Abstract
Background & aims
The intensity and duration of the catabolic phase in COVID-19 patients can differ between survivors and non-survivors. The purpose of the study was to assess the determinants of, and association between, nitrogen balance trajectories and outcome in critically ill COVID-19 patients.
Methods
This retrospective monocentric observational study involved patients admitted to the intensive care unit (ICU) of the University Hospital of Clermont Ferrand, France, from January 2020 to May 2021 for COVID-19 pneumonia. Patients were excluded if referred from another ICU, if their ICU length of stay was <72 h, or if they were treated with renal replacement therapy during the first seven days after ICU admission. Data were collected prospectively at admission and during ICU stay. Death was recorded at the end of ICU stay. Comparisons of the time course of nitrogen balance according to outcome were analyzed using two-way ANOVA. At days 3, 5, 7, 10 and 14, uni- and multivariate logistic regression analyses were performed to assess the impact of a non-negative nitrogen-balance on ICU death. To investigate the relationships between nitrogen balance, inflammatory markers and protein intake, linear and non-nonlinear models were run at days 3, 5 and 7, and the amount of protein intake necessary to reach a neutral nitrogen balance was calculated. Subgroup analyses were carried out according to BMI, age, and sex.
Results
99 patients were included. At day 3, a similar negative nitrogen balance was observed in survivors and non-survivors: −16.4 g/d [−26.5, −3.3] and −17.3 g/d [−22.2, −3.8] (p = 0.54). The trajectories of nitrogen balance over time thus differed between survivors and non-survivors (p = 0.01). In survivors, nitrogen balance increased over time, but decreased from day 2 to day 6 in non-survivors, and thereafter increased slowly up to day 14. At days 5 and 7, a non-negative nitrogen-balance was protective from death. Administering higher protein amounts was associated with higher nitrogen balance.
Conclusion
We report a prolonged catabolic state in COVID patients that seemed more pronounced in non-survivors than in survivors. Our study underlines the need for monitoring urinary nitrogen excretion to guide the amount of protein intake required by COVID-19 patients.
Keywords: Nutrition assessment, Nitrogen balance, Critical care, COVID-19, Hypercatabolism, Stress
1. Introduction
Around 5% of COVID-19 patients develop a severe form of the disease requiring intensive care unit (ICU) admission [1,2] not only because of lung degradation but also in relation to thoracic sarcopenia [3]. Inflammation and cytokine storm are common features in most of these ICU patients [4,5]. This initial inflammatory state in critically ill patients is usually accompanied by a state of hypercatabolism characterized by an activation of muscle proteolysis [6,7], resulting in rapid changes in body composition [8] and skeletal muscle wasting. It has been shown that skeletal muscle wasting in ICU contributes to poor clinical outcomes including persistence of organ failure, loss of autonomy, reduced quality of life, and an increased risk of death [9,10].
In COVID-19 patients, systematic administration of steroids can worsen this hypercatabolism by activating proteolytic systems [11]. The hypercatabolic phase is usually followed by an anabolic phase. The timing of the transition between these two periods is of paramount importance because nutritional interventions and their efficacy including amount of protein intakes differ from one phase to the next [12]. During the first phase, characterized by an activated endogenous production of numerous energy substrates, exogenous energy requirement is considered to be lower than in the anabolic phase. Moreover, both under- and over-nutrition during the hypercatabolic phase might be associated with poor prognosis, whereas increasing protein intake would be associated with a decreased mortality [13,14]. The dynamic changes in protein and energy needs should therefore be more accurately appraised in order to improve the nutritional management of patients [15,16] and their clinical outcomes [17,18]. The intensity and duration of this catabolic phase, especially in COVID-19 patients, might differ between survivors and non-survivors.
Nitrogen balance (NBAL) is known as one of the most widely used and robust indicators to identify body protein metabolic state, namely protein loss or gain [19]. If more dietary nitrogen (i.e. protein) is administered than nitrogen loss, then a positive NBAL is observed, reflecting a gain of total body protein, and so the patient is considered to be in net body protein anabolism. Conversely, a negative NBAL reflects net body protein catabolism with protein loss being higher than protein retention. To date, few studies have described the temporal changes in NBAL among COVID-19 patients. Several reported a prolonged catabolic state, which could be related to inflammation but also to the administration of steroids [[20], [21], [22]]. In contrast, very few assessed the association between negative NBAL and the increased risk of death in COVID-19 patients. Hence, the purpose of the present study was to assess temporal NBAL trajectories and the association of NBAL with outcomes in critically ill COVID-19 patients.
2. Materials and methods
2.1. Data source
This retrospective, single-center study was conducted in an intensive care unit (ICU) at Clermont-Ferrand University Hospital, France, from January 1, 2020 to April 31, 2021. All patients with COVID-19 admitted to intensive care were included in the Outcomerea™ database. The methods for data collection and quality of the database have been described in detail elsewhere [23]. All patients or their relatives received fair and relevant information. They gave their written informed consent for the storage and research use of residual blood from samples collected as part of routine care (IRB No. 20.03.20.56342 from CPP-Ile-de-France VI Groupe Hospitalier Pitié-Salpetriêre).
