevskii, Krasnoarmeiskii, Pozharskii and Chuguevskii districts. Each sample was placed in plastic jar that labeled with records of the animal’s age and sex. The age of each boar has been determined through examination of the teeth, as described by Sáez-Royuela et al. (1989) (Table 1, Fig. 1).
Table 1.
Wild boars investigated.
| Host parameter | Totally examined | Infected | Prevalence, % |
|---|---|---|---|
| Sex | |||
|
| |||
| Female | 32 | 10 | 31.3 |
| Male | 34 | 10 | 29.4 |
| Total | 66 | 20 | 30.3 |
|
| |||
| Age | |||
|
| |||
| Piglets | 23 | 5 | 21.7 |
| 1-2 years of ages | 13 | 7 | 53.8 |
| 2-3 years of ages | 7 | 2 | 28.6 |
| 3-4 years of ages | 21 | 6 | 28.6 |
| Elder than 4 years of ages | 2 | 0 | - |
|
| |||
| Year | |||
|
| |||
| 2017 | 38 | 9 | 23.7 |
| 2018 | 28 | 11 | 39.3 |
Fig. 1.
Map of research area (Primorye region).
Fecal examination
All the fecal samples were examined through light microscope for the presence of helminths eggs, proglottids and protozoan cysts. All of them placed to the Far Eastern Bank of Biological Materials (Shchelkanov et al., 2017). Before examination, the samples were stored at 6 °C during for 2 – 3 days. Each sample examined by means of the flotation technique using commercially available zinc sulfate solution (specific gravity, SG 1.18 – 1.2). The quantity of 3 – 5 gr of the sample was placed in a 10 mL glass jar with a saturated zinc sulfate solution and then mixed thoroughly using glass rod and stored for 10 – 15 min. Helminths eggs and protozoa cyst, that came to the surface, were collected using a copper loop and placed on a slide with some drops of glycerol. The classical sedimentation method used for detection of Trematoda eggs (Becker et al., 2016).
Statistical analysis
The data analyzed using Stata/MP 14.1. Correlations between infection and wild boars’ age and sex were determine using Fisher’s exact test. Bivariate logistic regression was used to assess associations between infection and the animals’ characteristics. Odds ratios (OR) and 95 % confidence intervals (CI) were calculated and p < 0.05 was considered to be statistically significant.
Ethical Approval and/or Informed Consent
This research relating to animals complied with all the relevant national regulations and institutional policies for the care and use of animals (Federal Act entitled “The animal world” (1995); Far Eastern Federal University ethical statement).
This study received approval from a research committee at the Far Eastern Federal University, under protocol number 612-004, dated December 10, 2017.
Results
Intestinal parasites were found in 20 (30.3 %) of the 66 wild boars studied. In total six parasite genera/species were found. The most parasites belongs to Nematoda (66.6 %) and some of them belongs to Protozoa (33.3 %). The most prevalent parasites were Metastrongylus spp. (13.6 %) follow by Trichuris suis (7.6 %). The other parasites found were Eimeria sp. (3 %), Ascaris suum (3 %), Capillaria sp. (1.5 %) and Cystoisospora suis (1.5 %).
Infection was present in 31.3 % of the females and 29.4 % of the males. In total six parasite species were found in the females. The most prevalent parasites in females were Metastrongylus spp. (9.4 %), followed by Sarcocystis sp. and Ascaris suum (6.3 %). The other species found had low prevalence (Table 2). In the males, the most prevalent parasites were Metastrongylus spp. (17.6 %) and Trichuris suis (11.7 %) (Table 2).
Table 2.
Results of parasitological examination.
| Species | Metastrongylus spp. | Sarcocystis sp. | Cystoisospora suis | Trichuris suis | Ascaris suum | Capillaria sp. |
|---|---|---|---|---|---|---|
| Sex | ||||||
|
| ||||||
| Female | 3(15.0%) | 2(10.0%) | 1(5.0%) | 1(5.0%) | 2(10.0%) | 1(5.0%) |
| Male | 6(30.0%) | 4 (20.0%) | ||||
|
| ||||||
| Age | ||||||
|
| ||||||
| Piglets | 4 (20.0%) | 1(5.0%) | ||||
| 1-2 years of ages | 2(10.0%) | 2(10.0%) | 1(5.0%) | 1(5.0%) | 1(5.0%) | |
| 2-3 years of ages | 1(5.0%) | 1(5.0%) | ||||
| 3-4 years of ages | 2(10.0%) | 2(10.0%) | 1 (5.0%) | 1 (5.0%) | ||
| Elder than 4 years of ages | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Year of research | ||||||
|
| ||||||
| 2017 | 7(35.0%) | 2(10.0%) | ||||
| 2018 | 2(10.0%) | 2(10.0%) | 1(5.0%) | 3(15.0%) | 2(10.0%) | 1(5.0%) |
The highest parasite prevalence was among wild boars aged 1 – 2 years (53.8 %), followed by those aged 2 – 3 years (28.6 %) and 3 – 4 years (28.6 %), then piglets (21.7 %). Parasite infections were not found in boars older than 4 years.
