Abstract
Objectives
In a context of COVID-19 vaccine shortages, this study sought to evaluate the safety and efficacy of receiving one dose of Gam-COVID-Vac rAd26 followed by a second COVID-19 vaccine dose of either Gam-COVID-Vac rAd5, ChAdOx1 nCoV-19 or BBIBP-CorV in a cohort of older adults.
Study design
Single-centre, randomised, open label, non-inferiority trial.
Methods
Adults aged ≥65 years who had received one dose of Gam-COVID-Vac rAd26 were randomised in a 1:1:1 ratio to receive a second-dose COVID-19 vaccination of either Gam-COVID-Vac rAd5, ChAdOx1 nCoV-19 or BBIBP-CorV. The primary outcome was the assessment of the humoral immune response to vaccination (i.e. antibody titres of SARS-CoV-2 spike protein at 28 days after second-dose vaccination). In addition, neutralising antibody titres at day 28 for the three schedules were measured.
Results
Of 85 participants who were enrolled in the study between 26 and July 30, 2021, 31 individuals were randomised to receive Gam-COVID-Vac rAd5, 27 to ChAdOx1 nCoV-19 and 27 to BBIBP-CorV. The mean age of participants was 68.2 years (SD 2.9) and 49 (57.6%) were female. Participants who received Gam-COVID-Vac rAd5 and ChAdOx1 nCoV1-19 showed significantly increased anti-S titres at 28 days after second-dose vaccination, but this magnitude of difference was not observed for those who received BBIBP-CorV. The ratio between the geometric mean at day 28 and baseline within each group was 11.8 (6.98–19.89) among patients assigned to Gam-COVID-Vac rAd26/rAd5, 4.81 (2.14–10.81) for the rAd26/ChAdOx1 nCoV-19 group and 1.53 (0.74–3.20) for the rAd26/BBIBP-CorV group. All of the schedules were shown to be safe.
Conclusions
The findings in this study contribute to the scarce information published on the safety and immunogenicity of Gam-COVID-Vac heterologous regimens and will help the development of guidelines and vaccine programme management.
Keywords: COVID-19, Vaccination, Older adults, Vaccines shortage
1. Introduction
Since December 2019, COVID-19 has rapidly spread throughout the world and has become a global public health problem [1].
The scientific community faced the challenge of quickly developing vaccines and political decision-makers had to implement accelerated vaccination campaigns to prevent COVID-19-related morbidity and mortality in the population.
To date, the National Administration of Medicines, Food and Medical Technology (ANMAT) have approved seven SARS-CoV-2 vaccines for emergency use in Argentina. Three of these vaccines [[2], [3], [4]] were included in the first phase of the national immunisation campaign, which took place in stages, prioritising target populations in accordance to risk and vulnerability.
It is well known that the elderly are at high risk of COVID-19-associated morbidity and mortality [6,7]. Thus, older adults were considered to be a priority and started vaccination schedules using non-replicative viral vector vaccines (Gam-COVID-Vac [SPUTNIK-V] and ChAdOx1 nCoV-19 [Oxford-AstraZeneca]) or inactivated virus vaccines, such as BBIBP-CorV (Sinopharm), according to availability of the resource. All of these vaccines have demonstrated efficacy and safety in both clinical trials and real-world settings [[2], [3], [4], [5]]. Complete vaccination schedules require two vaccine doses administered at an interval of at least 3 weeks.
However, in the second quarter of 2021, Argentina faced a shortage of the second component of Gam-COVID-Vac (rAd5), which resulted in a large number of elderly individuals who had received the first dose of Gam-COVID-Vac (rAd26) and were not able to complete their schedule after the recommended period of ≥21 days. This shortage, due to delays in the delivery of shipments committed by the Gam-COVID-Vac producer, caused a major public health problem as many of the vulnerable elderly population were not able to achieve full vaccination.
As heterologous prime-boost COVID-19 vaccine schedules can facilitate mass COVID-19 immunisation, the use of heterologous schemes is a source of active research in developed countries [[8], [9], [10], [11]]. However, most of these investigations are focused on the combination of a regimen that includes mRNA vaccines, which is a technology that is not affordable in many low- and middle-income countries.
To the best of the authors’ knowledge, there is currently no information about the safety and efficacy of the combination of Gam-COVID-Vac rAd26 (SPUTNIK-V) first dose with ChAdOx1 nCoV-19 (Oxford-AstraZeneca) or BBIBP-CorV (Sinopharm) second vaccine does in older adults.