2.2. Study population
Patients over 18 years old with severe COVID-19 confirmed by a positive SARS-CoV-2 test using reverse-transcriptase polymerase chain reaction (PCR) were eligible. Patients were excluded if referred from another ICU, if a decision was made to discontinue life-sustaining treatments during the first two days after ICU admission if their ICU length of stay was <72 h, or if they were treated with renal replacement therapy during the first seven days after ICU admission.
The primary objective was to compare the NBAL profile during the first two weeks after ICU admission between the patients with ICU death (non-survivors) and the other patients (survivors).
2.3. Data collection
All data were prospectively collected and comprised details on ICU admission (demographics, chronic disease/comorbidities as defined by the Knaus Scale [24], baseline severity indices: SAPS II [25] and SOFA [26] scores, treatments on admission). An array of variables was recorded throughout the ICU stay (clinical and biological variables including feeding modalities, nutritional intake, diuresis, urea and creatinine, and urinary levels, requirement for non-invasive ventilatory support and invasive mechanical ventilation (IMV) and other organ support (vasopressors, renal replacement therapy (RRT)), and outcomes (hospital length of stay (LOS), vital status at ICU and hospital discharge and at day 60 after ICU admission).
In patients not receiving invasive mechanical ventilation, all information on the amount of food and beverage consumption was prospectively collected by ICU nurses and entered into the ICU electronic patient record (the IntelliSpace Critical Care and Anaesthesia, Phillips). These data were retrospectively extracted to calculate daily protein intake (Table S1).
3. Definitions
NBAL was estimated from the following formula: NBAL (g ⁄d) = nitrogen intake (g⁄d) – nitrogen loss (g/d).
Nitrogen losses were estimated by urine urea, which makes up 80% of urinary nitrogen (the other 20% being nitrogen as urinary ammonia). Urine urea makes up 90% of total nitrogen losses (the other 10% being fecal losses).
The following formula to estimate nitrogen loss was used:
Nitrogen loss (g/d) = [urinary urea (mmol/24 h) x 0.06]/2.14 + 4.
The constant term of 4 g/d of nitrogen in the NBAL computation was an estimate of considering stool, integumentary, and other insensible nitrogen losses. We also took into account serum urea nitrogen concentration changes as proposed by Dickerson et al. [19,27].
Therefore, if plasma urea variation was higher than 5 mg/dL between day n and day n-1, then a corrective term is proposed, to be subtracted from the nitrogen balance to minimize bias in its measurement: Urea Nitrogen Variation (g/d) = (0.6 (L/kg) for male or 0.55 (L/kg) for female patients) × (weight (kg) on day n-1) × (Δ urea (mg/dL)) × 0.01 + urea (mg/dL) × (weight (kg) on day n – weight (kg) on day n-1) × 0.01 (ESM 1).
The formula to obtain protein loss is the following: Protein loss (g/d) = nitrogen loss (g/d) x 6.25.
NBAL less than −4 g/day was considered to be a ‘negative NBAL’ reflecting net protein catabolism. NBAL between −4 g/day and +4 g/day was considered to be a neutral NBAL reflecting an equilibrium between whole body protein anabolism and catabolism. NBAL over +4 g/day, was considered to be a ‘positive NBAL’ reflecting net protein anabolism. The protein intake needed to reach a neutral NBAL were determined in survivors and non-survivors at days 3, 5, 7, 10 and 14 [27]. Adjusted body weight was considered for obese patients if necessary [12](ESM 1).
In our ICU, patients are fed according to ESPEN Guidelines [12]. Thus, in most COVID patients, the objective was to rapidly administer 25 kcal/kg/day during the first week. The decision to increase calorie intake up to 30–35 kcal/kg/day was left at the discretion of the physician according to the clinical course.
4. Statistical analysis
Statistical analysis was performed in SAS version 9.4 (SAS Institute Inc., Cary, North Carolina) and R (version 3.6.3). Continuous data are presented as median with interquartile range (IQR) or range and were compared using the Wilcoxon rank sum test. Categorical data were compared using Fisher's test. Trajectories of all indices are displayed as rolling medians with 95% confidence interval of the median. Comparisons between the time course of these indices depending on vital status at the end of ICU stay were made using two-way analysis of variance. Subgroup analyses were carried out according to BMI, age, sex, and in the subgroup of patients with an ICU LOS of at least 14 days. Uni and multi logistic regression analyses were achieved to determine factors associated with ICU death at the landmark time day 3, 5, 7, 10 and 14, i.e: at day 3 among the patients still in ICU at day 3, at day 5 among the patients still in ICU at day 5 and similarly at day 7, 10 and 14.
Goodness of fit of the linear model between two variables was assessed from the Pearson product moment correlation coefficient (r) analysis. To represent the relationship between NBAL and protein intake at days 3 and 7, linear and non-nonlinear models in the whole cohort and depending on age ( ± 70 y), sex, and BMI ( ± 30 kg/m2) were run.
The protein intakes necessary to reach a zero NBAL were determined by linear regression between NBAL and protein intake.
