Abstract
In Pseudomonas aeruginosa, iron modulates gene expression through a cascade of negative and positive regulatory proteins. The master regulator Fur is involved in iron-dependent repression of several genes. One of these genes, pvdS, was predicted to encode a putative sigma factor responsible for the transcription of a subset of genes of the Fur regulon. PvdS appears to belong to a structurally and functionally distinct subgroup of the extracytoplasmic function family of alternative sigma factors. Members of this subgroup, also including PbrA from Pseudomonas fluorescens, PfrI and PupI from Pseudomonas putida, and FecI from Escherichia coli, are controlled by the Fur repressor, and they activate transcription of genes for the biosynthesis or the uptake of siderophores. Evidence is provided that the PvdS protein of P. aeruginosa is endowed with biochemical properties of eubacterial sigma factors, as it spontaneously forms 1:1 complexes with the core fraction of RNA polymerase (RNAP, α2ββ′ subunits), thereby promoting in vitro binding of the PvdS-RNAP holoenzyme to the promoter region of the pvdA gene. These functional features of PvdS are consistent with the presence of structural domains predicted to be involved in core RNAP binding, promoter recognition, and open complex formation. The activity of pyoverdin biosynthetic (pvd) promoters was significantly lower in E. coli overexpressing the multicopy pvdS gene than in wild-type P. aeruginosa PAO1 carrying the single gene copy, and pvd::lacZ transcriptional fusions were silent in both pfrI (the pvdS homologue) and pfrA (a positive regulator of pseudobactin biosynthetic genes) mutants of P. putida WCS358, while they are expressed at PAO1 levels in wild-type WCS358. Moreover, the PvdS-RNAP holoenzyme purified from E. coli lacked the ability to generate in vitro transcripts from the pvdA promoter. These observations suggest that at least one additional positive regulator could be required for full activity of the PvdS-dependent transcription complex both in vivo and in vitro. This is consistent with the presence of a putative activator binding site (the iron starvation box) at variable distance from the transcription initiation sites of promoters controlled by the iron starvation sigma factors PvdS, PfrI, and PbrA of fluorescent pseudomonads.
Iron deficiency is a key extracytoplasmic stimulus for many bacterial pathogens, heralding the entry into the vertebrate host (21). Pseudomonas aeruginosa is a classic example of an opportunistic pathogen which can cause serious disease in the compromised or predisposed host, predominantly through accidental transmission from the environment (35). In P. aeruginosa, the expression of relevant virulence factors, including iron assimilation systems, proteases and exotoxin A, is tightly controlled by the iron level (7, 21). Extracellular iron concentrations of ca. >10 μM cause a strong repression of iron-responsive genes through a regulatory cascade governed by the Fur repressor. In the presence of sufficient iron, the P. aeruginosa Fur protein binds the promoter-operator regions of a number of iron-repressible genes, thereby inhibiting their transcription (34). Under low-iron conditions, the Fur-mediated repression is relieved and positive transcriptional regulation can occur. One of the P. aeruginosa Fur-controlled genes, designated pvdS (for pyoverdin sigma), encodes a transcriptional activator required for the expression of the pyoverdin (the fluorescent siderophore) biosynthetic genes pvdA, pvdD, and pvdE (referred to as pvd genes) and of the regAB and ptxR genes, involved in the positive control of the exotoxin A (toxA) gene (7, 8, 33, 50). At present time there is little information on the mechanism(s) by which the PvdS protein activates transcription from pvd, regAB, and ptxR promoters. The promoters of pvd genes share common features in that they often contain multiple transcription initiation sites and an essential sequence motif, termed the iron starvation box, also present in the toxA promoter (20, 25, 27, 39). Similarities between relevant sequence elements were also reported for the iron-regulated regAB P2 promoter and the pvdA promoter (20). The amino acid sequence of the PvdS protein is similar (nearly 85% identity) to that of iron-responsive regulators from other fluorescent pseudomonads, i.e., PfrI of Pseudomonas putida WCS358 and PbrA of Pseudomonas fluorescens M114 (45, 51). These proteins are also similar in function, all being involved in the transcriptional activation of genes for the biosynthesis of fluorescent siderophores (pyoverdin or pseudobactins) in Pseudomonas spp. (20, 45, 51). PvdS, PbrA, and PfrI are distantly related to PupI and FecI, two activator proteins which direct the expression of the ferric-pseudobactin BN8 receptor gene (pupB) in P. putida WCS358 and of the ferric-dicitrate receptor gene (fecA) in Escherichia coli, respectively (1, 19). Remarkably, the expression of all of these proteins is directly controlled by the Fur repressor. Additional positive regulation has been reported for PfrI-, PupI-, and FecI-controlled genes (1, 19, 52).
Functional properties and primary structure analysis have related PvdS, PbrA, PfrI, PupI, and FecI to the ECF (extracytoplasmic function) family of alternative sigma factors (1, 19, 20, 45, 51). Sequence comparison of ς70 family proteins from different eubacteria led to the identification of four highly conserved primary structure domains (23). Regions 2 and 4 are the most conserved and are prevalently basic; regions 1 and 3 are less conserved and are prevalently acidic. A number of genetic and biochemical studies made it possible to assign specific functions to each of the conserved domains of ς70 family proteins (23). FecI was at first proposed to be a sigma factor belonging to the ECF family (22), and further biochemical studies confirmed that this protein was endowed with sigma factor activity (1). Based on sequence similarity to FecI, the PvdS, PfrI, PbrA, and PupI activator proteins have also been proposed to belong to the ECF family of alternative sigma factors (8, 53). Alternative sigmas display poor homology with the primary sigmas and can be highly divergent from each other, but they share an overall similarity with two or more of the conserved domains (23). As a general rule, the ECF alternative sigma factors typically lack much of the conserved regions 1 and 3 while retaining many of the conserved features of regions 2 and 4 (22).
In spite of the compelling evidence of the key role played by PvdS, PbrA, and PfrI in the transcriptional activation of genes for the biosynthesis of fluorescent siderophores, a direct interaction of these putative sigma factors with RNA polymerase (RNAP) and cognate promoters has not yet been proven. The present study was therefore undertaken to demonstrate that the PvdS protein of P. aeruginosa is endowed with structural, biochemical, and functional properties of eubacterial alternative sigma factors.
MATERIALS AND METHODS
Strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli was routinely grown in Luria-Bertani (LB) medium or in M9 minimal medium (41). P. aeruginosa was grown in NYB, SM9, or tryptic soy yeast-extract medium (TSY), containing 3 g of tryptic soy broth (TSB) and 5 g of yeast extract (Difco) per liter (20). DCAA and iron-free King B (IFKB) were used as the low-iron media for P. aeruginosa and P. putida WCS358 (20, 54). IFKB medium was obtained by treatment of a tryptone (10 g/liter)-Casamino Acids (5 g/liter) solution with 20 g of Chelex 100 resin (Bio-Rad) per liter under previously described conditions (54). After removal of the resin, the IFKB basal solution was supplemented with 1.5 g of K2HPO4, 1.5 g of MgSO4, and 10 g of glycerol per liter and then adjusted to pH 7.4 prior to autoclaving. Media were solidified with 1.2% agar N.1 (Unipath). To reduce iron availability, the iron chelator 2,2′-dipyridyl was added to the M9 minimal medium at 150 μM. Antibiotics were used in selective media at the following concentrations: tetracycline, 12.5 μg/ml for E. coli and 100 μg/ml for P. aeruginosa; chloramphenicol, 30 μg/ml for E. coli and 100 μg/ml for P. aeruginosa; kanamycin, 25 μg/ml for E. coli, 50 μg/ml for P. putida, and 300 μg/ml for P. aeruginosa; and ampicillin, (100 μg/ml), nalidixic acid (20 μg/ml), and streptomycin (25 μg/ml) for E. coli.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or relevant characteristics | Reference or source |
|---|---|---|
| E. coli | ||
| DH5αF′ | recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 Δ(lacZYA-argF)U169 [Φ80dlacZΔM15] Nalr | 44 |
| M15 | lac1qara-14 galK2 mtl-1 F− | 49 |
| MC4100 | araD139 rpsL150 relA1 flbB5301 deoC1 pstF25 rbsR Δ(lacZYA-argF)U169 Strr | 5 |
| P. aeruginosa | ||
| PAO1 (ATCC 15692) | Prototroph | American Type Culture Collection |
| 8830 | his-1 | 9 |
| 8830R2::Cm | his-1 algR2::Cm | 42 |
| P. putida | ||
| WCS358 | Prototroph | 14 |
| WCS358.E9 | pfrA::Tn3Gus | 52 |
| VM119 | pfrI::Tn5 | 51 |
| Plasmids | ||
| pQE60 | Expression vector; ColE1 replicon, PT5lacO RBSII Apr | Diagen |
| pDMI,1 | Repressor plasmid; p15A replicon, lac1q Kmr | 6 |
| pRK2013 | Helper plasmid; ColE1 replicon, Kmr Mob+ Tra+ | 12 |
| pMP190 | Broad-host-range, low-copy-number promoter probe vector; IncQ replicon, lacZ Cmr Tra− | 47 |
| pMP220 | Broad-host-range, low-copy-number promoter probe vector; IncP replicon, lacZ Tcr Tra− | 47 |
| pPV51 | Promoter of pvdA pyoverdin synthesis gene cloned into pMP220 | 20 |
| pMP190::PpvdD | Promoter of pvdD pyoverdin synthesis gene cloned into pMP190 | 8 |
| pMP190::PpvdE | Promoter of pvdE pyoverdin synthesis gene cloned into pMP190 | 8 |
| pBRXB | 1.8-kb XhoI-BamHI fragment containing pvdS ligated to pBR322 | 8 |
| pPvdS6H | The 564-bp pvdS coding sequence ligated to the NcoI-BglII sites of pQE60 in frame to the His6 coding sequence | This study |
| pPvdSF | The 564-bp pvdS coding sequence ligated to the NcoI-HindIII sites of pQE60 in frame to the FLAG coding sequence | This study |
| pPvdSWT | The 564-bp pvdS coding sequence ligated to the NcoI-HindIII sites of pQE60 | This study |
Sequence alignments and phylogenetic inference.
