Abstract
Neuropsychiatric symptoms are the most common sequelae of long-COVID. As accumulating evidence suggests an impact of survived SARS-CoV-2-infection on brain physiology, it is necessary to further investigate brain structural changes in relation to course and neuropsychiatric symptom burden in long-COVID.
To this end, the present study investigated 3T-MRI scans from long-COVID patients suffering from neuropsychiatric symptoms (n = 30), and healthy controls (n = 20). Whole-brain comparison of gray matter volume (GMV) was conducted by voxel-based morphometry. To determine whether changes in GMV are predicted by neuropsychiatric symptom burden and/or initial severity of symptoms of COVID-19 and time since onset of COVID-19 stepwise linear regression analysis was performed.
Significantly enlarged GMV in long-COVID patients was present in several clusters (spanning fronto-temporal areas, insula, hippocampus, amygdala, basal ganglia, and thalamus in both hemispheres) when compared to controls. Time since onset of COVID-19 was a significant regressor in four of these clusters with an inverse relationship. No associations with clinical symptom burden were found.
GMV alterations in limbic and secondary olfactory areas are present in long-COVID patients and might be dynamic over time. Larger samples and longitudinal data in long-COVID patients are required to further clarify the mediating mechanisms between COVID-19, GMV and neuropsychiatric symptoms.
Keywords: COVID-19, Long-COVID, Neuroimaging, VBM
1. Introduction
The current pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses an ongoing challenge to treatment of severe acute conditions, but also to treatment of survivors with long lasting symptoms following the SARS-CoV-2 infection. While many organs are affected after suffering from Coronavirus Disease 2019 (COVID-19), mental health problems are particularly prominent as up to 78% of survivors develop neuropsychiatric long-COVID symptoms lasting more than 4 weeks after acute infection (Groff et al., 2021; Schou et al., 2021; Zhao et al., 2021). Most common are symptoms of depression, anxiety, and cognitive deficits. These symptoms impair the individual´s quality of life and pose a general socioeconomic burden (Iqbal et al., 2021). While it is not known yet, what specifically causes these persisting neuropsychiatric symptoms of long-COVID, it has been originally speculated that people with initially severe symptoms of COVID-19 are likely to present with long-COVID symptoms in general for months (Yong, 2021). The severity of COVID-19 disease was defined by WHO and the severe symptoms include hospitalization and delivery of oxygen. Predicting factors of neuropsychiatric long-COVID symptoms are age, gender, and previous presence of psychiatric disorders, but the findings have been inconsistent until now (Schou et al., 2021). With respect to the time-course of neuropsychiatric long-COVID symptoms, studies indicate either spontaneous or symptomatic-therapy-induced remission (Matalon et al., 2021). However, in some patients cognitive dysfunctions might even worsen over time (Lu et al., 2020). Just the lack of knowledge of the pathology caused by SARS-CoV-2 and the long-lasting symptoms makes the treatment challenging. However, there is hope, that the understanding of the pathogenic factors causing these long-lasting symptoms will allow to identify targeted therapy options.
Up to date, most neuroimaging studies in patients with COVID-19 were carried out in patients in the acute stage. The methods applied were either just visual inspection or automated statistical analysis of neuroradiological images. Such studies report visible alterations spanning signs of cerebral hemorrhage, strokes, and cerebral swelling and structural alterations in olfactory system, corpus callosum, cingulate cortex, and insula (Najt et al., 2021).
So far, evidence for long-lasting brain structural changes, (i.e. on the neural correlates of the neuropsychiatric long-COVID syndrome), is scarce, and mediators of these long-lasting changes were not identified yet. One of the first voxel-based morphometry (VBM) study of structural changes in long-COVID (3 months after acute COVID-19) reported increased bilateral gray matter volumes (GMV) in olfactory cortices, hippocampi, insulas, left Rolandic operculum, left Heschl's gyrus, and right cingulate gyrus in patients compared to controls. Moreover, an association of GMV alterations with symptoms of memory impairment and loss of smell was reported (Lu et al., 2020). Another VBM study, carried out 6 months after acute COVID-19, identified increased GMV in bilateral hippocampus and amygdala, with GMV of left hippocampus and amygdala being negatively correlated with post-traumatic stress symptoms (Tu et al., 2021). Furthermore, a source-based morphometry (SBM) study using computed tomography scans (CT) in 120 patients (58 cases with COVID-19 vs. 62 non-infected, in neurological care during spring 2020) reported lower GMV in fronto-temporal areas at discharge and 6 months later to be associated with clinical factors like fever or lack of oxygen during intensive care in all patients. There were no GMV changes in the long-COVID group when compared to the non-infected group (Duan et al., 2021). A recently published longitudinal analysis reported reductions of cortical thickness in orbitofrontal and parahippocampal cortex in previously infected participants (Douaud et al., 2022). In sum, the existing literature implies relevant lingering effects of COVID-19 on cortical brain structure in the aforementioned regions. Yet, as the majority of previous neuroimaging studies did not account for neuropsychiatric symptoms in long-COVID patients, it appears widely unclear how brain structural alterations might be related to neuropsychiatric symptoms in long-COVID, and more specifically which brain structural correlates might characterize neuropsychiatrically affected long-COVID patients.
To address this gap in the literature, the present study compared GMV of 30 patients with neuropsychiatric long-COVID symptoms and 20 healthy controls. Based on the previously published studies, we expected brain structural group differences in areas relevant to cognition and emotion. We expected these alterations to be associated with neuropsychiatric symptom burden. Since long-COVID symptom burden was previously reported to depend on the severity of acute COVID-19 and time since onset of COVID-19, we hypothesized an association of GMV differences with these factors, too.