We assessed the association between laboratory features on admission, the administration of steroids and non-negative NBAL on days 3, 5 and 7. We also assessed the association of those biomarkers on admission, and administration of steroids with ICU death. Missing data were imputed linearly. For all tests, a two-sided α value of 0.05 was considered significant.
5. Results
5.1. Characteristics of the population
From February 15 to May 1, 2021, 116 patients with laboratory-confirmed COVID-19 were admitted to our ICU. Of these, 99 were included in the study (Fig. S1). Of these, 64 (64.6%) were male, with a median age (IQR) of 71 years [60.6; 76.2]. Obesity (n = 45,45.5%) and cardiovascular disease (n = 15,15.2%) were the most frequently observed comorbidities. On ICU admission, SAPS II was 32[22; 41], 7(7.1%) patients were under IMV, 58(58.6%) high-flow nasal cannula (HFNC) therapy; 84(84.8%) of the patients received steroids.
During ICU stay, 31(31.3%) were mechanically ventilated and 8(8.1%) needed RRT after day 7. The median ICU length of stay was 8 days[5; 14], and death at day 60 occurred in 30(30.1%) patients. The patients dying at the end of ICU stay were older, with more comorbidities, were more severely ill on admission and were more often ventilated and needed more RRT during ICU stay (Table 1 ). Up to day 8 of the ICU stay, more than 65% of patients were fed orally without any enteral or parenteral supplementation (Table S2).
Table 1.
Main characteristics and outcomes and comparison depending on vital status at the end of ICU stay.
| Variables | N(%) or median (IQR) | ICU survivors N = 69 |
ICU non-survivors N = 30 |
p |
|---|---|---|---|---|
| Age (years) | 71 [60.6; 76.2] | 68.9 [58.7; 75.9] | 73.9 [67.6; 78] | 0.02 |
| Sex (Male) | 64 (64.6) | 45 (65.2) | 19 (63.3) | 0.86 |
| BMI (kg/m2) | 29.4 [25.5; 32.7] | 29.7 [25.8; 32.9] | 29.2 [25.5; 32.4] | 0.92 |
| BMI >30 kg/m2 | 45 (45.5) | 31 (44.9) | 14 (46.7) | 0.87 |
| Comorbidities | ||||
| Cardio-vascular | 15 (15.2) | 6 (8.7) | 9 (30) | <0.01 |
| Respiratory | 5 (5.1) | 3 (4.3) | 2 (6.7) | 0.63 |
| Renal | 6 (6.1) | 2 (2.9) | 4 (13.3) | 0.05 |
| Liver | 1 (1) | 0 (0) | 1 (3.3) | 0.13 |
| Immunocompromised host | 15 (15.2) | 10 (14.5) | 5 (16.7) | 0.78 |
| Diabetes | 15 (15.2) | 12 (17.4) | 3 (10) | 0.35 |
| Time from first symptoms to ICU admission | 9 [7; 11] | 9 [8; 11] | 7.5 [5; 9] | <0 0.01 |
| On admission (Day 1 or Day 2) | ||||
| Steroids | 86 (86.9) | 60 (87) | 26 (86.7) | 0.97 |
| Diuretics | 84 (84.8) | 58 (84.1) | 26 (86.7) | 0.74 |
| SAPS II | 32 [22; 41] | 32 [22; 41] | 33.5 [22; 44] | 0.55 |
| SOFA score | 4 [3; 6] | 4 [3; 5] | 5 [4; 6] | 0.03 |
| PaO2/FiO2 | 86.9 [68; 138.8] | 92.7 [76.3; 140] | 86 [61.1; 109.2] | 0.24 |
| Invasive mechanical ventilation | 7 (7.1) | 6 (8.7) | 1 (3.3) | 0.34 |
| High Flow Nasal Canula | 58 (58.6) | 35 (50.7) | 23 (76.7) | 0.02 |
| Vasopressors | 7 (7.1) | 5 (7.2) | 2 (6.7) | 0.92 |
| Treatments during ICU stay | ||||
| Invasive mechanical ventilation | 31 (31.3) | 11 (15.9) | 20 (66.7) | <0.01 |
| Renal replacement therapy | 8 (8.1) | 2 (2.9) | 6 (20) | <0.01 |
| Vasopressors | 28 (28.3) | 10 (14.5) | 18 (60) | <0.01 |
| Diuretics | 96 (97) | 66 (95.7) | 30 (100) | 0.25 |
| ICU LOS | 8 [5; 14] | 7 [5; 10] | 15 [7; 24] | <0.01 |
| Hospital LOS | 17 [11; 28] | 17 [11.5; 29] | 15.5 [9; 24] | 0.30 |
| ICU Death | 30 (30.3) | |||
ICU: Intensive Care Unit; IQR: Interquartile; P: pvalue; BMI: Body Mass Index; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; LOS: Length of stay.
5.2. Time course of NBAL during ICU stay in the whole cohort
Median Nitrogen intakes ranged from 7.6 g/d [2.4; 17.9] at day 3–15.8 g/d [8.7; 21] at day 14. Nitrogen loss was 14.7 g/d [11.0; 22.0] on day 3, reached a maximum of 21 g/d [14.7; 28.3] on day 7 and finally was 12.1 g/d [5.9; 19] at day 14. NBAL ranged from −16.4 g/d [−25.6; −3.3] on day 3 to −1.6 g/d [−7.8; 13.6] on day 14 (Table S3).