Sequence similarity searches were performed by the use of the BLAST network service (nucleic acid databases from the National Center for Biotechnology Information). The alignment of PvdS, PfrI, and PbrA with representative members of the ECF subfamily and ς70 was performed by the use of the CLUSTALW program. Basically, three distinct clusters of aligned proteins were generated. The Pseudomonas highly homologous peptides PvdS, PbrA, and PfrI were aligned in group 1. The P. putida PupI and E. coli FecI proteins were aligned in group 2. The P. aeruginosa AlgU and the E. coli RpoE (ςE) sigma factors were aligned in group 3. Each of the three previously aligned groups and the ς70 sequence were then aligned on the guide of the previously published multiple alignments (22, 23) and by visually matching obvious signature residues constraining the alignment topology. Reliability of the alignment was confirmed by searching the binary alignments given by BLASTP for the presence or absence of the alignment schemes generated by the multialignment algorithms (or manually inferred). Phylogenetic trees were constructed by using maximum-parsimony and maximum-likelihood methods. The maximum-parsimony analyses used the program PROTPARS implemented in PHYLIP version 3.57c (11). The PHYLIP programs SEQBOOT, PROTPARS, and CONSENSE were used sequentially to generate a maximum-parsimony tree which was replicated in 100 bootstraps; on this basis, bootstrap confidence levels were determined. For maximum-likelihood analyses, we used the program PUZZLE version 4.0 (48) with the Jones Taylor-Thornton substitution model and a gamma-distributed model of site-to-site rate variation using eight rate classes to approximate the continuous gamma distribution, as well as a gamma distribution parameter α estimated from the data set. Protein secondary structure predictions were inferred by the use of the MacDNASIS Pro, version 1.0 software (Hitachi Software Engineering Co.).
DNA manipulations and genetic techniques.
All procedures for the handling of recombinant DNA have been described before (41). Transfer of plasmids from E. coli to P. aeruginosa was performed by triparental matings with the helper plasmid pRK2013 (12).
DNA and protein sequencing.
DNA sequencing reactions were performed with double-stranded preparations of plasmids pPvdSWT, pPvdS6H, and pPvdSF by the dideoxy-chain termination method using QIAexpress forward and reverse sequencing primers (Diagen) and a commercial T7 sequencing kit (Pharmacia Biotech). Primers were 5′ labeled with carbocyanin (Pharmacia Biotech), and sequencing products were analyzed with a Pharmacia Biotech ALFexpress automated DNA sequencer apparatus. The partial amino acid sequence of the overexpressed proteins was determined by automated Edman degradation. The protein samples to be sequenced were submitted to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (41) and then electrotransferred onto a ProBlott membrane (Applied Biosystem) and sequenced on a Perkin-Elmer/Applied Biosystem 476A peptide sequencer equipped with a Blott cartridge using an optimized liquid-phase fast program.
Construction of PvdS expression plasmids.
A 573-bp fragment containing the entire PvdS coding sequence was generated by PCR using plasmid pBRXB as the template (8) and primers FWpvdS6H (5′-CCCATGGCGGAACAACTGTCTACCCGCAGATGC-3′) and RVpvdS6H (5′-GGGAGATCTGCGGGCGCTGAGATGGGT-3′) annealing to the pvdS sequence from codons 2 to 10 and from codons 181 to 188, respectively. The introduced NcoI site (underlined) in FWpvdS6H restored the ATG first codon but introduced a conservative (Ala-to-Ser) change at codon 2. Introduction of the BglII site in RVpvdS6H (underlined) caused the substitution of the original stop codon with Arg. Amplification reactions were carried out by using 1 ng of circular template in a 100-μl reaction mixture containing 1× PCR buffer (Perkin-Elmer), 1.5 μM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, a 1 μM concentration of each primer, and 2.5 U of Taq DNA polymerase (Perkin-Elmer). Thirty cycles were performed in a Perkin-Elmer 480 thermal cycler, each cycle comprising 30 s at 95°C, 1 min at 60°C, and 45 s at 74°C. The amplification product was cloned into the NcoI-BglII sites of the expression vector pQE60 (Qiagen Inc., Valencia, Calif.) of the QIAexpress system, generating plasmid pPvdS6H. In this plasmid, the 3′ terminus of the pvdS open reading frame is cloned in frame to a 18-bp-long sequence encoding six histidines (His6 tag), and expression of the tagged protein is under the control of the PT5 lacO′ promoter-operator element. Plasmid pPvdSF was derived from pPvdS6H by replacing the His6 tag with the FLAG octapeptide. For this purpose, oligonucleotides containing the sequence for the FLAG tag epitope were constructed (5′-GAAGATCTGACTACAAGGACGACGATGACAAGTAAGCTTGGGG-3′ and 5′-CCCCAAGCTTACTTGTCATCGTCGTCCTTGTAGTCAGATCTTC-3′), annealed, and then ligated into the BglII-HindIII sites of pPvdS6H. To obtain a construct expressing the wild-type pvdS gene, a new fragment corresponding to the PvdS coding sequence was generated by PCR, using as the template the plasmid pBRXB, the primer FWpvdS6H, and the reverse primer RVpvdSWT, identical to the RVpvdS6H primer except for the replacement of the BglII site with the HindIII site. Consequently, the original stop codon at the 3′ terminus of pvdS coding sequence was restored. The amplification product obtained using the PCR conditions described above was cloned in the NcoI-HindIII sites of pQE60, originating plasmid pPvdSWT. The fragments cloned in pPvdS6H, pPvdSF, and pPvdSWT were sequenced to assess that no point mutations occurred during PCR. The pPvdS6H, pPvdSF and pPvdSWT constructs were used to transform E. coli M15 carrying the repressor plasmid pDMI,1 (49).
Purification of PvdS.
Overnight cultures of E. coli M15(pDMI,1) carrying alternately expression plasmids pPvdS6H and pPvdSF were diluted 1:1,000 in 500 ml of TSY medium containing ampicillin (100 μg/ml) and kanamycin (25 μg/ml). Growth at 37°C was monitored until the A600 was ≅0.5, at which stage cultures were induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After an additional 3 h of incubation at 37°C, the cells were harvested by centrifugation and resuspended in 10 ml of lysis buffer (50 mM Na2PO3, 300 mM NaCl). Cell lysis was achieved by sonication. Phenylmethylsulfonyl fluoride was added to 1 mM (final concentration) immediately after cell lysis.
The insoluble cell fraction containing PvdS6H was collected by centrifugation and stirred for 3 h in 20 ml of buffer A (6.0 M guanidine hydrochloride, 0.1 M Na2HPO4 [pH 8]). The suspension was centrifuged at 10,000 × g, and the supernatant was directly applied to a 10-ml nickel column (nitrilotriacetic acid-resin; Diagen). After an equilibration step with buffer B (8 M urea, 0.1 M Na2HPO4, 10 mM Tris [pH 8]), PvdS6H was eluted by stepwise lowering the pH of the urea solution to pH 4. The eluted PvdS6H protein was >90% pure but readily precipitated during rapid dialysis at urea concentrations of <3.0 M. Refolding by slow (12 days) dialysis against a linear urea gradient (8 to 0 M in 100 mM phosphate buffer [pH 7]) yielded a minor fraction of soluble PvdS6H which was used for further experiments.
The FLAG-tagged PvdS (PvdSF) protein, overexpressed in E. coli M15(pQEpvdSF; pDMI,1), was purified from the soluble cell fraction under nondenaturing conditions by the use of anti-FLAG M2 affinity gel chromatography as instructed by the supplier (Kodak IBI, Inc.). Briefly, 100 ml of the PvdSF-containing culture supernatant, recovered after sonication and centrifugation, was recirculated three times through a 1-ml anti-FLAG-M2 column. After three washes with 50-ml aliquots of Tris-buffered saline (TBS; 0.15 M NaCl, 0.05 Tris-HCl [pH 7.4]), the PvdSF protein was eluted in one step with 1 ml of TBS containing 50 μg of the FLAG octapeptide (Kodak IBI) per ml.
