Abstract
Coronavirus 2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) has had significant impacts worldwide since its emergence in December, 2019. Despite a high recovery rate, there is a growing concern over its residual, long-term effects. However, because of a lack of long-term data, we are still far from establishing a consensus on post-COVID-19 complications. The deposition of excessive extracellular matrix (ECM), known as fibrosis, has been observed in numerous survivors of COVID-19. Given the exceptionally high number of individuals affected, there is an urgent need to address the emergence of fibrosis post-COVID-19. In this review, we discuss the clinical relevance of COVID-19-associated fibrosis, the current status of antifibrotic agents, novel antifibrotic targets, and challenges to its management.
Keywords: COVID-19, SARS-CoV-2, Fibrosis, Extracellular matrix, Antifibrotic agents
Introduction
COVID-19 first emerged in December 2019 in Wuhan, China, and was declared a pandemic by the WHO on 11 March, 2020. It is caused by a novel virus, named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2, 2019-nCoV) because of its striking resemblance to SARS-CoV. By mid-2022, more than 6.36 million people had died from COVID-19 and more than 558 million active cases had been reported. SARS-CoV-2 primarily affects the pulmonary organs, although multiple organs can also be affected. The virus causes infections from mild cold, fever, and acute inflammation to severe acute respiratory distress syndrome (ARDS). Associated symptoms include headache, generalised weakness, vomiting and diarrhoea, and lower respiratory tract infection-related symptoms, such as dry cough and dyspnoea. In the acute phase, SARS-CoV-2 infection drastically increases the production of proinflammatory cytokines, resulting in severe lung damage, fibrosis and multiorgan dysfunction.1
However, one of the biggest concerns arises from the emergence of post COVID-19 fibrosis.2 Xu et al. provided early evidence that SARS-CoV-2 infection causes upregulation of the mRNA levels of fibrosis drivers, such as angiotensin-converting enzyme 2 (ACE2), transforming growth factor-β1 (TGFb1), connective tissue growth factor (CTGF), and fibronectin 1 (FN1).3 Later, it was established that around one-third of patients demonstrated SARS-COV-2-associated pulmonary fibrosis.4 The established fibrosis causes further deterioration of the already-compromised pulmonary functions in survivors of COVID-19. In addition, COVID-19 infection affects not only the lungs, but also other organs, such as liver and kidney, among others.5, 6 However, because of the limited treatment options, the management of organ fibrosis in survivors of COVID-19 remains a challenge. Therefore, a clear understanding of fibrosis progression is essential for fibrogenesis management in survivors of COVID-19, which would decrease the global health burden associated with the pandemic.
COVID-19-associated fibrosis: Clinical relevance
COVID-19 infection primarily affects the lungs, resulting in pneumonia, or ARDS-like conditions, in severe cases. ARDS is observed in almost 40% of cases. Despite a high recovery rate, 70–80% of patients do not recover fully and present with at least one symptom, even after becoming COVID-19 free. Given the wide impact of the disease, even a minor proportion of patients affected by fibrosis-like conditions warrants investigation of this condition. COVID-19-affected lungs develop fibrosis primarily as a result of viral- and immune-mediated mechanisms. In response to lung injury, persistent inflammation initiates fibrotic signalling, leading to compromised organ functions. Typical imaging features of fibrosis include ground glass opacities (GGOs), consolidation patterns, reticulations, and mixed lesions, as observed on computerised tomography (CT) scans. In addition, these CT scan results are correlated with histopathological findings, such as interstitial oedema, inflammatory infiltration, fibrin deposition, alveolar oedema, hyaline membrane deposition, diffuse alveolar damage, necrotizing and non-necrotizing vasculitis, capillary congestion, and collagen deposition.7, 8
Although COVID-19 infection primarily affects the lungs, the hyperactivated immune response increases the risk of multiple organ failure. Apart from lungs, COVID-19 infection also affects kidney function.9, 10 SARS-CoV-2 can directly infect kidney cells and induced fibrosis in human-induced pluripotent stem cell-derived kidney organoids.11 Meta-analysis studies showed that severe acute kidney injury (AKI) is a primary cause of mortality in patients with COVID-19,12 resulting from acute renal failure, increases in plasma creatinine, and rhabdomyolysis suggestive of severe renal injury.13, 14 Given the abundance of ACE2 in renal cells and its utilisation by the virus to gain entry to host cells, it is suggested that COVID-19 infection activates the renin–angiotensin–aldosterone system (RAAS) to enhance levels of angiotensin II, a profibrotic agent. Histopathological findings from clinical samples show tubular injury, obstruction of the peritubular and glomerular capillary loops, lymphocytic endothelialitis, and viral inclusion particles, which can aggravate fibrosis progression in the kidneys.
As another viral target of the virus, the liver is also affected by COVID-19. Clinical studies reported hepatocellular injury in 14–53% of patients hospitalised with severe COVID-19.15, 16, 17 In another study, 43 out of 99 patients with COVID-19 demonstrated varying degrees of liver damage along with increased levels of liver injury markers.18 In addition, elevated bilirubin levels in patients hospitalised with COVID-19 were also linked with severe alterations of liver function.19, 20 Although it is not known whether such liver damage was due to COVID-19 or to drug exposure, ACE2 expression in the liver suggests that this is an organ affected by COVID-19.21 Another study demonstrated that ACE2 occurs in cholangiocytes and that liver damage could be the result of the specific involvement of these cells rather than of hepatocytes.22 A recent study showed that 65% of individuals infected with COVID-19 had an increased liver fibrosis index (FIB-4).5 Thus, given such evidence, a new paradigm involving severe liver fibrosis in COVID-19 warrants research attention.
Basic mechanisms of fibrosis
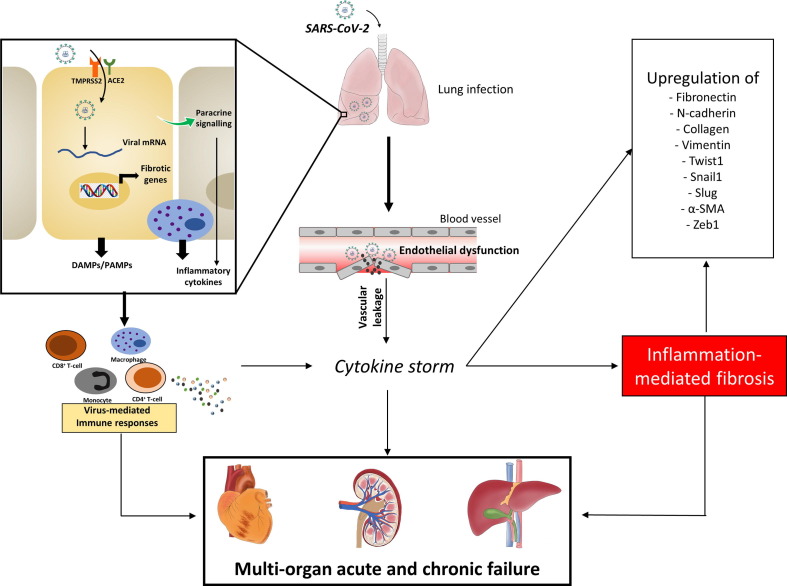
The establishment of fibrotic scars is a result of an uncontrolled wound-healing process. In normal wound healing, inflammatory pathways are activated in response to injury, resulting in the activation of wound-healing signals followed by the apoptotic death of activated fibroblasts or myofibroblasts. However, in fibrotic reactions, the wound-healing process never comes to a halt, primarily because of the persistent nature of the injury. The release of inflammatory cytokines/chemokines and growth factors from immune cells activates fibrotic signalling to cause interstitial fibrosis.23 In addition, paracrine signalling from injured cells fuels the recruitment of inflammatory cells and help to remodel the ECM. In addition, the reduced activity of matrix metalloproteinases (MMPs), which are matrix-degrading enzymes, aggravates this process and results in the formation of a highly stable ECM-rich matrix. Clinical cases of COVID-19 have also shown symptoms of remodelled matrices rich in collagens.24 Despite no concrete evidence of long-term pulmonary fibrosis in survivors, this remodelling and developed fibrosis compromise organ function and, thus, requires research attention. The intricate relationship between inflammation, cytokine storm and fibrosis is detailed in Fig. 1 .