NBAL was most often negative but changed over time in the whole cohort. NBAL first decreased from admission to around day 4 and then increased slowly. Around 70% of the patients displayed a negative NBAL during their ICU stay until day 10 (Table S3, Fig. S2a). Time course differed between obese and non-obese patients but not between younger or older patients nor between male and female (Figs. S2b–d).
5.3. Comparison of NBAL between survivors and non-survivors
The trajectories of nitrogen intakes and nitrogen loss including variations due to urea variation among survivors and non survivors are reported in Fig. S3 and Tables S3 and S4. There were no significant differences of nitrogen intakes between survivors and no survivors. In both group, nitrogen intakes didn't differ over time. Nitrogen loss were lower in survivors. For both survivors and non-survivors’ nitrogen loss first increased during the first week and then diminished during the second week.
The trajectories of NBAL are reported in Fig. 1 and S4. On day 3, a negative similar NBAL was observed in survivors and non-survivors: −16.4 g/d[−26.5;−3.3] and −17.3 g/d[−22.2;−3.8](p = 0.54), respectively (Table S3). The NBAL time course differed between survivors and non-survivors after day 3 (p = 0.01, Fig. S5a).
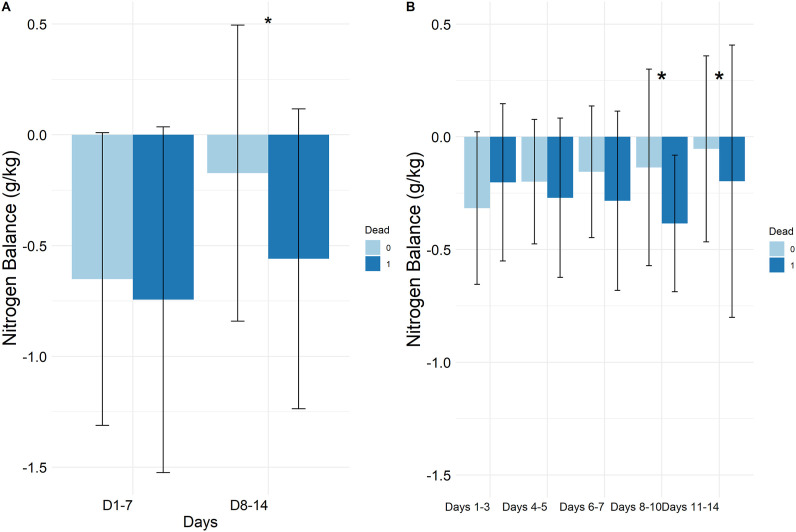
Fig. 1.
Trajectory of Nitrogen balance from day 2 to day 14 for survivors and non-survivors.
In survivors, NBAL increased over time. From day 3 to day 14, the percentage of patients with net protein catabolism decreased from 73.9% to 45.5% while the percentage of patients with net protein anabolism increased from 10.1% to 45.5%, respectively (Table S3).
In non-survivors, NBAL decreased from day 3 to day 6, and thereafter increased slowly up to day 14. Between day 3 and day 10, there was no decrease in the percentage of patients with net protein catabolism (73.3% at day 3 vs. 78.9% at day 10), or with net protein anabolism (13.3% at day 3 and 15.8% at day 10). Similar results could be observed in the subgroup of patients still admitted in ICU after day 7 (Fig. S5).
The cumulative NBAL were not different between survivors and non-survivors during the first week (−0.6 g/kg[−1; −0.2] vs. −0.7 g/kg[−1; −0.2], p = 0.48), whereas it was significantly higher in survivors than in non-survivors during the 2 nd week (−0.2 g/kg[−0.5; 0.1] vs. −0.6 g/kg[−0.9; −0.1], p = 0.03) (Fig. 2 , Table S4).
Fig. 2.
Cumulative nitrogen balance (g/kg): comparison between survivors and non-survivors.
Subgroup analyses showed a significant difference in NBAL trajectory between survivors and non-survivors only in the subgroup of patients older than 70 years, in female patients and in non-obese patients. In these three subgroups, NBAL in survivors was higher than in non-survivors and became positive earlier. There were no differences in nitrogen balance between survivors and non-survivors in patients younger than 70 years, in male patients, or in obese patients (Table S5, Figs. S6a–f).
In uni- and then multivariate analyses, NBAL > - 4 g/d was protective from death at day 5 and 7 (day 5: aOR = 0.29[0.09–0.96], pval = 0.043; day 7: aOR = 0.12[0.02–0.63], pval = 0.013, Fig. 3 , Table S5).
Fig. 3.
Association between a non-negative NBAL and ICU death at the landmark time: day 3, day 5, day 7, day 10 and day 14, multivariate logistic regression analyses.Adjustment on age, chronic cardio-vascular and kidney disease.
Among the inflammatory biomarkers measured on ICU admission, higher serum values of IL1, IL6 and ferritin were associated with a negative NBAL on day 3 (Table S7) and lower values of IL10 and HLA DR with ICU death (Table S8).