Protein concentrations were determined using the Bradford assay with bovine serum albumin as the standard (2).
SDS-PAGE and Western blot analysis.
Bacterial cultures were harvested by centrifugation and suspended in gel loading buffer (0.25 M Tris-HCl, 2% SDS, 10% 2-mercaptoethanol, 20% glycerol), heated at 100°C for 5 min, and analyzed on a 0.1% SDS–12.5% polyacrylamide gel (41). Electrophoresis was carried out at 10 V/cm in Tris-glycine buffer (25 mM Tris-HCl [pH 8.3], 192 mM glycine, 1% SDS). After electrophoresis, gels were stained with Coomassie brilliant blue, destained, and photographed. Alternatively, after electrophoresis, the protein samples were electrotransferred onto a nitrocellulose filter (Hybond C Extra; Amersham) using a semidry transfer unit (Hoefer Scientific Instruments) for 1 h at 100 mA. The filter was probed in TBS either with a polyclonal antiserum specific to the α subunit of the E. coli RNAP (a kind gift from A. Kolb, Institut Pasteur, Paris, France) at a dilution of 1:100 or with anti-FLAG monoclonal antibody M2 at 10 μg/ml (Kodak IBI). For signal detection, secondary anti-mouse immunoglobulin G antibodies conjugated to alkaline phosphatase (Promega) were used at a concentration of 1:7,500, and the reaction was visualized using 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium.
Enzymatic assays.
Plasmids pPV51, pMP190::PpvdD and pMP190::PpvdE, carrying the pvdA, pvdD, and pvdE promoters cloned into promoter probe vectors, respectively, have been previously described (8, 20). For reporter gene activity measurements, Pseudomonas strains harboring the pvd::lacZ transcriptional fusions were grown for 12 to 18 h at 37°C in DCAA or IFKB supplemented with tetracycline (100 μg/ml) and/or chloramphenicol (100 μg/ml). Cultures were then diluted 1:1,000 in the same medium with or without the addition of 100 μM FeCl3, and subcultured for 8 to 10 h with shaking until the A600 reached approximately 0.4. E. coli MC4100 (5) carrying both pBRXB and pvd::lacZ transcriptional fusions was grown for 18 h at 37°C in M9 minimal medium containing ampicillin (100 μg/ml) and either tetracycline (10 μg/ml) or chloramphenicol (30 μg/ml). Cultures were then diluted 1:1,000 in the same medium containing 100 μM FeCl3 or 200 μM 2,2′-dipyridyl for additional 8-h growth at 37°C (final A600 of ≅0.8 in low-iron medium and 1.2 in high-iron medium). Alternatively, E. coli MC4100 carrying plasmid pDMI,1 plus pQE60-derived plasmids (pPvdS6H, pPvdSF, and pPvdSWT) and pvd::lacZ transcriptional fusions was grown at 37°C in LB medium containing ampicillin (100 μg/ml) and kanamycin (30 μg/ml) and supplemented with tetracycline (10 μg/ml) or chloramphenicol (30 μg/ml). When the A600 reached ≅0.5, cultures were induced with 1 mM IPTG and grown for additional 3 h. The β-galactosidase (LacZ) activity was determined spectrophotometrically using o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate. Activity was normalized to the A600 of the bacterial culture and expressed in Miller units (28). Strains were assayed at least three different times, with duplicate assays each time.
Gel retardation assays.
The promoter region of the pvdA gene (PpvdA probe) was uniformly labeled with [α-32P]dCTP (10 mCi/ml) as previously described (20). Gel retardation assays were performed as described by Mencia et al. (26). The PpvdA probe (0.1 pmol) was mixed with purified PvdSF-core RNAP complex, purified PvdS6H, commercial core RNAP from E. coli (subunits α2ββ′; Epicentre Technologies), commercial RNAP holoenzyme from E. coli (subunits ς70α2ββ′, Epicentre Technologies), or various combination of these in 20 μl of DNA binding buffer (10 mM Tris-HCl [pH 8], 0.1 mM EDTA, 5 mM dithiothreitol, 10% glycerol). Proteins were used at the concentrations indicated in the legend to Fig. 6. Heparin (1 μg) and glycerol (30% [vol/vol]) were added to each reaction tube just before loading on prerun 5% acrylamide gels in 10 mM NaH2PO4 (pH 6). Electrophoresis was carried out at 4°C with recirculation of the buffer at 60 V for 20 h. Gels were dried and exposed to Kodak XAR film at room temperature.
FIG. 6.
Gel retardation assay for binding of PvdS and RNAP to the pvdA promoter in the presence of heparin (0.05 mg/ml). A 32P-labeled DNA fragment of 177 bp encompassing the pvdA promoter was used as the DNA probe. Lane 1, negative control (free DNA probe); lanes 2 to 4, vegetative E. coli RNAP holoenzyme (subunits ς70α2ββ′), 0.08 to 0.32 pmol; lanes 5 to 7, core RNAP (subunits α2ββ′), 0.08 to 0.32 pmol; lanes 8 to 10, PvdS6H protein, 0.25 to 1 pmol; lanes 11 to 15, PvdSF-core RNAP complex, 0.06 to 1 pmol; lanes 16 to 20, PvdS6H (0.06 to 1 pmol) and core RNAP (0.16 pmol). The amount of probe was 0.1 pmol for each sample. Arrows indicate the PpvdA probe.
RESULTS
PvdS belongs to a functionally distinct subgroup of the ECF family of alternative sigma factors.
BLASTP analysis of the PvdS sequence (8) retrieved seven proteins with significant similarity. PvdS displays the highest similarity to PbrA (89% identity) and PfrI (85% identity), which are activators of pseudobactin biosynthesis in P. fluorescens M114 (45) and P. putida WCS358 (51), respectively. Lower similarity was found to FecI (31% identity) and PupI (29% identity) (1, 19). Still significant similarity was also found to the products of three newly identified genes: fiuI (EMBL/GenBank accession no. AF051691; 34% identity) and pigD (EMBL/GenBank accession no. AF060193; 30% identity) from P. aeruginosa and rpoI from Rhizobium leguminosarum (EMBL/GenBank accession no. AJ238209; 34% identity). Remarkably, fiuI and pigD were isolated from the P. aeruginosa genome by cycle selection of Fur-regulated genes (34). Moreover, a search for PvdS homologues in the unfinished P. aeruginosa genome (http://www.pseudomonas.com) retrieved 10 putative protein sequences related to PvdS, besides FiuI and PigD.
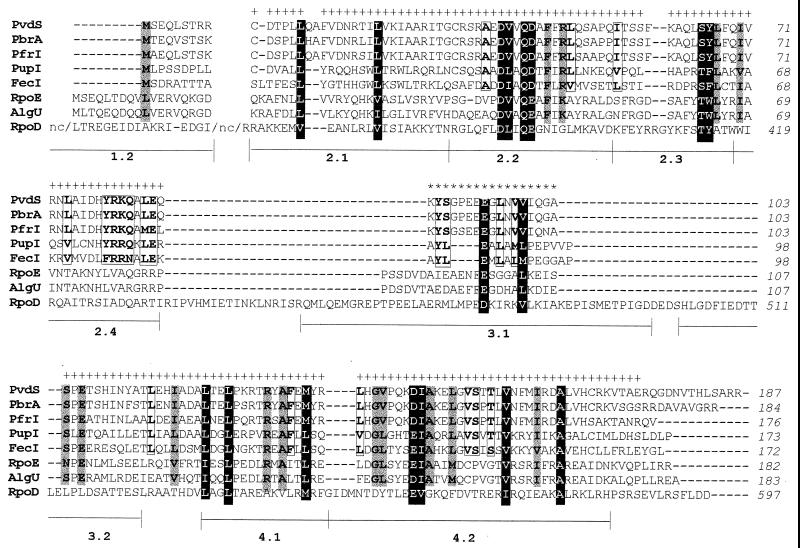
To highlight common features between PvdS and the ECF subfamily, the PvdS, PfrI, and PbrA proteins were aligned with representative members of the ECF subfamily and the ς70 factor (Fig. 1). Since RpoI, FiuI, PigD, and the putative PvdS-related P. aeruginosa peptides are predicted to be ECF sigma factors but functional evidence for their activity is missing, they were omitted from the alignment of Fig. 1.
FIG. 1.
