Abstract
Objective:
A bi-directional relationship between working memory and acute pain has long been assumed, but equivocal evidence exists regarding this relationship. The current study characterized the relationship between working memory and acute pain processing in healthy individuals using an adapted Sternberg working memory task.
Design:
Participants completed a Sternberg task while receiving noxious thermal stimulation. Participants received a pseudo-random presentation of 4 different temperatures (baseline temperatures and individually-determined low-, medium-, and high-temperature stimuli) and 4 levels of Sternberg task difficulty (0-, 3-, 6-, and 9-letter strings).
Subjects:
28 healthy participants were recruited from Stanford University and the surrounding community to complete the current study.
Results:
A non-linear interaction between intensity of thermal stimulation and difficulty of the Sternberg task was noted. Increased cognitive load from the Sternberg task resulted in increased perception of pain in low-intensity thermal stimulation but suppressed pain perception in high-intensity thermal stimulation. Thermal stimulation had no significant effect on participants’ response time or accuracy on the Sternberg task regardless of intensity level.
Conclusions:
Pain perception appears to decrease as a function of working memory load only for sufficiently noxious stimuli. However, increasing noxious stimuli did not affect cognitive performance. These complex relationships may reflect a shared cognitive space that can become “overloaded” with input of multiple stimuli of sufficient intensity.
Keywords: working memory, experimental pain, Sternberg task
Introduction
Pain processing involves more than a simple, discrete “stimulus to pain” pathway. Involving both affective and cognitive components [1], the experience of pain is subjective and variable between individuals. Limited processing resources and a shared cognitive space between cognitive and pain processing may account for some of this variation [2–4]. The empirical literature shows somewhat mixed results regarding the relationship between the resource demands of pain perception and cognition [5]. Several behavioral and neuroimaging studies have shown decreased pain perception during distraction or other cognitive tasks; when participants direct their attention away from a noxious thermal stimulus, they report lower levels of pain [6–10]. Similar effects of cognitive engagement on pain perception have also been noted in experimental paradigms like capsaicin pain models, which are designed to mimic chronic pain conditions [11]. This evidence highlights the malleable nature of pain experience and suggests that the variability in pain processing requires a closer examination of cognitive processes.
One process that is especially relevant for pain processing is working memory (WM), a cognitive system involved in the temporary storage of information needed to perform complex cognitive tasks [12]. Previous research has suggested that engagement of WM processes may modulate the experience of experimental pain [5]. This effect appears to be most evident in cases where a task is more cognitively demanding [7]. Additionally, mental rehearsal of information relevant to a task at hand, a process that is closely related to WM, has been found to inhibit the distracting effects of nociceptive inputs [13]. Further, individual differences in WM capacity predict differential reports of pain intensity during WM-related tasks [14].
Evidence also suggests a detrimental effect of acute pain on WM performance. If pain interferes with a person’s ability to attend to and temporarily hold information briefly in memory, then learning and retention of information may be affected. Evidence suggests that pain can adversely affect processing of visual information and can impair recognition of visual information at a later time, an effect that is modulated by engagement of WM processes [15]. Evidence for attentional and cognitive decrements due to acute pain has been somewhat equivocal, however; although some studies [5] have reported that increased pain intensity decreases performance on a WM task, this effect has not necessarily been robust across all experimental paradigms [5, 14, 16]. Given the relative inconsistency in the effects of acute pain on WM performance, this phenomenon warrants further attention in experimental studies.
The current study examined the bidirectional relationship between WM and pain using a modified version of the Sternberg task [17, 18]. The Sternberg Task requires participants to use maintenance and recognition processes of memory while being exposed to several chains of letters; this task is well-suited for experimental studies, as it does not require extensive training for the participant to switch between different levels of cognitive load within the task. We adapted the Sternberg by introducing a noxious thermal stimulus during the maintenance phase, when information is being rehearsed or maintained in WM. We varied the difficulty of the Sternberg task by manipulating the length of the letter string and applied 4 levels of individualized noxious thermal stimulation (no pain, low-intensity, medium-intensity, and high-intensity). Unlike many previous studies, which commonly employ only 2 levels within an experimental condition [11, 19], our study utilized a design that examined these effects across 4 levels of thermal stimulation and 4 levels of WM demand, thereby allowing us to examine the potential interaction of these factors in greater detail. We hypothesized that participants’ pain ratings during presentation of a noxious thermal stimulus would decrease as the cognitive demands of the Sternberg task increased. Furthermore, we hypothesized that as the noxious stimuli increased in intensity, participants’ accuracy and response time would decrease, thereby reflecting a negative impact of pain on WM performance through the disruption of the maintenance processes of WM.
Materials and Methods
All procedures were approved by the Institutional Review Board at the Stanford University School of Medicine, and all participants provided written informed consent.
Participants
We recruited and enrolled 39 healthy participants in our study. Individuals were not included in our study if they were taking any pain medications, were treated with an antidepressant medication, had a history or current diagnosis of a psychiatric disorder, were undergoing ongoing litigation regarding their pain, or experienced recurrent pain with an intensity of 4/10 for the previous 6 months or longer. Data from 8 participants were excluded after completing the experiment, as these participants did not remain within specific pain ranges (determined by pre-task pain testing) during trials with no cognitive task. One participant was excluded from analysis due to providing incorrect responses on the Sternberg task for more than half of the trials. Two participants were withdrawn because of equipment malfunction. Twenty-eight participants (14 females and 14 males, age range 18–57, mean 33.3 years) completed the study and were included in data analysis. Eleven were recruited from the local undergraduate college and 17 were identified from responses to local community advertising. Participants received compensation of $30.