This research was therefore aimed to provide good quality evidence to decision-making health authorities on the safely and efficacy of heterologous vaccine schemes.
2. Methods
2.1. Study design
This clinical trial was a single-centre, randomised, open, non-inferiority trial that aimed to determine immunogenicity and reactogenicity of heterologous COVID-19 vaccine schedules (registration no. NCT04983537). The study was designed and conducted by the Ministry of Health of the City of Buenos Aires, Argentina, and was supported by the Buenos Aires City Government. The clinical research site was the Emergent Diseases Research Unit at Hospital Jose Maria Ramos Mejia, which is a large facility with the capacity to develop clinical research, including early phase vaccine trials. The trial protocol was reviewed and approved by the institutional review board (IRB).
2.2. Study population
Adults aged ≥65 years who had received a dose of the first component of the SPUTNIK-V vaccine (Gam-COVID-Vac rAd26) at least 30 days before randomisation and were awaiting a second COVID-19 vaccine dose were eligible for this study. All participants provided written informed consent.
The main exclusion criteria were as follows: known history of COVID-19 in the last 6 months; known history of severe allergy to any vaccine ingredient; use of systemic corticosteroids in the last 30 days; history of anaphylaxis; known history of autoimmune disease; current use of anticoagulants or cancer treatment in the last 6 months; having any medical procedure scheduled that could jeopardise the 14- and 28-day post-randomisation visits; and/or any disease or condition that, in the investigator's opinion, could impact participation in the study.
2.3. Randomisation and masking
Individuals were randomized in a 1:1:1 ratio to receive the Gam-COVID-Vac (SPUTNIK-V) second component (rAd5) vaccine, ChAdOx1 nCoV-19 (Oxford-Astra Zeneca) or BBIBP-CorV (Sinopharm). For allocation of the participants, an ad-hoc software was used. After consent was obtained, the participant was randomised and immediately notified of the treatment allocation. Both participant and health-care personnel were aware of group allocation; however, the investigators who performed the full blood count and antibody measurements were blinded to the vaccine allocation.
2.4. Procedures
The trial was announced in the public mass media and an enrolment registry was set up on the Buenos Aires City Government's web portal. From this registry, a random selection of individuals were invited to participate through a telephone recruitment procedure, where inclusion and exclusion criteria were checked. Participants who accepted the invitation were sent information by text message, explaining the nature of the study and were invited to make a baseline visit (day 0). Participants who completed the final eligibility assessment and provided written informed consent were randomly assigned to a study group. At the time of treatment assignment, all participants were vaccinated and blood samples were taken to assess baseline full blood count, anti-S antibody measurements and neutralising antibody titres.
During the baseline visit, participants were instructed to record local and systemic adverse reactions in a paper diary that was provided to them.
Vaccines used in the trial included the Gam-COVID-Vac (SPUTNIK-V) vaccine consisting of two adenovirus vectors (recombinant Ad26 [rAd26] and Ad5 [rAd5]), both containing the gene coding for the SARS-CoV-2 glycoprotein [2]; the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine [3], which uses a modified adenovirus as a vector containing the full-length codon-optimised coding sequence of SARS-CoV-2 spike protein along with a tissue plasminogen activator (tPA) leader sequence; and the BBIBP-CorV (Sinopharm) vaccine, which uses an inactivated SARS-CoV-2 virus antigen [4].
All participants were contacted by telephone on days 1, 5, 10 and 20 after vaccination. During the telephone calls, trained investigators from the Ministry of Health collected information on the occurrence of local or systemic adverse reactions. In addition, participants were invited for two in-person visits on days 14 and 28 post-vaccination in order to perform a clinical evaluation and to take a blood sample to measure the full blood count (haematocrit, haemoglobin, white blood cells, platelets, creatinine and liver functions test including bilirubin, aspartate transaminase [SGOT] and alanine transaminase [SGPT]), antibody levels and neutralising antibody titres.
Antibodies to SARS-CoV-2 were detected using enzyme-linked immunosorbent microplate assay (Covidar IgG). This assay has plates coated with a mixture of spike and the receptor binding domain (RBD) and includes the WHO International Standard, allowing the quantitation in international units (UI/ml) [12]. For the construction of the calibration curve, serial dilutions of the WHO International Standard were read at 450 nm. The linear range used was 0.2–1.5 optical density (OD) 450 nm. The immunoglobulin concentration of each sample was obtained by extrapolation of the curve considering the dilution factor.