Alignment of the PvdS protein with Fur-controlled putative ECF sigma factors (PbrA, PfrI, PupI, and FecI), stress response sigma factors (AlgU and RpoE), and the primary sigma (RpoD). The sequences were translated from the GenBank entries given in parentheses: P. aeruginosa PvdS (U128919) and AlgU (L021119), P. fluorescens PbrA (X79908), P. putida PfrI (X82038) and PupI (X77918), and E. coli FecI (U14003), RpoE (U37089), and RpoD (J016887). For RpoD, the N-terminal 85 residues and a 254-residue nonconserved sequence (nc) between regions 1 and 2 are not shown. Regions 2, 3, and 4 of RpoD, conserved among the ς70 family (23), are shown below the alignment. Conserved residues among the entire data set are highlighted in reverse type. Shaded areas indicate conserved residues among the ECF family. Conserved residues among the Fur-controlled ECF sigma factors are boxed. Identical residues and the following sets of residues are considered matched: DE; NQ; RK; ST; FYW; ILVM. Plus signs and asterisks indicate amino acid positions selected for construction of maximum-likelihood trees.
The phylogenetic relationships among proteins aligned in Fig. 1 were analyzed by quartet puzzling, a maximum-likelihood algorithm which accounts for site-to-site rate variation and gives only fully resolved groupings. After removal of gap positions and regions of ambiguous or uncertain homology, a data set of 145 positions (topped by plus symbols in Fig. 1) was obtained. The maximum-likelihood tree inferred from the data set is shown in Fig. 2. Identical results and comparable robustness of the internal nodes were obtained by use of the maximum-parsimony analysis. Similar topology and comparable quartet puzzling reliability and bootstrap confidence level values were also obtained upon inclusion of 13 sites (topped by asterisks in Fig. 1) corresponding to part of conserved subregion 3.1 of ς70. The phylogenetic distance analysis confirmed that the PvdS, PfrI, and PbrA proteins belong to the ECF family and cluster, together with the iron-responsive PupI and FecI proteins, in a different subgroup with respect to the ςE-like sigma factors AlgU and RpoE (15, 37, 40, 43). This is consistent with the former resolution of the FecI protein in a different branch with respect to RpoE and AlgU (22).
FIG. 2.
Maximum-likelihood tree inferred from the primary structure alignment shown in Fig. 1. The analysis was limited to the 145 positions marked by the plus symbols in Fig. 1. The quartet puzzling method was used with a gamma-distributed model of site-to-site variation using eight rate categories. The gamma distribution parameter α estimated from the data set was 2.03, and the log-likelihood of the tree was −1,973.18. The scale bar represents 1.0 amino acid substitution per site. Numbers above nodes are quartet puzzling reliability values. Bootstrap confidence levels were comparable to quartet puzzling reliability values.
Since primary and alternative sigmas related to the ς70 family typically contain a helix-turn-helix (H-T-H) motif in the C-terminal region (23), a computational analysis of the PvdS secondary structure was performed, showing the presence of a possible H-T-H motif in the PvdS C-terminal region (from positions 113 to 149).
Expression of PvdS and in vitro binding to the core fraction of RNAP.
The coding region of the pvdS gene was cloned in the expression vector pQE60, in frame with the His6 coding sequence at the C terminus, yielding plasmid pPvdS6H. Alternatively, the His6 coding sequence was replaced by a sequence encoding the FLAG octapeptide, yielding plasmid pPvdSF. To obtain a pQE60-derived construct expressing the wild-type pvdS gene, a third plasmid, designed pPvdSWT, was generated by deleting the His6-coding sequence in pQE60 (see Materials and Methods). The pQE60 expression vector and its three derivatives were used to transform E. coli M15 carrying the repressor plasmid pDMI,1 (49). SDS-PAGE analysis of IPTG-induced bacterial lysates revealed that the wild-type and tagged PvdS proteins can be overexpressed in E. coli to similar extents (data not shown). The pvdS gene sequence predicts a protein of 21,230 kDa. The apparent masses of both the wild-type and tagged PvdS proteins on SDS-polyacrylamide gels were approximately 28 kDa (data not shown). This anomalous mobility has been previously reported for many RNAP sigma factors (22). Automated Edman degradation of the overexpressed protein samples electroblotted after SDS-PAGE gave, in all three cases, the expected N-terminal sequence (AEQLSTRR), which differs from the wild-type sequence (SEQLSTRR) by the A→S conservative substitution resulting from the cloning of pvdS into pQE60.
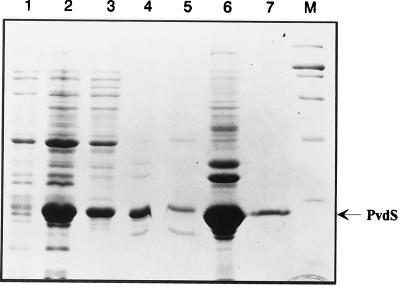
Analysis of supernatants and pellets of the whole cell lysates by SDS-PAGE revealed that the main fraction of overexpressed PvdS was recovered in the insoluble cell fraction (Fig. 3, lane 4) and could not be solubilized with the detergent N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (DNP) (Fig. 3, lanes 5 and 6), sodium deoxycolate, or Nonidet P-40 (data not shown). The His6-tagged PvdS (PvdS6H) was therefore purified by metal chelate (Ni-nitrilotriacetic acid) affinity chromatography under denaturing conditions (key purification steps are shown in Fig. 3).
FIG. 3.
Overexpression of the His6-tagged PvdS protein (PvdS6H) in E. coli M15(pDMI,1) carrying the pPvdS6H expression plasmid. The PvdS6H protein was purified under denaturing conditions from the insoluble cell fraction. SDS-PAGE analysis of protein samples from key steps of the purification are shown: lane 1, uninduced whole cell extract; lane 2, induced whole cell extract; lane 3, soluble cell extract; lane 4, insoluble cell extract; lane 5, supernatant of DNP-treated inclusion bodies; lane 6, precipitate of detergent-treated inclusion bodies; lane 7, renatured fraction of the eluted PvdS6H protein; M, protein size standards of 200.0, 116.2, 97.4, 66.2, 45.0, 31.0, and 21.0 kDa (high and medium range; Bio-Rad). The arrow indicates the PvdS6H protein.
Replacement of the His6 tag with the FLAG tag in our expression system did not affect significantly the induction parameters, nor did it alter protein yields, though it made it possible to purify by affinity chromatography the soluble fraction of the protein from the bacterial lysate. PvdSF, although overproduced (Fig. 4A, lane 2), was poorly recovered after purification from the soluble cell extract using anti-FLAG affinity gel chromatography. Interestingly, SDS-PAGE analysis of the purified fraction (Fig. 4A, lane 3) revealed that PvdSF (28-kDa protein band) coeluted with three additional proteins whose sizes were compatible with the molecular masses of the E. coli core RNAP α, β, and β′ subunits (37, 151, and 156 kDa, respectively [13]). Protein sequencing confirmed that the proteins of 151 and 156 kDa correspond to the β and β′ subunits of the RNAP, respectively. Western blot analyses with antibodies against FLAG (Fig. 4B) and the α subunit of the RNAP (Fig. 4C) demonstrated that copurification of PvdSF with the core fraction of RNAP had occurred. Densitometric scanning of the gel revealed an approximately 1:1 stoichiometry of the PvdSF-core (α2ββ′) RNAP complex.
FIG. 4.
Copurification of PvdS with the core fraction of E. coli RNAP. The FLAG-tagged PvdS protein (PvdSF) was overexpressed in E. coli M15(pDMI,1) carrying the pPvdSF expression plasmid and purified under nondenaturing conditions from the soluble cell fraction. Lane 1, whole cell extracts of the uninduced culture; lane 2, whole cell extracts of the induced culture; lane 3, PvdS-RNAP complex (subunits α2ββ′), eluted from the FLAG affinity column; lane 4, commercial vegetative RNAP (subunits ς70α2ββ′; Epicentre Technologies). Protein size markers (high and medium range; Bio-Rad) are shown on the left. (A) SDS-PAGE analysis of protein samples. (B) Western blot analysis with anti-FLAG antibodies. (C) Western blot analysis with antibodies against the α subunit of the E. coli RNAP.
The pvdS-dependent activity of pvd promoters in P. aeruginosa and E. coli.
The pvdA, pvdD, and pvdE promoters, independently examined by different laboratories, exhibit significantly lower activity in E. coli carrying the multicopy pvdS gene in trans than in wild-type P. aeruginosa (8, 20, 30). To rule out the possibility that the low activity of pvd promoters in E. coli may be due to reduced expression of the pvdS gene, plasmids carrying the pvdA, pvdD, and pvdE promoters fused to lacZ were introduced into the parental strain PAO1 and in the heterologous host E. coli MC4100 carrying the multicopy pvdS gene under the control of either the indigenous iron-repressible promoter (plasmid pBRXB) or the IPTG-inducible PT5 lacO promoter (plasmid pPvdSWT). The β-galactosidase activities were measured under repressing (100 μM FeCl3) or inducing (low-iron; 100 μM IPTG) growth conditions (see Materials and Methods for details). The results (Table 2) indicate that under low-iron conditions, the pvd promoters exhibit 20- to 100-fold-lower activity in E. coli MC4100 carrying the multicopy plasmid pBRXB than in PAO1. Remarkably, the activity of pvd promoters in induced MC4100 (pPvdSWT) was still 2- to 10-fold lower than in PAO1 grown under low-iron conditions. SDS-PAGE analysis of cell lysates showed that PvdS was overproduced upon induction of E. coli MC4100(pPvdSWT; pDMI,1) and that the levels of soluble protein were comparable to those previously detected in induced cultures of E. coli M15 (pPvdSWT; pDMI,1) (data not shown). This result demonstrates that the low activity of pvd promoters in E. coli is unlikely to be due to inefficient expression of the pvdS gene and raises the possibility that some additional regulator(s) may be required for full activity of these promoters.