2. Methods
2.1. Participants and assessment
From April 2021 to September 2021 we included 30 long-COVID patients with neuropsychiatric symptoms and 20 healthy controls with no prior infection with COVID-19. Both groups were matched regarding age and gender with no significant differences of mean values between groups. In addition, groups did not differ regarding years of education, crystalized IQ and body mass index, as confirmed by independent sample t-tests. An overview of demographic characteristics, results of the independent sample t-tests and long-COVID related information is given in Table 1 .
Table 1.
Demographic data and anamnesis.
| Basic characteristics | ||
|---|---|---|
| long-COVID patients (n = 30) | healthy controls (n = 20) | |
| Age in years, mean (SD) | 47.5 (±11.5) | 42.95 (±13.41) |
| Independent samples t-test (t, sig.) | t = 1.29, p = 0.202 | |
| Gender | 17 f, 13 m | 10 f, 10 m |
| Independent samples t-test (t, sig.) | t = −0.46, p = 0.651 | |
| Education in years, mean (SD) | 10.96 (±1.1) | 11 (±1.1) |
| Independent samples t-test (t, sig.) | t = −0.11, p = 0.912 | |
| BMI (kg/m2), mean (SD) | 23.8 (±4.4) | 22.4 (±4) |
| Independent samples t-test (t, sig.) | t = 1.02, p = 0.313 | |
| IQ (MWT-B), mean (SD) | 112.62 (±14) | 119.37 (±16.31) |
| range | 88–145 | 97–145 |
| Independent samples t-test (t, sig.) | t = −1.53, p = 0.133 | |
| Severity of COVID (WHO) (SD) | 2.45 (±1.06) | n.a. |
| range | 1–5 | |
| Time since COVID, in months | 8.65 | n.a. |
| range | 2–16 | |
| Psychometry at the time of scan | ||
| long-COVID patients (n = 30) | healthy controls (n = 20) | |
| MADRS, mean (SD) | 12.24 (± 8.46) | 2.55 (±1.79) |
| range | 2–30 | 0–6 |
| MoCa, mean (SD) | 26.21 (±2.54) | 28.05 (±1.82) |
| range | 20–30 | 24–30 |
| state anxiety (STAI X1), mean (SD) | 43 (±5.21) | 41.89 (±4.03) |
| range | 28–52 | 34–50 |
| trait anxiety (STAI X2), mean (SD) | 43.41 (±7.63) | 43. 53 (±6.22) |
| range | 14–56 | 34–56 |
Abbreviations: SD, Standard Deviation; f, female; m, male; IQ (MWT-B), premorbid IQ (measured by the German version of Multiple-Choice Vocabulary, called in German Mehrfachwahl-Wortschatz-Intelligenz test); MoCA, Montreal Cognitive Assessment (assessment of mild cognitive dysfunction); WHO, World Health Organization (provides guidelines for scores on severity symptoms and duration of COVID-19 disease); MADRS, Montgomery-Asberg Depression Rating Scale; STAI, State-Trait-Anxiety Inventory.
Characters in bold font indicate statistically significant difference between patients with long-COVID and non-infected controls.
Patients were recruited from the post-COVID outpatient clinic of the Department of Internal Medicine IV (Infectiology) and the Department of Neurology of Jena University Hospital. There they had undergone verification of their post-COVID condition (via real-time reverse transcriptase-polymerase chain reaction (RT-qPCR) at the time of acute infection), anamnesis covering timepoint and severity of their COVID-19 symptoms according to WHO by a board-certified physician. The WHO subdivides patients with COVID-19 into mild disease (ambulatory cases, WHO scores 1 to 3), moderate disease (hospitalised cases, score 4, no oxygen therapy, and score 5, oxygen by mask or nasal prongs) severe and critical disease (hospitalised cases scores 6 to 9, cared for with intensive ventilation and intensive care procedures)(World Health Organization-Global Forum for Health Research - Mental Health Research Mapping Project, 2020). Among our patients two received therapy (score of 5), two were hospitalised without oxygen therapy (score of 4) and all others were treated ambulatory with scores of 2 and 3. Anamnesis also covered subject's symptoms of fatigue, depression and cognitive impairment as leading neuropsychiatric symptoms in long-COVID.
In line with the study´s specific focus on neuropsychiatric impairment in long-COVID patients, inclusion criterion for the study was reported symptoms of fatigue and/or depressed mood and/or impairment of memory and concentration at their appointment with the physician in the post-COVID outpatient clinic. Symptoms were determined by clinical interview and screening via Patient Health Questionnaire (PHQ-9), Fatigue Assessment Scale (FAS) and Montreal Cognitive Assessment (MoCA). To keep a naturalistic study design, we did not define a certain age range or time since COVID-19 as inclusion criteria. We included men and women between age 18–70 and a range of 2–16 months of post-acute COVID-19.
At the day of scanning all patients received comprehensive psychiatric diagnostic via Mini-International Neuropsychiatric Interview (MINI-Interview for DSM-5) (Sheehan et al., 1998) by trained interviewers. The resulting diagnoses were confirmed by a board-certified psychiatrist. None of the included patients had a history of psychiatric disorders prior to their COVID-19. Healthy controls were recruited from the community via press releases and contacted the laboratory via e-mail or phone. They were first screened for history of positive SARS-CoV-2 test or typical COVID-19 symptoms since December 2019. Additionally, at the day of scanning, they were serologically tested for the presence of IgG antibodies against SARS-CoV-2. If the participant was vaccinated against COVID-19, the Western blot differentiated between antibodies due to vaccination (only present antibodies against spike and/or receptor binding antigens) or previous infection (present antibodies against nucleocapsid antigen). Healthy control participants also received psychiatric screening according to the MINI-Interview for DSM-5 (Sheehan et al., 1998) which confirmed absence of any present or lifetime psychiatric disorder.