Seven out of ten of the areas investigated were positive for infected wild boars. The highest parasite prevalences appeared in Anuchinskii (80 %), Pogranichnii (62.5 %) Spasskii (55.5 %) and Chuguevskii (50 %) districts. Metastrongylus spp. was found in five areas: Anuchinskii, Partizanskii, Pogranichnii, Spasskii and Ussuriiskii. Another frequently found parasite was Trichuris suis, which was recorded in four areas: Anuchinskii, Spasskii, Chuguevskii and Yakovlevskii, among which the highest prevalence was in Spasskii district (33.3 %) (Fig. 2).
Fig. 2.
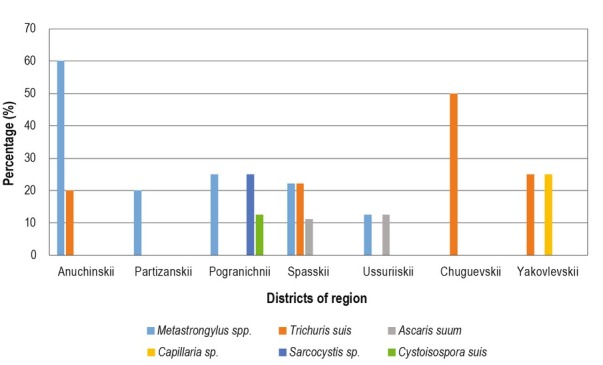
The prevalence of wild boar intestinal parasites in Primorye region.
Higher parasite prevalences were recorded in 2018 (Table 2). A positive correlation found between parasite prevalence and sex (p < 0.05). The prevalence was higher in males.
No found correlations between prevalence and other parameters (age and area investigated).
Discussion
The aim of the present study was to investigate wild boar parasites in the Primorye region. There are few researches about wild boar parasites in Russia. Our study was the first to highlight the prevalence of parasites in wild boars in Primorsky Krai.
The six genera of parasites found, included four genera/species of nematodes and twor protozoan genera/species. Some have zoonotic potential such as Capillaria sp. and Ascaris suum. Significant zoonotic endoparasites of wild boar, such as cysticercosis, trichinosis and cestodoses, which were not studied in this work.
Previously found the lungworm Metastrongylus spp. is the most prevalent parasite of wild boars in Europe (Castagna et al., 2018, García-González et al., 2013; Senlik et al., 2013; Panayotova-Pencheva et al., 2018). These results can be explained by the wide geographical distribution of earthworm species, which act as intermediate hosts for Metastrongylus spp. and form part of wild boars’ diet (Antipov et al., 2018).
The species Ascaris suum and Trichuris suis were previously recorded in wild boars in Poland (Popiolek et al., 2010), Spain (Gasso et al., 2015), Brazil (da Silva et al., 2013) and Finland (Hälli et al., 2010). Capillaria spp. was previously recorded in wild boars in Slovakia (Kanka et al., 2017) and Tunisia (Lahmar et al., 2009).
Although fecal examination is a simple method, it is also less effective than parasitological autopsy. Some studies based on examination of fecal samples from wild boars have been conducted (Moretta et al., 2010).
In Bulgaria, eleven parasite genera were found based on fecal examination. Metastrongylus sp. was the most prevalent species (28.8 %) (Panayotova-Pencheva et al., 2018). The most prevalent species in wild boars in Italy, based on coproscopic examination, was Ascaris suum (Castagna et al., 2019). In the research that conducted in Umbria, central Italy, the most prevalent parasite was also Metastrongylus spp. (Castro et al., 2018).
Eimeria spp. are frequently found as parasites in Sus scrofa worldwide (Calero-Bernal et al., 2016; Berger-Schoch et al., 2011; Castro et al., 2018). In our study, Eimeria sp. had low prevalence due to the low sensitivity of the fecal flotation method. Because of the low concentration of Eimeria oocysts in fecal material, these species were not identified in the present study.