TABLE 2.
PvdS-dependent transactivation of pvd::lacZ fusions in P. aeruginosa and E. coli
| Plasmids | LacZ activitya
|
|||||
|---|---|---|---|---|---|---|
|
P. aeruginosa
|
E. coli(pBRXB)
|
E. coli (pPvdSWT)
|
||||
| DCAAb | DCAA + 100 μM FeCl3 | M9 + 150 μM 2,2′- dipyridyl | M9 + 100 μM FeCl3 | LB | LB + IPTGc | |
| pPV51 | 10,508 | 322 | 312 | 138 | 58 | 1,905 |
| pMP190::PpvdD | 1,272 | 262 | 62 | 21 | 81 | 585 |
| pMP190::PpvdE | 4,056 | 236 | 37 | 9 | 75 | 413 |
Expressed in Miller units (28). The reported values correspond to means of three independent determinations.
Low-iron medium (54).
Cultures (A600 ≅ 0.4) were induced with 1 mM IPTG, followed by a 3-h incubation at 37°C prior to testing for β-galactosidase activity. Uninduced cultures in LB were used as control.
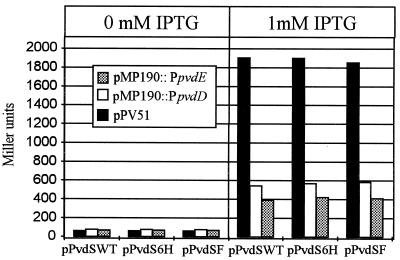
To investigate whether the C-terminal tagging could affect the functional integrity of PvdS, we compared the abilities of wild-type and tagged PvdS proteins to transactivate the pvd promoters in E. coli. The pvd::lacZ promoter probe plasmids pPV51, pMP190::PpvdD, and pMP190::PpvdE were introduced in E. coli MC4100(pDMI,1) carrying plasmids pPvdSWT, pPvdS6H, and pPvdSF, respectively. The β-galactosidase activity levels were measured in uninduced or IPTG-induced cultures in LB. The results in Fig. 5 demonstrate that neither C-terminal extension affects the in vivo activity of PvdS, as the extents of transactivation of pvd promoters are comparable for wild-type and both tagged PvdS proteins.
FIG. 5.
Expression of pvd::lacZ fusions (plasmids pPV51, pMP190::PpvdD, and pMP190::PpvdE) in E. coli MC4100(pDMI,1) harboring alternatively plasmids pPvdSWT, pPvdS6H, and pPvdSF. Cultures in LB medium (A600 = ≅0.4) were induced with 1 mM IPTG and incubated for additional 3 h at 37°C prior to testing for β-galactosidase activity (expressed in Miller units [28]). Uninduced cultures (0 mM IPTG) were used as controls.
Formation of the PvdS-RNAP complex is essential for binding to the pvdA promoter.
To investigate the in vitro interaction between the PvdSF-RNAP complex and the pvdA promoter, a 32P-labeled DNA fragment of 177 bp encompassing the pvdA promoter (PpvdA) was used as the probe for gel retardation experiments. Preliminary assays indicated that the affinity chromatography-purified PvdSF-RNAP complex (1 pmol) was able to bind the PpvdA probe (0.1 pmol) even in the presence of unspecific competitors such as sonicated salmon sperm DNA (5 μg/ml) or poly(dI-dC) (2 μg/ml), thereby retarding the electrophoretic mobility of the DNA probe (data not shown). To gain insight into the properties of the protein-DNA complex, binding experiments were carried out using the polyanion heparin as competitor (Fig. 6). Heparin is known to release the RNAP from the weak (heparin-sensitive) closed complexes which initially occur between RNAP and the target promoter (10, 31, 38), and addition of heparin to the RNAP-DNA reaction mixture makes it possible to specifically detect the formation of stable (heparin-resistant) open complexes. Our results show that the PpvdA probe freely migrates through the gel when preincubated with commercial E. coli ς70-RNAP complex or the core RNAP fraction, prior to heparin addition (Fig. 6, lanes 2 to 4 or 5 to 7, respectively). Likewise, the PvdS6H protein does not cause retardation of the DNA probe when tested under the same conditions (Fig. 6, lanes 8 to 10). On the other hand, both the affinity-purified PvdSF-RNAP 1:1 complex (lanes 11 to 15) and the PvdS6H protein, premixed with commercial core RNAP fraction from E. coli (lanes 16 to 20), display heparin-resistant PpvdA binding ability. Control assays confirmed that gel shifts of PvdS-RNAP-PpvdA complexes were no longer detectable upon addition of a 125-fold excess of unlabeled PpvdA probe to the reaction mixture (data not shown). Although the PvdS-RNAP holoenzyme engages the pvdA promoter in a heparin-resistant way, the DNA-protein complex is formed at low efficiency. Indeed, most of the probe freely migrates through the gel in the presence of an equimolar concentration of PvdS-RNAP holoenzyme. Low DNA binding activity could be due either to a poor quality of our PvdS-RNAP preparations or to the requirement of additional factors for efficient binding. It is clear, however, that neither PvdS nor the core RNAP fraction independently associates with PpvdA, but physical interaction between these two components is required for specific recognition and open complex formation at the pvdA promoter.
Additional positive regulatory factors may be required for full activity of PvdS-dependent promoters.
Repeated attempts to detect in vitro transcription using the PvdS-RNAP holoenzyme and a linear pvdA template were unsuccessful. Since pvd promoters were found to be weakly active in E. coli carrying the pvdS gene in trans, it was speculated that the inability of the PvdS-RNAP holoenzyme to direct transcription of pvdA promoter in vitro could be due to the requirement of additional positive regulator(s). In P. putida WCS358, besides PfrI, the PvdS homologue, a second positive regulator, PfrA, is required for pseudobactin biosynthesis. We reasoned that this could also be the case for P. aeruginosa. Search for PfrA homologues in the P. aeruginosa genomic sequence revealed that the protein with the highest similarity to PfrA was AlgR2, the product of the algR2 gene (also known as algQ [18, 42]). AlgR2, a positive regulator of alginate biosynthesis, is 58% identical to PfrA, and the two proteins are partially interchangeable between P. putida and P. aeruginosa (52). On this basis, the iron-dependent activity of pvd transcriptional fusions was measured in wild-type P. putida strain WCS358 and in its derivatives VM119 and WCS358.E9, in which the pfrI and pfrA genes are inactivated by Tn5 and Tn3Gus insertions, respectively (51, 52). Results (Table 3) demonstrate that the pvd promoters are normally iron regulated in P. putida WCS358 but completely silent in both pfrI and pfrA mutants, demonstrating that in P. putida, both PfrI and PfrA are essential for expression of pvd genes. Remarkably, the β-galactosidase levels determined for pvd::lacZ fusions were comparable in wild-type P. putida WCS358 (Table 3) and P. aeruginosa PAO1 (Table 2), indicating that very similar, probably interchangeable, regulatory pathways direct the expression of pyoverdins and pseudobactins in fluorescent pseudomonads.
TABLE 3.
Effects of pfrA and pfrI mutations on the activity of pvd::lacZ gene fusions in P. putida WCS358
| Strain | Genomic mutation | Plasmid | LacZ activitya
|
|
|---|---|---|---|---|
| −Fe(III) | +Fe(III) | |||
| WCS358 | pMP220 | 168 | 132 | |
| pMP190 | 52 | 38 | ||
| pPV51 | 11,621 | 141 | ||
| pMP190::PpvdD | 2,161 | 35 | ||
| pMP190::PpvdE | 4,230 | 41 | ||
| WCS358.E9 | pfrA::Tn3Gus | pMP220 | 131 | 110 |
| pMP190 | 98 | 75 | ||
| pPV51 | 92 | 101 | ||
| pMP190::PpvdD | 101 | 113 | ||
| pMP190::PpvdE | 116 | 109 | ||
| VM119 | pfrI::Tn5 | pMP220 | 129 | 113 |
| pMP190 | 95 | 78 | ||
| pPV51 | 93 | 101 | ||
| pMP190::PpvdD | 110 | 118 | ||
| pMP190::PpvdE | 120 | 115 | ||
Determined in culture lysates of P. putida grown overnight in DCAA [−Fe(III)] and DCAA supplemented with 100 μM FeCl3 [+Fe(III)]. Units of activity are as defined by Miller (28) and correspond to means of four determinations.