To confirm a significant neuropsychiatric symptom burden in patients compared to controls at the day of scanning all participants were rated for symptoms of depression with Montgomery-Asberg Depression Rating Scale (MADRS, (Montgomery et al., 1985) and completed the State-Trait-Anxiety Inventory (STAI, (Spielberger and Vagg, 1984) as a self-rating assessment for symptoms of anxiety. Furthermore, participants were screened for cognitive impairment with the Montreal Cognitive Assessment (Nasreddine et al., 2005).
Exclusion criteria for all participants were: exclusion criteria for an MRI scan, history of major neurological diseases and unmedicated internal medical conditions, especially chronic inflammatory conditions, and a history of or current substance abuse disorder. All participants completed the Multiple Choice Vocabulary Test B (MWT-B), which is available in German (Antretter et al., 2013), to estimate crystallized IQ and confirm the inclusion criterion of IQ higher 80.
All participants gave written informed consent to participate in the study. The study protocol was approved by the local Ethics Committee of Jena University Medical School.
2.2. Magnetic resonance imaging (MRI)
All subjects underwent high–resolution T1-weighted MRI on a 3 Tesla Siemens Tim Trio scanner (Siemens, Erlangen, Germany) using a standard quadrature head coil and an axial 3-dimensional magnetization prepared rapid gradient echo (MPRAGE) sequence (TR 2400 ms, TE 2.22 ms, α 9°, 208 contiguous sagittal slices, FoV 256 mm, voxel resolution 0.8 × 0.8 × 0.8 mm; acquisition time 6:38 min). The scan was part of an MRI protocol of 60 min total duration. All scans were checked to exclude imaging artefacts.
2.3. Voxel-based morphometry
For voxel-based morphometry (VBM), we used the CAT12 toolbox (Computational Anatomy Toolbox 12) of the Structural Brain Mapping group, Jena University Hospital, Jena, Germany, which is running as toolbox in SPM12 (Statistical Parametric Mapping, Institute of Neurology, London, UK). All T1-weighted images were corrected for bias–field inhomogeneities. All images were then segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) (Ashburner and Friston, 2005). The images were spatially normalised using the DARTEL algorithm (Ashburner, 2007). The segmentation process was further extended by accounting for partial volume effects (Tohka et al., 2004), applying adaptive maximum a posteriori estimations (Rajapakse et al., 1997). After pre-processing and in addition to visual checks for artefacts, all scans passed an automated quality check protocol. Scans were smoothed with a Gaussian kernel of 8 mm (FWHM). To guarantee that only gray matter areas were considered in the statistical analysis, we applied an absolute gray matter threshold of 0.1.
2.4. Statistics
To confirm neuropsychiatric symptom burden of long-COVID patients at the day of scanning we performed 2-sample t-tests (IBM SPSS Statistics, Version 27) comparing mean values of MADRS, STAI and MoCA between groups.
To compare GMV between the patients and the control group, we applied the general linear model (GLM) approach implemented in SPM12. We performed an ANOVA on the whole-brain level and included total intracranial volume (TIV), age, and gender as nuisance variables in order to remove the related variance in all our analyses.
Results from voxel-based morphometry were corrected by application of threshold-free cluster enhancement (TFCE) (Spisak et al., 2019) with 20.000 permutations and then corrected for multiple comparisons with the family-wise error method (FWE) applying a significance level of p<0.05. Anatomical labeling of clusters was performed using Hammers atlas (Hammers et al., 2003).
To explore possible clinical and biological contributors to the volume differences we extracted adjusted raw values of maximum voxels (point of strongest effect size) of the significant clusters to IBM SPSS Statistics version 27 and performed stepwise linear regression to determine which of the following variables contributed to the variance of cortical volumes in patients: MADRS, STAI state and trait scales, MoCA, initial severity of COVID-19 and/or time since onset of COVID-19. Age and gender were also included in the model as GMV is strongly influenced by both. Age and gender were not significantly correlated to initial severity or time since onset of COVID-19 in our patient group as determined by correlation analysis.
3. Results
At the time of scanning groups differed significantly with respect to depressive symptoms measured by MADRS (p < 0.001, Cohen´s d = 1.46) and cognitive symptoms measured by MoCA (p = 0.008, Cohen´s d = −0.81) with higher symptom burden in long-COVID patients. The groups did not differ regarding symptoms of state and trait anxiety as measured by the STAI. Descriptive statistics of sample properties and psychometric data is presented in Table 1.
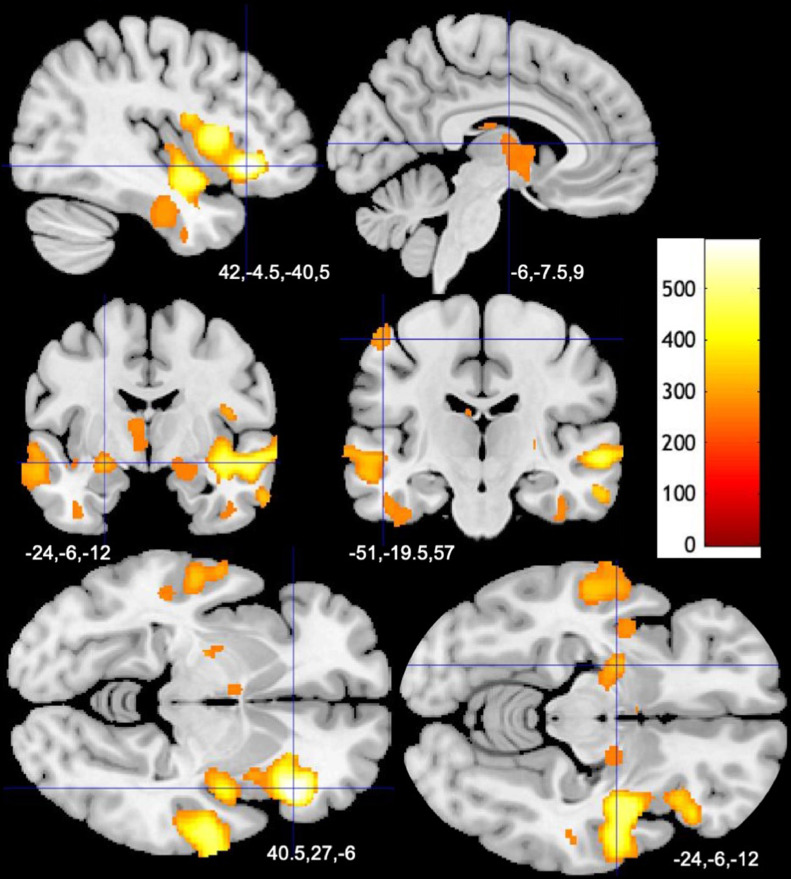
The group analysis in SPM yielded several clusters of significantly larger GMV (significance level p<0.05, FWE-corrected) in patients spanning fronto-temporal areas, insula, hippocampus, amygdala, basal ganglia, and thalamus in both hemispheres when compared to controls. There were few clusters of smaller GMV, mostly in bilateral lingual gyri (Table 2 , Fig. 1 ).
