Abstract
Among the multiple SARS-CoV-2 variants identified since summer 2020, several have co-circulated, creating opportunities for coinfections and potentially genetic recombinations that are common in coronaviruses. Viral recombinants are indeed beginning to be reported more frequently. Here, we describe a new SARS-CoV-2 recombinant genome that is mostly that of a Omicron 21L/BA.2 variant but with a 3′ tip originating from a Omicron 21K/BA.1 variant. Two such genomes were obtained in our institute from adults sampled in February 2022 in university hospitals of Marseille, southern France, by next-generation sequencing carried out with the Illumina or Nanopore technologies. The recombination site was located between nucleotides 26,858-27,382. In the two genomic assemblies, mean sequencing depth at mutation-harboring positions was 271 and 1362 reads and mean prevalence of the majoritary nucleotide was 99.3 ± 2.2% and 98.8 ± 1.6%, respectively. Phylogeny generated trees with slightly different topologies according to whether genomes analyzed were depleted or not of the 3′ tip. This 3′ terminal end brought in the Omicron 21L/BA.2 genome a short transposable element of 41 nucleotides named S2m that is present in most SARS-CoV-2 except a few variants among which the Omicron 21L/BA.2 variant and may be involved in virulence. Importantly, this recombinant is not detected by currently used qPCR that screen for variants in routine diagnosis. The present observation emphasizes the need to survey closely the genetic pathways of SARS-CoV-2 variability by whole genome sequencing, and it could contribute to gain a better understanding of factors that lead to observed differences between epidemic potentials of the different variants.
Keywords: SARS-CoV-2, Recombinant, Variant, Lineage, Omicron, 21L/BA.2, 21K/BA.1, Epidemic
1. Introduction
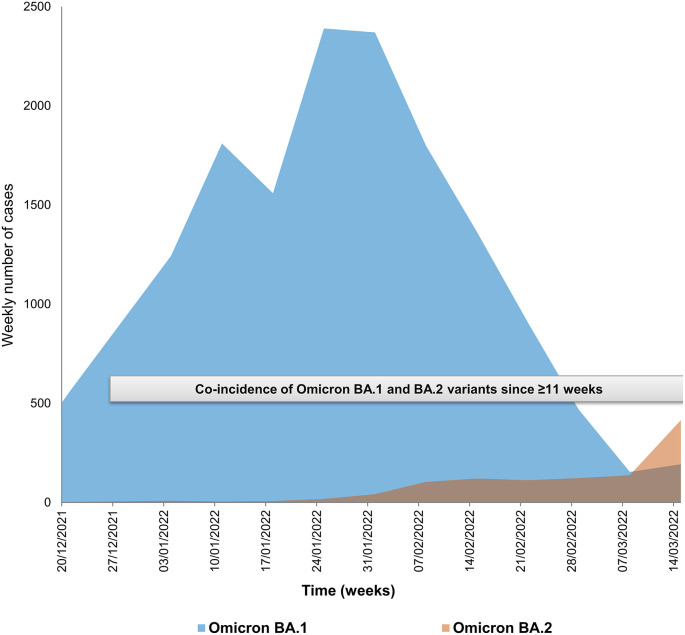
Multiple SARS-CoV-2 variants have been identified since summer 2020 (Lemey et al., 2021; Colson et al., 2022a; Hodcroft et al., 2021; Harvey et al., 2021). There were periods during which two distinct variants co-circulated with a crossing of their incidence when an old variant was vanishing and a new one was rising. Substantial rates of co-incidence over periods of several weeks have thus been reported recently worldwide for the Delta [WHO denomination (https://www.who.int/fr/activities/tracking-SARS-CoV-2-variants)]/21J [Nextclade classification (Aksamentov et al., 2021) (https://nextstrain.org/)] and Omicron 21K/BA.1 [Pangolin classification (Rambaut et al., 2020) (https://cov-lineages.org/resources/pangolin.html)] variants, and thereafter, to a lesser extent, for the Omicron BA.1 and BA.2 variants (https://covariants.org/per-country) (Hodcroft, 2021; Hadfield et al., 2018), including in our geographical area (Fig. 1 ). This created the opportunity for coinfections (Rockett et al., 2022; Hosch et al., 2022; Bolze et al., 2022; Belén Pisano et al., 2022), and consequently for homologous genetic recombinations, which constitute a major and very common mechanism of evolution in viruses (Bentley and Evans, 2018). Such genetic recombinations are extremely frequent for viruses of family Coronaviridae, and they have already been identified for endemic human coronaviruses (Lai, 1996; Zhang et al., 2015; So et al., 2019; Gribble et al., 2021). Regarding SARS-CoV-2, the occurrence of recombinations has been reported or suspected (Yi, 2020; Yeh and Contreras, 2020; Haddad et al., 2021; Ignatieva et al., 2022; Jackson et al., 2021; Taghizadeh et al., 2021; Varabyou et al., 2021; Kreier, 2022; Wertheim et al., 2022; He et al., 2022; Sekizuka et al., 2022; Colson et al., 2022b; Lacek et al., 2022; Lohrasbi-Nejad, 2022; Bolze et al., 2022; Ou et al., 2022; Belén Pisano et al., 2022; Burel et al., 2022). Very recently, we described the identification and culture of two SARS-CoV-2 recombinants, one between the B.1.160 and Alpha/20I variants in a patient chronically-infected with SARS-CoV-2 (Burel et al., 2022), and another between the Delta/21J AY.4 and Omicron 21K/BA.1 variants in patients infected approximately 10 weeks after the start of the period of co-detection of these two variants in our geographical area (Colson et al., 2022b). Here, we describe a new hybrid genome, which consists of a Omicron 21L/BA.2 genome whose 3′ tip originates from a Omicron 21K/BA.1.