To investigate the role of the AlgR2 protein in the regulation of iron-dependent P. aeruginosa promoters, the β-galactosidase levels expressed under low- and high-iron conditions by plasmid pPV51 were compared in P. aeruginosa 8830 and 8830R2::Cm, which are isogenic except for an algR2::Cm mutation in the latter. Table 4 shows that the levels of iron-regulated expression of the reporter gene are comparable in both the parental strain and the algR2 mutant, demonstrating that the AlgR2 protein is not involved in regulation of the pvdA gene. This conclusion is in line with the results of cross-complementation studies between the pfrA gene of P. putida and the algR2 gene of P. aeruginosa, which showed that pfrA could functionally replace algR2 for alginate production in P. aeruginosa, while the defect in siderophore expression by the pfrA mutant of P. putida was very poorly complemented by the algR2 gene (52).
TABLE 4.
Effect of the algR2 mutation on the activity of the pvdA::lacZ gene fusion in P. aeruginosa 8830
| Strain | Genomic mutation | Plasmid | LacZ activitya
|
|
|---|---|---|---|---|
| −Fe(III) | +Fe(III) | |||
| 8830 | his-1 | pMP220 | 48 | 50 |
| pPV51 | 2,151 | 38 | ||
| 8830R2::Cm | his-1 algR2::Cm | pMP220 | 38 | 35 |
| pPV51 | 2,200 | 34 | ||
Determined in culture lysates of P. aeruginosa grown in IFKB [−Fe(III)] and IFKB supplemented with 100 μM FeCl3 [+Fe(III)]. Units of activity are as defined by Miller (28) and correspond to means of four determinations.
DISCUSSION
Members of the ECF family of alternative sigma factors display remarkable diversity at the level of primary structure. However, all sigma factors included in this family are endowed with two main properties; first, they are responsible for the expression of genes or operons involved in extracytoplasmic functions (e.g., biosynthesis and/or uptake of siderophores); second, their own expression is responsive to specific extracytoplasmic stimuli (e.g., iron limitation) (15, 22, 29). In Pseudomonas spp., the PvdS, PbrA, PfrI, and PupI transcriptional activators are primarily involved in iron uptake, and their expression is controlled in response to iron availability through the Fur repressor protein (7, 53).
In this study we provide genetic and biochemical evidence for the inclusion of PvdS within the ECF family of alternative sigma factors. An alignment of the iron uptake regulators PvdS, PbrA, and PfrI from fluorescent Pseudomonas spp. with the ECF sigma factors RpoE (ςE) and FecI from E. coli, AlgU from P. aeruginosa, and the primary sigma RpoD (ς70) from E. coli (1, 15, 22) highlighted significant conservation of relevant residues in regions 2 and 4 (Fig. 1). Region 2 consists of four subregions: 2.1, 2.2, 2.3, and 2.4. Subregions 2.1 and 2.3 are critical for high-affinity binding of ς70 to the core RNAP and for promoter melting, respectively (23). Subregion 2.2 is the hydrophobic core of region 2, being sandwiched between the prevalently helical 2.1, 2.3 and 2.4 subregions (23, 24). PvdS shares common traits with subregions 2.1, 2.2, and 2.3 of ς70, while it is highly divergent at the level of subregion 2.4. This domain of ς70 is implicated in recognition of the −10 promoter region and is typically variant among alternative sigma factors which recognize a variety of −10 sequences (22, 23). However, conservation of region 2.4 can be observed within subgroups of closely related sigma factors, consistent with group-specific promoter preferences. In fact, AlgU of P. aeruginosa and RpoE of E. coli are functionally interchangeable between the two species (29, 57). Likewise, the P. putida pfrI gene can complement a pvdS mutation in P. aeruginosa and vice versa (L. Leoni and P. Visca, unpublished data). In contrast, neither RpoE nor FecI can activate the P. aeruginosa PvdS-responsive promoters, which are known to be silent in iron-limited E. coli cells (8, 20). The lack of conserved residues in the 2.4 subregion of the ECF sigma factors could also account for the existence of multiple members of the ECF subfamily in the same species (29). The ECF sigma factors, including PvdS, were found to lack most of region 3 which has been implicated in the binding of core RNAP by ς70 (23). The domain corresponding to region 4 of ς70 is present and fairly conserved in PvdS and the related ECF sigma factors. This region is involved in recognition of the −35 promoter sequence (23) and contains a putative H-T-H motif which was also predicted for PvdS.
Interestingly, the topology of the maximum-likelihood tree (Fig. 2) correlates members of each subgroup by both function and mode of regulation. In fact, the Fur-dependent sigma factors PvdS, PbrA, PfrI, PupI, and FecI cluster in a distinct branch with respect to the stress response ECF factors RpoE and AlgU (15, 29). Within the Fur-controlled cluster, the PvdS, PbrA, and PfrI proteins, which activate the expression of siderophore biosynthesis genes, branch in a different subgroup with respect to PupI and FecI, which direct the expression of ferric chelator receptors and undergo similar regulatory controls (7). These findings are in line with the previous observation that members of a subgroup are more similar than primary and alternative sigma factors in the same organism (23). The functional rather than phylogenetic correlation between different subgroups of sigma factors could also imply that regulation of similar activities requires common functional constraints.
The ECF alternative sigmas are connected to complex regulative pathways. Members of the ςE-like subgroup are generally negatively controlled by anti-sigma factors (29). In contrast, the sigma factors belonging to the iron-related group are negatively controlled at the transcriptional level by the Fur repressor, and most of them require additional positive regulators to direct transcription (7, 53). The modes of positive regulation seem to differ between the two subgroups of Fur-controlled sigma factors. PupI and FecI behave as response regulators in a novel two-component regulatory system in which PupR and FecR act as signal transducers (1, 19, 32). PfrI-dependent promoters require the positive regulator PfrA for full activity. PfrA displays no similarity to PupR or FecR, suggesting that the PfrI/PfrA system may act differently from the PupI/PupR and FecI/FecR systems (52, 53).
Our investigation also provides robust but still incomplete biochemical evidence that PvdS behaves like a sigma factor sensu stricto, since we demonstrated in vitro that PvdS is able to form a complex with the core fraction of RNAP, thereby promoting binding of the PvdS-RNAP holoenzyme to the pvdA promoter. The PvdS protein was expressed as fusion peptide with either the His6 tag or the FLAG epitope at the C terminus and purified by affinity chromatography. C-terminal tagging was preferred because of the structural and functional constraints predicted for the PvdS N-terminal domain. In fact, a region of significant homology with the ς70 region 2 is located in proximity to the N-terminal region of the PvdS protein, while the extreme C-terminal region of ECF factors is highly divergent both in extension and in sequence, predicting structural tolerance for this protein domain. In line with these predictions, in vivo transactivation assays of pvd promoters indicated that C-terminal extensions do not affect the activity of PvdS compared to the wild-type counterpart (Fig. 5). Most interestingly, the PvdS protein expressed in E. coli was copurified with the core fraction of RNAP (subunits α2ββ′ [Fig. 4]) under nondenaturing conditions. Since the stoichiometry determined for the PvdS-core RNAP complex was approximately 1:1 and there was no evidence for the presence of ς70 in copurified fraction (Fig. 4), it can be deduced that PvdS can replace ς70 for core RNAP binding. These results are in agreement with alignment data showing that PvdS and ς70 are endowed with similar structural features at the level of subregion 2.1 and parts of region 3, which have been implicated in binding to the core fraction of RNAP. Although large amounts of PvdSF were present in the induced whole cell extracts, only a minor portion was eluted after affinity chromatography, suggesting that only the fraction of PvdSF capable of interacting with the core RNAP can be successively purified from the soluble fraction. A possible explanation is that binding of the overexpressed PvdSF to the available core RNAP could facilitate the proper folding or increase the stability of the protein in the cytosol.
The primary structure of the core RNAP subunits is highly conserved in E. coli and P. aeruginosa, and both enzymes have been reported to recognize and direct transcription from heterologous strong promoters with the same efficiency in vitro (13). For these reasons, protein-DNA interaction experiments were conducted with commercial core RNAP and ς70-dependent RNAP holoenzyme from E. coli. Gel retardation assays clearly show that PvdS is by itself unable to associate with the pvdA promoter in the presence of heparin, while it is able to do so in combination with the core RNAP fraction. Heparin releases weak protein-DNA interactions, and only promoters engaged with RNAP in a stable (open) complex are expected to be heparin resistant (10, 31).
Because PvdS is essential for the heparin-resistant binding of the core RNAP fraction to the pvdA promoter, the formation of stable open complexes between the PvdS-RNAP holoenzyme and PpvdA can be hypothesized. Binding was far from being stoichiometric, suggesting that only a small portion of our PvdS-RNAP preparation is functionally active. Since the C-terminal tagging does not affect the in vivo activity of PvdS, plausible reasons for the low binding efficiency could be the requirement of either posttranslational modifications or cooperating factors capable of increasing the affinity of the PvdS-RNAP complex for the pvdA promoter.