Figure 1.
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infection, cytokine storm, and fibrosis induction. Pulmonary infection with SARS-CoV-2 can result in the activation of immune responses and inflammatory pathways, leading to an exaggerated release of cytokines in acute manner, also known as a cytokine storm. Once the virus enters the cell, it releases its genetic material into the cytoplasm and causes pyroptotic cellular injury, which activates paracrine signalling in nearby cells. The affected cells, nearby cells, and tissue macrophages release cytokines/chemokines, giving rise to a cytokine storm. By contrast, when the virus is absorbed in the systemic circulation, it can directly affect other organs, such as liver, heart, and kidneys. The increased immune response along with inflammation results in the induced transcription of fibrotic genes causing organ fibrosis. Abbreviations: ACE2, angiotensin-converting enzyme 2; DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern.
Myofibroblasts
Fibroblasts are one of the most dominant cells in the interstitial matrix and help to maintain the structural support and rigidity by secreting ECM components. However, activated fibroblasts, also known as myofibroblasts, secrete excessive ECM, increasing tissue stiffness and forming a scar. Myofibroblasts acquire mesenchymal characteristics, such as increased actin stress fibres and vimentin fibres, induced transcription factors (Snail1 and Slug), and increased motility, among others. In addition, myofibroblasts have microfilament bundles, which form a fibronexus, a specialised adhesion complex that connects the internal microfilaments of myofibroblast with fibronectin in the extracellular environment. This bridging helps these cells to produce and transmit contractile forces to the surrounding ECM, which can be strengthened by collagen deposition under fibrotic conditions.25 The myofibroblast population is well correlated with fibrosis severity and, accordingly, strategies to reduce the myofibroblast population or inhibit their activation are beneficial in reducing fibrosis. The clinical presentation of patients with severe COVID-19 also includes excessive proliferation of myofibroblasts,26, 27 suggestive of their crucial involvement in fibroprogression in these patients.
Epithelial–mesenchymal transition
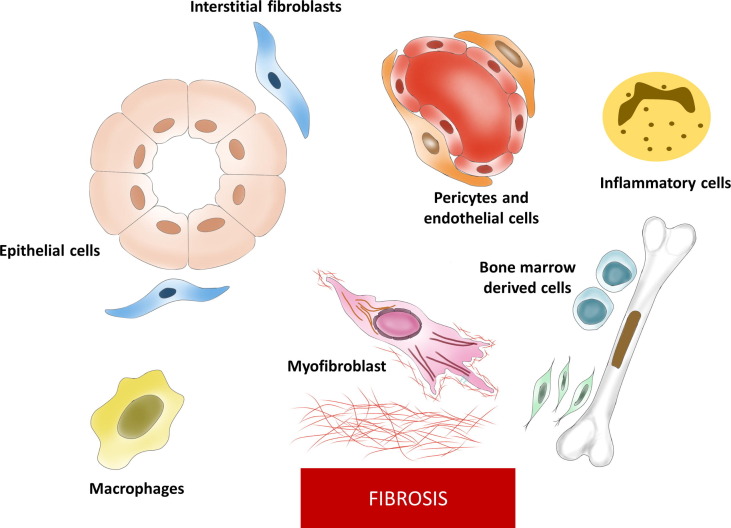
Epithelial–mesenchymal transition (EMT) is one of the basic programs primarily activated during the initial stages of life. However, EMT reactivation is observed in multiple disorders, particularly in cancer and fibrosis. EMT activation leads to the transformation of epithelial cells to a mesenchymal subtype through a series of events. When the EMT program is activated, epithelial cells start to lose cell–cell junctions, causing them to detach from the basement membrane, gain migratory properties, and overexpress mesenchymal markers. This transformation favours the deposition of ECM proteins secreted by activated fibroblasts/myofibroblasts. Although the contributing role of epithelial cells to EMT program has been a topic of debate because of the involvement of several cell types,28 there is evidence that EMT program activation is crucial for fibrogenesis, irrespective of the cell type involved.29, 30 It is now believed that epithelial cells mainly undergo partial EMT activation during fibrosis. The major players involved in EMT program and deposition of fibrotic matrix are shown in Fig. 2 .
Figure 2.
Major cells involved in epithelial–mesenchymal transition (EMT) and eventual deposition of fibrosis. Several cell types can undergo phenotypic transition to give rise to an increased population of myofibroblasts. These cells then release excessive extracellular matrix (ECM) proteins, contributing to fibrosis.
TGFβ1
TGFβ, a cytokine involved in an array of physiological functions, is mainly secreted by immune cells and generally present in plasma and ECM proteins. The abnormal activation of TGFβ signalling has been associated with fibrosis. Following injury, inflammatory and epithelial cells produce numerous profibrogenic mediators, including TGFβ1. Induced TGFβ1 signalling directly activates fibrotic responses through downstream canonical and non-canonical signalling. The former is mediated by the activation of Smad proteins. Phosphorylation of Smad2/3 leads to the formation of a complex with Smad4, which, after nuclear translocation, induces the transcription of fibrotic genes, such as SNAI1, SLUG, and Zeb1. By contrast, non-canonical signalling involves three different pathways: ERK1/2, p38, and AKT. TGFβ1 can affect multiple pathways implicated in fibrogenesis, such as myofibroblast proliferation, EMT activation, ECM expansion, inflammation, and induction of transcription factors.
Matrix metalloproteinases
MMPs are members of a family of extracellular endopeptidase that are mainly involved in the degradation of ECM substrates. The family has 25 members with contrasting effects on the establishment of fibrotic ECM. However, in general, MMPs have a proteolytic effect on ECM substrates. By contrast, tissue inhibitors of metalloproteinases (TIMPs) can block the catalytic activity of MMPs. Hence, the net effect on ECM is the result of the difference in the expression of MMPs versus that of TIMPs. MMP2 is activated in liver fibrosis and it highly expressed on myofibroblasts.31 However, Mmp2 –/– mice showed a higher degree of fibrosis compared with wild-type, suggesting the antifibrotic behaviour of MMP2.32 In addition, expression of MMP9 is downregulated in fibrotic rigidities.33 By contrast, MMP9 is profibrotic in nature,as shown in experimental fibrosis.34 Patients with COVID-19 also showed dysregulated levels of MMP2/MMP9.35 In line with this, targeting of MMPs has been suggested for COVID-19 management.36 However, because of the contradictory roles of MMPs under different disease conditions, it will be necessary to thoroughly dissect the involvement of MMPs before reaching any firm conclusion.