Procedure
Each 1.5-hour session consisted of: (1) noxious thermal stimuli calibration, (2) a practice session, and [20] a main experimental task.
Noxious Thermal Stimuli Calibration.
First, we determined temperatures corresponding to each participant’s 3/10 (low pain), 5/10 (medium pain), and 7/10 (high pain) ratings of pain intensity. A Medoc (Medoc Advanced Medical Systems, Durham, North Carolina) Advanced Thermal Stimulator 3 × 3 cm Peltier contact thermode was used to deliver thermal stimulation on the thenar eminence of the left hand. Starting at 40 degrees Celsius, each participant was exposed to 15-second thermal heat blocks, and then asked to report verbally the degree of pain on an 11-point scale (0 = no pain at all, 10 = worst pain imaginable). Each successive heat block was increased by 1 degree Celsius until participants reached their 10/10 (maximum) pain score. Selected temperatures were then re-tested to verify which temperature elicited 7/10 (high) pain. Two degrees and four degrees were subtracted from the high pain temperature, respectively, to establish the 5/10 (medium) and 3/10 (low) level stimuli used in the experimental task. Only temperatures corresponding to a participant’s 7/10 (high), 5/10 (medium), 3/10 (low), and 0/10 (no pain) levels were used during the task. 0/10 pain was held constant at 32 degrees Celsius for all participants.
Practice Session.
Before beginning the practice session, participants were given both oral instructions and written instructions on a computer screen. All visual stimuli and data acquisition were controlled using E-Prime software package version 1.2 (Psychology Software Tools, Inc.). Visual stimuli were presented on a computer screen, and responses entered using a keyboard. Participants initially completed a 10-trial practice run to familiarize themselves with the task before proceeding to the 64-trial main task.
The task paradigm is illustrated in Figure 1. Each trial consisted of 1) a presentation of a trial of sequenced letters from the Sternberg task [21]; 2) a thermal stimulus; 3) participants rating their pain on a Visual Analog Scale (VAS) into the study computer; and 4) a letter sequence recognition prompt. Using a computer monitor, participants were presented with a string of pseudo-randomized letters, which varied in difficulty level: zero-, three-, six-, or nine-letter strings. Each letter in the string was displayed for 1 second with a 2-second delay between letters. After the letter string had been presented, we presented participants with a pseudo-randomized thermal stimulus for 8 seconds (randomized to either no stimulus or low, medium, or high temperature stimuli determined from their pre-task session). During the thermal heat application, the computer screen was blank. Following the heat presentation, participants were given 5 seconds to rate their pain with their right hand using a keyboard to control a cursor on a VAS from “no pain at all” on the far left to “worst pain imaginable” on the far right. Finally, participants were given 5 seconds to indicate whether a given letter was present in the previous series of letters by pushing the number 1 for “yes” and 2 for “no.” There was a 1.5-second delay between each trial described above.
Figure 1.
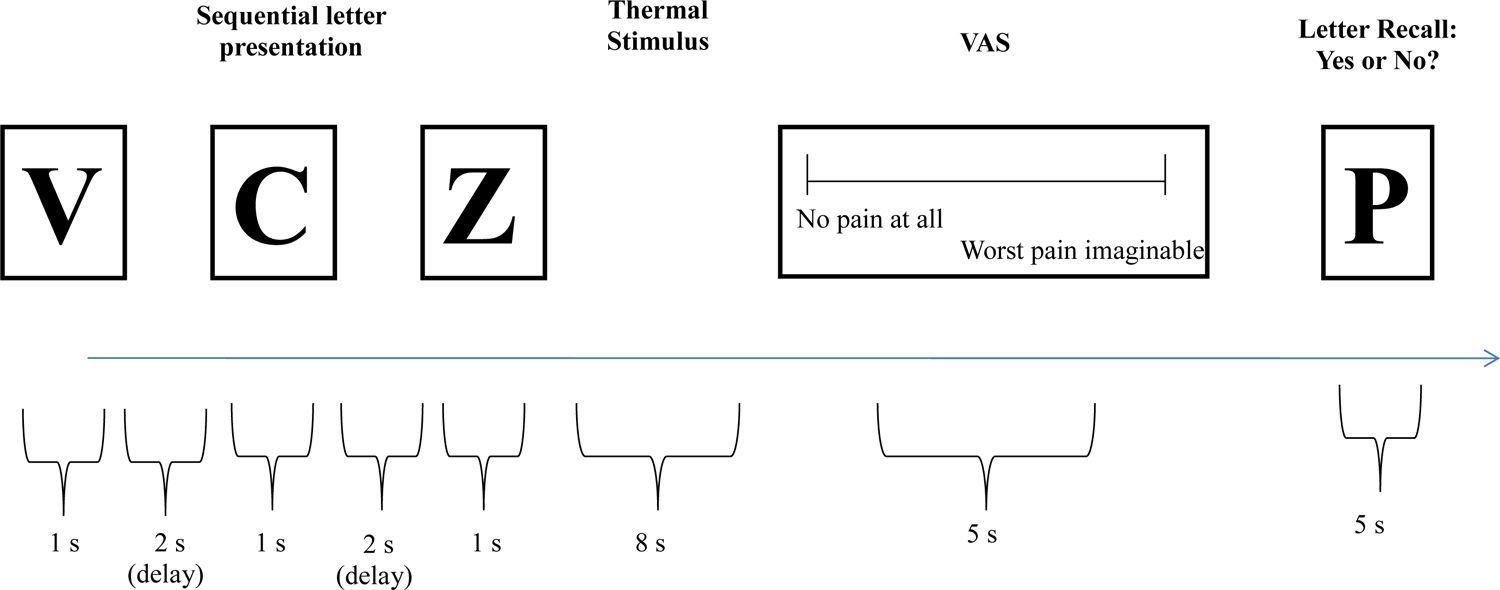
Representation of the presentation of Sternberg task and thermal pain induction and rating within a single trial.