The hypothesis that PvdS mediates promoter recognition and DNA melting at the pvdA promoter is consistent with the presence of a well-conserved 2.3 region, responsible for promoter melting, in all ECF sigma factors (Fig. 1). Moreover, the observation that PvdS alone cannot bind the pvdA promoter, though as PvdS-RNAP holoenzyme it can, makes it reasonable that PvdS could interact with the core RNAP fraction in the cytosol prior to promoter recognition. Lack of binding to target promoter sequences is a common feature of primary and alternative sigma factors (23).
Although many lines of evidence support the hypothesis that PvdS is an alternative sigma factor specific for pvd genes, conclusive proof of the transcriptional activity of the PvdS-dependent RNAP complex is still missing. A role in transcription initiation in vitro has not yet been demonstrated for PvdS, PfrI, and PbrA (8, 45, 53), while it has been documented for several ECF sigma factors, including FecI and RpoE from E. coli, AlgU from P. aeruginosa, SigE from Streptomyces coelicolor, SigE from Mycobacterium tuberculosis, and SigX and SigW from Bacillus subtilis (1, 3, 17, 22, 40, 43, 56). In our hands, repeated attempts to obtain runoff transcripts with in vitro-reconstituted PvdS6H-core RNAP complex or the copurified PvdSF-RNAP complex and the pvdA promoter as a linear template were unsuccessful, probably due to the requirement of some still unidentified positive regulator(s), an issue which will be the subject of further studies.
The strongest indication of the involvement of an additional factor in the activation of pvd genes comes from the observation that the pvd::lacZ fusions are silent in both pfrA and pfrI mutants of P. putida WCS358, while in wild-type WCS358 they are expressed at the same level as in PAO1. The phylogenetic and functional relationships between PfrI and PvdS (reference 53 and our results), combined with the evidence that pvd promoters are silent in both pfrA and pfrI mutants of P. putida, argue for the existence of similar regulatory mechanisms of fluorescent siderophore genes in P. putida and P. aeruginosa (7, 39, 46, 53). However, the mechanism by which PfrA stimulates transcription from pseudobactin 358 biosynthetic promoters is still obscure, nor it is clear whether this protein can interact with PfrI.
Many ECF sigma factors coexist in P. aeruginosa, and some of them are controlled by cognate activators (15, 34). Therefore, it is plausible that an additional activator, functionally related to PfrA but not to AlgR2, may be involved in the positive regulation of pyoverdin genes in concert with PvdS. Further evidence for the existence of common activation pathways of pyoverdin and pseudobactin genes in fluorescent pseudomonads is provided by the presence of the iron starvation box in the pvd and toxA promoters of P. aeruginosa and in PbrA- and PfrI-dependent promoters of the fluorescent Pseudomonas strains M114 and WCS358, respectively (4, 39, 51, 52). The iron starvation box, a cis-acting element probably involved in binding of a trans-acting positive regulatory protein, is a distinctive element of promoters directly or indirectly controlled by the iron starvation sigma factors (39). Since the iron starvation box is located at variable distance from the transcription start points of different pvd promoters and is absent on the PvdS-dependent regAB promoter, it is unlikely that it constitutes the DNA recognition site for a sigma factor like PvdS (25, 27, 39). Typical features of activation-responsive promoters transcribed by vegetative RNAP are the deviation of the recognition sequence from the typical −10/−35 consensus and the presence of multiple transcription initiation sites (16, 36). The failure to retrieve a consensus for PvdS-dependent promoters (20) and the presence of multiple transcription start sites in pvdA, pvdD, and regAB genes (20, 33, 39, 55) are further arguments suggesting the requirement of ancillary activating factors for the PvdS-dependent gene expression.
Since the PvdS-RNAP holoenzyme engages the pvdA promoter at low efficiency but in heparin-resistant fashion, a hypothetical transcriptional activator could act at different stages during the formation of the transcription initiation complex (38). It is also possible that posttranscriptional modifications of PvdS may be required for full-efficiency open complex formation or switch from abortive to processive elongation. A model summarizing the proposed regulatory pathway of pvd genes is shown in Fig. 7. Further searches for an additional iron-responsive regulator(s) will provide additional insight into the complex regulatory network of iron-controlled genes in P. aeruginosa.
FIG. 7.
Proposed regulatory cascade for pyoverdin (pvd) biosynthetic genes. In the presence of iron, the Fur protein binds the Fur box on the pvdS promoter, thereby repressing PvdS expression. In the absence of iron, Fur repression is relieved and pvdS is transcribed. The PvdS protein is an alternative sigma factor which confers to the core RNAP (cRNAP) specificity for pvd promoters. Additional positive regulator(s) may also be involved in the regulatory network.
ACKNOWLEDGMENTS
We are very grateful to G. Bertoni, CSIC, Madrid, Spain; P. Cammarano, University of Rome “La Sapienza,” Rome, Italy; A. Chakrabarty, University of Illinois College of Medicine, Chicago; I. Lamont, University of Otago, Otago, New Zealand; and V. Venturi, ICGEB, Trieste, Italy, for the gifts of strains and plasmids and for helpful discussions. We thank A. Petrucca and G. Baiocchi for valuable technical assistance.
This work was supported by grants from “Istituto Pasteur - Fondazione Cenci Bolognetti” and from the Italian Ministry of University and Scientific Research.
REFERENCES
- 1.Angerer A, Enz S, Ochs M, Braun V. Transcriptional regulation of ferric citrate transport in Escherichia coli K12. FecI belongs to a new subfamily of ς70-type factors that respond to extracytoplasmatic stimuli. Mol Microbiol. 1995;18:163–174. doi: 10.1111/j.1365-2958.1995.mmi_18010163.x. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Brutsche S, Braun V. SigX of Bacillus subtilis replaces the ECF sigma factor FecI of Escherichia coli and is inhibited by RsiX. Mol Gen Genet. 1997;256:416–425. doi: 10.1007/s004380050585. [DOI] [PubMed] [Google Scholar]
- 4.Callanan M, Sexton R, Dowling D N, O'Gara F. Regulation of the iron uptake genes in Pseudomonas fluorescens M114 by pseudobactin M114: the pbrA sigma factor does not mediate the siderophore regulatory response. FEMS Microbiol Lett. 1996;144:61–66. doi: 10.1111/j.1574-6968.1996.tb08509.x. [DOI] [PubMed] [Google Scholar]
- 5.Casabadan M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 6.Certa U, Bannwarth W, Stueber D, Gentz R, Lanzer M, Le Grice S, Giullot F, Wendler I, Hunsmann G, Bujard H, Mous J. Subregions of a conserved part of the HIV gp41 transmembrane protein are differentially recognized by antibodies of infected individuals. EMBO J. 1986;5:3051–3056. doi: 10.1002/j.1460-2075.1986.tb04605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crosa J H. Signal transduction and transcriptional and posttranscriptional control of iron regulated genes in bacteria. Microbiol Mol Biol Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunliffe H E, Merriman T R, Lamont I L. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa. PvdS is probably an alternative sigma factor. J Bacteriol. 1995;177:2744–2750. doi: 10.1128/jb.177.10.2744-2750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darzins A, Chakrabarty A M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate from Pseudomonas aeruginosa. J Bacteriol. 1984;159:9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escolar L, de Lorenzo V, Martin J P. Metalloregulation in vitro of the aerobactin promoter of Escherichia coli by the Fur (ferric uptake regulation) protein. Mol Microbiol. 1997;26:799–808. doi: 10.1046/j.1365-2958.1997.6211987.x. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.5c. Seattle: Department of Genetics, University of Washington; 1993. . (Distributed by the author.) [Google Scholar]
- 12.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J, Gussin G N. RNA polymerases from Pseudomonas aeruginosa and Pseudomonas syringae respond to Escherichia coli activator proteins. J Bacteriol. 1991;173:394–397. doi: 10.1128/jb.173.1.394-397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geels F P, Schippers B. Reduction in yield depression in high frequency potato cropping soil after seed tuber treatments with antagonistic fluorescent Pseudomonas spp. Phytopathology. 1983;108:201–214. [Google Scholar]
- 15.Govan J R W, Deretic W. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoopes B C, McClure W R. Strategies in regulation of transcription initiation. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 1231–1240. [Google Scholar]