Inflammation
Inflammation is an integral component of fibrosis, given the causal link between inflammation and established fibrosis. The persistent activation of inflammatory pathways leads to uncontrolled wound healing, resulting in establishment of fibrotic foci. The exaggerated immune response mediated by sudden release of circulating proinflammatory cytokines, also called a ‘cytokine storm’, is an influential proinflammatory mechanism involved in the pathogenesis of COVID-19 and its associated complications.37 The activation of immune responses after SARS-CoV-2 infection results in increased recruitment of inflammatory cells, such as macrophages, monocytes, and neutrophils, which results in the massive release of cytokines. Patients with COVID-19 have demonstrated induced levels of multiple cytokines/chemokines, including IL2, IL7, IP10, MIP1α, MCP1, and TNFα. Given the stringent role of proinflammatory pathways in COVID-19-associated abnormalities, several therapeutic strategies have been proposed, such as steroids, monoclonal antibodies, JAK inhibitors, and NLRP3 inflammasome inhibitors. The partial success of anti-inflammatory agents in COVID-19 suggests that regulating proinflammatory mechanisms could be helpful to both contain the virus-mediated damage and provide antifibrotic effects.
Macrophages
Macrophages are one of the earliest cells to reach the site of injury to prepare for repair and wound healing. Locally released signals, such as damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs), chemokines, cytokines, and other chemotactic proteins trigger macrophage recruitment at the injury site. Macrophages then release several types of cytokine and other secreted factors necessary to remodel the matrix. Macrophage infiltration appears to be one of the most commonly observed processes in almost all types of fibrotic reaction. In addition, the degree of macrophage infiltration closely associates with the extent of fibrosis.38 Broadly, there are two subtypes of macrophage based on their phenotypes: classically activated M1 and alternatively activated M2 macrophages.39 Inflammatory agents, such as IFNγ and lipopolysaccharide (LPS), activate proinflammatory M1 macrophages, whereas IL4/13 induce the M2 phenotype. However, macrophage polarisation, a term given to the phenotypic conversion of the M1 subtype to M2, is associated with the profibrotic activity of macrophages. On a broader scale, the change from an M1 to an M2 subtype was demonstrated to induce fibrosis by releasing profibrotic mediators, such as TGFβ, IGF1, FGF2, and PDGF.40
Therapeutic options for fibrosis management
Despite a continuous increase in the number of patients with fibrosis, only limited therapeutic agents are available for their management. Only two drugs are currently approved for the pulmonary fibrosis: nintedanib and pirfenidone.
Pirfenidone works by inhibiting expression of multiple fibrotic and inflammatory mediators, primarily TGFβ1, TNFα, IL1β, and IL6. Inhibition of TGFβ1 and its downstream signalling by pirfenidone blocks fibroblast proliferation, myofibroblast differentiation, and, ultimately,collagen deposition. It can also upregulate Regulator of G-protein Signaling 2 (RGS2) to inhibit thrombin-dependent collagen deposition.41 Furthermore, it can be helpful in COVID-19 because of its broad-spectrum activity, including inhibition of CTGF, PDGF, TNFα, and reactive oxygen species. In addition, it can also downregulate the expression of ACE2 receptors, which are used by SARS-CoV-2 to gain cellular entry, thereby making it a valuable drug to use to inhibit viral entry. Furthermore, the anti-inflammatory activity of pirfenidone, notably against TNFα and the NLRP3 inflammasome, might also inhibit the cytokine storm. A recent trial reported that 4 weeks of treatment with pirfenidone improved lung inflammation and interstitial damage, and reduced the duration of hospitalisation, suggesting it as a viable drug for cases of severe COVID-19.42 Furthermore, 2 months of pirfenidone therapy improved dyspnoea and fibrosis symptoms, helping the patient to return to daily activities by the seventh month of treatment.43 Deupirfenidone (LYT-100), a deuterium-substituted analogue of pirfenidone, is in a clinical trial (NCT04652518) for its efficacy against post-acute COVID-19 respiratory diseases.
Nintedanib is an ATP-competitive inhibitor of receptor tyrosine kinase and has also proved to be beneficial in the management of COVID-19-associated pulmonary function decline.44, 45 A recent report showed that six months of nintedanib treatment reduced pulmonary fibrosis and restored lung functions, as shown by a reduced Borg score, improved total lung capacity, diffusive lung capacity for carbon monoxide (DLCO), CT scoring, and a 6-min walk test score.46 The adverse effects reported included mild dysphagia, frontal headache, and upper-extremity numbness, which eventually resolved a few weeks after treatment without causing any treatment discontinuation. Furthermore, two ongoing clinical trials (NCT04619680 and NCT04338802) aim to investigate the potential of nintedanib against COVID-19 associated pulmonary fibrosis.
In addition, various other investigational agents are in clinical trials for the management of fibrotic conditions, as detailed in Table 1 .
Table 1.