Table 2.
- Brain regions of differences in gray matter volumes between patients with long-COVID and controls.
| Anatomical regions corresponding to clusters of differences in gray matter | Co-ordinates of cluster peak | k | p (FWE-corrected at p<0.05) | TFCE |
|---|---|---|---|---|
| Long-COVID patients < healthy controls | ||||
| right lingual gyrus, cerebellum, left lingual gyrus | 8 −80 −8 | 400 | 0.024 | 305 |
| right lateral occipital lobe, cuneus, lingual gyrus | 16 −92 9 10 −93 15 | 177 | 0.044 0.045 | 231 229 |
| Long-COVID patients > healthy controls | ||||
| right inferior frontal gyrus, lateral orbital gyrus, insula anterior short gyrus, posterior orbital gyrus insula anterior inferior | 40 27 −6 32 34 0 26 27 2 | 9664 | 0.005 0.006 0.008 | 592 562 512 |
| left middle and inferior temporal gyrus, anterior temporal lobe lateral part, superior temporal gyrus anterior part, superior temporal gyrus middle part | −57 0 −22 −57 −15 −14 −57 −20 −4 | 3026 | 0.019 0.027 0.027 | 382 335 333 |
| right superior middle frontal gyrus, middle frontal gyrus | 16 6 62 9 21 60 14 16 69 | 1142 | 0.027 0.027 0.028 | 336 333 332 |
| left amygdala, putamen, pallidum, hippocampus, insula posterior long gyrus, insula anterior long gyrus | −24 −6 −12 −21 −14 −8 | 339 | 0.028 0.033 | 328 307 |
| left postcentral gyrus, precentral gyrus | −51 −20 57 | 162 | 0.036 | 295 |
| right fusiform gyrus, middle and inferior temporal gyrus, parahippocampal and ambient gyrus, hippocampus, superior temporal gyrus middle part | 40 −15 −30 42 −4 −40 51 −8 −38 | 514 | 0.037 0.046 0.049 | 291 263 255 |
| left lateral occipital lobe, superior parietal gyrus, angular gyrus | −26 −69 32 −20 −64 42 | 282 | 0.039 0.044 | 284 269 |
| left middle and inferior temporal gyrus, fusiform gyrus | −50 −20 −27 −42 −10 −32 39 −18 −33 | 839 | 0.040 0.041 0.042 | 281 279 276 |
| left thalamus, right thalamus, left caudate nucleus | −6 −8 9 −4 4 6 −6 −2 −4 | 543 | 0.044 0.045 0.046 | 269 267 264 |
| right amygdala, pallidum, hippocampus, substantia nigra, parahippocampal and ambient gyrus | 16 −8 −10 22 −6 −18 | 244 | 0.046 0.047 | 263 260 |
Abbreviations: k, cluster size; p, probability value; FWE, family-wise error; TFCE, Threshold Free Cluster Enhancement. Anatomical labeling according to Hammers atlas.
Fig. 1.
Larger Gray Matter Volumes in Patients with long-COVID Compared to Controls, (A) Results of GMV comparison (p<0.05, FWE-corrected) between long-COVID patients and healthy controls are presented as overlays. Color bar represents TFCE-value.
Results of the stepwise multiple regression carried out in patients with long-COVID and controls are shown in Table 3 . For all clusters with larger GMV in patients the R² for the overall model ranged from 0.537 to 0.805, indicating a high goodness-of-fit according to Cohen (1988). The whole model included gender, age, MADRS, STAI state and trait scales, MoCA, severity score of COVID-19 according to WHO, and time since onset of COVID-19 as regressors and predicted significantly GMV in the clusters with larger GMV in patients.
Table 3.
– Duration of long-COVID, gender and age are significant regressors of increased GMV in patients.