Serum neutralising capacity was assessed using the ancestral SARS-CoV-2 reference strain 2019 B.1 (GISAID Accession ID: EPI_ISL_499083). Serum samples were heat-inactivated (56 °C, 30 min) and serial dilutions from 1/2 to 1/8192 were incubated for 1 h at 37 °C with the ancestral variant of SARS-CoV-2 in DMEM 2% FBS. Then, 50 μl of the mixtures were cultured with Vero cell monolayers for 1 h at 37 °C (MOI = 0.01). The media was then removed and replaced by DMEM 2% FBS. After 72 h, cells were fixed with PFA 4% (4 °C, 20 min) and stained with crystal violet solution in methanol. The cytopathic effect of the virus on the cell monolayer was then analysed and the neutralisation titre was defined as the highest serum dilution capable of preventing any cytopathic effect.
2.5. Outcomes
The primary outcome was the humoral immune response to vaccination (assessed by antibody titres against the SARS-CoV-2 spike protein, measured by immunoassay at 28 days) of ChadOx1 or BBIBP-CorV in patients who had received a first dose of Gam-COVID-Vac compared with the standard two Gam-COVID-Vac-dose regimen. A secondary immunogenicity outcome measure was neutralising antibody titres, measured by a virus neutralisation assay at day 28 for the three schedules.
Secondary endpoints included the rate of adverse events of any type, moderate or severe, in each of the study arm.
In addition, this study analysed total SARS-CoV-2 anti-spike binding IgG concentrations at 0, 14 and 28 days after randomisation, and antibody neutralisation titres at days 0, 14 and 28.
2.6. Statistical analyses
Baseline patient characteristics are given as absolute counts and proportions for categorical variables, and means and standard deviations for numerical variables. Chi square tests and independent sample t tests were used to compare categorical and numerical variables, respectively.
The primary analysis of SARS-CoV-2 anti-spike IgG was performed at day 28 in a per-protocol analysis. The per-protocol analysis population consisted of all participants who completed the study at day 28 and who did not have any protocol deviations (e.g. development of COVID-19 during study participation).
The geometric mean concentration (GMC) ratio was calculated as the antilogarithm of the difference between the mean of the log10 transformed SARS-CoV-2 anti-spike IgG in the heterologous vaccine groups and that in the rAd26/rAd5 (as reference category) vaccine group. The GMC ratios were reported separately for participants who received rAd26/rAd5 and for those who received each of the alternative vaccines separately, with a one-sided 97.5% confidence interval (CI) to adjust for multiple testing. The criterion for non-inferiority of alternative second-dose vaccines compared with rAd26/rAd5 was for the lower limit of the one-sided 97.5% CI of the GMC ratio to be greater than 0.67. Hence, the sample size was calculated taking in to account a non-inferiority analysis, with 90% power, a one-sided alpha error of 0.025, a SD for the Log10 antibody concentration of 0.3, and a lower limit of the GMC ratio greater than 0.67 (according to WHO recommendations for vaccine non-inferiority trials), with a geometric mean ratio (GMR) between the rAd26/rAd5 and alternative second-dose vaccines assumed to be 1. Following these assumptions, the study needed to recruit at least 66 participants per group. Neutralising antibody titres were analysed after conversion to a logarithmic scale and differences in titre levels at 28 days were calculated using the same procedures as described for anti-S antibody levels.
The alternative second-dose vaccine schedules were considered superior to rAd26/rAd5 if the lower limit of the two-sided 95% CI was >1, and the rAd26/rAd5 group was considered superior to the alternative treatments if the upper limit of the two-sided 95% CI was <1. Censored data reported below the lower limit of detection or lower limit of quantification were imputed with a value equal to one-fifth of the threshold before transformation. If a normal distribution could not be rendered after transformation, the Mann-Whitney U test was used. Correlations between different immunological outcomes were evaluated by Pearson correlation coefficients (data not shown).
Participants who received at least one dose of a study vaccine were included in the safety analysis. The proportion of participants with at least one safety event was reported for each vaccine schedule separately. The Fisher's exact test was used to compare the difference between groups if expected counts were <5 per cell. All statistical analyses were performed using R version 4.1.0.