Molecules in clinical trials for pulmonary fibrosis, COVID-19-induced fibrosis, and associated conditions.a
| S. no. | Drug | Indication | Mechanism of action | Status | Clinical trial identifier |
|---|---|---|---|---|---|
| Potential molecules for COVID-19-associated fibrosis | |||||
| 1 | Pirfenidone | COVID-19-induced pulmonary fibrosis (PF) | Inhibits TGFβ | Phase II | NCT04607928 |
| 2 | Nintedanib | COVID-19-induced PF | Inhibits FGFRs, PDGFRs, and VEGFRs | Phase II | NCT04338802 |
| 3 | Deupirfenidone (Lyt-100) | COVID-19-induced PF | Inhibits IL6, TNFα, and TGFβ | Phase II | NCT04652518 |
| 4 | Fuzheng Huayu | COVID-19-induced PF | Inhibits hematopoietic stem cell activation and inflammation | Phase II | NCT04279197 |
| 5 | Sirolimus | COVID-19 pneumonia or post-COVID fibrosis | Inhibits T lymphocyte activation | Phase II/III | NCT04948203 |
| 6 | Canrenoate potassium | COVID-19-induced PF | Mineralocorticoid receptor antagonist | Phase IV | NCT04912011 |
| 7 | Longidaze (bovhyaluronidase azoxymer) | COVID-19-induced PF | Hyaluronic acid degradation | NA | NCT04645368 |
| 8 | Collagen-polyvinylpyrrolidone | Cytokine storm | Decreases inflammation and TGFβ | Phase I/II | NCT04517162 |
| 9 | Antifibrotic monocyte (MON002) | COVID-19-induced PF | Clears partially degraded collagen fragments | Phase I/II | NCT04805086 |
| 10 | Treamid | COVID-19 pneumonia | Metal ion chelator | Phase II | NCT04527354 |
| 11 | Tetrandrine | COVID-19 | Calcium channel blocker | Phase IV | NCT04308317 |
| 12 | Genistein nanoparticles (BIO300) | COVID-19 | Inhibits tyrosine kinase and topoisomerase II | Phase II | NCT04482595 |
| Antifibrotic agents under clinical trials | |||||
| 1 | HZN-825 | IPF, diffuse cutaneous scleroderma | Lysophosphatidic acid receptor 1 antagonist | Phase II | NCT05032066 |
| 2 | Pamrevlumab | IPF | Monoclonal antibody against CTGF | Phase III | NCT03955146 |
| 3 | Taladegib | IPF | Hh pathway inhibitor | Phase II | NCT04968574 |
| 4 | Lansoprazole | IPF | Proton pump inhibitor | Phase III | NCT04965298 |
| 5 | TRK-250 | IPF | Single-strand long-chain nucleic acid against TGFβ1 | Phase I | NCT03727802 |
| 6 | Inhaled nitric oxide | IPF | Soluble guanylate cyclase activator | Early Phase I | NCT05052229 |
| 7 | Vismodegib | IPF | Hh inhibitor | Phase I | NCT02648048 |
| 8 | GLPG1690 | IPF | Autotaxin inhibitor | Phase II | NCT02738801 |
| 9 | N-acetylcysteine | IPF | Antioxidant | Phase III (PRECISIONS trial) | NCT04300920 |
| 10 | CC-90001 | IPF | JNK inhibitor | Phase II | NCT03142191 |
| 11 | Autoantibody reductive therapy | IPF | Reduces autoantibodies | Phase II | NCT03286556 |
| 12 | Umbilical cord mesenchymal stem cells | COPD | Immunomodulation | Phase I | NCT05016817 |
| 13 | Saracatinib | IPF | Inhibits Src and Bcr-Abl tyrosine kinase | Phase II | NCT04598919 |
| 14 | Belumosudil | Systemic sclerosis | Inhibits ROCK2 | Phase II | NCT02688647 |
| 15 | ORIN1001 | IPF | Inhibits IRE1 | Phase I | NCT04643769 |
| 16 | Jaktinib | IPF | Inhibits JAK1–3 | Phase II | NCT04312594 |
| 17 | Morphine | IPF-associated cough | Opioid cough suppressant | Phase III | NCT04429516 |
| 18 | BMS-986278 | IPF | LPA1 antagonist | Phase II | NCT04308681 |
| 19 | Ifenprodil (NP120) | IPF and associated cough | NMDA antagonist | Phase II | NCT04318704 |
| 20 | GKT137831 | IPF | Inhibits NOX1/4 | Phase II | NCT03865927 |
| 21 | PLN-74809 | IPF | Dual-selective inhibitor of αVβ6 and αVβ1 | Phase II | NCT04396756 |
| 22 | GB0139 | IPF | Inhibits Galectin-3 | Phase II | NCT03832946 |
Source:https://www.clinicaltrials.gov.
Novel pharmacological targets for COVID-19-associated fibrosis
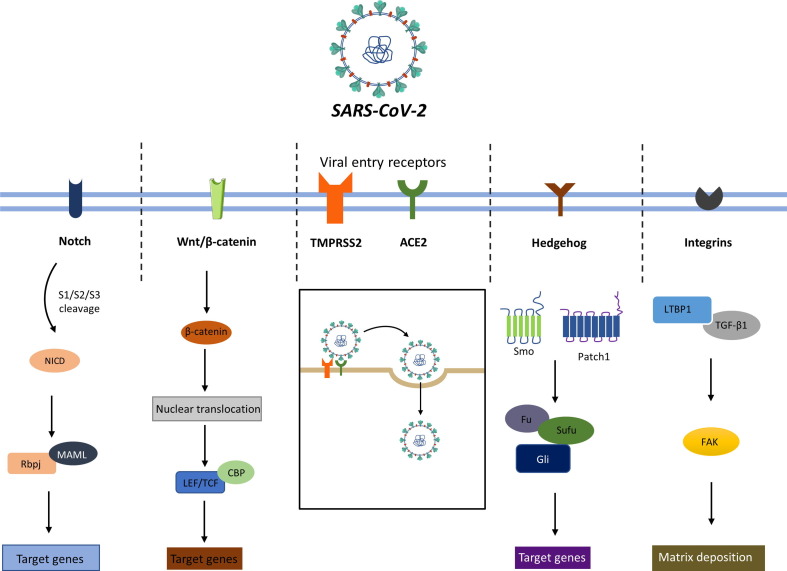
Although various pharmacological targets have already been proposed for the management of fibrosis, these are oriented mainly toward the management of COVID-19 infection. Here, we discuss novel targets for the prevention/resolution of the underlying fibrotic matrix to aid the management of interstitial fibrosis (Fig. 3 ).
Figure 3.
Entry of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) into the host cell using angiotensin-converting enzyme 2 (ACE2) and TMPRSS2 receptors. Viral infection activates certain important signalling pathways, including Notch, Wnt/β-catenin, Hedgehog (Hh) and integrins, which results in the induced transcription of target genes and increased matrix deposition. Furthermore, the crosstalk of these pathways with other fibrotic pathways augments the fibrotic sequalae and fuels the deposition of extracellular matrix (ECM) in the interstitial space in tissues.
Notch signalling
Notch is a well-known signalling pathway that involves four notch transmembrane receptors. Notch signalling has an important role in embryonic development and cell–cell communication. Activated Notch signalling promotes myofibroblast proliferation in the fibrosis of various organs, including lungs,47 liver,48 kidney,49 and heart.50 In addition, Notch signalling also promotes EMT activation, leading to phenotypic transition of epithelial cells to a mesenchymal subtype, further aggravating fibrogenesis.51 Increased Notch expression correlates with structural fibrotic anomalies in the lungs.52 A preclinical study showed the induction of Notch signalling in SARS-CoV-2 infection in macaques.53 Using computational tools, another study also suggested a link between Notch2 signalling and SARS-CoV-2 infection.54 Although no studies have performed direct manipulation of Notch signalling in SARS-CoV-2 infection, there might be an indirect link with Notch signalling because of the crucial role of Furin and ADAM17 in COVID-19 infection.55
Wnt/β-catenin
Wnt/β-catenin is involved in the regulation of various crucial cellular events,56 whereas abnormal Wnt/β-catenin signalling is implicated in inflammation, fibrosis, and cancer.57 Wnt ligands control immune cell modulation, inflammatory response, and tissue damage and repair.58 The upregulation of Wnt/β-catenin is observed in lung injury and ARDS.59 Given that Wnt/β-catenin signalling is crucial for the renewal of taste bud cells and taste perception,60 the loss of taste and smell observed in some patients with COVID-19 suggests a direct involvement of Wnt/β-catenin in COVID-19 infection. Furthermore, the increased release of TGFβ activates Wnt/β-catenin signalling and increases risk of pulmonary infection and fibrosis in COVID-19.61 Taylor et al. analyzed samples from patients with COVID-19 and observed alterations in the expression of nine genes that directly interact with either Wnt or β-catenin in the signalling pathway.62 This suggests that genetic or pharmacological blockade of Wnt/β-catenin as a useful therapeutic approach for the management of COVID-19-associated complications and fibrosis.