Fig. 1.
Incidence and co-incidence of Omicron 21K/BA.1 and Omicron 21L/BA.2 variants among cases diagnosed in our institute.
2. Materials and methods
Nasopharyngeal samples were tested for the presence of SARS-CoV-2 RNA by real-time reverse transcription-PCR (qPCR) using the BGI real-time fluorescent RT-PCR assay (BGI Genomics, Shanghai Fosun Long March Medical Science Co., Ltd., Shenzhen, China), as previously described (Burel et al., 2022). Subsequently, qPCR assays that screen for SARS-CoV-2 variants were carried out with the detection of mutations among which spike substitution K417N (Thermo Fisher Scientific, Waltham, USA) and the targeting of viral genes S (spike), N (nucleocapsid) and ORF1 with the TaqPath COVID-19 kit (Thermo Fisher Scientific), as previously reported (Colson et al., 2022a; Colson et al., 2022b).
SARS-CoV-2 genomes were obtained and analyzed as described previously (Colson et al., 2022a; Colson et al., 2022b). Briefly, next-generation sequencing was carried out with the Illumina COVID-seq protocol on the NovaSeq 6000 instrument (Illumina Inc., San Diego, CA, USA), or with the Oxford Nanopore technology (ONT) on a GridION instrument (Oxford Nanopore Technologies Ltd., Oxford, UK) following multiplex PCR amplification with the ARTIC nCoV-2019 Amplicon Panel v4.1 of primers (IDT, Coralville, IA, USA) and the ARTIC procedure (https://artic.network/).
Sequence read processing and genome analysis were performed as described previously (Colson et al., 2022a; Colson et al., 2022b). Briefly, for Illumina NovaSeq reads, base calling was carried out using the Dragen Bcl Convert pipeline [v3.9.3; https://emea.support.illumina.com/sequencing/sequencing_software/bcl-convert.html (Illumina Inc.)], mapping was carried out using the bwa-mem2 tool (v2.2.1; https://github.com/bwa-mem2/bwa-mem2) on the Wuhan-Hu-1 isolate genome (GenBank accession no. NC_045512.2) before cleaning with the SAMtools program (v. 1.13; https://www.htslib.org/) (Danecek et al., 2021). Variant calling was performed using FreeBayes (v1.3.5; https://github.com/freebayes/freebayes) (Garrison and Marth, 2012), and consensus genomes were built with the Bcftools program (v1.13; https://samtools.github.io/bcftools/bcftools.html). ONT reads were processed with the ARTIC-nCoV-bioinformaticsSOP pipeline (v1.1.0; https://github.com/artic-network/fieldbioinformatics). Nucleotide and amino acid changes compared to the Wuhan-Hu-1 isolate genome were determined using the Nextclade tool (https://clades.nextstrain.org/) (Hadfield et al., 2018; Aksamentov et al., 2021). Nextstrain clades and Pangolin lineages were identified with the Nextclade web application (https://clades.nextstrain.org/) (Hadfield et al., 2018; Aksamentov et al., 2021) and the Pangolin tool (https://cov-lineages.org/pangolin.html) (Rambaut et al., 2020), respectively.
Phylogenetic analyses were performed with the MEGA X software (v10.2.5; https://www.megasoftware.net/) (Kumar et al., 2018) following sequence alignment with MAFFT (https://mafft.cbrc.jp/alignment/server/) (Katoh et al., 2002), and trees were visualized with MEGA X. We built two separate trees, a first one for the whole recombinant genome and a second one for its part originating from an Omicron 21L/BA.2 variant. The 10 genomes the most similar to these sequences among genomes of the Omicron 21L/BA.2 and 21K/BA.1 variants of the sequence database of our institute were selected by a BLAST search (Altschul et al., 1990) then incorporated in the phylogenies together with the sequence of the Wuhan-Hu-1 isolate.
This study has been approved by the ethics committee of the University Hospital Institute Méditerranée Infection (No. 2022–008). Access to the patients' biological and registry data issued from the hospital information system was approved by the data protection committee of Assistance Publique-Hôpitaux de Marseille (APHM) and was recorded in the European General Data Protection Regulation registry under number RGPD/APHM 2019–73. Genome sequences obtained and analyzed here were deposited in the NCBI GenBank nucleotide sequence database (https://www.ncbi.nlm.nih.gov/genbank/) (Sayers et al., 2022) (Accession no. OM993515 and OM993473), on the IHU Méditerranée Infection website (https://www.mediterranee-infection.com/sars-cov-2-recombinant/) (IHUCOVID-063942 and IHUCOVID-068136), and in the GISAID database (https://www.gisaid.org/) (Elbe and Buckland-Merrett, 2017; Alm et al., 2020) (EPI_ISL_10843457, EPI_ISL_10047082).
