Abstract
Respiratory viruses stimulate the release of antiviral interferons from the airway epithelium. Previous studies have shown that asthmatic patients show diminished release of type I and type III interferons from bronchial epithelia. However, the mechanism of this suppression is not understood. Here we report that extracellular nucleotides and histamine, which are elevated in asthmatic airways, strongly inhibit release of type 1 and type III interferons from human bronchial airway epithelial cells (AECs). Specifically, ATP, UTP, and histamine all inhibited the release of type I and type III IFNs from AECs induced by activation of TLR3, RIG-I, or cGAS-STING. This inhibition was at least partly mediated by Gq signaling through purinergic P2Y2, and H1 receptors, but did not involve store-operated calcium entry. Pharmacological blockade of PKC partially reversed inhibition of IFN production. Conversely, direct activation of PKC with phorbol esters strongly inhibited TLR3- and RIG-I-mediated IFN production. Inhibition of type 1 and type III IFNs by ATP, UTP, histamine, and the PAR2 receptor agonist SLIGKV also occurred in differentiated airway epithelial cells grown at an air-liquid interface, indicating that the suppression is conserved following mucociliary differentiation. Importantly, histamine, and more strikingly ATP, inhibited type I interferon release from human airway cells infected with live influenza A virus or rhinovirus 1B. These results reveal an important role for extracellular nucleotides and histamine in attenuating the induction of type I and III interferons from airway epithelial cells and help explain the molecular basis of the suppression of interferon responses in asthmatic patients.
Introduction
Respiratory viruses take a major toll on society. In the United States alone, estimates are that 29–59 million people are infected yearly with influenza virus and approximately 36,000 die (1). However, these numbers can significantly increase in a pandemic such as in 1918 when an estimated 50 million deaths occurred (2) and more recently with the emergence of the SARS-CoV-2 pandemic (3). Other respiratory viruses that cause the common cold, such as human rhinovirus, also exact a significant economic burden on society (4). Although vaccines do exist for some of these viruses, new therapies based on an understanding of molecular events underlying the course of infection are necessary.
Airway epithelial cells (AECs) are primary host target cells for infection by respiratory viruses (5, 6). Although AECs were once thought to be exclusively involved in barrier function, they have increasingly been understood to orchestrate downstream immune and inflammatory responses through the production of various classes of inflammatory mediators (7). In response to viral infections, AECs produce and secrete interferons (6, 8, 9), an essential class of innate immune cytokines to combat the infections. Interferons (IFNs) are classified as type 1 (IFN-α/β), 2 (IFN-γ), or 3 (IFN-λ). Respiratory virus-infected AECs produce type 1 and type 3 IFN (10) and while both have antiviral activity, important distinctions exist. Type 3 IFN receptors are restricted to mucosal surfaces while type 1 IFN receptors are considered ubiquitous (6, 8). Although AECs generally make higher quantities of type 3 IFN than type 1 IFN following infection (10), type 1 has more potent antiviral activity (11). Both type 1 and type 3 IFN signaling induce expression of interferon-stimulated genes (ISGs) that ultimately mount strong antiviral defenses (6, 8). Thus, improved understanding of the signaling checkpoints regulating AEC IFN production could advance the development of novel antiviral therapies as well elucidate the basis of why certain individuals show impaired interferon production.
A puzzling feature of acute respiratory viral infections is that they frequently aggravate underlying airway diseases like asthma. For example, there is a strong correlation between children experiencing wheezing episodes early in life induced by rhinovirus (RV) or respiratory syncytial virus (RSV) with later development of asthma (12). Additionally, acute RV or RSV infections are responsible for most exacerbations of asthma (4, 12–14). Conversely, asthma is implicated as a key risk factor for severe influenza A virus (IAV) infection during IAV pandemics (5), and children with asthma are reported to have increased susceptibility to IAV infection (15). While asthmatics appear to be not prone to greater incidence of respiratory tract infections, they are prone to more severe and longer lasting lower respiratory tract symptoms during the course of infection compared to healthy individuals (16). One mechanism proposed to explain this clinical observation is that cells and tissues such as bronchial epithelial cells derived from patients with asthma often have an intrinsic defect in IFN production following viral infections (13, 17–24). However, the underlying mechanisms of why asthmatics are deficient in IFN production are poorly understood.