Lysyl oxidase
Lysyl oxidase (LOX) enzymes belong to a family of copper-dependent, amine oxidase enzymes that has five members (LOX and LOXL1–4). Their primary function is to crosslink collagen and elastin molecules, resulting in the formation of highly interlinked ECM. Given their crucial role in collagen stabilisation, these enzymes have attracted research attention. The dysregulated levels of these enzymes have been observed in the fibrosis of multiple organs, including lungs, kidneys, liver, heart, and pancreas. In addition, a non-competitive monoclonal antibody against LOXL2 (simtuzumab) was investigated for its efficacy in patients with idiopathic pulmonary fibrosis (IPF), although the results were not promising.63 Nevertheless, it is evident that these enzymes are involved in the establishment of fibrotic foci and targeting them could limit fibrosis progression.64 An in silico study proposed interactions between LOX protein and ORF8 of COVID-19.65 Thus, the influential role of LOX proteins in fibrosis warrants their exploration for the management of COVID-19 and associated fibrosis.
Hedgehog pathway
The Hedgehog (Hh) pathway is essential for proper embryonic development, organogenesis, homeostasis, and regeneration. Disrupted Hh signalling contributes significantly to the progression of cancer and fibrosis. Hh activation can induce fibrosis in preclinical66, 67 and clinical settings.68 Baratella et al. suggested the involvement of Shh signalling in patients with COVID-19-related pneumomediastinum, a rare complication of ARDS.69 In addition, the involvement of the Hh pathway in multiple physiological functions highlights a possible role in COVID-19-associated fibrosis. Hence, targeting Hh signalling could lead to the development of clinically effective therapies for the management of COVID-19-associated complications.
Integrins
Although ACE2 was identified as the primary receptor for SARS-CoV-2 binding and cell entry, recent evidence also suggests the involvement of other pathways, such as integrins. These are heterodimers used by animal cells as transmembrane linkers between the ECM and the cellular actin cytoskeleton. The spike (S) protein of SARS-CoV-2 has an arginine-glycine-aspartate (RGD) motif, which suggests the involvement of integrins in viral entry.70 The RGD sequence found in ECM proteins, including fibronectin and laminin, acts as a cell attachment site and helps to bind these ECM components with their integrin receptors. Integrin signalling is involved in fibrogenesis and demonstrates two-way interactions with TGFβ. Depletion of αv-integrins in hepatic stellate cells and myofibroblasts protected mice from CCl4-induced liver fibrosis.71 In addition, several viruses use RGD motifs to bind with the ECM domain of integrins and gain entry to host cells.72, 73 Furthermore, Sigrist et al., discussed the possibility of integrins as a route for host cell entry by SARS-CoV-2.70. To further strengthen the case for the effectiveness of integrin blockers, the recovery from COVID-19 was reported of a patient with multiple sclerosis who was treated with natalizumab (an α4 integrin antagonist) and who then demonstrated negative results in five consecutive microbiological studies.74 Such evidence suggests that protection could be provided by integrin blockers and highlights inhibition of integrins as potential therapeutic targets for COVID-19-associated fibrosis.
COVID-19-associated fibrotic complications: Reality or myth?
The clinical relevance of fibrotic disorders in COVID-19 is clear. Previous SARS-CoV and Middle East respiratory syndrome (MERS)-CoV pandemics have taught us that the residual effects of the infection can persist for years.75, 76 Studies have shown a significant persistence of abnormalities in survivors of COVID-19, raising the concerns of long-term residual effects. One case study revealed pulmonary abnormalities in the form of septal thickening and traction bronchiectasis suggestive of progressive pulmonary fibrosis post infection.77 Carfì et al. reported the persistence of symptoms in patients with COVID-19 and suggested that continue monitoring of patients is necessary even after recovery.78 A significant proportion of patients with SARS-CoV-2 pneumonitis had inflammatory lung disease and functional deficits at 4 weeks after discharge.79 A recent report demonstrated that 72% (85 out of 118) patients showed fibrotic-like changes and 42% (49/118) showed GGOs on a 6-month follow-up chest CT.80 Similarly, another 6-month follow-up study also demonstrated similar results whereby 40 of 114 participants with severe COVID-19 pneumonia showed fibrotic-like symptoms, while the remaining 74 participants showed either complete radiological resolution, residual GGOs, or interstitial thickening.81 Li et al., reported abnormal lung functions in more than half of a total of 462 patients after 90 days from onset. They found that 62.03% of patients developed pulmonary fibrosis after >120 days, whereas 48.98% of patients showed a reversal of fibrosis.82 Schwensen and associates documented the development of fatal lung fibrosis even after eradication of COVID-19.83 When the CT scans were compared, it was observed that the patients developed characteristic fibrotic symptoms in previously healthy lungs. Another study reported the development of fibrotic-like changes correlated with lung function, cough, and measures of frailty, 4 months after hospitalisation.84 A 6-month-long follow-up study also reported fibrotic-like changes in the lungs of more than one-third of survivors of severe COVID-19.81
However, there is also evidence to suggest that COVID-19-associated complications can resolve with time. Although SARS-CoV-2 shares 79.5% and 50% genomic homology with SARS-CoV and MERS-CoV, respectively,85 predicting a similar type of persistence in COVID-19 as seen with the former two would not be appropriate. Available evidence also points to the ‘self-resolutory’ nature of COVID-19-associated complications, including fibrosis, which can spontaneously reverse during the recovery phase. A 4-week follow-up study of 51 patients suggested significant resolution of pulmonary abnormalities post infection.86 The patients showed varying percentages of improvement in different parameters, such as focal GGOs, multiple GGOs, reticular patterns, and consolidation and septal thickening suggestive of gradual recovery of pulmonary functions. However, the follow-up period was short (up to 4 weeks), which might have affected the results because the development of reversible fibrosis occurs over a period of time. A large population cohort study of 8 256 161 patients reported that the risk of COVID-19 in patients with asthma was relatively small. Furthermore, the risk of death in patients with chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD) was far lower than the risk of death from other causes,87 suggesting a lower risk of pulmonary fibrosis in COVID-19. In addition, the landscape of immunological and inflammatory events, such as circulating lymphocytes, monocytes, and concentration of inflammatory mediators, which show exaggerated levels in acute cases, also reduces during the recovery phase.88, 89 Moreover, clinical assessment of patients with COVID-19 at 1 year post discharge revealed a significant decline in immune cell infiltration and improvement in CT abnormalities compared with 3–6-months post discharge, demonstrating a long-term resolution of lung pathology.90 However, the contradictions observed among studies add to the complexity of establishing a direct association between COVID-19 and the persistence of fibrotic symptoms. Nevertheless, this should not compromise the search for a potential antifibrotic drug useful for the management of fibrosis of diverse organs.