Note: A 1.5-second delay was included between each trial.
Experimental task.
The experimental task contained 64 trials. Throughout the 64 trials, the combinations of difficulty and temperature level were pseudo-randomly assigned and counterbalanced to avoid any artificial effects of stimulus presentation.The first four trials from each participant were removed from data analysis in order to account for the time necessary for participants to familiarize themselves with the task. Letter presentations were chosen by randomly sorting a set of available letters and checked to ensure that there was no repetition of letters within a single presentation set. Participants whose overall recognition accuracy fell below 50% were excluded from the analyses; one participant’s data were excluded for this reason.
Measures.
VAS pain responses were converted to a numerical score on an 11-point scale (0= no pain at all, cursor marked on far left, 10 = worst pain imaginable, cursor marked on far right). The WM task yielded two performance measures: (1) accuracy, and (2) reaction time on individual trials. We recorded accuracy as a binary (correct or incorrect) outcome for each trial. We measured reaction time, in milliseconds, as the latency between presentation of the target stimulus and the participant’s key-press response.
Statistical analysis.
To evaluate the effect of increasing memory load on pain ratings, we constructed multilevel models (MLM) using the SAS PROC MIXED program,[22] in which thermal stimulus temperature and Sternberg task difficulty, as well as the interaction of these variables, were modeled as predictors of pain ratings at each trial. Both the thermal stimulus intensity predictor (none, low, medium, high) and the task difficulty predictor (no task, 3 letters, 6 letters, and 9 letters) contained 4 levels in models examining pain ratings as the outcome. Statistical significance was set at a level of p < 0.05. MLM allows for the construction of statistical models that involve clustered data and accounts for the statistical non-independence of observations, thereby preventing alpha inflation.[23]
To evaluate the effect of increasing pain levels on task performance (represented by reaction time and accuracy), we created 2 separate models; (1) MLM and (2) General Estimating Equations (GEE), respectively. In the first model, thermal stimulus temperature, task difficulty, and the interaction of temperature and task difficulty were entered as predictors of reaction time at each trial using SAS PROC MIXED. In the second model, we employed a generalized estimating equation (GEE) to predict accuracy rates from thermal stimulus intensity, task difficulty, and the interaction of thermal stimulus intensity and task difficulty using SPSS version 19 (SPSS, Chicago, IL). The GEE model was specified to use logistic regression using a binary distribution and included age, gender, and trial number as covariates; these same covariates were used in MLM models, as well. We excluded trials with a 0-letter memory load from all performance analyses.
Results
Experimental effects on subjective pain ratings
Average pain ratings for each level of thermal stimulation in the no-letter Sternberg task condition can be found in Table 1. Visual examination of the ratings of pain ratings at each level of thermal stimulus intensity suggested the possible presence of a curvilinear effect (see Figure 2), so a quadratic effect of thermal stimulation (i.e., the squared effect of thermal stimulation) was modeled in interaction with task difficulty. Consistent with previous studies [24], significant quadratic (B2 = .441, p < .001) and linear (B = .914, p < .001) effects of thermal stimulus intensity on pain ratings were noted. Analysis results revealed a significant interaction between both the quadratic term for thermal stimulus intensity and task difficulty (B2 = −.139, p < .001), along with a significant interaction between the linear effect of thermal stimulus intensity and task difficulty (B = .309, p < .001). When the potential quadratic effect of task difficulty was tested in interaction with a linear effect of thermal stimulus intensity, this interaction was found to be non-significant (B2 = −.045, p = .153). Inclusion of covariates representing participant age, gender, and trial number did not change the direction or significance of any of the examined effects.
Table 1.
Average pain ratings at each level of thermal stimulus intensity in trials with no Sternberg task.
| No Stimulus | Low Intensity | Medium Intensity | High Intensity | |
|---|---|---|---|---|
| No task | 0.10(0.33) | 1.48(1.24) | 3.48(2.03) | 7.33(1.41) |
Note: Pain ratings were rated on a 0–10 VAS rating scale.
Figure 2.