Host factors such as age and sex can influence the presence of parasite infection (Krasnov et al., 2005; Marwaha et al., 2019; Moretta et al., 2011). For example, young animals are frequently affected by parasites due to immature immune system and perinatal infection of some parasite species. Animals’ diet and activity also influences on parasite infection rates. For example, piglets are often infected by Metastrongylus spp. due to eating more earthworms than adults do (Senlik et al., 2011). In the current study, male sex was associated with infection. This can relate to cold season of the year when boars aren’t eating earthworms.
In conclusion, the present study showed that wild boars are infected with a broad spectrum of parasites. This is one of the first studies to investigate the parasites of wild boars in Primorsky Krai. Therefore, further comparative studies to determine the population dynamics, prevalence, intensity and abundance of helminths would help in assessing the relationship between these parasite communities and their host populations.
Acknowledgement
The present study was conducted without any supporting funds.
Footnotes
Conflict of Interest
Conflict of Interest Statement: The authors declare that they did not have any conflict of interest in conducting this study. Moreover, the authors do not have any potential conflict of interest pertaining to this submission to Helminthologia.
References
- Antipov A.A., Bakhur T.I., Feshchenko D.V., Romanishina T.A., Avramenko N.V., Goncharenko V.P., Zghozinska O.A., Solovyova L.M., Koziy N.V., Pidborska R.V. Earthworms (Lumbricidae) as Intermediate Hosts of Lung Nematodes (Metastrongylidae) of Swine in Kyiv and Zhytomyr Regions of Ukraine. Ves Zool. 2018;52(1):59–64. doi: 10.2478/vzoo-2018-0008. et al . [DOI] [Google Scholar]
- Berger-Schoch A.E., Bernet D., Doherr M.G., Gottstein B., Frey C.F.. Toxoplasma gondii in Switzerland: a serosurvey based on meat juice analysis of slaughtered pigs, wild boar, sheep and cattle. Zoonoses Public Hlth. 2011;58(7):472–478. doi: 10.1111/j.1863-2378.2011.01395.x. [DOI] [PubMed] [Google Scholar]
- Becker A.C., Kraemer A., Epe C., Strube C.. Sensitivity and efficiency of selected coproscopical methods—sedimentation, combined zinc sulfate sedimentation-flotation, and McMaster method. Parasitol Res. 2016;115(7):2581. doi: 10.1007/s00436-016-5003-8. –. [DOI] [PubMed] [Google Scholar]
- Calero-Bernal R., Perez-Martin J.E., Reina D., Serrano F.J., Frontera E., Fuentes I., Dubey J.P.. Detection of zoonotic protozoa Toxoplasma gondii and Sarcocystis suihominis in wild boars from Spain. Zoonoses Public Hlth. 2016;63(5):346–350. doi: 10.1111/zph.12243. [DOI] [PubMed] [Google Scholar]
- Castagna F., Musella V., Esposito L., Poerio A., Rinaldi L., Bosco A., Cringoli G, Britti, D.. Helminths of Wild Boar (Sus scrofa) in the Calabrian Region of Southern Italy. J Wildl Dis. 2019;55(2):416–420. doi: 10.7589/2018-02-028. [DOI] [PubMed] [Google Scholar]
- Castro V., Cruciani D., Cambiotti V., Crotti S., Manuali E., Consalvi F., Ciavarella R. XVIII Congresso Nazionale SI Di. LV, Perugia (PG), Italia, 7–9 Novembre 2018. Società Italiana di Diagnostica di Laboratorio Veterinaria (SIDiLV); 2018. Sarcocystis miescheriana in wild boar “Sus scrofa” diaphragms in Umbria: histological characterization and molecular identification [Conference poster] pp. 121–122. [Google Scholar]