3. Results
The recombinant genomes were obtained from two adult patients sampled in February 2022 in the university hospitals of Marseille, southern France. Cycle threshold values (Ct) of the diagnosis qPCR performed in our institute were 13 and 19. The TaqPath COVID-19 assay showed positivity for all targeted genes including the S gene, and the spike K417N mutation was detected, which led to suspect an infection with the Omicron 21L/BA.2 variant. However, next-generation sequencing performed in our laboratory allowed obtaining SARS-CoV-2 Omicron 21L/BA.2-21K/BA.1 genomic hybrid forms. These hybrid genomes have a Omicron 21L/BA.2 backbone but their approximately 2500–3000 nucleotide-long 3′ terminal region is that of a Omicron 21K/BA.1 (Fig. 2 ). The recombination site is located between nucleotides 26,858 and 27,382 (in reference to the genome of the Wuhan-Hu-1 isolate). This 3′ terminal end in the genomes of the Omicron BA.1 variant does not harbor any signature mutations, and therefore a “gain” of mutation could not be observed here. However, we noted the presence and integrity of a sequence corresponding to a short transposable element of 41 nucleotides named S2m (Tengs et al., 2021), which is present in the Omicron 21K/BA.1 variant but is truncated of 26 nucleotides in its central part in the Omicron 21L/BA.2 variant. Current strategies of variant screening by qPCR therefore fail to detect this recombinant as they target mostly mutations in the spike gene, or other genomic regions that do not allow identifying it either. In the two genomic assemblies, the mean (±standard deviation) sequencing depth at positions harboring mutations relatively to the genome of the Wuhan-Hu-1 isolate was 271 ± 164 and 1362 ± 1146 reads and the mean prevalence of the majoritary nucleotide was 99.3 ± 2.2% and 98.8 ± 1.6%, respectively, which rules out the concommitant presence of the sequences of two variants in the samples, either due to co-infection or to contamination.
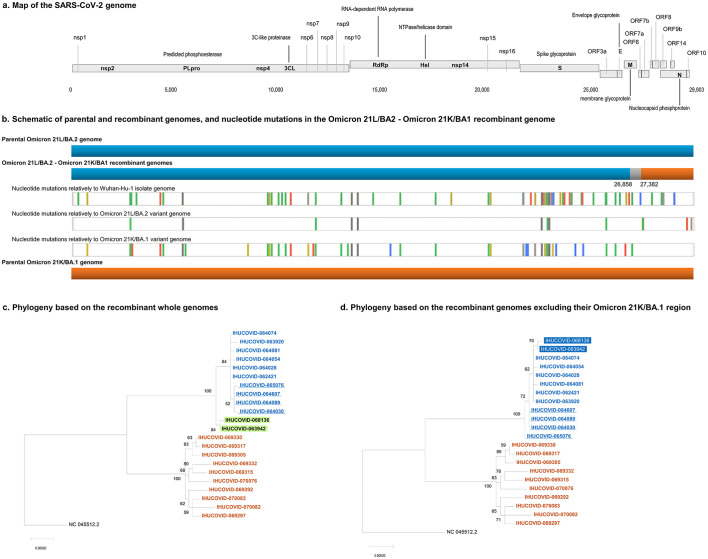
Fig. 2.
Schematic of the SARS-CoV-2 Omicron 21L/BA.2-21K/BA.1 recombinant genomes (a, b) and phylogeny reconstruction based on SARS-CoV-2 Omicron 21L/BA.2 and Omicron 21K/BA.1 genomes (c, d).
a. Map of the SARS-CoV-2 genome.