5.4. Relationship between NBAL and protein intakes (Fig. S5)
Although the data were variable, the relationships between NBAL and protein intake were more nonlinear over time. As shown in Figs. S7a and b, at day 3 and day 7, in the overall population, there was a small increase in NBAL so long as protein intakes remained lower than 1.2 g/kg. Administering higher protein amounts was associated with higher NBALs. The neutral NBAL balances were achieved at days 3 and 7 for protein intakes of 2.1 g/kg and 1.64 g/kg respectively in the whole cohort.
The association between higher protein intakes and higher NBAL was observed in nearly all the subgroups of patients at days 3 and 7 except for those older than 70 years, in female patients and in obese patients at day 7 for whom a near plateau was observed for the higher protein intakes (Fig. S7). The estimated protein intakes to achieve a neutral NBAL at days 3 and 7 for each of these subgroups are reported in Table S9. For each subgroup, it was always higher at day 3 than at day 7. Younger patients, non-obese patients, and male patients needed more protein intakes to reach a neutral NBAL at days 3 and 7.
Because of nonlinearity, the estimated protein intakes to achieve a neutral NBAL could only be estimated in survivors at days 3, 5, 7, 10 and 14 and in non-survivors at days 3 and 7. In survivors, it decreased roughly linearly from 2.26 g/kg at day 3 to 0.97 g/kg at day 14. In non-survivors it decreased from 1.65 g/kg at day 3 to 1.29 g/kg at day 7.
6. Discussion
This study was designed to assess the temporal trajectories of NBAL in critically ill COVID-19 patients. In the cohort, we observed: (i) a high level of protein catabolism during the first 10 days after ICU admission, which tended to be less marked for the survivors than for the non-survivors after day 3, (ii) a much more negative NBAL for the obese than for the non-obese patients, (iii) a different time course of NBAL between survivors and non-survivors mostly for the older patients, female patients and non-obese patients, and (iv) a higher increase in NBAL for protein intakes higher than 1.2 g/kg/day and a neutral NBAL obtained with protein intakes of 2 g/kg and 1.64 g/kg at days 3 and 7. These results elicit several remarks.
6.1. A net negative NBAL
First, we report a net negative NBAL for the COVID-19 critically ill patients during the first 10 days after ICU admission. This net negative NBAL is multifactorial and is related to inadequate protein intakes together with high levels of muscle protein catabolism. Such prolonged catabolism was already highlighted in ICU patients. For instance, a negative nitrogen balance was found during the first two weeks after ICU admission, despite high protein intakes, indicating that protein catabolism remained elevated through 14 days follow-up [28]. In COVID-19 patients, several studies reported this prolonged catabolic state, which could be related to inflammation but also to the administration of steroids [[20], [21], [22]] [[20], [21], [22]] [[20], [21], [22]]. For instance, Formenti et al. showed that even on meeting supposed nutrition targets, nitrogen balance remained negative [29]. In our study, we also observed that inflammation, characterized by higher serum levels of IL1, IL6 and ferritin on admission, was associated with a negative NBAL at day 3, suggesting that inflammation is a key determinant of protein loss in these COVID-19 patients.
6.2. Obese patients and negative NBAL
Obese COVID patients underwent a more severe catabolic phase which can have various explanations. Obese patients have a higher energy expenditure [30,31], together with an increased protein turnover and amino acid oxidation [32,33]. In such cases, higher absolute protein intakes should be recommended for obese patients. For instance, for critically ill patients with COVID-19, ASPEN recommended a protein intake of 2–2.5 g/kg/day based on ideal body weight for patients with obesity, whereas ESPEN recommended >1 g/kg/day adjusted body weight for those with obesity [16,34]. In addition, other authors have suggested that the equation for calculating protein amount in obese patients, even taking into account adjusted body weight, might underestimate the real need for protein intake and so minimized their NBAL [35].
6.3. A more positive NBAL among survivors
Survivors increased their NBAL significantly more than non-survivors who kept a net negative balance during the first 10 days after ICU admission. We also report that non-negative NBALs at day 5 and day 7 were protective from death. The persistence of a net negative NBAL generally leads to early, rapid muscle wasting, which has been linked to increased morbidity and mortality [20,35,36]. Furthermore, the persistence of hypercatabolism is also generally associated with depressed immune functions, mainly due to its negative effects on both the amount of functional immunoglobulins and gut-associated lymphoid tissue (GALT), which have also been linked to increased risk of nosocomial infections and death [37,38]. In our study, we showed that patients with poor outcome, i.e. those who tended to have a lower NBAL and longer negative NBAL, had lower serum levels on admission of HLA DR and IL10, which are both biomarkers of immunosuppression and monocyte dysregulation. This result is in agreement with the above hypothesis. We also underline heterogeneity between subgroups depending on age, sex, and BMI. Other studies also focused on these subgroups. For instance, Dickerson et al. reported different time course profiles between older sarcopenic patients and younger patients and underlined the variability in NBAL response to varying protein intakes among both older and younger patient groups. In this study, older patients had worse outcomes [27].