It is well established that asthmatic airways exhibit increased levels of histamine and extracellular nucleotides (25, 26). Histamine was the first mediator causally linked to allergic disease as it recapitulated many of the symptoms of allergy including bronchoconstriction. In asthmatic airways, histamine is implicated in a range of effects including bronchconstriction, mucus secrection, and numerous immunological roles mediated by histamine receptors on T cells, B cells, macrophages, and dendritic cells (25, 27). Likewise, allergen exposure in human airways and viral exposure of AECs induces dramatic increases in levels of extracellular nucleotides including increases in the concentrations of ATP in the broncholavage fluid (26) (28–31). These nucleotides, which include ATP and UTP, are linked to allergic sensitization (26, 32). However, whether nucleotides and histamine modulate interferon production from AECs is unknown. Here we examined the effects of histamine and extracellular nucleotides in regulating type I and type III interferons from primary human airway epithelial cells and find that these mediators significantly suppress the release of IFN-β and IFN-λ from AECs. The attenuation of IFN-β and IFN-λ release is mediated in part by H1 and P2Y2 receptors and involves the activation of Gq-PKC signaling. These findings uncover a potential mechanism by which ligands found in asthmatic airways diminish AEC IFN responses.
Materials and Methods
Cells, media and solutions:
Normal human bronchial epithelial (NHBE) cells from human donors were purchased from Lonza (CC-2540) and were grown in bronchial epithelial growth media (BEGM, CC-3170). All experiments that used media for the stimulation phase utilized Lonza BEBM (CC-3171) media supplemented with Ca2+ to bring the total concentration up to 2mM. Cells were grown in 37°C and 5% CO2. Ringers solutions were as follows: 2mM Ca2+ Ringers solution: 155mM NaCl, 4.5mM KCl, 10mM D-glucose, 5mM HEPES, 2mM CaCl2, 1mM MgCl2. 0mM Ca2+ Ringers solution: 155mM NaCl, 4.5mM KCl, 10mM D-glucose, 5mM HEPES, 1mM EGTA, 3mM MgCl2.
Antibodies and Pharmacological Tools:
Primary antibodies were as follows: P-STAT1 (CST 7649), β-actin (CST 3700), T-STAT1 (CST 9172), IRF1 (CST 8478), P-TBK1 (CST 5483), IRF3 (CST 11904), p65 (CST 8242), LaminA/C (CST 4777), T-TBK1 (Abcam ab40676), α-Tubulin (Abcam ab52866). Pharmacological tools were as follows: UTP (Sigma U6875), SLIGKV-NH2 (Tocris 4153), ATP (Sigma A6419), Adenosine (Tocris 3624), AR-C 118925XX (Tocris 4890), histamine (Tocris 3545), BTP2 (Sigma 203890), cetirizine (Tocris 2577), YM-254890 (Cayman Chemical 29735), Gö 6983 (Tocris 2285), GF 109203X (Tocris 0741), PDBu (Tocris 4153), PMA (Tocris 1201), ruxolitinib (Tocris 7054), ARL 67156 (Tocris 1283), NECA (Tocris 1691), IFN-β (R & D systems 8499-IF), poly(I:C) (Invivogen tlrl-picw), 2,3 cGAMP (Invivogen tlrl-nacga23), ISD (Invivogen tlrl-isdn), 3p-hpRNA (Invivogen tlrl-hprna).
shRNA knockdowns:
On day 0, NHBE cells were thawed into T-25 flasks at approximately 25,000 cells/flask. On day 1, cells were infected with lentivirus harboring shCon, shTLR3_1 or shTLR3_2 at a MOI of 10. On day 2, fresh BEGM media was given to each flask. On day 3, puromycin was added to the culture media at a final dose of 4μg/ml and selection was allowed to occur for 3 days. Nontransduced cells were always handled in parallel to confirm puromycin’s ability to induce selection. On day 6 or 7, cells were then plated for experiments and maintained in 1μg/ml puromycin until the time of stimulation when puromycin was removed from the culture media and cells were stimulated in BEBM without growth factors. Lentiviral particles expressing shRNA were purchased from Sigma: shCon (SHC202V), shTLR3_1 (TRCN0000056851), shTLR3_2 (TRCN0000358585).