Anti-spike antibody determinations were carried out at the Hospital Francisco Javier Muñiz, División Análisis Clínicos, Unidad Virología, Buenos Aires, Argentina. Serum neutralising capacity assays were performed at the Instituto de Investigaciones Biomédicas en Retrovirus y SIDA, Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina.
3. Results
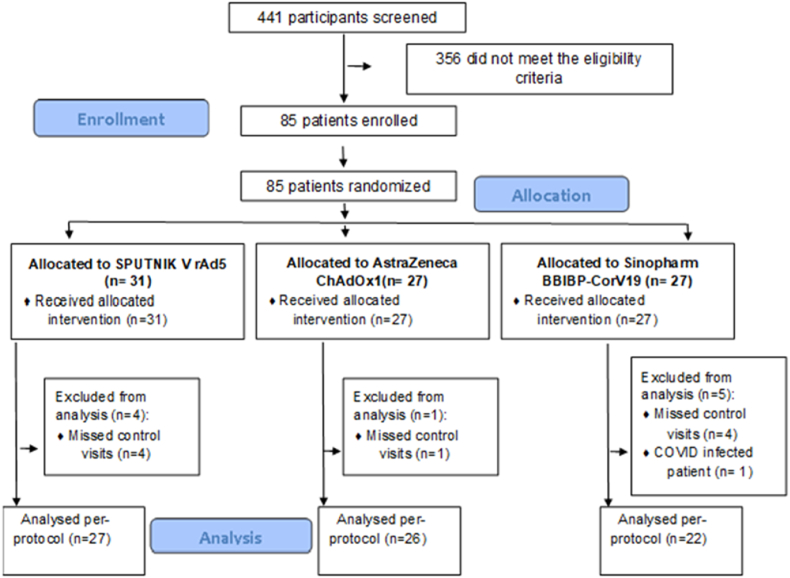
A total of 441 individuals registered on the Buenos Aires City Ministry of Health web portal as possible volunteers and were contacted. Finally, 85 participants were enrolled between 26 and July 30, 2021. In total, 31 individuals received the Gam-COVID-Vac rAD5 (SPUTNIK-V) second-dose vaccine, 27 were randomised to receive ChAdOx1 nCoV-19 (Oxford-AstraZeneca) and 27 to BBIBP-CorV (Sinopharm).
The final per-protocol analysis included 75 participants because nine individuals (12%) had no results at day 28 as they did not attend the scheduled appointments and one individual (1.33%) was diagnosed with COVID-19 before the end of the trial and therefore could not be analysed on an intention-to-treat basis. Of the nine participants who missed the control visits, four had been allocated to BBIBP-CorV, four to Gam-COVID-Vac rAD5 and one to ChAdOx1 nCoV-19 (see Fig. 1). Of note, the SARS-CoV-2-infected patient was a 67-year-old woman with a history of meningioma (diagnosed in 2018), in good state of health, who had been allocated to the BBIBP-CorV group. COVID-19 infection was confirmed 16 days after vaccination. The patient had mild symptoms and did not require hospitalisation.
Fig. 1.
CONSORT flow chart of the participants.
Overall, the mean age of participants was 68.2 years (SD 2.9) and 49 (57.6%) were female. Baseline characteristics were similar across the three groups (see Table 1).
Table 1.
Baseline characteristics of the trial participants (n = 85), according to vaccination schedule assigned (rAd26/ChAdOx1, rAd26/BBIBP-CorV or rAd26/rAd5).
| Characteristic | Vaccine schedule |
||
|---|---|---|---|
| rAd26/ChAdOx1 (n = 27) | rAd26/BBIBP-CorV (n = 27) | rAd26/rAd5 (n = 31) | |
| Mean age [years (SD)] | 68 (3) | 68 (3) | 69 (3) |
| Body mass Index [kg/m2 (SD)] | 29.22 (4.99) | 27.49 (3.59) | 28.43 (5.41) |
| Days since first vaccination [days (range)] | 76 (64–89) | 74 (58–88) | 71 (38–87) |
| Sex female [n (%)] | 16 (59) | 16 (59) | 17 (55) |
| Coexisting condition [n (%)] | |||
| Diabetes mellitus | 6 (22) | 4 (15) | 3 (10) |
| COPD | 1 (4) | 1 (4) | 2 (6) |
| Cardiovascular disease | 13 (48) | 15 (56) | 14 (45) |
SD: Standard deviation. COPD: Chronic obstructive pulmonary disease.