6.4. Increasing protein intake could improve NBAL
We observed that increasing protein intakes was able to improve NBAL and might therefore limit muscle wasting. Previous investigations of the association of protein intake with nitrogen loss in critically ill patients suggested that increased protein intake might improve nitrogen balance [36,39,40]. Similarly, in older critically ill patients, monitoring NBAL allowed the identification of an “anabolic resistance” state and underlined the need for an increased protein intake with close nitrogen monitoring to avoid potential azotemia in this population at risk of renal failure [27]. Prospectively, it was confirmed that higher protein intakes could minimize negative NBAL. Results of an observational study indicated a significant improvement in NBAL with a protein intake of 1.5 g/kg/day vs. a protein intake of 1.1 g/kg/day [41]. In COVID-19 patients, a study found that 1.5 g/kg/day of protein would be required to achieve nitrogen equilibrium [42]. NBAL was improved over 7 days in critically ill patients randomized to a protein-fortified diet versus those on a standard diet [43].
We underline that during the first week after ICU admission, protein intake to reach neutral NBAL should be close to or slightly above the recommended targets. According to ASPEN guidelines [44], protein intake should be at 1.2–2.0 g/kg/day and ESPEN guidelines recommend progressive administration of protein at 1.3 g/kg/day [45]. Our data also suggest that the severity of the net protein catabolism could be at least partially improved by aggressive nutrition therapy.
6.5. Minimizing negative NBAL to improve outcome of the patients
All our results showed therefore that 1) NBAL does vary over time and between subgroups, 2) it was possible to obtain a non-negative NBAL thanks to higher protein intakes and 3) a non-negative NBAL was associated with better prognosis. Minimizing the negative NBAL by administration of higher protein intakes and/or optimizing protein intakes by monitoring 24 h urinary nitrogen excretion could consequently be recommended to enhance patient prognosis. This strategy has already yielded a benefit in neurocritically ill patients in terms of survival [46]. More recently, Singer et al. demonstrated that a formula containing a high protein content but with moderate energy load could provide a sufficient protein intake while preserving nitrogen balance and preventing overfeeding in critically ill, mechanically ventilated, hypercatabolic patients during the acute phase of illness (in the first 7 days) [47]. As a result, high protein intakes would be recommended to improve outcomes of critically ill patients. However, the actual metabolic fate of muscle-derived amino acids during critical illness is still unclear [48]. Whether exogenous nutrients in the form of dietary protein are also diverted away from the muscle during critical illness or if their amino acids enter the muscle and stimulate muscle protein synthesis and inhibit proteolysis during critical illness remains poorly studied [49] and is a major unresolved question with potential for intervention [50].
So, our results underline not only the need for higher protein intakes but also, because of heterogeneity of the patients, the importance of estimating each patient's protein requirements using 24 h urinary nitrogen excretion and associated NBAL. Moreover, monitoring energy expenditure by indirect calorimetry to avoid under- or overnutrition, despite high protein intakes, is of key relevance.
6.6. Advantage and limits
This study has the main advantage of being one of the first in COVID patients to report multiple serial NBAL determinations to examine overall protein balance.
This study has several limits. It was a monocentric retrospective observational study. Hence, more than half of the patients during the first week in ICU were not intubated and were fed orally, making the measurements of protein amount more difficult. Only descriptive and univariate and multivariate analyses are reported, and no causal conclusions can be drawn from this study. Because of the significant reduction of study subjects in whom NBAL were measured between day 7 and day 14, the trajectories of NBAL during the second week should be interpreted with caution. Unfortunately, as we could not monitor inflammation of our patients throughout the whole ICU stay, the link between inflammation and NBAL trajectories during ICU stay was only analyzed using the inflammatory data at admission.
7. Conclusion
We report a prolonged catabolic state in COVID patients, which seemed more pronounced in non-survivor than in survivor patients. Our study underlines the need for monitoring both urinary nitrogen excretion and energy expenditure to individualize nutritional needs in COVID-19 patients. Whether catabolism and nitrogen losses can be reversed by enhanced individualized nutritional support, and whether this improves outcome, remain to be investigated.
Contributors
CD, AB, YB, BS designed the study, assisted in data collection and analysis, and drafted and edited the manuscript. AJ, OG, RB, LD, GH, OM, LC, FT, KG, PC, FK, GL, MA assisted in data collection and edited the manuscript.
Funding
There was no funding for this study.
Conflict of interest
We declare that we have no conflicts of interest.