Intracellular Ca2+ measurements:
NHBE cells were grown on poly-L-lysine coated glass-bottom dishes purchased from MatTek. Cells were loaded with 2μM Fura-2-AM (Thermofisher F1221) in BEGM with 5% FBS added to increase loading efficiency. Cells were loaded for 40 min at room temperature in the dark. Cells were washed 3X with 2mM Ca2+ Ringers solution and then incubated for an additional 15 min in the dark before initiating Ca2+ imaging. Experiments were performed at room temperature. Dishes were mounted on the stage of an Olympus IX71 inverted microscope. Images were acquired every 6 seconds at excitation wavelengths of 340nm and 380nm and an emission wavelength of 510nm. Image acquisition and analysis were performed using SlideBook software. For data analysis, regions of interest were drawn around individual cells, background fluorescence was subtracted, and the F340/F380 ratios were calculated for each time point. An increase in the ratio of F340/F380 indicates a rise in [Ca2+]i. The F340/F380 ratios were then converted to an estimate of [Ca2+]i through the equation: [Ca2+]i = β*Kd*(R-Rmin)/(Rmax-R), where R is the F340/F380 ratio and the values of β, Rmin, Rmax were determined from an in vitro calibration with Fura-2 pentapotassium salt. β is determined from the Fmin/Fmax ratio at 380nm and Kd is the dissociation constant of Fura-2 binding to Ca2+ (135nM). The determined values were β= 23.152, Rmin=0.2092, Rmax=6.954.
Transfection of NHBEs:
NHBEs were transfected with 2,3 cGAMP, ISD, or 3p-hpRNA using Lipofectamine 2000 (Thermofisher Scientific 11668019) at a constant volume of 2.5μl Lipofectamine/well of 24 well plate. Transfection cocktails were mixed containing ligand (2,3 cGAMP, ISD, 3p-hpRNA), Lipofectamine 2000, and Opti-MEM media (Thermofisher Scientific 31985062) and were gently vortexed and allowed to sit 10 minutes prior to transfecting NHBEs. Using 24 well plates, 50μl of transfection cocktail was added to 450μl of BEBM media (containing 2mM Ca2+) to initiate transfection. Cells were incubated with transfection cocktail for the length of the experiment.
Influenza A and RV1B virus infection of NHBEs:
Influenza A virus strain A/WSN/33(H1N1) was used for all IAV experiments. NHBEs were plated onto 24 well plates and infected at MOI 0.1, 0.5 or 1.0 for 24 hours. Cells were washed with PBS prior to infection, 200μl of BEBM (containing 2mM Ca2+) with IAV was added to the respective wells and cells were incubated on shaker (approximately 30 rpm) inside an incubator set to 37°C and 5% CO2. Infection was allowed to occur for 1 hour then virus was aspirated and fresh BEBM (containing 2mM Ca2+) was added to the respective wells. RV1B was purchased from ATCC (VR-1645) and was used at an MOI of 10 for all experiments. Cells were washed with PBS prior to infection, 200μl of BEBM (containing 2mM Ca2+) with RV1B was added to the respective wells and cells were incubated on shaker (approximately 30 rpm) inside an incubator set to 33°C and 5% CO2. Infection was allowed to occur for 2 hours then virus was aspirated and fresh BEBM (containing 2mM Ca2+) was added to the respective wells. For treatments including UTP, ATP, or histamine, agonist (100μM) was added at the time of initial infection and fresh agonist was added following infection.