Pitfalls and future directions
Despite concrete evidence of fibrosis development in patients with COVID-19, we are still far from knowing whether to use antifibrotic therapy for COVID-19-associated fibrosis. There have been concerns raised over the use of antifibrotic agents in different conditions. One of the first questions associated with the use of antifibrotic therapy in COVID-19 is whether we should administer antifibrotic drugs at all, given the self-resolving nature of COVID-19-associated fibrotic-like reactions. In addition, fibrosis is generally regarded as an irreversible process and antifibrotic therapy is mainly used to slow functional decline. Hence, antifibrotic therapy is unlikely to a definitive cure for established fibrosis, limiting the use of these agents. In addition, both available drugs are given by oral route, which hampers their use in patients critically ill with COVID-19. Furthermore, complications associated with these drugs reduce their usefulness for the management of COVID-19-associated fibrotic complications. For instance, the two replicate 52-week, randomised, double-blind, Phase III trials (INPULSIS-1 and INPULSIS-2) documented that nintedanib showed incidence rates of 61.5 and 63.2%, respectively.91 By contrast, a long-term safety trial with pirfenidone (PAASPORT) observed that, out of 1009 patients, 73.4% experienced adverse drug reactions, most commonly nausea (20.6%) and fatigue (18.5%), with a treatment discontinuation rate of 28.7%.92 In addition, pirfenidone is also contraindicated in renal failure, which needs to be kept in mind. Furthermore, continuous mutations of the virus further complicate the picture, resulting in changes to the transmissibility, virulence and clinical presentation of the infection, which make it difficult to choose therapeutic options and other measures.
Another major concern is the impaired wound-healing process in the patients undergoing antifibrotic treatment. Theoretically, long-term treatment with antifibrotic agents is expected to impair the overall wound-healing capabilities of the body. However, there are no reports for such observations in clinical settings and it remains is highly unlikely. In fact, patients taking pirfenidone up to 1 month before transplant showed no symptoms of impaired wound healing.93 In addition, pirfenidone, as an immunosuppressant, also increases the risk of infections and superinfections. From the lessons of cancer research, antifibrotic treatment is suspected to enhance the cellular growth of tissues and disrupt tissue homeostasis. However, there is a lack of data on the mentioned complications associated with the use of antifibrotic drugs, although these could be a major concern in patients with COVID-19 complications. However, these adverse events can be easily managed with suitable approaches and, thus, the effectiveness of antifibrotic agents in COVID-19-associated complications cannot be ignored.
Concluding remarks
The significant improvement in pulmonary functions following treatment with antifibrotic agents indicates the viability of these agents. Numerous studies have shown their effectiveness in ameliorating fibrosis-like conditions and improving the quality of life of patients. Given the benefits of antifibrotic agents, it makes more sense to add these agents to therapeutic regimen for COVID-19. In addition, the relatively safer profiles of these drugs also strengthen their case. However, it is advisable to monitor liver and kidney functions closely to avoid compromising patient health. A temporary suspension, dose reduction, and a judiciously prepared antifibrotic regimen could be beneficial for patients with COVID-19. Currently, there is uncertainty over the use of antifibrotic agents in COVID-19. However, with an increasing number of clinical trials and comprehensive studies, it is possible that the underlying pathological mechanisms will become clearer. Nevertheless, the timely regulation of fibrosis progression will lead to positive outcomes and will improve the quality of life of survivors of COVID-19.
Acknowledgments
Acknowledgements
All the authors thank the Director, NIPER Hyderabad, Department of Pharmaceuticals and Ministry of Chemicals and Fertilizers, Government of India, for support.
Declaration of interests
None declared by authors.
Data availability
No data was used for the research described in the article.
References
- 1.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wendisch D., Dietrich O., Mari T., von Stillfried S., Ibarra I.L., Mittermaier M., et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184:6243–6261. doi: 10.1016/j.cell.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J., Xu X., Jiang L., Dua K., Hansbro P.M., Liu G. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir Res. 2020;21:1–12. doi: 10.1186/s12931-020-01445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasarmidi E., Tsitoura E., Spandidos D.A., Tzanakis N., Antoniou K.M. Pulmonary fibrosis in the aftermath of the COVID-19 era. Exp Ther Med. 2020;20:2557–2560. doi: 10.3892/etm.2020.8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolesova O., Vanaga I., Laivacuma S., Derovs A., Kolesovs A., Radzina M., et al. Intriguing findings of liver fibrosis following COVID-19. BMC Gastroenterol. 2021;21:1–9. doi: 10.1186/s12876-021-01939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legrand M., Bell S., Forni L., Joannidis M., Koyner J.L., Liu K., et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17:751–764. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pannone G., Caponio V.C.A., De Stefano I.S., Ramunno M.A., Meccariello M., Agostinone A., et al. Lung histopathological findings in COVID-19 disease–a systematic review. Infect Agent Cancer. 2021;16:1–18. doi: 10.1186/s13027-021-00369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montero-Fernandez M.A., Pardo-Garcia R. Histopathology features of the lung in COVID-19 patients. Diagnostic Histopathol. 2021;27:123–127. doi: 10.1016/j.mpdhp.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan L., Chaudhary K., Saha A., Chauhan K., Vaid A., Zhao S., et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen J., Reimer K.C., Nagai J.S., Varghese F.S., Overheul G.J., de Beer M., et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell. 2022;29:217–231. doi: 10.1016/j.stem.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim J.H., Park S.H., Jeon Y., Cho J.H., Jung H.Y., Choi J.Y., et al. Fatal outcomes of COVID-19 in patients with severe acute kidney injury. J Clin Med. 2020;9:1718. doi: 10.3390/jcm9061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L., Hsu C., Tian Y., Fang J. Rhabdomyolysis associated with acute renal failure in patients with severe acute respiratory syndrome. Int J Clin Pract. 2005;59:1162–1166. doi: 10.1111/j.1368-5031.2005.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26:1618. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., van Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol A. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. Published online February 4, 2020. 10.1101/2020.02.03.931766. [DOI]
- 23.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J Pathol A. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guizani I., Fourti N., Zidi W., Feki M., Allal-Elasmi M. SARS-CoV-2 and pathological matrix remodeling mediators. Inflamm Res. 2021;70:847–858. doi: 10.1007/s00011-021-01487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dugina V., Fontao L., Chaponnier C., Vasiliev J., Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114:3285–3296. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- 26.Wang S., Yao X., Ma S., Ping Y., Fan Y., Sun S., et al. A single-cell transcriptomic landscape of the lungs of patients with COVID-19. Nat Cell Biol. 2021;23:1314–1328. doi: 10.1038/s41556-021-00796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delorey TM, Ziegler CGK, Heimberg G, Normand R, Yang Y, Segerstolpe A, et al. A single-cell and spatial atlas of autopsy tissues reveals pathology and cellular targets of SARS-CoV-2. bioRxiv. Published online February 26, 2021. 10.1101/2021.02.25.430130. [DOI]