| Anatomical regions corresponding to clusters of differences in gray matter | R2 | F (df) | significant predictors (unstandardized B, standardized B, significance) |
|---|---|---|---|
| Post-COVID patients < healthy controls | |||
| right lingual gyrus, cerebellum, left lingual gyrus | n.a. | n.a. | No variables were entered into the equation. |
| right lateral occipital lobe, cuneus, lingual gyrus | 0.201 | F(1,25)=6.306, p = 0.019 | MADRS (0.003; 0.449; p = 0.019) |
| Post-COVID patients > healthy controls | |||
| right inferior frontal gyrus, lateral orbital gyrus, insula anterior short gyrus, posterior orbital gyrus, anterior inferior insula | 0.727 | F(1,25)=20.4, p<0.001 | gender (0.11, 0.45 p = 0.001) age (−0.008, −0,8, p<0.001) time since COVID-19 (−0.008, −0.26, p = 0.034) |
| left middle and inferior temporal gyrus, anterior temporal lobe lateral part, superior temporal gyrus anterior part, superior temporal gyrus middle part | 0.713 | F(3,23)=19.07, p<0.001 | gender (0.118, 0,47, p = 0.001) age (−0.008, −0.78, p<0.001) time since COVID-19 (−0.009, −0.275, p = 0.029) |
| right superior middle frontal gyrus, middle frontal gyrus | 0.734 | F(2,24)=33.1 p<0.001 | gender (0.049, 0,41, p = 0.001) age (−0.004, −0.82, p<0.001) |
| left amygdala, putamen, pallidum, hippocampus, insula posterior long gyrus, insula anterior long gyrus | 0.789 | F(2,24)=44.96, p<0.001 | gender (0.093, 0.399, p = p<0.001) age (−0.008, −0.86, p<0.001) |
| left postcentral gyrus, precentral gyrus | 0.738 | F(3,23)=21.65, p<0.001 | gender (0.081, 0.51, p<0.001) age (−0.005, −0.78, p<0.001) time since COVID-19 (−0.005, −0.26, p = 0.03) |
| right fusiform gyrus, middle and inferior temporal gyrus, parahippocampal and ambient gyrus, hippocampus, superior temporal gyrus middle part | 0.805 | F(3,23)=14.08, p = 0.001 | gender (0.118, 0.43, p = 0.004) age (−0.009, −0.74, p<0.001) time since COVID-19 (−0.01, −0.3, p = 0.033) |
| left lateral occipital lobe, superior parietal gyrus, angular gyrus | 0.537 | F(2,24)=13.9, p<0.001 | gender (0.063,0.35, p = 0.022) age (−0.005, −0.7, p<0.001) |
| left middle and inferior temporal gyrus, fusiform gyrus | 0.549 | F(2,24)=14.62, p<0.001 | gender (0.07, 0.38, p = 0.012) age (−0.006, −0.7, p<0.001) |
| left thalamus, right thalamus, left caudate nucleus | 0.677 | F(2,24)=25.14, p<0.001 | gender (0.095, 0.41, p = 0.002) age (−0.008, −0.78, p<0.001) |
| right amygdala, pallidum, hippocampus, substantia nigra, parahippocampal and ambient gyrus | 0.794 | F(2,24)=46.3, p<0.001 | gender (0.044, 0.41, p<0.001) age (−0.004, −0.86, p<0.001) |
Abbreviations: R2, coefficient of multiple determination, representing the goodness-of-fit for the overall model; F, ratio of the mean sum of squares to the mean square error, df, number of degrees of freedom, p, significance level, unstandardized B, value for the regression equation for predicting the dependent variable from the independent variable. Rows in bold fond indicate clusters with significant associations between time elapsed since COVID-19 and GMV alterations. Anatomical labeling according to Hammers atlas.
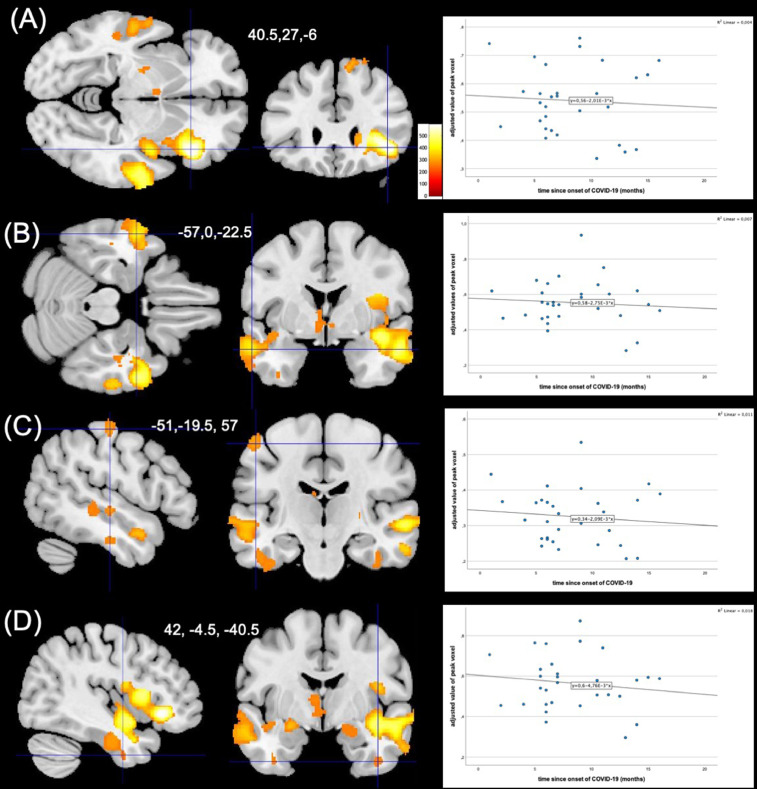
Among the variables related to COVID-19, time since onset of COVID-19 was a significant regressor in four of these clusters (anatomically located in right inferior frontal gyrus, lateral and posterior orbital gyrus, anterior parts of the insula, left superior, middle and inferior temporal gyrus and left postcentral and precentral gyrus, right fusiform gyrus, middle and inferior temporal gyrus, parahippocampal gyrus, hippocampus, superior temporal gyrus middle part). The relationship was inverse, suggesting higher GMV with shorter time since onset of COVID-19 (Fig. 2 ). Age and gender were significant predictors in all clusters, suggesting a linear relationship with GMV. Psychometric values (MADRS, STAI and MoCA) did not significantly predict GMV values.
Fig. 2.
Time elapsed since COVID-19 is a significant regressor of GMV values in long-COVID patients, Four clusters of larger gray matter volume predicted by time since onset of COVID-19 (A-D), cluster peak is marked by crosshairs. Color bar represents TFCE-value. Scatter plots show the association of adjusted value of peak voxel with time since onset of COVID-19 in each participant of respective cluster.