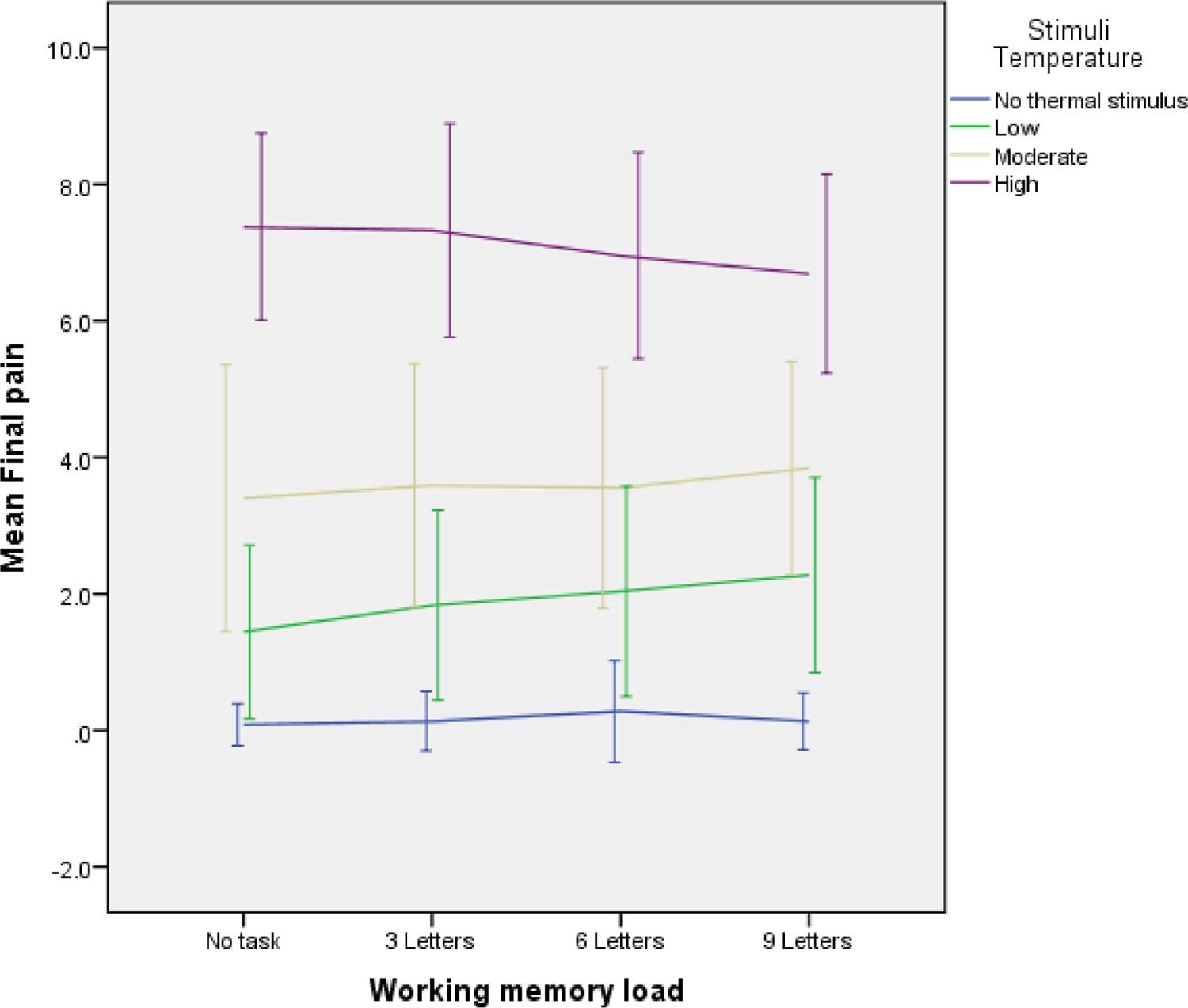
Mean ratings of pain intensity across Sternberg task difficulty levels and across thermal stimulus intensity levels.
Note: Error bars represent 1 standard deviation in scores at each condition.
In order to more effectively represent these results, simple slopes were computed for the effect of Sternberg Task difficulty on pain ratings at each level of thermal stimulation intensity. When these effects were examined separately according to stimulus intensity level, there was no effect of task difficulty on pain ratings when no thermal stimulation was present (B = .014, p = .316), but greater task difficulty predicted greater pain ratings when participants received low-intensity thermal stimulation (B = .246, p < .001). The relationship between task difficulty and pain ratings was non-significant when participants were receiving medium-intensity thermal stimulation (B = .087, p = .136). Within trials of high-intensity thermal stimulation, there was a significant inverse relationship between task difficulty and pain ratings (B = −.282, p < .001). These results indicate the following: (1) increases in thermal stimulus intensity significantly predicted increases in pain report; (2) increases in Sternberg task difficulty were positively related to pain ratings in trials where participants were exposed to a low-intensity thermal stimulus, but uncorrelated to pain ratings in trials in which there was no thermal stimulation or medium-intensity thermal stimulation; and [20] in conditions where both task difficulty and thermal stimulus intensity were high (i.e., trials in which participants were exposed to letter chains of 6 or 9 letters while being exposed to high-intensity thermal stimulus intensity), the combination of high-intensity thermal stimulation and high task difficulty significantly suppressed pain ratings.