IFN ELISAs:
On day 0, NHBE cells were plated onto 24 well or 48 well plates. On day 1, BEGM media was replaced with BEGM media lacking hydrocortisone. On day 2, cells were stimulated. If cells were pretreated with drugs, half of the BEGM media lacking hydrocortisone was taken off the cells, the drug was added to that media at 2X final concentration, vortexed, and added back to the relevant wells. At the time of stimulation, media was removed and cells were stimulated in BEBM media lacking all growth factors. Poly(I:C) and GPCR agonists were added simultaneously. Following this stimulation phase, the supernatants were collected and stored at −80°C until the time of analysis. To perform analysis of IFN-β levels, the PBL Assay Science kit (41435–1) was used and the manufacturers protocols were followed. To perform analysis of IFN-λ1/3 levels, the R & D systems kits (DY1598B and DY008) were used and the manufacturers protocols were followed. Samples that were compared statistically had the same concentration of organic solvents (DMSO).
Air-liquid interface (ALI) differentiation and stimulation:
NHBEs were plated onto costar 3460 transwells at 110,000 cells/well in BEGM media on day 0. BEGM media was on both apical and basolateral sides from day 0 until day 2. On day 1, fresh BEGM media was given on both apical and basolateral sides. On day 2, the media was removed from the apical side and the basolateral side media was replaced with differentiation media. Differentiation media was purchased from StemCell Technologies (05001) supplemented with hydrocortisone (07925) and heparin (07980) and Gibco gentamicin/amphotericin (R-015–10). Differentiation media was replaced Monday, Wednesday, and Friday (days 3, 6, 8, 10, etc) and differentiated was allowed to occur for at least 28 days until experiments were performed. Once cells began releasing mucus around day 14, the apical surface was washed once weekly with PBS to remove excess mucus until the time of the experiment. On the day of the experiment, the apical side was washed 3X with BEBM media and cells were stimulated with all drugs on both the apical side (300μl) and the basolateral side (800μl). The apical side was stimulated for 2 hours, and at this time point, the apical side media was aspirated out to reestablish the air interface because long-term media on the apical side has been shown to cause dedifferentiation (33). The basal compartment was stimulated for 20 hours. At the end of this stimulation period, the apical side was washed with BEBM (250μl/well) and the secreted cytokines were collected. The basolateral media containing secreted cytokines was also collected at this time. Supernatants were then used for IFN analysis by ELISA.
Western Blots:
Following stimulation, cells were lysed using 1X Cell Signaling Lysis Buffer (9803S) containing protease and phosphatase inhibitors (PPIs) (Thermofisher 78440). Lysates were boiled for 5 min in 1X Laemmli Sample Buffer (Bio-Rad 1610747) containing 2-ME. Samples were then subjected to SDS-PAGE. Transfer occurred at 4°C for 1.5 hrs and at 100V. PVDF membranes were used for the transfer. Following transfer, membranes were washed in TBST (0.1% Tween 20), blocked for 1 hr at RT using 5% BSA dissolved in TBST, then incubated overnight at 4°C with primary antibodies. All dilutions for primary antibodies were 1:1000 besides anti-β-actin: 1:2000 and anti-α-Tubulin: 1:5000. The following day, membranes were washed 3X for 5 min each using TBST and incubated with secondary antibodies for 1 hr at RT in 5% BSA dissolved in TBST. Li-Cor secondary antibodies were used (IRDye 800 CW or IRDye 680 RD) at dilutions of 1:10000. Membranes were washed 3X for 5 min each using TBST and immediately imaged using an Odyssey CLx imaging system.
Nuclear and Cytosolic Fractionation assays:
On day 0, NHBE cells were plated onto 6 well plates. On day 1, BEGM media was replaced with BEGM media lacking hydrocortisone. On day 2, cells were stimulated. If cells were pretreated with drugs, half of the BEGM media lacking hydrocortisone was taken off the cells, the drug was added to that media at 2X final concentration, vortexed, and added back to the relevant wells. This protocol was adapted from Chang et al. (34). Cells were stimulated for 5 minutes in BEBM media lacking all growth factors. Media was immediately aspirated, cells were placed on ice, and cells were scrapped in hypotonic lysis buffer containing protease and phosphatase inhibitors (PPIs) (Thermofisher 78440) and collected into prechilled eppendorf tubes. Lysates from two wells were combined into one tube to ensure sufficient protein content. Samples were incubated on ice for 10min. Samples were then spun at 1,000rpm for 4min at 4°C. The supernatant was collected as the crude cytosolic fraction and the pellet was resuspended in hypertonic lysis buffer containing PPIs. Both the crude cytosolic fractions and the resuspended pellet were incubated on ice for another 30min, and periodically vortexed every 10min to ensure full lysis. Next, both crude cytosolic fraction samples and the resuspended pellet samples were centrifuged at 12,000rpm for 10min at 4°C and the supernatants were collected and termed “cytosolic fraction” and “nuclear fraction”. The hypotonic buffer consisted of: 10mM Hepes pH 7.9, 10mM KCl, 1.5mM MgCl2, 0.5mM EDTA, PPIs added fresh. The hypertonic buffer consisted of: 20mM Hepes pH 7.9, 420mM NaCl, 1.5mM MgCl2, 0.2mM EDTA, 25% glcyerol, PPIs added fresh.