- 28.LeBleu V.S., Taduri G., O'Connell J., Teng Y., Cooke V.G., Woda C., et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovisa S., LeBleu V.S., Tampe B., Sugimoto H., Vadnagara K., Carstens J.L., et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015;21:998. doi: 10.1038/nm.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grande M.T., Sánchez-Laorden B., López-Blau C., De Frutos C.A., Boutet A., Arévalo M., et al. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015;21:989. doi: 10.1038/nm.3901. [DOI] [PubMed] [Google Scholar]
- 31.Préaux A., Mallat A., Van Nhieu J.T., d’Ortho M., Hembry R.M., Mavier P. Matrix metalloproteinase-2 activation in human hepatic fibrosis regulation by cell-matrix interactions. Hepatology. 1999;30:944–950. doi: 10.1002/hep.510300432. [DOI] [PubMed] [Google Scholar]
- 32.Onozuka I., Kakinuma S., Kamiya A., Miyoshi M., Sakamoto N., Kiyohashi K., et al. Cholestatic liver fibrosis and toxin-induced fibrosis are exacerbated in matrix metalloproteinase-2 deficient mice. Biochem Biophys Res Commun. 2011;406:134–140. doi: 10.1016/j.bbrc.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Lachowski D., Cortes E., Rice A., Pinato D., Rombouts K., del Rio H.A. Matrix stiffness modulates the activity of MMP-9 and TIMP-1 in hepatic stellate cells to perpetuate fibrosis. Sci Rep. 2019;9:1–9. doi: 10.1038/s41598-019-43759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Zhou Y., Tan R., Xiong M., He W., Fang L., et al. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2010;299:F973–F982. doi: 10.1152/ajprenal.00216.2010. [DOI] [PubMed] [Google Scholar]
- 35.Avila-Mesquita D., Couto A.E.S., Campos L.C.B., Vasconcelos T.F., Michelon-Barbosa J., Corsi C.A.C., et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed Pharmacother. 2021;142:112067. doi: 10.1016/j.biopha.2021.112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solun B., Shoenfeld Y. Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19. Med Drug Discov. 2020;7:100052. doi: 10.1016/j.medidd.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirawat R., Saifi M.A., Godugu C. Targeting inflammatory cytokine storm to fight against COVID-19 associated severe complications. Life Sci. 2021;267:118923. doi: 10.1016/j.lfs.2020.118923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eardley K.S., Kubal C., Zehnder D., Quinkler M., Lepenies J., Savage C.O., et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74:495–504. doi: 10.1038/ki.2008.183. [DOI] [PubMed] [Google Scholar]
- 39.Porta C., Riboldi E., Ippolito A., Sica A. Molecular and epigenetic basis of macrophage polarized activation. Seminars Immunol. 2015;27:237–248. doi: 10.1016/j.smim.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Hou J., Shi J., Chen L., Lv Z., Chen X., Cao H., et al. M2 macrophages promote myofibroblast differentiation of LR-MSCs and are associated with pulmonary fibrogenesis. Cell Commun Signal. 2018;16:1–14. doi: 10.1186/s12964-018-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Y., Jiang H., Zhang Q., Mehrotra S., Abel P.W., Toews M.L., et al. Upregulation of RGS2: a new mechanism for pirfenidone amelioration of pulmonary fibrosis. Respir Res. 2016;17:1–14. doi: 10.1186/s12931-016-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F., Wei Y., He L., Zhang H., Hu Q., Yue H., et al. A trial of pirfenidone in hospitalized adult patients with severe coronavirus disease 2019. Chin Med J (Engl) 2022;135:368–370. doi: 10.1097/CM9.0000000000001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X., Yang D., Kong X., Wei C., LvQiu S., Wang L., et al. Case report: pirfenidone in the treatment of post-COVID-19 pulmonary fibrosis. Front Med. 2022;9:925703. doi: 10.3389/fmed.2022.925703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umemura Y., Mitsuyama Y., Minami K., Nishida T., Watanabe A., Okada N., et al. Efficacy and safety of nintedanib for pulmonary fibrosis in severe pneumonia induced by COVID-19: an interventional study. Int J Infect Dis. 2021;108:454–460. doi: 10.1016/j.ijid.2021.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bussolari C., Palumbo D., Fominsky E., Nardelli P., De Lorenzo R., Vitali G., et al. Case report: nintedaninb may accelerate lung recovery in critical coronavirus disease 2019. Front Med. 2021;8:766486. doi: 10.3389/fmed.2021.766486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lomanta J.M.J., Quinto M.L., Urquiza S.C., Santiaguel J.M. Pulmonary function and chest computed tomography (CT) scan findings after antifibrotic treatment for COVID-19-related pulmonary fibrosis. Am J Case Rep. 2022;23:e934830-1. doi: 10.12659/AJCR.934830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nie J., Wu Q., Liu W., Zhu F., Qiu F., Zhou Q., et al. Ectopic expression of Ligand-of-Numb protein X promoted TGF-β induced epithelial to mesenchymal transition of proximal tubular epithelial cells. Biochim Biophys Acta Molecular Basis Dis. 2009;1792:122–131. doi: 10.1016/j.bbadis.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X., Du G., Xu Y., Li X., Fan W., Chen J., et al. Inhibition of notch signaling pathway prevents cholestatic liver fibrosis by decreasing the differentiation of hepatic progenitor cells into cholangiocytes. Lab Investig. 2016;96:350–360. doi: 10.1038/labinvest.2015.149. [DOI] [PubMed] [Google Scholar]
- 49.Sweetwyne M.T., Tao J., Susztak K. Kick it up a notch: Notch signaling and kidney fibrosis. Kidney Int Suppl. 2014;4:91–96. doi: 10.1038/kisup.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nemir M., Metrich M., Plaisance I., Lepore M., Cruchet S., Berthonneche C., et al. The Notch pathway controls fibrotic and regenerative repair in the adult heart. Eur Heart J. 2014;35:2174–2185. doi: 10.1093/eurheartj/ehs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gopalakrishnan N., Sivasithamparam N.D., Devaraj H. Synergistic association of Notch and NFκB signaling and role of Notch signaling in modulating epithelial to mesenchymal transition in colorectal adenocarcinoma. Biochimie. 2014;107:310–318. doi: 10.1016/j.biochi.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 52.Jespersen K., Liu Z., Li C., Harding P., Sestak K., Batra R., et al. Enhanced Notch3 signaling contributes to pulmonary emphysema in a murine model of Marfan syndrome. Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-67941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosa B.A., Ahmed M., Singh D.K., Choreño-Parra J.A., Cole J., Jiménez-Álvarez L.A., et al. IFN signaling and neutrophil degranulation transcriptional signatures are induced during SARS-CoV-2 infection. Commun Biol. 2021;4:1–14. doi: 10.1038/s42003-021-01829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vandelli A., Monti M., Milanetti E., Armaos A., Rupert J., Zacco E., et al. Structural analysis of SARS-CoV-2 genome and predictions of the human interactome. Nucleic Acids Res. 2020;48:11270–11283. doi: 10.1093/nar/gkaa864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizzo P., Dalla Sega F.V., Fortini F., Marracino L., Rapezzi C., Ferrari R. COVID-19 in the heart and the lungs: could we ‘Notch’ the inflammatory storm? Basic Res Cardiol. 