Experimental effects on Sternberg task performance
Accuracy scores and reaction times for each level of Sternberg task difficulty in the absence of thermal stimulation can be found in Table 2. No significant interactions were noted between Sternberg task difficulty and thermal stimulus intensity in predicting either accuracy (Wald χ2(6) = 3.105, p = .796) or reaction time (B = 17.819, p = .44). There were significant linear effects of task difficulty on both accuracy ratings and reaction times for each trial, such that accuracy ratings decreased (Wald χ2(2) = 44.212, p < .001) and reaction times increased (B = 150.56, p < .001) when participants were presented with a longer chains of letters during the Sternberg task. However, changes in thermal stimulus intensity did not predict differential rates of accuracy during the Sternberg task (Wald χ2(3) = 3.371, p = .338) or reaction times during the Sternberg task (B = −17.583, p = .71).
Table 2.
Sternberg task performance indices in trials with no thermal stimulation.
| 3-Letter Load | 6-Letter Load | 9-Letter Load | |
|---|---|---|---|
| Accuracy (%) | .94(.24) | .88(.33) | .76(.42) |
| Reaction Time (ms) | 1657(696) | 1947(674) | 2006(729) |
Note: Reaction time was measured in milliseconds, and accuracy scores are presented as a percentage of correct responses for each trial type.
Discussion
The current study examined the bidirectional relationship between WM processing and pain. Using a modified Sternberg task concurrently with a thermal pain induction, we found that the intensity of nociceptive inputs and cognitive load interact in a non-linear fashion in their effects on subjective pain ratings. Unsurprisingly, participants reported greater pain when exposed to greater thermal stimulation. At low levels of nociceptive inputs, high WM load increased the subjective experience of pain. However, when thermal stimulation reached a high level of intensity, moderate or high levels of cognitive load served to suppress subjective pain ratings. These results may suggest that active engagement in a WM task leaves fewer cognitive resources available for pain perception,[13] thereby decreasing pain ratings.[25, 26] Though previous studies have demonstrated a non-linear effect of noxious stimulus presentation on pain perception,[24], the current study is the first to demonstrate a statistically significant non-linear interaction between WM load and noxious thermal stimulation in altering pain perception.
These findings may be explained by a shared cognitive space between working memory and pain processing [27]. Several of the brain regions activated during WM tasks have been found to be activated during painful tasks. More specifically, the experience of induced pain has been found to activate areas of the dorsolateral prefrontal cortex (DLPFC), which have been implicated in the modulation of heat pain [28], along with regions of the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC), which are involved in the direction of attentional resources and pain perception [7]. Similarly, previous evidence suggests that the Sternberg task has only been found to activate DLPFC at the higher cognitive loads [29]. These results mirror our current findings, and may reflect a maximal capacity of the DLPFC that, once reached, manifest in dampened pain responses. Supplementation of our findings with neuroimaging data under similar experimental conditions may help to further clarify the mechanisms underlying this effect.
Our findings of increased pain ratings under conditions of high WM load but low thermal stimulus intensity were somewhat unexpected, however. It is possible that this finding reflects a consequence of a negative emotional response, such as frustration, that may be more likely when both a more demanding cognitive task and a noxious physical stimulus are present. Indeed, negative moods have been shown to affect pain ratings in experimental settings [30]. However, as we did not measure emotional responses concurrently with pain ratings during the procedure, we offer this conclusion only as a possible explanation that would require future replication.
However, it is also possible that the non-linear effects of WM load on pain ratings reflect an interference effect of WM engagement on pain discriminability. Under this assumption, higher task difficulty may suppress ascending nociceptive inputs as they are processed in the brain, such that participants may not be able to accurately identify the true intensity of the thermal stimulus, leading their reported levels of pain to converge towards their average pain ratings for the experiment. This interpretation of the data may best explain both the increased level of pain ratings noted in the low-difficulty levels of the Sternberg task and the dampened pain ratings in the high WM load conditions. Our results do not allow us to make distinctions between these interpretations of the data, though both appear to be viable. These issues warrant greater attention in future studies.
Our pattern of results may also reflect prioritization of processing through executive functioning processes, such that higher-order cognitive functions can prioritize processing of WM-relevant information through inhibition of pain processing through descending mechanisms, as opposed to ongoing engagement of WM processes leaving an insufficient number of cognitive resources to adequately process nociceptive input. This “shielding” effect of cognition against nociceptive input through engagement of WM processes has been noted in previous studies [31]. As participants were already engaged in the Sternberg task when thermal stimulation occurred, the thermal stimulation may have been processed secondarily to the cognitive task itself, which showed no decrements at any level of thermal intensity. This interpretation appears reasonable, given the nature of our results, but requires validation in future studies. It would be of value, for example, to examine whether temporal ordering of the WM task versus thermal stimulus modulates these effects. Had thermal stimulation occurred before the cognitive task, priority of cognitive processing may have been given to pain processing, rather than engagement in the Sternberg task. This phenomenon may be akin to conscious attempts at modulating attention, as in mindfulness meditation [32] or attentional training paradigms [33], which have been shown to alter afferent nociceptive signals. Though we did not assess the extent to which participants were expending mental effort in order to remain focused on the task at hand, variability in pain ratings might be replicated in other situations where individuals explicitly attempt to modulate their WM engagement.