CellTox and CellTiter-Glo assays:
CellTox Green Cytotoxicity Assay (G8741) and CellTiter-Glo 2.0 Assay (G9241) were purchased from Promega and manufacturers instructions were followed for experimentation. NHBEs were plated in 96 well plates (costar 3610) and infected with IAV at an MOI of 0.5 as described in the virus infection methods section. The sequential multiplexing protocol was followed such that both CellTox and CellTiter-Glo could be performed using the same plate of cells.
RT-qPCR analysis:
Total RNA was extraction was performed using RNeasy Plus Mini Kit (Qiagen 74134). cDNA generation was performed using iScript Reverse Transcription Supermix for RT-qPCR (Biorad 1708841). qPCR was performed using PowerUp SYBR Green Master Mix (A25741). For qPCR, final concentration of primers was 500nM and cDNA was used at 6ng/well. Primer sequences used were as follows: RPLP0 Forward 5’ AGCCCAGAACACTGGTCTC 3’, RPLP0 Reverse 5’ ACTCAGGATTTCAATGGTGCC 3’, IFN-Β1 Forward 5’ GAAACTGAAGATCTCCTAGCCT 3’, IFN-Β1 Reverse 5’ GCCATCAGTCACTTAAACAGC 3’ (IDT Assay Name Hs.PT.58.39481063.g), TLR3 (IDT Assay Name Hs.PT.58.25887499.g).
Data analysis:
All bar graphs summarizing data are represented as mean ± SEM. Individual points are always indicative of biological replicates. For a data set involving more than two groups, an initial one-way ANOVA was performed followed by Tukey’s multiple comparison tests. Dose-response curves were created using a four parameter (variable slope) nonlinear regression. Statistical analysis and data analysis was performed using Prism 9 (GraphPad Software). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 when statistical comparisons are made.
Results
Nucleotides and histamine inhibit TLR3-induced IFN release
In response to viral challenge, airway epithelial cells produce and release types I and III IFNs (5, 6). To study the regulation of this process, we began our studies by stimulating primary AECs obtained from human donors with poly(I:C), a double-stranded synthetic RNA (dsRNA) widely used as an analog of dsRNA viruses (8), and measured the release of type 1 and type 3 IFNs via ELISA. Poly(I:C) challenge induced the release of both IFN-β and IFN-λ1/3 from human AECs, but with distinct kinetics (Supplemental Fig. 1A–B). IFN-β release occurred largely within the first 6 hours while IFN-λ1/3 induction required overnight poly(I:C) stimulation. Further, in agreement with published results (10), we found that AECs released 10–20 fold more IFN-λ1/3 than IFN-β over the same stimulation period (Supplemental Fig. 1A–B) (10). Poly(I:C) is thought to mediate its effects primarily via signaling initiated by toll-like receptor 3 (TLR3) (35). Consistent with this requirement for TLR3, shRNA knockdown of TLR3 abrogated poly(I:C)-induced release of both IFN-β and IFN-λ1/3 (Supplemental Fig. 1C–E).