Among participants who were randomised to receive Gam-COVID-Vac rAd5, anti-S titres (IU/mL) went from 46 IU/mL at day 0–544 IU/mL at day 28 (GMC ratio = 1.00 reference category). In participants receiving ChAdOx1 nCoV-19 anti-S titres went from 129 to 620 IU/mL [GMC ratio = 1.14 (0.69–1.88)] and among those randomised to BBIBP-CorV anti-S titres went from 63 to 101 IU/mL [GMC ratio = 0.18 (0.11 to 0.30)] (Table 2).
Table 2.
Immunogenicity at baseline, 14- and 28-days post second dose (rAd25, ChAdOx1 or BBIBP-CorV).
| Vaccine schedule | Day |
Geometric mean concentration day 28–0 | ||
|---|---|---|---|---|
| 0 | 14 | 28 | ||
| Geometric mean (GM) anti-S titres (IU/mL) | ||||
| rAd26/rAd5 | 46.1 | 417.0 | 543.6 | 11.78 (7.05–19.67) |
| rAd26/ChAdOx1 | 128.9 | 670.0 | 619.7 | 4.81 (2.14–10.81) |
| rAd26/BBIBP-CorV | 63.5 | 101.3 | 97.5 | 1.53 (0.74–3.20) |
| GMC ratioa | ||||
| rAd26/rAd5 | 1.00 | 1.00 | 1.00 | |
| rAd26/ChAdOx1 | 2.79 (1.30–6.03) | 1.61 (0.94–2.74) | 1.14 (0.69–1.88) | |
| rAd26/BBIBP-CorV | 1.38 (0.70–2.72) | 0.24 (0.14–0.42) | 0.18 (0.11–0.30) | |
| No. of participants | ||||
| rAd26/rAd5 | 31 | 30 | 27 | |
| rAd26/ChAdOx1 | 24 | 24 | 26 | |
| rAd26/BBIBP-CorV | 27 | 27 | 22 | |
GMC ratio was calculated as the antilogarithm of the difference between the mean of the log10 transformed SARS-CoV-2 anti-spike IgG in the heterologous groups and that in rAd26/rAd5 (as reference category).
Second-dose vaccination with Gam-COVID-Vac rAd5 and ChAdOx1 nCoV-19 significantly increased anti-S titres at 28 days, but this was not observed on those who received BBIBP-CorV as their second-dose COVID-19 vaccine.
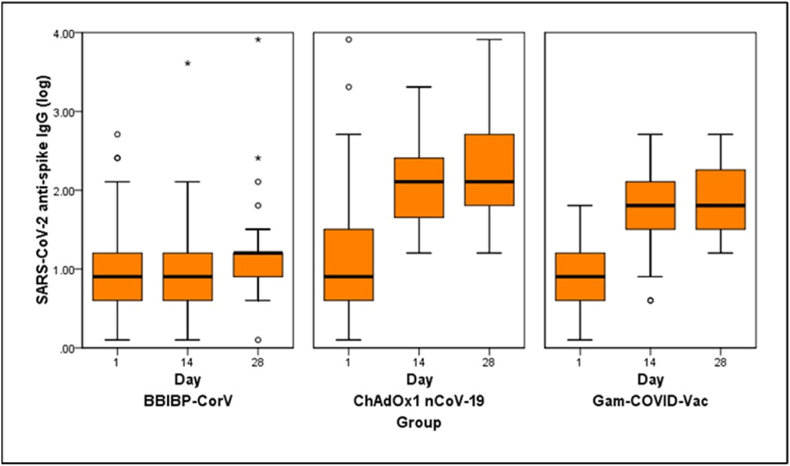
The geometric mean concentration ratio between day 28 and baseline within each treatment group was 11.8 (6.98–19.89) among patients assigned to Gam-COVID-Vac rAd5, 4.81 (2.14–10.81) in the rAd26/ChAdOx1 group and 1.53 (0.74–3.20) among those who received rAd26/BBIBP-CorV (Table 2 and Fig. 2).
Fig. 2.