For the two clusters with smaller GMV in patients only one model included one factor as a significant predictor (MADRS). The model showed a low goodness-of-fit (R²=0.201). This small cluster was anatomically located in right lateral occipital lobe, cuneus, lingual gyrus.
4. Discussion
To our best knowledge, this is the first study reporting regionally increased gray matter in long-COVID patients with neuropsychiatric symptoms such as fatigue, depression and cognitive deficits and a mean time since disease onset of 8 months (range 2–16 months). Volumetric changes in patients were statistically predicted by age, gender and the time since infection by the SARS-CoV-2 virus.
The longitudinal landmark study by Douaud et al. reported decreased cortical thickness in orbitofrontal and parahippocampal gyrus in participants previously infected with SARS-CoV-2 compared to their pre-infection status (Douaud et al., 2022). This seemingly conflicting finding might result from several methodical differences between both studies. First of all, measures of cortical thickness and cortical volume, although often intercorrelated, deliver different information about changes in local cortical gray matter. Cortical thickness is a more sensitive marker of age-related decline in gray matter making it important to note, that the UK Biobank sample was 10 years older on average. VBM on the other hand amalgamates aspects of cortical thickness, surface area and folding (Hutton et al., 2009; Storsve et al., 2014). Also, participants in UK Biobank were described as mild cases, which apart from report of greater cognitive decline than controls did not receive any further neuropsychiatric characterization. Furthermore, the second scan was performed 141 days after infection on average compared to an average of 8 months in the present study. Pathophysiological mechanisms related to neuropsychiatric symptoms might follow differing temporal trajectories. But even if we ruled out a history of psychiatric conditions in retrospect, the cross-sectional design of the present study cannot entirely eliminate effects of pre-existing symptoms before COVID-19, which is a great strength of the UK Biobank study.
In line with our hypothesis, we report GMV alterations in the limbic and secondary olfactory system in patients with long-COVID in comparison to non-infected participants. The VBM analyses showed significant larger GMV in clusters covering bilateral fronto-temporal areas, insula, hippocampus, amygdala, basal ganglia, and thalamus in long-COVID patients compared to controls. The current study therefore replicates findings of previous VBM-studies that were carried out at 3 months after infection (Lu et al., 2020) and 6 months after infection (Tu et al., 2021) reporting GMV increase in patients compared to controls as well. However, our findings go beyond these published findings, first, by demonstrating persistent alterations in patients with a mean time of 8 months after COVID-19 and, second, by documenting an association of GMV alterations with time elapsed since the acute phase of the disease.
The presence of larger rather than smaller GMV in long-COVID patients when compared to controls, might indicate compensatory or recovery effects. This is in line with the findings by Lu et al. (2020). Compensatory or recovery effects could be considered especially for areas belonging to and connected with the olfactory system, which is suspected to be the system first infected by SARS-CoV on its way into the central nervous system using retrograde neuronal transport mechanisms (Netland et al., 2008). The primary olfactory cortex (piriform cortex) was not affected in our analysis, but structures belonging to the secondary olfactory cortex such as thalamus, insula, striatum, hippocampus, and amygdala were (Han et al., 2019). In cases of infection of the upper respiratory tract with subsequent loss of smell in patients, GMV in the central olfactory system has been found to be decreased in the acute phase of COVID-19 due to lack of stimulation. During the time of recovery, the volume of the system was found to be enlarged (Gellrich et al., 2017). One mechanism of volume increases during recovery could be neurogenesis with migration of neuroblasts from the subventricular zone or the subgranulate layer of the hippocampus’ dentate gyrus into the affected olfactory areas replacing interneurons like periglomerular and granular cells (Curtis et al., 2007). Another mechanism of recovery could be an increased functional activity resulting in hypertrophy of neurons and amplification of dendritic connections, as suggested in sensory deprivation models (Karstensen et al., 2018).
Several areas belonging to the secondary olfactory system are also part of the structural and functional limbic system enabling cognitive functioning such as emotion processing, learning, and memory (Rolls, 2015). Since, and representative for long-COVID patients (Schou et al., 2021), our patient cohort was significantly affected by depression and cognitive dysfunctions, structural alterations in this network might mediate these symptoms. Respective evidence for the relevance of structural and functional alterations in the medial prefrontal cortex, the medial and caudolateral orbital cortex (medial prefrontal network), the amygdala, the hippocampus, and ventromedial parts of the basal ganglia for psychiatric symptoms has been reported in various studies on patients with major depressive disorder (Banasr et al., 2021). Comparably, a significant role of gray matter alterations, globally and in hippocampus, mediotemporal lobe structures in particular has been documented in patients with mild cognitive impairment at risk for developing dementia (Raji et al., 2019).
Therefore, the finding of structural alterations in the brain areas decisive for psychological and cognitive health in post-COVID-19 patients might imply that these contribute to the variety of respective symptoms in COVID-19 survivors (Putri et al., 2021). Against our expectation clinical symptom burden measured by MADRS, STAI and MoCA was not associated with GMV in any of the altered clusters. An exception was MADRS being a predictor in a small cluster with lower GMV in patients than in controls located in right lateral occipital lobe, cuneus, lingual gyrus. Overall, this might hint towards clinical subtypes of long- and post-COVID syndromes and/or a regionally diverse relationship between gray matter volume and symptom burden. These relationships should be explored further with region-of-interest analyses in larger samples.