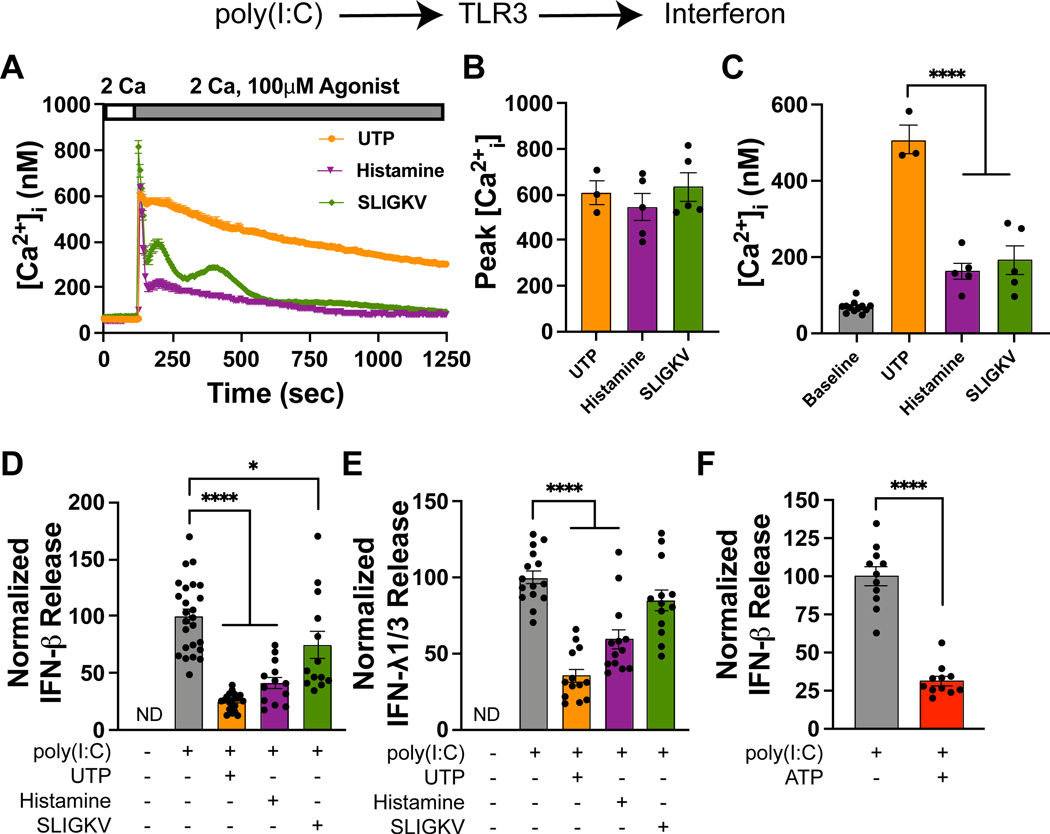
Asthmatic airways harbor elevated levels of both histamine and extracellular nucleotides (25, 26). However, the effects of these mediators on type I and III interferon responses is unknown. To study this regulation, we first examined whether AECs express function histamine and nucleotide receptors using rises in [Ca2+]i as a readout for activation of the corresponding G protein coupled receptors (GPCRs). Activation of the proteinase-activated receptor 2 (PAR2) in human AECs has been shown to blunt IFN-β production elicited by toll-like receptors and fungi (36, 37). Hence, we utilized PAR2 modulation of the IFN response as a positive control for these studies. In agreement with our prior reports and other published literature (38–42), stimulation of AECs with UTP, histamine, or the PAR2 activating peptide SLIGKV caused rises in Ca2+ [Ca2+]i (Fig. 1A). Although the peak Ca2+ response was similar across all three agonists, the amplitude of the UTP-evoked sustained [Ca2+]i rise was consistently largest relative to the other two ligands (Fig. 1B–C), suggesting that downstream signaling linked to Ca2+ store depletion and store-operated calcium entry is stronger with UTP.
Figure 1. UTP and histamine inhibit TLR3-induced IFN release.