Acknowledgments
The OutcomeRea™ study group. We thank JeffreyWatts for advice on the English manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2022.08.023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Xie J., Wu W., Li S., Hu Y., Hu M., Li J., et al. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Intensive Care Med. 2020;46(10):1863–1872. doi: 10.1007/s00134-020-06211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koehler J., Boirie Y., Bensid L., Pereira B., Ghelis N., Dupuis C., et al. Thoracic sarcopenia as a predictive factor of SARS-COV2 evolution. Clin Nutr Edinb Scotl. 2022;22:S0261–S5614. doi: 10.1016/j.clnu.2022.01.022. 00032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batlle D., Soler M.J., Sparks M.A., Hiremath S., South A.M., Welling P.A., et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol JASN. 2020;31:1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puthucheary Z.A., Rawal J., McPhail M., Connolly B., Ratnayake G., Chan P., et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 7.Mansoor O., Beaufrere B., Boirie Y., Ralliere C., Taillandier D., Aurousseau E., et al. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci U S A. 1996;93:2714–2718. doi: 10.1073/pnas.93.7.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cava E., Carbone S. Coronavirus disease 2019 pandemic and alterations of body composition. Curr Opin Clin Nutr Metab Care. 2021;24:229–235. doi: 10.1097/MCO.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermans G., Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care Lond Engl. 2015;19:274. doi: 10.1186/s13054-015-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weijs P.J.M., Looijaard W.G.P.M., Beishuizen A., Girbes A.R.J., Oudemans-van Straaten H.M. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care Lond Engl. 2014;18:701. doi: 10.1186/s13054-014-0701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dos Santos C., Hussain S.N.A., Mathur S., Picard M., Herridge M., Correa J., et al. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. A pilot study. Am J Respir Crit Care Med. 2016;194:821–830. doi: 10.1164/rccm.201512-2344OC. [DOI] [PubMed] [Google Scholar]
- 12.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Villet S., Chiolero R.L., Bollmann M.D., Revelly J.-P., Cayeux R.N.M.-C., Delarue J., et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr Edinb Scotl. 2005;24:502–509. doi: 10.1016/j.clnu.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Zusman O., Theilla M., Cohen J., Kagan I., Bendavid I., Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care Lond Engl. 2016;20:367. doi: 10.1186/s13054-016-1538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr Edinb Scotl. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barazzoni R., Bischoff S.C., Busetto L., Cederholm T., Chourdakis M., Cuerda C., et al. Nutritional management of individuals with obesity and COVID-19: ESPEN expert statements and practical guidance. Clin Nutr Edinb Scotl. 2021;21:S0261–S5614. doi: 10.1016/j.clnu.2021.05.006. 00248-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamrin-Gripenberg L., Sundström-Rehal M., Olsson D., Grip J., Wernerman J., Rooyackers O. An attenuated rate of leg muscle protein depletion and leg free amino acid efflux over time is seen in ICU long-stayers. Crit Care Lond Engl. 2018;13:22. doi: 10.1186/s13054-017-1932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pertzov B., Bar-Yoseph H., Menndel Y., Bendavid I., Kagan I., Glass Y.D., et al. The effect of indirect calorimetry guided isocaloric nutrition on mortality in critically ill patients-a systematic review and meta-analysis. Eur J Clin Nutr. 2022;76(1):5–15. doi: 10.1038/s41430-021-00919-0. [DOI] [PubMed] [Google Scholar]
- 19.Dickerson R.N., Tidwell A.C., Minard G., Croce M.A., Brown R.O. Predicting total urinary nitrogen excretion from urinary urea nitrogen excretion in multiple-trauma patients receiving specialized nutritional support. Nutrition. 2005;21:332–338. doi: 10.1016/j.nut.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Umbrello M., Guglielmetti L., Formenti P., Antonucci E., Cereghini S., Filardo C., et al. Qualitative and quantitative muscle ultrasound changes in patients with COVID-19-related ARDS. Nutr Burbank Los Angel Cty Calif. 2021;111449:91–92. doi: 10.1016/j.nut.2021.111449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittle J., Molinger J., MacLeod D., Haines K., Wischmeyer P.E. Persistent hypermetabolism and longitudinal energy expenditure in critically ill patients with COVID-19. Crit Care. 2020;24:581. doi: 10.1186/s13054-020-03286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakenman P.L.M., van der Hoven B., Schuijs J.M., Eveleens R.D., van Bommel J., Olieman J.F., et al. Energy expenditure and feeding practices and tolerance during the acute and late phase of critically ill COVID-19 patients. Clin Nutr ESPEN. 2021;43:383–389. doi: 10.1016/j.clnesp.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahar J.-R., Timsit J.-F., Garrouste-Orgeas M., Français A., Vesim A., Descorps-Declere A., et al. Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med. 2011;39:1886–1895. doi: 10.1097/CCM.0b013e31821b827c. [DOI] [PubMed] [Google Scholar]