- Coelho C., Gomes J., Inácio J., Amaro A., Mesquita J.R., Pires I., Lopes A.P., Vieira-Pinto M.. Unraveling Sarcocystis miescheriana and Sarcocystis suihominis infections in wild boar. Vet Parasitol. 2015;212(3-4):100–104. doi: 10.1016/j.vetpar.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Da Silva D., Muller G.. Parasites of the respiratory tract of Sus scrofa scrofa (wild boar) from commercial breeder in southern Brazil and its relationship with Ascaris suum. Parasitol Res. 2013;112(3):1353–1356. doi: 10.1007/s00436-012-3214-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalez A.M., Perez-Martin J.E., Gamito-Santos J.A., Calero-Bernal R., Alonso M.A., Carrion E.M.F.. Epidemiologic study of lung parasites (Metastrongylus spp.) in wild boar (Sus scrofa) in southwestern Spain. J Wildl Dis. 2013;49(1):157–162. doi: 10.7589/2011-07-217. [DOI] [PubMed] [Google Scholar]
- Gasso D., Feliu C., Ferrer D., Mentaberre G., Casas-Diaz E., Velarde R., Fernández-Aguilar X., Colom-Cadena A., Navarro-Gonzalez N., López-Olvera J.R. Uses and limitations of faecal egg count for assessing worm burden in wild boars. Vet Parasitol. 2015;209(1-2):133–137. doi: 10.1016/j.vetpar.2015.02.006. et al . [DOI] [PubMed] [Google Scholar]
- Hälli O., Ala-Kurikka E., Peltoniemi O., Heinonen M.. The prevalence of internal parasites in wild boar farms in Finland. Acta Vet Scand. 2010;52(1):29. doi: 10.1186/1751-0147-52-S1-S29. [DOI] [Google Scholar]
- Kanka T., Rolinec M., Kasarda R., Imr ich I., Bučko O.. Endoparasites prevalence of wild boar (Sus scrofa) in CHKO Štiavnické vrchy. Res Pig Breed. 2017;11(1):18–21. [Google Scholar]
- Krasnov B.R., Morand S., Hawlena H., Khokhlova I.S., Shenbrot G.I.. Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia. 2005;146(2):209–217. doi: 10.1007/s00442-005-0189-y. [DOI] [PubMed] [Google Scholar]
- Lahmar S., Torgerson P.R., Mhemmed H., Tizaoui L., Mhadhbi N., Bani A., Dhibi M.. Cystic echinococcosis and other helminth infections of wild boar in northeastern and northwestern regions of Tunisia. Parasitology. 2019;146(10):1263–1274. doi: 10.1017/S0031182019000532. [DOI] [PubMed] [Google Scholar]
- Marwaha J., Aase H., Geist J., Stoeckle B.C., Kuehn R., Jakobsen P.J.. Host (Salmo trutta) age influences resistance to infestation by freshwater pearl mussel (Margaritifera margaritifera) glochidia. Parasitol Res. 2019;118(5):1519–1532. doi: 10.1007/s00436-019-06300-2. [DOI] [PubMed] [Google Scholar]
- Moretta I., Veronesi F., Paola R.D., Battistacci L., Moretti A.. Parasitological survey on wild boar (Sus scrofa) shot in the hunting season 2009-2010 in Umbria (central Italy) Large Anim Rev. 2011;17(5):187–192. [Google Scholar]
- Panayotova-Pencheva M., Dakova V.. Studies on the gastrointestinal and lung parasites fauna of wild boards (Sus scrofa scrofa L.) from Bulgaria. Ann Parasitol. 2018;64(4):379–384. doi: 10.17420/ap6404.174. [DOI] [PubMed] [Google Scholar]
- Popiolek Marcin., Knecht D., Szczesna-Staskiewicz Justyn. A., Czerwinska-Rozalow A. Gnieszka.. Helminths of the wild boar (Sus scrofa L.) in natural and breeding conditions. Bull Vet Inst Pulawy. 2010;54(2):161–166. [Google Scholar]
- Ramayo Y., Shemeret’eva I.N., Pérez‐Enciso M.. Mitochondrial DNA diversity in wild boar from the Primorsky Krai Region (East Russia) Anim Gen. 2011;42(1):96–99. doi: 10.1111/j.1365-2052.2010.02074.x. [DOI] [PubMed] [Google Scholar]
- Sáez-Royuela C., Gomariz R.P., Tellería J.L.. Age determination of European wild boar. Wildl Soc Bull. 1989;17(3):326–329. [Google Scholar]
- Senlik B., Cirak V.Y., Girisgin O., Akyol C.V.. Helminth infections of wild boars (Sus scrofa) in the Bursa province of Turkey. J Helminthol. 2011;85(4):404–408. doi: 10.1017/S0022149X1000074X. [DOI] [PubMed] [Google Scholar]
- Shchelkanov M.Yu., Galkina I.V., Fomenko P.V., Aramilev S.V., Surovyi A.L., Zhuravlev Yu.N.. Far Eastern bank of biological materials (FE BBM) from big cats (Pantherinae) as an improvement tool of the practice of law-enforcement of 226.1 and 258.1 articles in the criminal code of Russian Federation. Russ. J. Criminol. 2017;11(1):146–153. doi: 10.17150/25004255.2017.11(1).146-153(InRussian). [DOI] [Google Scholar]