b. Schematic representation of parental and recombinant genomes and mutations in the recombinant genomes. Adapted from screenshots of the nextclade web application output (https://clades.nextstrain.org) (Aksamentov et al., 2021; Hadfield et al., 2018). Color codes for nucleotide mutations are as follows: Green: U; yellow: G; blue: C; red: A; light grey: deletions; dark grey: regions uncovered by sequencing reads.
c, d. Phylogeny reconstructions based on the whole genomes (c) or only on the region of these genomes that corresponds to the region of the recombinant genome that originates from the Omicron 21L/BA.2 variant. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
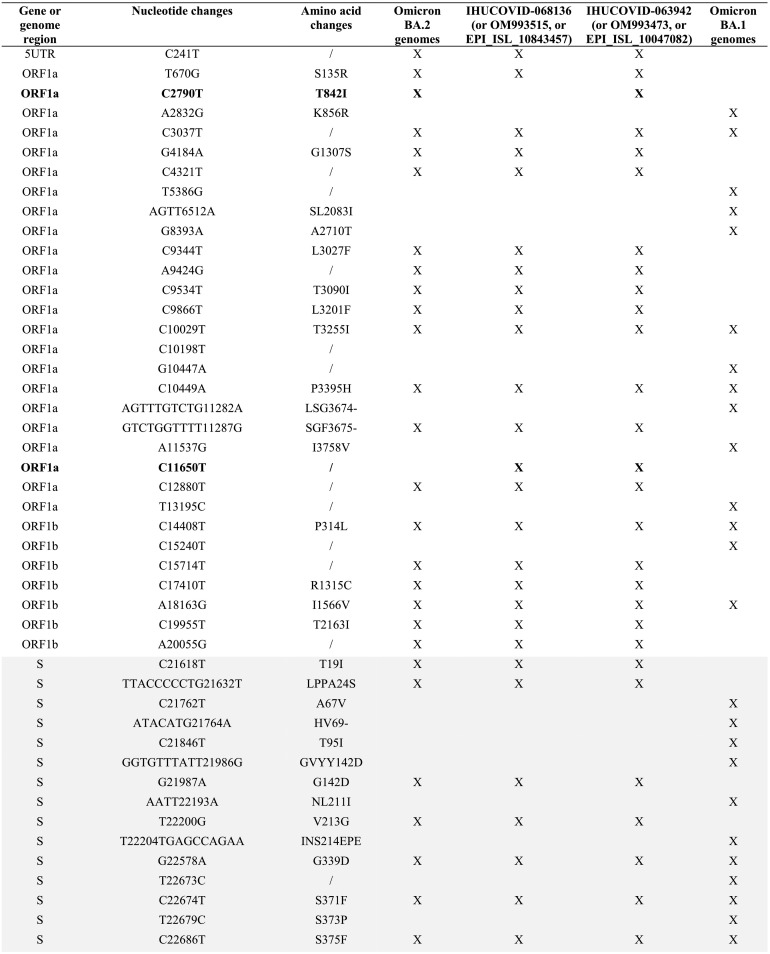
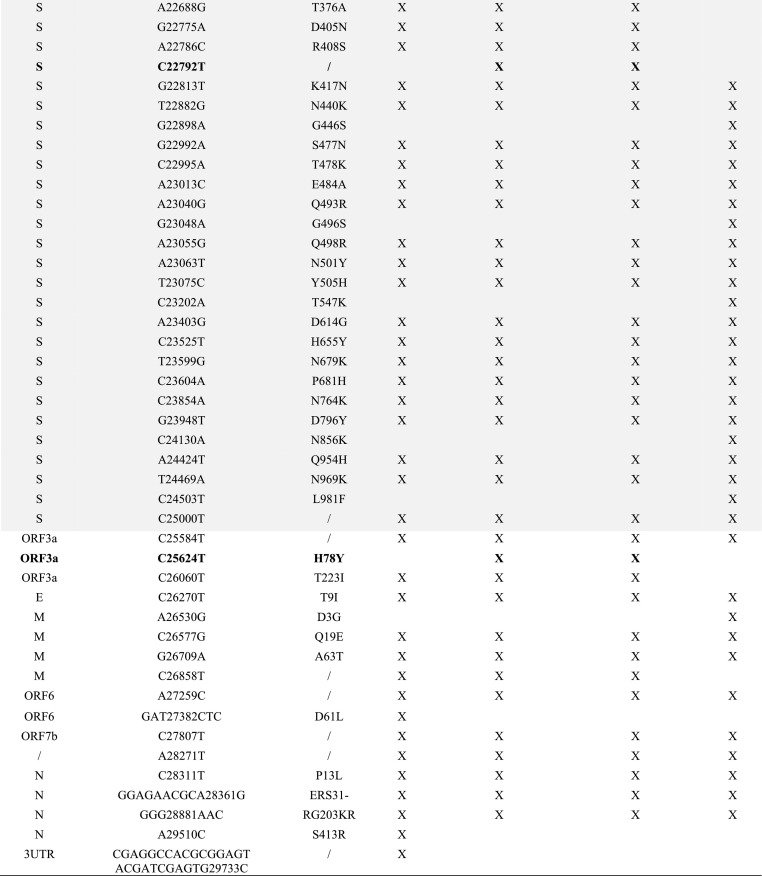
The two recombinant genomes harbor 65 mutations relatively to the genome of the Wuhan-Hu-1 isolate, including 3 or 4 that are not signature mutations of the parental Omicron 21L/BA.2 or Omicron 21K/BA.1 genomes (Table 1 ). The spike genes of the two genomes only harbor signature mutations of the Omicron 21L/BA.2 variant, apart from a synonymous mutation in one genome. Besides, these two genomes differ between each other by a single mutation in the ORF1 gene (C2790U; T841I).
Table 1.
Nucleotide and amino acid changes in the MixOmicron recombinant according to their presence/absence in the Omicron 21L/BA.2 and Omicron 21K/BA.1 variants.
/, no change; UTR, untranslated region; S gene region is indicated by a grey background; X, present.
Mutations with a bold font are those that are not signature mutations of the Omicron BA.1 or Omicron BA.2 variants.
The two phylogenetic trees that were built, based either on the whole genomes or only on the region of these genomes that originate from the Omicron 21L/BA.2 variant in the recombinants, did not show exactly the same topology (Fig. 2c, d). The trees incorporated the 10 genomes of Omicron 21L/BA.2 and 21K/BA.1 variants from the sequence database of our institute that were the most similar to the sequences of the recombinants. In both trees, sequences from the recombinants were clustered with sequences of the Omicron 21L/BA.2 variant. Nonetheless, whole recombinant genomes were clustered separately, apart from their best hits, in the Omicron 21L/BA.2 clade that encompassed two sister groups. In contrast, the two partial recombinant genomes were clustered together but nested inside the Omicron 21L/BA.2 clade.