Apart from compensatory processes, an alternative explanation for larger GMV in patients with COVID-19 could be ongoing inflammatory activity that results in endothelial activation, microvascular dysfunction, and vasogenic increase of tissue water (Whitmore and Kim, 2021). This hypothesis is worth testing, since there is some evidence of systemically increased inflammatory markers in long-COVID patients. Those markers were associated with long-term symptoms of depression and anxiety. Also, reduced levels of IL-6 predicted improvement of depressive symptoms (Mazza et al., 2020, 2021). In immunometabolic major depression, which is clinically similar to neuropsychiatric long-COVID, dysregulation of both the innate and adaptive immune system was reported. There is a measurable increase of pro-inflammatory markers both on a systemic as well as CNS level. Long-term neuroinflammatory activity appears to be associated with cortical volume loss rather than increase though (Beurel et al., 2020; Frodl and Amico, 2014). Therefore the very pathway between systemic inflammation and increase of cortical volume in long-COVID remains unknown. A component specific to acute and long-term effects of SARS-CoV-2 might be disruption of the blood-brain-barrier (BBB) by infection and destruction of endothelium and pericytes by the virus (Theoharides, 2022). This might subsequently lead to perivascular inflammation and disruption of glymphatic drainage, but data on this mechanism is still scarce (Langan et al., 2022).
Another novel finding of our study is that the time since onset of COVID-19 is associated with changes in volumes of gray matter. In four clusters (anatomically located in right inferior frontal gyrus, lateral and posterior orbital gyrus, anterior parts of the insula, left superior, middle and inferior temporal gyrus and left postcentral and precentral gyrus, right fusiform gyrus, middle and inferior temporal gyrus, parahippocampal gyrus, hippocampus, superior temporal gyrus middle part) time since onset of COVID-19 was significant as an inverse predictor of the volume, suggesting that larger GMV might decrease over time. It is quite possible that decreasing volumes are part of recovery from COVID-19. This hypothesis would be best to test in a longitudinal data set.
Our study documents that patients with long-COVID, who are suffering from the typical neuropsychiatric symptoms, show brain alterations in gray matter volumes that are predicted by patient's age, gender and time since disease onset but not by symptom burden.
Apart from the cross-sectional design our study is limited by the heterogeneity between the initial clinical assessment the recruiting was based on and the assessments performed at the day of scanning. Also there is considerable heterogeneity of the individual time frames between infection, appointment in the post-COVID clinic and scanning as a part of the study. Even though this naturalistic approach suggested to test for associations of brain structural alterations with time since infection, this might obscure diverse clinical trajectories of subgroups.
Further analyses stratifying long-COVID patients with neuropsychiatric symptoms according to severity of depressive or cognitive symptoms could yield further insight into the process of recovery. Unfortunately, our sample size is not suited for that analysis yet. The identification of neural correlates for symptom burden in people with long-COVID paves the way towards a better understanding of the disease and targeted therapy.
Author statement
BB as the project leader planned and organized the study, VBM analysis, creation and correction of the manuscript. MM, MT, AT, AZ, TR and CH were responsible for data acquisition (recruiting, MRI scanning, neuropsychiatric testing, data management) and critically editing the manuscript. ZK and NO contributed substantially to editing the manuscript. SB and KF organized recruiting of post-COVID patients from the outpatient clinic of the department of Neurology (UKJ) and offered valuable comments on study organization and the manuscript. PAR and AS organized recruiting of post-COVID patients from the outpatient clinic of the department of Internal Medicine IV (UKJ) and offered valuable comments on study organization and the manuscript. MW and CG oversaw conceptualization of the overall study, VBM analysis and creation of the manuscript.
Funding statement
The ongoing project (Post-COVID Brain) is in part funded by IZKF Jena (advanced clinician scientist grant to B.B.). Apart from that this research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Declaration of Competing Interest
Outside of the study, PAR received consulting and lecture fees from CSL Behring, Dr. Wilmar Schwabe, Boston Scientific, Pfizer, Bristol Myers Squibb and travel grants from Merz Pharma. All other authors state, that they have no conflict of interests.
Acknowledgments
We are grateful to Daniel Güllmar for his help setting up MRI sequences for the project, as well as Ines Krumbein for overseeing most of the MRI measurements.
References
- Antretter E., Dunkel D., Haring C. [The assessment of cognitive abilities in psychiatric patients: are widely used psychological tests still up-to-date?] Psychiatr Prax. 2013;40(3):120–129. doi: 10.1055/s-0032-1332988. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Banasr M., Sanacora G., Esterlis I. Macro- and microscale stress-associated alterations in brain structure: translational link with depression. Biol. Psychiatry. 2021;90(2):118–127. doi: 10.1016/j.biopsych.2021.04.004. [DOI] [PubMed] [Google Scholar]
- Beurel E., Toups M., Nemeroff C.B. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Hillsdale N.J. 2nd ed. L. Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Curtis M.A., Kam M., Nannmark U., Anderson M.F., Axell M.Z., Wikkelso C., Holtas S., van Roon-Mom W.M., Bjork-Eriksson T., Nordborg C., Frisen J., Dragunow M., Faull R.L., Eriksson P.S. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., Jbabdi S., Taschler B., Keating P., Winkler A.M., Collins R., Matthews P.M., Allen N., Miller K.L., Nichols T.E., Smith S.M. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022 doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Premi E., Pilotto A., Cristillo V., Benussi A., Libri I., Giunta M., Bockholt H.J., Liu J., Campora R., Pezzini A., Gasparotti R., Magoni M., Padovani A., Calhoun V.D. Alterations of frontal-temporal gray matter volume associate with clinical measures of older adults with COVID-19. Neurobiol. Stress. 