- 17.Huang X, Frederick K L, Helmann J D. Promoter recognition by Bacillus subtilis ςW: autoregulation and partial overlap with the ςX regulon. J Bacteriol. 1998;180:3765–3770. doi: 10.1128/jb.180.15.3765-3770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato J, Chu L, Kitano K, DeVault J D, Kimbara K, Chakrabarty A M, Misra T K. Nucleotide sequence of a regulatory region controlling alginate synthesis in Pseudomonas aeruginosa: characterization of the algR2 gene. Gene. 1989;84:31–38. doi: 10.1016/0378-1119(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 19.Koster M W, van Klompenburg, Bitter W, Leong J, Weisbeek P. Role of the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO J. 1994;13:2805–2813. doi: 10.1002/j.1460-2075.1994.tb06574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leoni L, Ciervo A, Orsi N, Visca P. Iron-regulated transcription of the pvdA gene in Pseudomonas aeruginosa: effect of Fur and PvdS on promoter activity. J Bacteriol. 1996;178:2299–2313. doi: 10.1128/jb.178.8.2299-2313.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litwin C M, Calderwood S B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lonetto M A, Gribskov M, Gross C A. The ς70 family: sequence conservation and evolutionary relationship. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra A, Severinova E, Darst S A. Crystal structure of a ς70 subunit fragment from Escherichia coli RNA polymerase. Cell. 1996;87:127–136. doi: 10.1016/s0092-8674(00)81329-x. [DOI] [PubMed] [Google Scholar]
- 25.McMorran B J, Merriman M E, Rombel I T, Lamont I L. Characterization of the pvdE gene which is required for pyoverdine synthesis in Pseudomonas aeruginosa. Gene. 1996;176:55–59. doi: 10.1016/0378-1119(96)00209-0. [DOI] [PubMed] [Google Scholar]
- 26.Mencia M, Salas M, Rojo F. Residues of the Bacillus subtilis phage φ29 transcriptional activator required both to interact with RNA polymerase and to activate transcription. J Mol Biol. 1993;233:695–704. doi: 10.1006/jmbi.1993.1546. [DOI] [PubMed] [Google Scholar]
- 27.Merriman T R, Merriman M E, Lamont I L. Nucleotide sequence of pvdD, a pyoverdine biosynthetic gene from Pseudomonas aeruginosa: PvdD has similarity to peptide synthases. J Bacteriol. 1995;177:252–258. doi: 10.1128/jb.177.1.252-258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 252–255. [Google Scholar]
- 29.Missiakas D, Raina S. The extracytoplasmic function sigma factors: role and regulation. Mol Microbiol. 1998;28:1059–1066. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki H, Kato H, Nakazawa T, Tsuda M. A positive regulatory gene, pvdS, for expression of pyoverdin biosynthetic genes in Pseudomonas aeruginosa PAO. Mol Gen Genet. 1995;248:17–24. doi: 10.1007/BF02456609. [DOI] [PubMed] [Google Scholar]
- 31.Ninfa A J, Reitzer L J, Magasanik B. Initiation of transcription at the bacterial glnAp2 promoter by purified Escherichia coli components is facilitated by enhancers. Cell. 1987;50:1039–1046. doi: 10.1016/0092-8674(87)90170-x. [DOI] [PubMed] [Google Scholar]
- 32.Ochs, Veitinger M S, Kim I, Weltz D, Angerer A, Braun V. Regulation of citrate-dependent iron transport of Escherichia coli: fecR is required for transcription activation by FecI. Mol Microbiol. 1995;15:119–132. doi: 10.1111/j.1365-2958.1995.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 33.Ochsner U A, Johnson Z, Lamont I L, Cunliffe H E, Vasil M L. Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor. Mol Microbiol. 1996;21:1019–1028. doi: 10.1046/j.1365-2958.1996.481425.x. [DOI] [PubMed] [Google Scholar]
- 34.Ochsner U A, Vasil M. Gene repression by the ferric-uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc Natl Acad Sci USA. 1996;93:4409–4414. doi: 10.1073/pnas.93.9.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollack M. Pseudomonas aeruginosa. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone Inc.; 1995. pp. 1980–2003. [Google Scholar]
- 36.Raibaud O, Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- 37.Raina S, Missiakas D, Georgopoulos C. The rpoE gene encoding the ςE (ς24) heat shock sigma factor of Escherichia coli. EMBO J. 1995;14:1043–1055. doi: 10.1002/j.1460-2075.1995.tb07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Record M T, Reznikoff Jr, W S, Craig M L, McQuade K L, Schlax P. Escherichia coli RNA polymerase (Eς70), promoters, and the kinetics of the steps of transcription initiation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 792–820. [Google Scholar]
- 39.Rombel I T, McMorran B J, Lamont I L. Identification of a DNA sequence motif required for expression of iron-regulated genes in pseudomonads. Mol Gen Genet. 1994;246:519–528. doi: 10.1007/BF00290456. [DOI] [PubMed] [Google Scholar]
- 40.Rouviere P, Delas Penas A, Mecsas J, Lu C Z, Rudd K E, Gross C A. rpoE, the gene encoding the second heat shock sigma factor, ςE, Escherichia coli. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 42.Schlictman D, Kavanaugh-Black A, Shankar S, Chakrabarty A M. Energy metabolism and alginate biosynthesis in Pseudomonas aeruginosa: role of the tricarboxylic acid cycle. J Bacteriol. 1994;176:6023–6029. doi: 10.1128/jb.176.19.6023-6029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schurr M J, Yu H, Martinez-Salazar M, Hibler N S, Deretic V. Biochemical characterization and posttranslational modification of AlgU, a regulator of stress response in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1995;216:874–880. doi: 10.1006/bbrc.1995.2703. [DOI] [PubMed] [Google Scholar]
- 44.Schweizer H P. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene. 1991;97:109–112. doi: 10.1016/0378-1119(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 45.Sexton R, Gill P R, Jr, Callanan M J, O'Sullivan D J, Dowling D N, O'Gara F. Iron-responsive gene expression in Pseudomonas fluorescens M114: cloning and characterization of a transcription-activating factor, PbrA. Mol Microbiol. 1995;15:297–306. doi: 10.1111/j.1365-2958.1995.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 46.Sexton R, Gill P R, Jr, Dowling D N, O'Gara F. Transcriptional regulation of the iron-responsive sigma factor gene pbrA. Mol Gen Genet. 1996;250:50–58. doi: 10.1007/BF02191824. [DOI] [PubMed] [Google Scholar]
- 47.Spaink H P, Okker R J H, Wijffelman C A, Pees E, Lugtenberg B J J. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- 48.Strimmer K, Von Haeseler A. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol. 1996;13:964–969. [Google Scholar]
- 49.Stuber D, Matile H, Garotta G. System for high level production in E. coli and rapid purification of recombinant proteins: application to epitope mapping, preparation of antibodies and structure/function analysis. In: Lafkovitz I, Pernis B, editors. Immunological methods. Vol. 4. Orlando, Fla: Academic Press; 1990. pp. 121–152. [Google Scholar]
- 50.Vasil M L, Ochsner U A, Johnson Z, Colmer J A, Hamood A N. The Fur-regulated gene encoding the alternative sigma factor PvdS is required for iron-dependent expression of the LysR-type regulator PtxR in Pseudomonas aeruginosa. Mol Gen Genet. 1998;180:6784–6788. doi: 10.1128/jb.180.24.6784-6788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venturi V, Ottewanger C, Bracke M, Weisbeek P J. Iron regulation of siderophore biosynthesis and transport in Pseudomonas putida WCS358: involvement of a transcriptional activator and of the Fur protein. Mol Microbiol. 1995;15:1081–1093. doi: 10.1111/j.1365-2958.1995.tb02283.x. [DOI] [PubMed] [Google Scholar]
- 52.Venturi V, Ottewanger C, Leong J, Weisbeek P J. Identification and characterization of a siderophore regulatory gene (pfrA) of Pseudomonas putida WCS358: homology to the alginate regulatory gene algQ of Pseudomonas aeruginosa. Mol Microbiol. 1993;10:63–73. doi: 10.1111/j.1365-2958.1993.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 53.Venturi V, Weisbeek P, Koster M. Gene regulation of siderophore mediated iron acquisition in Pseudomonas: not only the Fur repressor. Mol Microbiol. 1995;17:603–610. doi: 10.1111/j.1365-2958.1995.mmi_17040603.x. [DOI] [PubMed] [Google Scholar]
- 54.Visca P, Ciervo A, Sanfilippo V, Orsi N. Iron-regulated salicylate synthesis by Pseudomonas spp. J Gen Microbiol. 1993;139:1995–2001. doi: 10.1099/00221287-139-9-1995. [DOI] [PubMed] [Google Scholar]
- 55.West S E, Kaye S A, Hamood A N, Iglewski B H. Characterization of Pseudomonas aeruginosa mutants that are deficient in exotoxin A synthesis and are altered in expression of regA, a positive regulator of exotoxin A. Infect Immun. 1994;62:897–903. doi: 10.1128/iai.62.3.897-903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Q L, Kong D, Lam K, Husson R M. A mycobacterial extracytoplasmatic function sigma factor involved in survival following stress. J Bacteriol. 1997;179:2922–2929. doi: 10.1128/jb.179.9.2922-2929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu H, Schurr M J, Deretic V. Functional equivalence of Escherichia coli ςE and Pseudomonas aeruginosa AlgU: E. coli rpoE restores mucoidy and reduces sensitivity to reactive oxygen intermediates in algU mutants of P. aeruginosa. J Bacteriol. 1995;177:3259–3268. doi: 10.1128/jb.177.11.3259-3268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]