4. Discussion
In the present work we show a recombination between Omicron BA.2 and BA.1 variant viruses. Cases of detection of recombinant genomes have been increasingly reported in 2022 (Wertheim et al., 2022; Sekizuka et al., 2022; Colson et al., 2022b; Lacek et al., 2022; Bolze et al., 2022; Ou et al., 2022; Belén Pisano et al., 2022; Burel et al., 2022) illustrating that recombination is a significant evolutionary pathway in SARS-CoV-2 (Rochman et al., 2022). The SARS-Cov2 recombination rate has been estimated in previous studies. The proportion of recombinant genomes was 0.006% (16 out of a set of 279,000 genomes) (Jackson et al., 2021); 0.219% (1175 of 537,360) (VanInsberghe et al., 2021); 0.257% (225 of 87,695) (Varabyou et al., 2021); or 2.7% (43,104 of approximately 1.6 million with 589 recombination events) (Turakhia et al., 2022). In addition, VanInsberghe et al. reported that up to 5% of SARS-CoV-2 that circulated in the USA and UK might have been recombinants (VanInsberghe et al., 2021). Turkahia et al. also reported that the ratio of variable positions contributed by recombination versus that contributed by de novo mutation was 0.00264, which is about 100-times lower than estimated for MERS-coronavirus (Turakhia et al., 2022). Muller et al. reported that the recombination rate of SARS-like viruses including SARS-CoV-1, SARS-CoV-2 and related bat and pangolin coronaviruses was about 2 × 10−6 per site per year, which is about 0.06 recombination event per lineage per year, being a rate approximately 200 times lower than the evolutionary rate (Müller et al., 2021). In comparison, recombination rates for human seasonal coronaviruses was reported to be a little higher, about 1 × 10−5 per site per year, being approximately 10–20 times lower than the evolutionary rate. Ignatieva et al. estimated that the recombination rate was >4 × 10−5 per site per year (Ignatieva et al., 2022).
As a matter of fact, the SARS-CoV-2 recombination rate depends of several parameters. Recombinations require co-circulation of parental viruses and co-infection of same host cells to occur. Thus recombinants are all the more likely to be generated when different variants co-circulate with high incidence levels. In our geographical area, >15,000 infections with the Omicron 21K/BA.1 variant and > 1000 infections with the Omicron 21L/BA.2 variant were diagnosed over the same period of 11 weeks between late December and mid-March (Fig. 1). The frequency of intra-host recombination is also potentially enhanced when the duration of viral carriage is elongated, which is the case in immunocompromised individuals who can remain infected for several months (Burel et al., 2022). Moreover, recombinants become more easily identifiable during bioinformatic analyses due to the accumulation over time of mutations along SARS-CoV-2 genomes, at intervals of increasingly reduced size. Indeed, the detection of recombinants often uses the identification of combinations of mutations that are hallmarks of a given lineage. Hence, the greater the number of such hallmark mutations within the genomes, the greater the ability to identify combinations of mutations from different lineages indicating recombination events.
In the present work, it is worthy to note that the two phylogeny reconstructions based on whole genomes or on their region that originate from the Omicron 21L/BA.2 variant in the recombinants exhibited slightly different topologies. This emphasizes that phylogenetic analyses do not accurately handle genomes that are hybrids of sequences with different evolutionary histories, which prompts building separate trees for sequences of different origins in the case of recombinants.
The spike gene of the recombinant virus identified here is typical of that of the Omicron 21L/BA.2 variant, which suggests similar phenotypic features regarding immune escape (Yu et al., 2022; Iketani et al., 2022). However, the acquisition by a Omicron 21L/BA.2 genome of the 3′ terminal part of a Omicron 21K/BA.1 genome is of very particular interest as this Omicron 21K/BA.1 fragment contains a short transposable element named S2m.
S2m is a 41-nucleotide long stem loop motif located in the 3′ untranslated region of the SARS-CoV-2 genome. Interestingly, it is present in four different distantly-related families of positive-sense single-stranded RNA viruses including Astroviridae, Caliciviridae, Picornaviridae, and Coronaviridae (Robertson et al., 2005; Imperatore et al., 2022). Among coronaviruses, it is present in sarbecoviruses, notably in SARS-CoV-1 and in most of the SARS-CoV-2 genomes available; its sequence in SARS-CoV-1 and SARS-CoV-2 differ by only two nucleotides. Strikingly, s2m also shows high levels of similarity with sequences of multiple insect species among which some belonging to the Araneae order (Tengs et al., 2021). Sequence conservation has been deemed to be related to a requirement for the elaboration of the three-dimensional structure (Lulla et al., 2021). The function of the S2m element is currently unclear. It was proposed to be involved in RNA interference pathways through its processing into a mature microRNA (Tengs et al., 2013), and in hijacking of host protein synthesis via interactions with ribosomal proteins (Robertson et al., 2005). The S2m of SARS-CoV-2 was recently reported to interact with the human cellular miRNA-1307-3p, which is predicted to regulate the translation of some interleukins (among which IL18), interleukin receptors, and the interferon alpha receptor, being putatively able to manipulate the host immune response (Imperatore et al., 2022). It was also reported that antisense oligonucleotides may interact with this s2m element, inhibit SARS-CoV-2 replication in culture (Lulla et al., 2021), decrease astrovirus titers and reduce the activity of an astrovirus replicon (Lulla et al., 2021; Janowski et al., 2022). However, Janowsky et al. did not observe that deletion or mutation of s2m of SARS-CoV-2 altered viral growth in vitro (Janowski et al., 2022). It was also hypothesized that s2m could intervene in the protection of the viral genome from degradation by host ribonucleases (Tengs and Jonassen, 2016). Otherwise, s2m was reported to form a homodimeric structure with the aid of the chaperone activity of the SARS-CoV-2 nucleocapsid protein, which may stabilize a dimerized form of the RNA genome (Imperatore et al., 2022). Also, it was hypothesized that s2m may have a role in genome circularization that was found to be essential for flavirus RNA replication (Tengs and Jonassen, 2016). Finally, S2m was reported to be involved in RNA recombination events (Gallaher, 2020). Although conserved in almost all SARS-CoV-2, s2m is absent or truncated in a few variants including the Eta (B.1.525), Iota (B.1.526) and B.1.640.1 lineages, which all had a low epidemic spread; and it is also truncated in the Omicron 21L/BA.2 variant. Taken together these data suggest that s2m could be considered as the equivalent of a virulence factor. This mobile genetic element could have initiated viral infection and pathogenicity in various animal hosts post-transfer. The consequence of the s2m acquisition by an Omicron 21L/BA.2 genome as reported here is unknown. A possibility would be a gain in transmissibility leading to a larger epidemic, which should be investigated by genotypic surveillance among SARS-CoV-2 diagnoses and phenotypic in vitro experiments. Hence, the present observation may contribute to gain a better understanding of factors that enhance SARS-CoV-2 spread and lead to the observed differences between the epidemic potential of the variants (Tao et al., 2021; Campbell et al., 2021).