2021;14 doi: 10.1016/j.ynstr.2021.100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:295–303. doi: 10.1016/j.pnpbp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Gellrich J., Stetzler C., Oleszkiewicz A., Hummel T., Schriever V.A. Olfactory threshold and odor discrimination ability in children - evaluation of a modified "Sniffin' Sticks" test. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-01465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff D., Sun A., Ssentongo A.E., Ba D.M., Parsons N., Poudel G.R., Lekoubou A., Oh J.S., Ericson J.E., Ssentongo P., Chinchilli V.M. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw. Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A., Allom R., Koepp M.J., Free S.L., Myers R., Lemieux L., Mitchell T.N., Brooks D.J., Duncan J.S. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003;19(4):224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Zang Y., Akshita J., Hummel T. Magnetic resonance imaging of human olfactory dysfunction. Brain Topogr. 2019;32(6):987–997. doi: 10.1007/s10548-019-00729-5. [DOI] [PubMed] [Google Scholar]
- Hutton C., Draganski B., Ashburner J., Weiskopf N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48(2):371–380. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal A., Iqbal K., Arshad Ali S., Azim D., Farid E., Baig M.D., Bin Arif T., Raza M. The COVID-19 sequelae: a cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus. 2021;13(2) doi: 10.7759/cureus.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karstensen H.G., Vestergaard M., Baare W.F.C., Skimminge A., Djurhuus B., Ellefsen B., Bruggemann N., Klausen C., Leffers A.M., Tommerup N., Siebner H.R. Congenital olfactory impairment is linked to cortical changes in prefrontal and limbic brain regions. Brain Imaging Behav. 2018;12(6):1569–1582. doi: 10.1007/s11682-017-9817-5. [DOI] [PubMed] [Google Scholar]
- Langan M.T., Smith D.A., Verma G., Khegai O., Saju S., Rashid S., Ranti D., Markowitz M., Belani P., Jette N., Mathew B., Goldstein J., Kirsch C.F.E., Morris L.S., Becker J.H., Delman B.N., Balchandani P. Semi-automated segmentation and quantification of perivascular spaces at 7 Tesla in COVID-19. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.846957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Li X., Geng D., Mei N., Wu P.Y., Huang C.C., Jia T., Zhao Y., Wang D., Xiao A., Yin B. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon N., Dorman-Ilan S., Hasson-Ohayon I., Hertz-Palmor N., Shani S., Basel D., Gross R., Chen W., Abramovich A., Afek A., Ziv A., Kreiss Y., Pessach I.M., Gothelf D. Trajectories of post-traumatic stress symptoms, anxiety, and depression in hospitalized COVID-19 patients: a one-month follow-up. J. Psychosom. Res. 2021;143 doi: 10.1016/j.jpsychores.2021.110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., group C.-B.O.C.S., Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., group C.-B.O.C.S., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Smeyatsky N., de Ruiter M., Montgomery D.B. Profiles of antidepressant activity with the montgomery-asberg depression rating scale. Acta Psychiatr. Scand. 1985;320:38–42. doi: 10.1111/j.1600-0447.1985.tb08073.x. Suppl. [DOI] [PubMed] [Google Scholar]
- Najt P., Richards H.L., Fortune D.G. Brain imaging in patients with COVID-19: a systematic review. Brain Behav. Immun. Health. 2021;16 doi: 10.1016/j.bbih.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putri C., Arisa J., Hananto J.E., Hariyanto T.I., Kurniawan A. Psychiatric sequelae in COVID-19 survivors: a narrative review. World J. Psychiatry. 2021;11(10):821–829. doi: 10.5498/wjp.v11.i10.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse J.C., Giedd J.N., Rapoport J.L. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans. Med Imaging. 1997;16(2):176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- Raji C.A., Ly M., Benzinger T.L.S. Overview of MR imaging volumetric quantification in neurocognitive disorders. Top Magn. Reson. Imaging. 2019;28(6):311–315. doi: 10.1097/RMR.0000000000000224. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Limbic systems for emotion and for memory, but no single limbic system. Cortex. 2015;62:119–157. doi: 10.1016/j.cortex.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Schou T.M., Joca S., Wegener G., Bay-Richter C. Psychiatric and neuropsychiatric sequelae of COVID-19 - a systematic review. Brain Behav. Immun. 2021;97:328–348. doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Spielberger C.D., Vagg P.R. Psychometric properties of the STAI: a reply to Ramanaiah, Franzen, and Schill. J. Pers Assess. 1984;48(1):95–97. doi: 10.1207/s15327752jpa4801_16. [DOI] [PubMed] [Google Scholar]
- Spisak T., Spisak Z., Zunhammer M., Bingel U., Smith S., Nichols T., Kincses T. Probabilistic TFCE: a generalized combination of cluster size and voxel intensity to increase statistical power. Neuroimage. 2019;185:12–26. doi: 10.1016/j.neuroimage.2018.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storsve A.B., Fjell A.M., Tamnes C.K., Westlye L.T., Overbye K., Aasland H.W., Walhovd K.B. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J. Neurosci. 2014;34(25):8488–8498. doi: 10.1523/JNEUROSCI.0391-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides T.C. Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome? Mol. Neurobiol. 2022;59(3):1850–1861. doi: 10.1007/s12035-021-02696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J., Zijdenbos A., Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23(1):84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Tu Y., Zhang Y., Li Y., Zhao Q., Bi Y., Lu X., Kong Y., Wang L., Lu Z., Hu L. Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol. Psychiatry. 2021 doi: 10.1038/s41380-021-01223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore H.A.B., Kim L.A. Understanding the role of blood vessels in the neurologic manifestations of coronavirus disease 2019 (COVID-19) Am. J. Pathol. 2021;191(11):1946–1954. doi: 10.1016/j.ajpath.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization-Global Forum for Health Research - Mental Health Research Mapping Project, G., 2020. Clinical management of COVID-19: interim guidance, 27 May 2020. 10665/26723.
- Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021;53(10):737–754. doi: 10.1080/23744235.2021.1924397. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.J., Jin Y., Rao W.W., Li W., Zhao N., Cheung T., Ng C.H., Wang Y.Y., Zhang Q.E., Xiang Y.T. The prevalence of psychiatric comorbidities during the SARS and COVID-19 epidemics: a systematic review and meta-analysis of observational studies. J. Affect. Disord. 2021;287:145–157. doi: 10.1016/j.jad.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]