2020;115:1–8. doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loh K.M., van Amerongen R., Nusse R. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev Cell. 2016;38:643–655. doi: 10.1016/j.devcel.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Staal F.J.T., Luis T.C., Tiemessen M.M. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 59.Newman D.R., Sills W.S., Hanrahan K., Ziegler A., Tidd K.M., Cook E., et al. Expression of WNT5A in idiopathic pulmonary fibrosis and its control by TGF-β and WNT7B in human lung fibroblasts. J Histochem Cytochem. 2016;64:99–111. doi: 10.1369/0022155415617988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu F., Thirumangalathu S., Gallant N.M., Yang S.H., Stoick-Cooper C.L., Reddy S.T., et al. Wnt-β-catenin signaling initiates taste papilla development. Nat Genet. 2007;39:106–112. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- 61.Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182:59–72. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor K, Das S, Pearson M, Kozubek J, Pawlowski M, Jensen CE, et al. Analysis of genetic host response risk factors in severe COVID-19 patients. medRxiv. Published online 20 June, 2020. 10.1101/2020.06.17.20134015. [DOI]
- 63.Raghu G., Brown K.K., Collard H.R., Cottin V., Gibson K.F., Kaner R.J., et al. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: a randomised, double-blind, controlled, phase 2 trial. Lancet Respir Med. 2017;5:22–32. doi: 10.1016/S2213-2600(16)30421-0. [DOI] [PubMed] [Google Scholar]
- 64.Saifi M.A., Godugu C. Inhibition of lysyl oxidase ameliorates renal injury by inhibiting CD44-mediated pericyte detachment and loss of peritubular capillaries. Life Sci. 2020;117294 doi: 10.1016/j.lfs.2020.117294. [DOI] [PubMed] [Google Scholar]
- 65.Seethy A.A., Singh S., Mukherjee I., Pethusamy K., Purkayastha K., Sharma J.B., et al. Potential SARS-CoV-2 interactions with proteins involved in trophoblast functions–an in-silico study. Placenta. 2021;103:141–151. doi: 10.1016/j.placenta.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fabian S.L., Penchev R.R., St-Jacques B., Rao A.N., Sipilä P., West K.A., et al. Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol. 2012;180:1441–1453. doi: 10.1016/j.ajpath.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolaños A.L., Milla C.M., Lira J.C., Ramírez R., Checa M., Barrera L., et al. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am J Physiol Cell Mol Physiol. 2012;303:L978–L990. doi: 10.1152/ajplung.00184.2012. [DOI] [PubMed] [Google Scholar]
- 68.Syn W.K., Choi S.S., Liaskou E., Karaca G.F., Agboola K.M., Oo Y.H., et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baratella E., Bussani R., Zanconati F., Marrocchio C., Fabiola G., Braga L., et al. Radiological–pathological signatures of patients with COVID-19-related pneumomediastinum: is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. 2021;7 doi: 10.1183/23120541.00346-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sigrist C.J.A., Bridge A., Le Mercier P. A potential role for integrins in host cell entry by SARS-CoV–2. Antiviral Res. 2020;177:104759. doi: 10.1016/j.antiviral.2020.104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henderson N.C., Arnold T.D., Katamura Y., Giacomini M.M., Rodriguez J.D., McCarty J.H., et al. Targeting of α v integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barczyk M., Carracedo S., Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hussein H.A.M., Walker L.R., Abdel-Raouf U.M., Desouky S.A., Montasser A.K.M., Akula S.M. Beyond RGD: virus interactions with integrins. Arch Virol. 2015;160:2669–2681. doi: 10.1007/s00705-015-2579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aguirre C., Meca-Lallana V., Barrios-Blandino A., Del Río B., Vivancos J. Covid-19 in a patient with multiple sclerosis treated with natalizumab: may the blockade of integrins have a protective role? Mult Scler Relat Disord. 2020;44:102250. doi: 10.1016/j.msard.2020.102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hui D.S., Wong K.T., Ko F.W., Tam L.S., Chan D.P., Woo J., et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ngai J.C., Ko F.W., Ng S.S., To K., Tong M., Hui D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tale S., Ghosh S., Meitei S.P., Kolli M., Garbhapu A.K., Pudi S. Post-COVID-19 pneumonia pulmonary fibrosis. QJM An Int J Med. 2020;113:837–838. doi: 10.1093/qjmed/hcaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Myall K.J., Mukherjee B., Castanheira A.M., Lam J.L., Benedetti G., Mak S.M., et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18:799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caruso D., Guido G., Zerunian M., Polidori T., Lucertini E., Pucciarelli F., et al. Postacute sequelae of COVID-19 pneumonia: 6-month chest CT follow-up. Radiology. 2021;301:E396–E405. doi: 10.1148/radiol.2021210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han X., Fan Y., Alwalid O., Li N., Jia X., Yuan M., et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li X., Shen C., Wang L.-F., Majumder S., Zhang D., Deen M.J., et al. Pulmonary fibrosis and its related factors in discharged patients with new coronavirus pneumonia: a cohort study of 90–150 days follow-up after onset. Respir Res. 2021;22:203. doi: 10.1186/s12931-021-01798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwensen H.F., Borreschmidt L.K., Storgaard M., Redsted S., Christensen S., Madsen L.B. Fatal pulmonary fibrosis: a post-COVID-19 autopsy case. J Clin Pathol. 2021;74:400–402. doi: 10.1136/jclinpath-2020-206879. [DOI] [PubMed] [Google Scholar]
- 84.McGroder C.F., Zhang D., Choudhury M.A., Salvatore M.M., D'Souza B.M., Hoffman E.A., et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax. 2021 doi: 10.1136/thoraxjnl-2021-217031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu C., Ye L., Xia R., Zheng X., Yuan C., Wang Z., et al. Chest computed tomography and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann Am Thorac Soc. 2020;17:1231–1237. doi: 10.1513/AnnalsATS.202004-324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aveyard P., Gao M., Lindson N., Hartmann-Boyce J., Watkinson P., Young D., et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med. 2021;9:909–923. doi: 10.1016/S2213-2600(21)00095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodriguez L., Pekkarinen P.T., Lakshmikanth T., Tan Z., Consiglio C.R., Pou C., et al. Systems-level immunomonitoring from acute to recovery phase of severe COVID-19. Cell Reports Med. 2020;1:100078. doi: 10.1016/j.xcrm.2020.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mann E.R., Menon M., Knight S.B., Konkel J.E., Jagger C., Shaw T.N., et al. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci Immunol. 2020;5:eabd6197. doi: 10.1126/sciimmunol.abd6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vijayakumar B., Boustani K., Ogger P.P., Papadaki A., Tonkin J., Orton C.M., et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55:542–556. doi: 10.1016/j.immuni.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Richeldi L., du Bois R.M., Raghu G., Azuma A., Brown K.K., Costabel U., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 92.Cottin V., Koschel D., Günther A., Albera C., Azuma A., Sköld C.M., et al. Long-term safety of pirfenidone: results of the prospective, observational PASSPORT study. ERJ Open Res. 2018;4:00084–02018. doi: 10.1183/23120541.00084-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mortensen A., Cherrier L., Walia R. Effect of pirfenidone on wound healing in lung transplant patients. Multidiscip Respir Med. 2018;13:1–5. doi: 10.1186/s40248-018-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.