(A) Administration of UTP, histamine, and the PAR2 activator SLIGKV elicit [Ca2+]i elevations. [Ca2+]i was measured using Fura-2 AM as previously described (42, 96). Data are mean ± SEM of n = 40–50 cells. (B) Summary of the peak [Ca2+]i following agonist addition. Each data point is the mean peak Ca2+ signal (averaged over approximately 30–50 cells) for a given experiment (one dish) and the bar graph is mean ± SEM of n = 3–5 independent experiments. (C) Quantification of [Ca2+]i measured 5 minutes after agonist addition, reflecting Orai1-mediated SOCE. Each data point is the mean [Ca2+]i (averaged over 30–50 cells) for a given experiment (one dish) and the bar graph is the mean ± SEM of n = 3–5 independent experiments. (D) UTP (100μM), histamine (100 μM), and to a lesser degree SLIGKV inhibit poly(I:C)-induced (10μg/mL) IFN-β release into the supernatant (24-hour time point). Data are mean ± SEM of n = 13–25 samples. (E) Similarly, UTP (100 μM) and histamine (100 μM) inhibit poly(I:C)-induced (10μg/mL) IFN-λ1/3 release (24-hour time point). Data are mean ± SEM of n = 13–15 samples. (F) ATP (10μM) inhibits poly(I:C)-induced (10μg/mL) IFN-β release into the supernatant (6-hour time point). Data are mean ± SEM of n = 11 samples. This data also appears in Supplemental Fig. 1H, 3A. (A-F) All experiments were performed in primary human airway epithelial cells (NHBEs) growing in submerged cultures. *p<0.05, ****p<0.0001
To examine whether UTP and histamine modulate TLR3-mediated IFN-β production, we stimulated AECs with poly(I:C), and measured the release of IFN-β and IFN-λ1/3 in the supernatant by ELISA. These experiments revealed that both UTP and histamine strongly inhibited IFN-β release (Fig. 1D). The blunting of IFN-β induction by these two ligands was significantly stronger than that elicited by the PAR2 peptide, SLIGKV (Fig. 1D). Likewise, UTP and histamine also strongly inhibited the secretion of IFN-λ1/3, but PAR2 activation had no effect on the induction of IFN-λ1/3 (Fig. 1E). We observed that UTP consistently inhibited IFN release to a greater extent than histamine (Fig. 1D–E). This stronger inhibition mirrored the larger Ca2+ response elicited by UTP (Fig. 1A), suggesting that the degree of inhibition may be related to stronger Gq signaling elicited by UTP compared to histamine (Fig. 1A–C). Likewise, ATP (10 μM) also strongly suppressed IFN-β release (Fig. 1F), indicating that nucleotides that are elevated in the astmatic airways may suppress IFN production from AECs. However, a dose-response curve comparing ATP- and UTP-mediated inhibition of IFN release demonstrated that low doses of extracellular ATP (less than 10μM) strongly suppressed IFN-β release while higher doses of ATP (100μM), potentiated IFN-λ1/3 release (Supplemental Fig. 1F–G). As AECs are known to express multiple classes of purinergic receptors (40, 41, 43, 44), the reversal of ATP-mediated inhibition of IFN production at high ATP doses suggests that distinct subsets of purinergic receptors are activated at low and higher doses, which could account for the loss of inhibition of the IFN response at high ATP doses. We did not further investigate the basis of this effect, in part because (as shown below) these differences were lost upon mucociliary differentiation (Fig. 2), but rather focused on the mechanism(s) by which nucleotides and histamine blunted the IFN response. Taken together, these results show that the GPCR agonists UTP, histamine, and to a lesser degree ATP and PAR2 activators all inhibit dsRNA-induced interferon release from AECs.
Figure 2. ATP, UTP, and histamine inhibit dsRNA-induced interferon induction following mucociliary differentiation.