The design of the Sternberg task is well-suited to studies using the Peltier thermode, in that stimulation can be presented at different points during the task: during encoding, maintenance, recall or continuously throughout the entire task. In our study, we presented the noxious stimulus after the letters were displayed but before the recognition phase, which restricts the interpretation of our results to the maintenance phase. Noxious heat applied at different time points in the task would provide information about the differential effects of noxious heat during processing, encoding, and recognition of visual memory. If engagement of executive functioning processes is simply devoted to the first sensory stimulus that occurs, then a non-linear effect of WM load on pain perception might not be expected if thermal stimulation is presented before a cognitive task. At present, it appears sufficient to state that this pain suppression effect necessitates the presence of both a significantly demanding WM task and the presence of a sufficiently intense physical stimulus. However, we note that our pattern of findings may have been different if pain ratings had occurred after the completion of the cognitive task. Our experimental design does not specifically address the issue of degree of cognitive effort required to complete each aspect of the Sternberg task, so it is possible that our pattern of results would be different if the order of Sternberg stimulus presentation and thermal stimulation had varied.
As expected, participants’ accuracy and reaction time decreased when the demands of the Sternberg task increased. However, our second hypothesis, that increasingly noxious thermal stimuli would decrease response accuracy and increase reaction time, was not supported. These results are divergent from previous studies of the effects of induced pain on cognitive performance, which have reported a significant bidirectional relationship between pain and a working memory task [5, 19]. As in our study, Buhle and Wager [5] found that increasing task difficulty attenuated pain. However, they also found that increasingly painful stimuli decreased task performance, an effect that was not significant in our results.
There are a few potential explanations for the lack of an effect of thermal stimulation on WM task performance. It is possible that the Sternberg task, which functions primarily as a more passive recognition task, may not have been challenging enough to show the expected decrements in performance when thermal stimulation occurred. More specifically, participants appeared able to remain engaged in the task, even when the task difficulty and thermal stimulus intensity were at their maximum levels. Previous studies have noted that the aspects of attention most relevant to pain processing appear to be the same aspects that are involved in directing attention between multiple sources of information [34]. Thus, tasks that require manipulation of verbal information using the working memory, such as an ongoing mental calculation task or an n-back task (which also requires engagement of executive functioning processes), appear to require a greater level of cognitive effort and could therefore show decrements in task performance when noxious thermal stimulation is introduced [19]. It is also possible that the binary nature of our accuracy variable may not have yielded sufficient variability in performance to reflect subtler changes in cognitive processing caused by noxious stimulation. Unlike an 11 point- VAS that allowed us to measure more nuanced differences in pain, a binary response to our task may not adequately gauge intermediate changes in cognitive processing.
Additionally, our procedure introduced thermal stimulation after the Sternberg letter strings had already been introduced, and the thermal stimulation had ceased by the time participants began the recognition task itself. Thus, we did not assess the potential consequences of thermal stimulation during the encoding phase of memory, nor did we assess the potential interference of thermal stimulation during the recognition phase of cognitive testing. As these phases of memory may be more salient in determining cognitive task performance, our findings should interpreted only as an indication that potential disruption of mental rehearsal through noxious stimulation may not be sufficient to interfere with working memory processes.
Additionally, some previous studies [35, 36] have also identified distinct profiles of cognitive performance during a painful task, noting that some individuals demonstrate enhanced performance in some cognitive domains in the presence of a concomitant painful stimulus, while others show poorer performance in the same domain. Though we did not identify any notable differences in profiles of pain-related WM performance, this factor is worth considering in the context of future studies.
Future Directions
The current study findings are informative, but several promising areas of future elaboration remain. For example, it is possible that the necessary threshold for cognitive load to reach a point of pain suppression may be lower if the nociceptive input is of higher intensity or, conversely, a task that demands an even higher level of cognitive engagement could demonstrate a suppressing effect on a nociceptive stimulus of a somewhat lower intensity. Clarification of these aspects of the interaction between cognitive load and nociceptive input would yield a more flexible and more ecologically valid model of pain reporting, as daily life will likely expose individuals to a much broader range of intensity for both cognitively demanding and noxious stimuli. Further, the current study did not specifically measure the magnitude of suppression in pain ratings; identification of differential levels of suppression of pain ratings, along with potential modifiers of this relationship, would provide the basis for a greater understanding of mechanisms underlying the modulation of pain. Similarly, as there is evidence that the cognitive demand and nature of neural activation of pain experience may vary according to the intensity of the noxious stimulus [37, 38], this issue constitutes another area of potential inquiry that was not addressed in the current study.