Overall, the increasing identification of recombinant SARS-CoV-2 genomes worldwide highlights the unpredictable nature of the genetic variability of this virus. The recombinant described here is not detected by current strategies that screen for variants in routine diagnosis by qPCR systems that target a single or at best a few mutations, often very close to each other in the genome, which does not allow detecting hybrid genomes. This emphasizes the interest of the most exhaustive whole-genome based surveillance possible to allow deciphering the genetic pathways of the variability and investigating their phenotypic consequences regarding transmissibility, clinical severity, and escape from neutralizing antibodies.
Author contributions
Conceptualization: Philippe Colson, Pierre-Edouard Fournier, Bernard La Scola, Didier Raoult. Methodology, Investigation, Formal analysis: Philippe Colson, Jeremy Delerce, Elise Marion-Paris, Jean-Christophe Lagier, Anthony Levasseur. Data analyses: Philippe Colson, Jeremy Delerce, Elise Marion-Paris, Jean-Christophe Lagier, Anthony Levasseur, Pierre-Edouard Fournier, Bernard La Scola, Didier Raoult. Writing -original draft of the manuscript: Philippe Colson, Pierre-Edouard Fournier, Bernard La Scola, Didier Raoult. Writing - review & editing: all authors.
Funding
This work was supported by the French Government under the “Investments for the Future” program managed by the National Agency for Research (ANR) (Méditerranée-Infection 10-IAHU-03), by the Région Provence Alpes Côte d'Azur and European funding FEDER PRIMMI (Fonds Européen de Développement Régional-Plateformes de Recherche et d'Innovation Mutualisées Méditerranée Infection) (FEDER PA 0000320 PRIMMI), and by the French Ministry of Higher Education, Research and Innovation (Ministère de l'Enseignement supérieur, de la Recherche et de l'Innovation) and the French Ministry of Solidarity and Health (Ministère des Solidarités et de la Santé).
Data availability
Genome sequences generated and analyzed in the present study are available from the NCBI GenBank nucleotide sequence database (https://www.ncbi.nlm.nih.gov/genbank/) (Sayers et al., 2022) (Accession no. OM993515 and OM993473), from the IHU Méditerranée Infection website (https://www.mediterranee-infection.com/sars-cov-2-recombinant/) (IHUCOVID-063942 and IHUCOVID-068136), and from the GISAID database (https://www.gisaid.org/) (Elbe and Buckland-Merrett, 2017; Alm et al., 2020) (EPI_ISL_10843457, EPI_ISL_10047082).
Conflicts of interest
The authors have no conflicts of interest to declare relative to the present study. Didier Raoult was a consultant for the Hitachi High-Technologies Corporation, Tokyo, Japan from 2018 to 2020. He is a scientific board member of the Eurofins company and a founder of a microbial culture company (Culture Top). Funding sources had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, and the preparation, review, or approval of the manuscript.
Ethics
This study has been approved by the ethics committee of the University Hospital Institute Méditerranée Infection (No. 2022–008). Access to the patients' biological and registry data issued from the hospital information system was approved by the data protection committee of Assistance Publique-Hôpitaux de Marseille (APHM) and was recorded in the European General Data Protection Regulation registry under number RGPD/APHM 2019–73.
Acknowledgments
We are very grateful to Raphael Tola, Ludivine Bréchard, and Claudia Andrieu for their technical help.