Box plot of log10 neutralising antibody titres at baseline, day 14 and day 28 post second-dose vaccination, according to schedule assigned (from left to right: rAd26/BBIBP-CorV, rAd26/ChAdOx1, rAd26/rAd5).
Neutralising antibody titres increased significantly between day 0 and day 28 among participants receiving Gam-COVID-Vac rAd26/rAd5 [GMC ratio between day 28 and day 0 = 9.77 (5.26–18.14)] and those receiving rAd26/ChAdOx1 Vac [GMC ratio between day 28 and day 0 = 8.98 (2.87–28.09)] but not for participants receiving rAd26/BBIBP-CorV [GMC ratio between day 28 and day 0 = 1.64 (0.14–4.14)] (Table 3).
Table 3.
Log 10 Neutralising antibody titres at baseline, 14- and 28-days post second-dose vaccination (rAd5, ChAdOx1 or BBIBP-CorV).
| Vaccine schedule | Day |
Geometric mean concentration day 28–0 | ||
|---|---|---|---|---|
| 0 | 14 | 28 | ||
| Geometric mean (GM) | ||||
| rAd26/rAd5 | 0.726 | 1.587 | 1.834 | 9.77 (5.26–18.14) |
| rAd26/ChAdOx1 | 0.881 | 1.972 | 2.131 | 8.98 (2.87–28.09) |
| rAd26/BBIBP-CorV | 0.782 | 0.870 | 1.041 | 1.64 (0.14–4.14) |
| GMC ratioa | ||||
| rAd26/rAd5 | 1.00 | 1.00 | 1.00 | |
| rAd26/ChAdOx1 | 2.30 (0.79–6.68) | 2.34 (1.14–4.81) | 2.12 (0.99–4.51) | |
| rAd26/BBIBP-CorV | 1.26 (0.60–2.62) | 0.24 (0.11–0.52) | 0.21 (0.09–0.47) | |
| No. of participants | 30 | 30 | 28 | |
| 25b | 23c | 27 | ||
| 27 | 26 | 25 | ||
GMC ratio was calculated as the antilogarithm of the difference between the mean of the log10 transformed SARS-CoV-2 anti-spike IgG in the heterologous groups and that in rAd26/rAd5 (as reference category).
Blood samples could not be collected for 2 participants.
Blood samples could not be collected for 4 participants.
The primary endpoint, GMC ratio at 28 days compared to the rAd26/rAd5 schedule (1.00), was 2.12 (0.99–4.51) with rAd26/ChAdOx1 and 0.21 (0.09–0.47) with rAd26/BBIBP-CorV.
After considering the differences in immunogenicity outcomes, participants in the rAd26/BBIBP-CorV group were offered an additional vaccination with Gam-COVID-Vac rAd5.
All vaccination schedules were found to be safe in this trial. Injection-site reactions (pain and local erythema) at day 1 were more common in participants who were randomised to rAd26/rAd5 (n = 14) and rAd26/ChAdOx1 (n = 14) than rAd26/BBIBP-CorV (n = 3). Only one patient who was allocated to the rAd26/ChAdOx1 group reported moderate feverishness and chills at day 5.
During the 28-day duration of the trial, there were no serious adverse events in any of the study arms.
4. Discussion
This study found that in patients aged ≥65 years, the standard two doses of Gam-COVID-Vac (rA26/rdA5) [SPUTNIK-V] and the combination of rAd26/ChAdOx1 (Oxford-Astra Zeneca) significantly increased anti-S titres at 28 days post second-dose vaccination; however, this result was not found for the rAd26/BBIBP-CorV (Sinopharm) combination. This result is important and should be considered when the need to accelerate vaccination is imperative and in a context of vaccine shortage when many low- and middle-income countries have limited access to initiate or complete immunisation schedules [13,14].
The safety and efficacy of some heterologous regimens has been widely studied in several trials [[8], [9], [10], [11]], most of which use mRNA-based platforms that are preferable options in high-income economies. However, data on Gam-COVID-Vac-based schedules are lacking, even though they have been authorised for emergency use in >70 nations and have been administered to millions of individuals all over the world.
All three vaccine combinations used in the current trial were safe and well tolerated. There were no serious adverse events in this study population in any of the study arms. In line with previous data [15,16], nearly all reported reactions were mild or moderate, most of them related to local injection-site pain and lasted <5 days. BBIBP-CorV recipients described less adverse events than Gam-COVID-Vac and ChAdOx1 allocated participants.