It is also worthwhile to consider whether the suppressing effects of cognitive load may be modified by enjoyment gleaned from tasks while enduring pain. From a clinical perspective, psychological approaches for pain management suggest that engagement in enjoyable activities improves adjustment to pain, as in the case of cognitive-behavioral therapy [39]. Positive emotions may also serve to attenuate the experience of pain [40], though it is unclear to what extent this effect is distinct from cognitive load or distraction. The Sternberg task, though sufficient for the purposes of increasing WM load, was unlikely to be deemed an enjoyable activity for participants in the current study. However, examination of more enjoyable tasks (e.g., carrying on a conversation with a loved one, or listening to an enjoyable story) may further serve to clarify whether there is a distinct contribution of the appetitive or aversive nature of engagement in a given task on pain perception. Understanding of these phenomena may be enhanced by examining the potentially unique roles of both cognitive load and enjoyment in altering the experience of pain.
Finally, additional research is needed to understand the extent to which these effects may differ in chronic pain populations. As high levels of WM engagement have been implicated in decreased pain perception in an experimental pain paradigm, it is worthwhile to examine these processes in individuals who face chronic pain. Meta-analytic studies have reported moderately worse performance on WM tasks [41] and increased reaction times on cognitive tasks [42] in individuals with chronic pain, compared to healthy controls. Divergent findings with regard to interactive effects of WM engagement and noxious stimulus intensity in a chronic pain sample, such as a lack of suppression at high levels of pain and WM engagement, could suggest a distinct cognitive context of pain perception in chronic pain. Similarly, it may be worthwhile to examine these effects in experimental conditions other than superficial thermal stimulation, as a majority of experimental pain research has been devoted to the study of exogenous nociceptive input (e.g., noxious thermal stimulation), which may have limited interpretability when applied to pain disorders that are experientially distinct, such as visceral or dental pain.[43]
Limitations
The current study also exhibited some limitations that should be noted. Our thermal induction procedure, while apparently effective in affecting pain ratings, did not specifically address individual differences in pain perception. More specifically, we utilized a thermal induction procedure that established a “high” level of pain, and then subtracted a set number of degrees from this temperature to determine “low” and “moderate” levels of pain. As the relationship between thermal stimulation and pain perception is often non-linear [44], using a thermal induction procedure that took additional steps towards individual calibration of pain ratings at each level of pain might have improved the interpretability of our findings. Further, our sample was comprised of healthy adults, which suggests that these findings require replication in other samples (such as individuals with chronic pain) in order to determine the generalizability of the findings. Further, given the wide age range of our sample, it is worth noting that there are age-related differences in perception of noxious thermal stimuli [45], which may be attributable to differential levels of regional brain activations [46]. Though we included age as a covariate in all analyses, our sample was likely not sufficiently distributed to test age-specific effects in our presented findings, and should thus be examined by future studies.
Conclusions
The current study is the first to demonstrate that nociceptive input and WM engagement affect pain perception in a non-linear fashion. These results serve to highlight the complex nature of pain perception and suggest a threshold of cognitive input that may modify the experience and report of pain. Additional work remains to verify this shared cognitive space through neuroimaging studies of prefrontal areas, as well as replication and extension of our findings using higher-intensity cognitive and nociceptive input and chronic pain populations. Nevertheless, these results highlight the value of experimental data in greater articulation of clinically-relevant phenomena and provide the basis for development of more ecologically valid models of pain and cognitive processes.
Disclosures:
Sean Mackey MD, PhD acknowledges support from K24DA029262 and the Chris Redlich Pain Research Endowment.
Footnotes
Disclosures
The authors have no conflict of interest related to the present article.
References
- 1.Keefe FJ, Lumley M, Anderson T, Lynch T, Studts JL, Carson KL. Pain and emotion: new research directions. J Clin Psychol. 2001;57:587–607. [DOI] [PubMed] [Google Scholar]
- 2.Norman DA, Bobrow DG. On data-limited and resource-limited processes. Cognit Psychol. 1975;7:44–64. [Google Scholar]
- 3.Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956;63:81. [PubMed] [Google Scholar]
- 4.Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114; discussion −85. [DOI] [PubMed] [Google Scholar]
- 5.Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain. 2010;149:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–9. [DOI] [PubMed] [Google Scholar]
- 8.Bushnell M, Duncan G, Hofbauer R, Ha B, Chen J-I, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain. 2003;106:101–8. [DOI] [PubMed] [Google Scholar]
- 10.Crombez G, Eccleston C, Van den Broeck A, Van Houdenhove B, Goubert L. The effects of catastrophic thinking about pain on attentional interference by pain: no mediation of negative affectivity in healthy volunteers and in patients with low back pain. Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2002. [DOI] [PubMed] [Google Scholar]