Data availability
Genome sequenGenome sequences generated and analyzed in the present study are available from the NCBI GenBank nucleotide sequence database (Accession no. OM993515 and OM993473)
References
- Aksamentov I., Roemer C., Hodcroft E.B., Neher R.A. Nextclade: clade assignment, mutation calling and quality control for viral genomes. Zenodo. 2021 doi: 10.5281/zenodo.5607694. [DOI] [Google Scholar]
- Alm E., Broberg E.K., Connor T., et al. Geographical and temporal distribution of SARS-CoV-2 clades in the WHO European Region, January to June 2020. Euro Surveill. 2020;25:2001410. doi: 10.2807/1560-7917.ES.2020.25.32.2001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Belén Pisano M., Sicilia P., Zeballos M., et al. SARS-CoV-2 genomic surveillance enables the identification of Delta/Omicron co-infections in Argentina. medRxiv. 2022 doi: 10.1101/2022.03.08.22270920. [DOI] [Google Scholar]
- Bentley K., Evans D.J. Mechanisms and consequences of positive-strand RNA virus recombination. J. Gen. Virol. 2018;99:1345–1356. doi: 10.1099/jgv.0.001142. [DOI] [PubMed] [Google Scholar]
- Bolze A., White S., Basler T., et al. Evidence for SARS-CoV-2 Delta and Omicron co-infections and recombination. medRxiv. 2022 doi: 10.1101/2022.03.09.22272113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burel E., Colson P., Lagier J.C., et al. Sequential appearance and isolation of a SARS-CoV-2 recombinant between two major SARS-CoV-2 variants in a chronically infected immunocompromised patient. medRxiv. 2022 doi: 10.1101/2022.03.21.22272673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F., Archer B., Laurenson-Schafer H., et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26:2100509. doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Fournier P.E., Chaudet H., et al. Analysis of SARS-CoV-2 variants from 24,181 patients exemplifies the role of globalization and zoonosis in pandemics. Front. Microbiol. 2022;12 doi: 10.3389/fmicb.2021.786233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Fournier P.E., Delerce J., et al. Culture and identification of a “Deltamicron” SARS-CoV-2 in a three cases cluster in southern France. medRxiv. 2022 doi: 10.1101/2022.03.03.22271812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P., Bonfield J.K., Liddle J., et al. Twelve years of SAMtools and BCFtools. GigaScience. 2021;10 doi: 10.1093/gigascience/giab008. giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbe S., Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Global Chall. 2017;1:33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W.R. A palindromic RNA sequence as a common breakpoint contributor to copy-choice recombination in SARS-CoV-2. Arch. Virol. 2020;165:2341–2348. doi: 10.1007/s00705-020-04750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison E., Marth G. Haplotype-Based Variant Detection from Short-Read Sequencing. 2012. https://arxiv.org/abs/1207.3907 arXiv.org.
- Gribble J., Stevens L.J., Agostini M.L., et al. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad D., John S.E., Mohammad A., et al. SARS-CoV-2: possible recombination and emergence of potentially more virulent strains. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield J., Megill C., Bell S.M., et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics. 2018;34:4121–4123. doi: 10.1093/bioinformatics/bty407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Ma W., Dang S., et al. Possible recombination between two variants of concern in a COVID-19 patient. Emerg. Microbes Infect. 2022;11:552–555. doi: 10.1080/22221751.2022.2032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodcroft E. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. 2021. https://covariants.org/https://covariants.org/ Available from:
- Hodcroft E.B., Zuber M., Nadeau S., et al. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature. 2021;595:707–712. doi: 10.1038/s41586-021-03677-y. [DOI] [PubMed] [Google Scholar]
- Hosch S., Mpina M., Nyakurungu E., et al. Genomic surveillance enables the identification of co-infections with multiple SARS-CoV-2 lineages in Equatorial Guinea. Front. Public Health. 2022;9 doi: 10.3389/fpubh.2021.818401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatieva A., Hein J., Jenkins P.A. Ongoing recombination in SARS-CoV-2 revealed through genealogical reconstruction. Mol. Biol. Evol. 2022;39:msac028. doi: 10.1093/molbev/msac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iketani S., Liu L., Guo Y., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. 2022 Mar 3Nature. doi: 10.1038/s41586-022-04594-4. Epub ahead of print. PMID: 35240676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperatore J.A., Cunningham C.L., Pellegrene K.A., et al. Highly conserved s2m element of SARS-CoV-2 dimerizes via a kissing complex and interacts with host miRNA-1307-3p. Nucleic Acids Res. 2022;50:1017–1032. doi: 10.1093/nar/gkab1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B., Boni M.F., Bull M.J., et al. Generation and transmission of interlineage recombinants in the SARS-CoV-2 pandemic. Cell. 2021;184:5179–5188. doi: 10.1016/j.cell.2021.08.014. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski A.B., Jiang H., Fujii C., et al. The highly conserved stem-loop II motif is important for the lifecycle of astroviruses but dispensable for SARS-CoV-2. bioRxiv. 2022 doi: 10.1101/2022.04.30.486882. 2022.04.30.486882. [DOI] [Google Scholar]