There are several limitations to the current data that must be taken into account. First, the small study population may limit generalisation of the results; however, the difference in antibody titres observed with the two doses of Gam-COVID-Vac (rAd26/rAd5) or rAd26/ChAdOx1 versus rAd26/BBIPB-CorV groups was evident. All patients who had been assigned to the BBIBP-CorV (Sinopharm) second-dose vaccination were offered an additional dose of the Gam-COVID-Vac (rAd5) as soon as the trial results were available.
Second, although participants with a known history of COVID-19 in the last 6 months were excluded, the higher baseline antibody titres in the group allocated to ChAdOx1 may be as a result of a higher percentage of these participants having previous asymptomatic or unrecognised infection and, therefore, influencing results in this study arm. Unfortunately, because of the need for rapid enrolment, the study did not have anti-SARS-CoV-2 serology test results available in real time to exclude these patients from participation.
This study provides valuable information about the humoral immune responses after heterologous Gam-COVID-Vac/ChAdOX1 vaccination compared with the Gam-COVID-Vac complete regimen or Gam-COVID-Vac/BBIBP-CorV vaccination. Results show that heterologous Gam-COVID-Vac/ChAdOX1 vaccination appears to be safe and induces an adequate humoral immune response against SARS-CoV-2 spike protein fragments with high neutralising antibody capacity in a cohort of older adults; however, the Gam-COVID-Vac/BBIBP-CorV vaccination schedules did not result in a sufficient humoral immune response. These findings contribute to the scarce information published on the safety and immunogenicity of Gam-COVID-Vac heterologous regimens and will help with the development of guidelines and vaccine programme management.
Ethical approval
Ethical approval was provided from the Hospital J.M. Ramos Mejía Research Ethics Board.
Funding
Funding was provided from the Ministry of Health of Buenos Aires City Government, Argentina. The Ministry of Health of Buenos Aires City designed and conducted the trial, and provided the vaccines used in this trial.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank the study participants for their contribution to this research, and would specifically like to thank Patricia Burgoa, Angel Parlante, Elena Aldea, Blanca Touceda, Sabrina Francavilla, Clara Torchia, Myriam Scherer, Silvia Margalejo and Cecilia Abela for their invaluable research and administrative support.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folegatti P.M., Ewer K.J., Aley P.K., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia S., Zhang Y., Wang Y., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macchia A., Ferrante D., Angeleri P., et al. Evaluation of a COVID-19 vaccine campaign and SARS-CoV-2 infection and mortality among adults aged 60 Years and older in a middle-income country. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhama K., Patel S.K., Natesan S., Vora K.S., Iqbal Yatoo M., Tiwari R., Saxena S.K., Singh K.P., Singh R., Malik Y.S. COVID-19 in the elderly people and advances in vaccination approaches. Hum. Vaccines Immunother. 2020 Dec 1;16:2938–2943. doi: 10.1080/21645515.2020.1842683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanez N.D., Weiss N.S., Romand J.A., Treggiari M.M. COVID-19 mortality risk for older men and women. BMC Publ. Health. 2020;20:1742. doi: 10.1186/s12889-020-09826-8. Published 2020 Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Shaw R.H., Stuart A.S.V., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borobia A.M., Carcas A.J., Pérez-Olmeda M., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [published correction appears in Lancet. 2021 Aug 14;398(10300):582] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt T., Klemis V., Schub D., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillus D., Schwarz T., Tober-Lau P., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir. Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojeda D.S., Gonzalez Lopez Ledesma M.M., et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peacocke E.F., Heupink L.F., Frønsdal K., et al. Global access to COVID-19 vaccines: a scoping review of factors that may influence equitable access for low and middle-income countries. BMJ Open. 2021 Sep 30;11 doi: 10.1136/bmjopen-2021-049505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz A.B., Raqib R., Khan W.A., et al. Integrated control of COVID-19 in resource-poor countries. Int. J. Infect. Dis. 2020;101:98–101. doi: 10.1016/j.ijid.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathioudakis A.G., Ghrew M., Ustianowski A., et al. Self-reported real-world safety and reactogenicity of COVID-19 vaccines: a vaccine recipient survey. Life (Basel) 2021;11:249. doi: 10.3390/life11030249. Published 2021 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menni C., Klaser K., May A., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]