- 11.Wiech K, Seymour B, Kalisch R, Enno Stephan K, Koltzenburg M, Driver J, et al. Modulation of pain processing in hyperalgesia by cognitive demand. NeuroImage. 2005;27:59–69. [DOI] [PubMed] [Google Scholar]
- 12.Baddeley A. Working memory. Science. 1992;255:556–9. [DOI] [PubMed] [Google Scholar]
- 13.Legrain V, Crombez G, Verhoeven K, Mouraux A. The role of working memory in the attentional control of pain. Pain. 2011;152:453–9. [DOI] [PubMed] [Google Scholar]
- 14.Nakae A, Endo K, Adachi T, Ikeda T, Hagihira S, Mashimo T, et al. The influence of working memory capacity on experimental heat pain. The journal of pain : official journal of the American Pain Society. 2013;14:1088–96. [DOI] [PubMed] [Google Scholar]
- 15.Bingel U, Rose M, Glascher J, Buchel C. fMRI reveals how pain modulates visual object processing in the ventral visual stream. Neuron. 2007;55:157–67. [DOI] [PubMed] [Google Scholar]
- 16.Paris TA, Misra G, Archer DB, Coombes SA. Effects of a force production task and a working memory task on pain perception. The journal of pain : official journal of the American Pain Society. 2013;14:1492–501. [DOI] [PubMed] [Google Scholar]
- 17.Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. NeuroImage. 2003;18:247–56. [DOI] [PubMed] [Google Scholar]
- 18.Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–8. [DOI] [PubMed] [Google Scholar]
- 19.Moore DJ, Keogh E, Eccleston C. The effect of threat on attentional interruption by pain. Pain. 2013;154:82–8. [DOI] [PubMed] [Google Scholar]
- 20.Seebach CL, Kirkhart M, Lating JM, Wegener ST, Song Y, Riley LH 3rd, et al. Examining the role of positive and negative affect in recovery from spine surgery. Pain. 2012;153:518–25. [DOI] [PubMed] [Google Scholar]
- 21.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–4. [DOI] [PubMed] [Google Scholar]
- 22.Littell RC. SAS for mixed models: SAS institute; 2006. [Google Scholar]
- 23.Raudenbush SW, & Bryk AS Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 24.Torebjork HE, LaMotte RH, Robinson CJ. Peripheral neural correlates of magnitude of cutaneous pain and hyperalgesia: simultaneous recordings in humans of sensory judgments of pain and evoked responses in nociceptors with C-fibers. J Neurophysiol. 1984;51:325–39. [DOI] [PubMed] [Google Scholar]
- 25.Van Damme S, Legrain V, Vogt J, Crombez G. Keeping pain in mind: a motivational account of attention to pain. Neuroscience and biobehavioral reviews. 2010;34:204–13. [DOI] [PubMed] [Google Scholar]
- 26.Legrain V, Bruyer R, Guerit JM, Plaghki L. Involuntary orientation of attention to unattended deviant nociceptive stimuli is modulated by concomitant visual task difficulty. Evidence from laser evoked potentials. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005;116:2165–74. [DOI] [PubMed] [Google Scholar]
- 27.Baddeley A. Working memory: looking back and looking forward. Nature reviews Neuroscience. 2003;4:829–39. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–91. [DOI] [PubMed] [Google Scholar]
- 29.Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. NeuroImage. 1999;9:216–26. [DOI] [PubMed] [Google Scholar]
- 30.Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–9. [DOI] [PubMed] [Google Scholar]
- 31.Legrain V, Crombez G, Plaghki L, Mouraux A. Shielding cognition from nociception with working memory. Cortex. 2013;49:1922–34. [DOI] [PubMed] [Google Scholar]
- 32.Zeidan F, Gordon NS, Merchant J, Goolkasian P. The effects of brief mindfulness meditation training on experimentally induced pain. The Journal of Pain. 2010;11:199–209. [DOI] [PubMed] [Google Scholar]
- 33.McGowan N, Sharpe L, Refshauge K, Nicholas M. The effect of attentional re-training and threat expectancy in response to acute pain. Pain. 2009;142:101–7. [DOI] [PubMed] [Google Scholar]
- 34.Moore DJ, Keogh E, Eccleston C. The interruptive effect of pain on attention. Quarterly journal of experimental psychology. 2012;65:565–86. [DOI] [PubMed] [Google Scholar]
- 35.Seminowicz D, Mikulis D, Davis K. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 2004;112:48–58. [DOI] [PubMed] [Google Scholar]
- 36.Erpelding N, Davis KD. Neural underpinnings of behavioural strategies that prioritize either cognitive task performance or pain. PAIN®. 2013;154:2060–71. [DOI] [PubMed] [Google Scholar]
- 37.Seminowicz DA, Davis KD. Interactions of pain intensity and cognitive load: the brain stays on task. Cerebral cortex. 2007;17:1412–22. [DOI] [PubMed] [Google Scholar]
- 38.Kong J, White NS, Kwong KK, Vangel MG, Rosman IS, Gracely RH, et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27:715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keefe FJ. Cognitive behavioral therapy for managing pain. Clin Psychol. 1996;49:4–5. [Google Scholar]
- 40.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;73:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berryman C, Stanton TR, Jane Bowering K, Tabor A, McFarlane A, Lorimer Moseley G. Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain. 2013;154:1181–96. [DOI] [PubMed] [Google Scholar]
- 42.Grisart JM, Plaghki LH. Impaired selective attention in chronic pain patients. European journal of pain. 1999;3:325–33. [DOI] [PubMed] [Google Scholar]
- 43.Moore DJ, Keogh E, Crombez G, Eccleston C. Methods for studying naturally occurring human pain and their analogues. Pain. 2013;154:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol. 2009;101:875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harkins SW, Price DD, Martelli M. Effects of age on pain perception: thermonociception. J Gerontol. 1986;41:58–63. [DOI] [PubMed] [Google Scholar]
- 46.Cole LJ, Farrell MJ, Gibson SJ, Egan GF. Age-related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiol Aging. 2010;31:494–503. [DOI] [PubMed] [Google Scholar]