- Katoh K., Misawa K., Kuma K., et al. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreier F. Deltacron: the story of the variant that wasn't. Nature. 2022;602:19. doi: 10.1038/d41586-022-00149-9. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacek K.A., Rambo-Martin B.L., Batra D., et al. Identification of a novel SARS-CoV-2 Delta-Omicron recombinant virus in the United States. bioRxiv. 2022 doi: 10.1101/2022.03.19.484981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C. Recombination in large RNA viruses: coronaviruses. Semin. Virol. 1996;7:381–388. doi: 10.1006/smvy.1996.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemey P., Ruktanonchai N., Hong S.L., et al. Untangling introductions and persistence in COVID-19 resurgence in Europe. Nature. 2021;595:713–717. doi: 10.1038/s41586-021-03754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrasbi-Nejad A. Detection of homologous recombination events in SARS-CoV-2. Biotechnol. Lett. 2022;17:1–16. doi: 10.1007/s10529-021-03218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lulla V., Wandel M.P., Bandyra K.J., et al. Targeting the conserved stem loop 2 motif in the SARS-CoV-2 genome. J. Virol. 2021;95(14) doi: 10.1128/JVI.00663-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N.F., Kistler K.E., Bedford T. Recombination patterns in coronaviruses. bioRxiv. 2021 doi: 10.1101/2021.04.28.441806. 2021.04.28.441806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J., Lan W., Wu X., et al. Tracking SARS-CoV-2 Omicron diverse spike gene mutations identifies multiple inter-variant recombination events. bioRxiv. 2022 doi: 10.1101/2022.03.13.484129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A., Holmes E.C., O’Toole A., et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020;5:1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M.P., Igel H., Baertsch R., et al. The structure of a rigorously conserved RNA element within the SARS virus genome. PLoS Biol. 2005;3:86–94. doi: 10.1371/journal.pbio.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman N.D., Wolf Y.I., Koonin E.V. Molecular adaptations during viral epidemics. EMBO Rep. 2022;23(8) doi: 10.15252/embr.202255393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett J.D., Gall M., Sim E.M., et al. Co-infection with SARS-CoV-2 Omicron and Delta variants revealed by genomic surveillance. medRxiv. 2022 doi: 10.1101/2022.02.13.22270755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers E.W., Cavanaugh M., Clark K., et al. GenBank. Nucleic Acids Res. 2022;50:D161–D164. doi: 10.1093/nar/gkab1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekizuka T., Itokawa K., Saito M., et al. Genome recombination between Delta and Alpha variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Jpn. J. Infect. Dis. 2022 Feb 28 doi: 10.7883/yoken.JJID.2021.844. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- So R.T.Y., Chu D.K.W., Miguel E., et al. Diversity of dromedary camel coronavirus HKU23 in African camels revealed multiple recombination events among closely related Betacoronaviruses of the subgenus Embecovirus. J. Virol. 2019;93 doi: 10.1128/JVI.01236-19. e01236–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghizadeh P., Salehi S., Heshmati A., et al. Study on SARS-CoV-2 strains in Iran reveals potential contribution of co-infection with and recombination between different strains to the emergence of new strains. Virology. 2021;562:63–73. doi: 10.1016/j.virol.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K., Tzou P.L., Nouhin J., et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021;22:757–773. doi: 10.1038/s41576-021-00408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengs T., Jonassen C.M. Distribution and evolutionary history of the mobile genetic element s2m in coronaviruses. Diseases. 2016 Jul 28;4(3):27. doi: 10.3390/diseases4030027. PMID: 28933407; PMCID: PMC5456283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengs T., Kristoffersen A.B., Bachvaroff T.R., Jonassen C.M. A mobile genetic element with unknown function found in distantly related viruses. Virol. J. 2013;10:132. doi: 10.1186/1743-422X-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengs T., Delwiche C.F., Monceyron J.C. A genetic element in the SARS-CoV-2 genome is shared with multiple insect species. J. Gen. Virol. 2021;102 doi: 10.1099/jgv.0.001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turakhia Y., Thornlow B., Hinrichs A., et al. Pandemic-scale phylogenomics reveals the SARS-CoV-2 recombination landscape. Nature. 2022 Aug 11 doi: 10.1038/s41586-022-05189-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanInsberghe D., Neish A.S., Lowen A.C., Koelle K. Recombinant SARS-CoV-2 genomes are currently circulating at low levels. bioRxiv. 2021 doi: 10.1101/2020.08.05.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varabyou A., Pockrandt C., Salzberg S.L., Pertea M. Rapid detection of inter-clade recombination in SARS-CoV-2 with Bolotie. Genetics. 2021;218:iyab074. doi: 10.1093/genetics/iyab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim J.O., Wang J.C., Leelawong M., et al. Capturing intrahost recombination of SARS-CoV-2 during superinfection with Alpha and Epsilon variants in New York City. medRxiv. 2022 doi: 10.1101/2022.01.18.22269300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh T.Y., Contreras G.P. Emerging viral mutants in Australia suggest RNA recombination event in the SARS-CoV-2 genome. Med. J. Aust. 2020;213:44–44.e1. doi: 10.5694/mja2.50657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H. 2019 novel coronavirus is undergoing active recombination. Clin. Infect. Dis. 2020;71:884–887. doi: 10.1093/cid/ciaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Collier A.Y., Rowe M., et al. Neutralization of the SARS-CoV-2 Omicron BA.1 and BA.2 variants. 2022, Mar 16N. Engl. J. Med. doi: 10.1056/NEJMc2201849. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li J., Xiao Y., et al. Genotype shift in human coronavirus OC43 and emergence of a novel genotype by natural recombination. J. Inf. Secur. 2015;70:641–650. doi: 10.1016/j.jinf.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Genome sequences generated and analyzed in the present study are available from the NCBI GenBank nucleotide sequence database (https://www.ncbi.nlm.nih.gov/genbank/) (Sayers et al., 2022) (Accession no. OM993515 and OM993473), from the IHU Méditerranée Infection website (https://www.mediterranee-infection.com/sars-cov-2-recombinant/) (IHUCOVID-063942 and IHUCOVID-068136), and from the GISAID database (https://www.gisaid.org/) (Elbe and Buckland-Merrett, 2017; Alm et al., 2020) (EPI_ISL_10843457, EPI_ISL_10047082).
Genome sequenGenome sequences generated and analyzed in the present study are available from the NCBI GenBank nucleotide sequence database (Accession no. OM993515 and OM993473)