- 24.Knaus W.A., Wagner D.P., Draper E.A., Zimmerman J.E., Bergner M., Bastos P.G., et al. The Apache III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 25.Le Gall J.R., Lemeshow S., Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 26.Vincent J.L., de Mendonça A., Cantraine F., Moreno R., Takala J., Suter P.M., et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Dickerson R. Nitrogen balance and protein requirements for critically ill older patients. Nutrients. 2016;8:226. doi: 10.3390/nu8040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wandrag L., Brett S.J., Frost G.S., Bountziouka V., Hickson M. Exploration of muscle loss and metabolic state during prolonged critical illness: implications for intervention? PLoS One. 2019;14 doi: 10.1371/journal.pone.0224565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Formenti P., Bichi F., Castagna V., Pozzi T., Chiumello D. Nutrition support in patients with acute respiratory distress syndrome COVID-19. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2021;36:500–501. doi: 10.1002/ncp.10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillet C., Masgrau A., Walrand S., Boirie Y. Impaired protein metabolism: interlinks between obesity, insulin resistance and inflammation. Obes Rev off J Int Assoc Study Obes. 2012. [DOI] [PubMed]
- 31.Preiser J.-C., van Zanten A.R.H., Berger M.M., Biolo G., Casaer M.P., Doig G.S., et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care Lond Engl. 2015;19:35. doi: 10.1186/s13054-015-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeevanandam M., Young D.H., Schiller W.R. Obesity and the metabolic response to severe multiple trauma in man. J Clin Invest. 1991;87:262–269. doi: 10.1172/JCI114980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tieland M., van Dronkelaar C., Boirie Y. Sarcopenic obesity in the ICU. Curr Opin Clin Nutr Metab Care. 2019;22:162–166. doi: 10.1097/MCO.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 34.Chapple L.-A.S., Tatucu-Babet O.A., Lambell K.J., Fetterplace K., Ridley E.J. Nutrition guidelines for critically ill adults admitted with COVID-19: is there consensus? Clin Nutr ESPEN. 2021;44:69–77. doi: 10.1016/j.clnesp.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vest M.T., Newell E., Shapero M., McGraw P., Jurkovitz C., Lennon S.L., et al. Energy balance in obese, mechanically ventilated intensive care unit patients. Nutrition. 2019;66:48–53. doi: 10.1016/j.nut.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickerson R.N., Pitts S.L., Maish G.O., Schroeppel T.J., Magnotti L.J., Croce M.A., et al. A reappraisal of nitrogen requirements for patients with critical illness and trauma. J Trauma Acute Care Surg. 2012;73:549–557. doi: 10.1097/TA.0b013e318256de1b. [DOI] [PubMed] [Google Scholar]
- 37.Beier K., Eppanapally S., Bazick H.S., Chang D., Mahadevappa K., Gibbons F.K., et al. Elevation of blood urea nitrogen is predictive of long-term mortality in critically ill patients independent of “normal” creatinine. Crit Care Med. 2011;39:305–313. doi: 10.1097/CCM.0b013e3181ffe22a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Quintela A., Milton-Laskibar I., Trepiana J., Gómez-Zorita S., Kajarabille N., Léniz A., et al. Key aspects in nutritional management of COVID-19 patients. J Clin Med. 2020;9 doi: 10.3390/jcm9082589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strack van Schijndel R.J.M., Weijs P.J.M., Koopmans R.H., Sauerwein H.P., Beishuizen A., Girbes A.R.J. Optimal nutrition during the period of mechanical ventilation decreases mortality in critically ill, long-term acute female patients: a prospective observational cohort study. Crit Care Lond Engl. 2009;13 doi: 10.1186/cc7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Japur C.C., Monteiro J.P., Marchini J.S., Garcia R.W.D., Basile-Filho A. Can an adequate energy intake be able to reverse the negative nitrogen balance in mechanically ventilated critically ill patients? J Crit Care. 2010;25:445–450. doi: 10.1016/j.jcrc.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Allingstrup M.J., Esmailzadeh N., Wilkens Knudsen A., Espersen K., Hartvig Jensen T., Wiis J., et al. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr Edinb Scotl. 2012;31:462–468. doi: 10.1016/j.clnu.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Buckley C.T., Prasanna N., Mays A.L., Tinsley J.M., Dickerson R.N. Protein requirements for critically ill ventilator-dependent patients with COVID-19. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2021;36:984–992. doi: 10.1002/ncp.10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danielis M., Lorenzoni G., Azzolina D., Iacobucci A., Trombini O., De Monte A., et al. Effect of protein-fortified diet on nitrogen balance in critically ill patients: results from the OPINiB trial. Nutrients. 2019;11 doi: 10.3390/nu11050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClave S.A., Taylor B.E., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N. J Parenter Enteral Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 45.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr Edinb Scotl. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 46.Kim T.J., Park S.-H., Jeong H.-B., Ha E.J., Cho W.S., Kang H.-S., et al. Optimizing nitrogen balance is associated with better outcomes in neurocritically ill patients. Nutrients. 2020;12:3137. doi: 10.3390/nu12103137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer P., Bendavid I., BenArie I., Stadlander L., Kagan I. Feasibility of achieving different protein targets using a hypocaloric high-protein enteral formula in critically ill patients. Crit Care Lond Engl. 2021;25:204. doi: 10.1186/s13054-021-03625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeds P.J., Fjeld C.R., Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994;124:906–910. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- 49.Liebau F., Wernerman J., van Loon L.J.C., Rooyackers O. Effect of initiating enteral protein feeding on whole-body protein turnover in critically ill patients. Am J Clin Nutr. 2015;101:549–557. doi: 10.3945/ajcn.114.091934. [DOI] [PubMed] [Google Scholar]
- 50.Liebau F., Deane A.M., Rooyackers O. Protein absorption and kinetics in critical illness. Curr Opin Clin Nutr Metab Care. 2021;24:71–78. doi: 10.1097/MCO.0000000000000707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.