(A) NHBEs were differentiated at the air-liquid interface (ALI) for 4 weeks and stimulated with 10μg/mL poly(I:C) and with or without the GPCR agonists (UTP, ATP, histamine, or PAR2 peptide, 100 μM each) on both apical and basolateral sides. (B and C) ALI cultures were stimulated with poly(I:C), and apical and basolateral supernatant samples were collected at the indicated time points and assessed for IFN-β (B) and IFN-λ1/3 release (C) via ELISA. Because the volumes of the apical and basolateral compartments are significantly different (~3.2-fold), the basolateral concentration was normalized to the apical side by multiplying values by 3.2. Data are mean ± SEM of n = 4–5 samples. (D-G) UTP, ATP, and histamine inhibit IFN release from dsRNA-stimulated ALI cultures. ALI cultures were stimulated with simultaneously with 10μg/mL poly(I:C) and the indicated agonists (100 μM each) on both apical and basolateral sides. The apical and basolateral supernatant samples were collected 20 hours later and measured for IFN-β (D-E) or IFN-λ1/3 (F-G) release via ELISA. Basolateral cytokine levels are shown in (D,F) while basolateral levels are shown in (E,G). Data are mean ± SEM of n = 11–21 samples. (H) ATP and UTP inhibit the release of IFN-β to both apical and basolateral compartments whereas histamine and SLIGKV exclusively inhibit basolateral IFN-β. In contrast, all agonists inhibit basolateral release of IFN-λ1/3 but not apical IFN-λ1/3 release. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
ATP, UTP, and histamine inhibit dsRNA-induced interferon following mucociliary differentiation
To examine whether the regulation of interferon secretion from AECs by nucleotides and histamine also occurs in a more physiologically relevant in vitro preparation, we next examined the effects of these mediators in primary airway epithelial cells that have undergone mucociliary differentiation at an air-liquid interface (ALI). Following ALI differentiation, we stimulated cells with the dsRNA mimic poly(I:C) and measured interferon release into both the apical and basolateral compartments (Fig. 2A). In order to minimize de-differentiation, poly(I:C) was pulsed onto the apical (air) surface for 2 hours only but was left in the basolateral compartment for 20 hours (see Methods). A time course analysis revealed that the release of IFN-β in the two compartments occurred at different rates, with apical IFN-β rising faster than the basolateral, although the basolateral concentrations reached higher peak levels (Fig. 2B). As seen in submerged cultures (Supplemental Fig. 1A–B), the absolute quantities of IFN-λ1/3 released were substantially higher than release of IFN-β, and the release of IFN-λ1/3 occurred more slowly (Fig. 2C). For both type 1 and type 3 IFN, the vast majority of cytokine was released into the basolateral compartment (Fig. 2B–C).
Do nucleotides and histamine modulate these interferon responses? To address this question, we co-administered poly(I:C) and the GPCR agonist on both apical and basolateral sides, and examined interferon concentrations at the 20-hour time point. In the basolateral supernatant, UTP, ATP, histamine, and SLIGKV all inhibited IFN-β release (Fig. 2D). On the apical side, however, whereas ATP and UTP inhibited IFN-β release, histamine and the PAR2 activator, SLIGKV, showed no effect (Fig. 2E). Likewise, all agonists inhibited the basolateral release of IFN-λ1/3 (Fig. 2F), whereas none were effective in suppressing apical IFN-λ1/3 levels (Fig. 2G). Thus, in contrast to the results seen in the submerged AEC cultures (Supplemental Fig. 1F–G), in ALI cultures, ATP phenocopied the effects of UTP in all endpoints examined. The switch in the effect of ATP in submerged cultures versus ALI cultures suggests that the purinergic receptor profile is likely altered upon differentiation. Nevertheless, the consistency of all three ligands (ATP, UTP, histamine) in suppressing interferon release from AECs in ALI cultures that have undergone mucociliary differentiation indicates that this is a conserved, physiologically relevant phenomenon relevant for human airways (Fig. 2H).
P2Y2 receptors and H1 receptors inhibit TLR3-mediated IFN release
To further dissect the molecular identity of the UTP and histamine receptors that mediate these inhibitory effects, we first compared the interferon inhibition dose-response curves for UTP and histamine. UTP was more potent than histamine at inhibiting both type 1 and type 3 interferons (IC50 of 0.34μM vs 3.4μM for IFN-β and 1μM vs 6μM for IFN-λ1/3 for UTP and histamine, respectively) (Fig. 3A–B). However, histamine had a much more pronounced Hill slope than UTP (Fig. 3A–B). We do not know what the significance of this difference in apparent cooperativity may imply, and suspect it may arise from arise from differences in signaling downstream of the receptors for these agonists. UTP is an agonist at P2Y2 and P2Y4 receptors (45). Because P2Y2 is expressed in airway epithelial cells and we have recently shown that this receptor stimulates mobilization of Ca2+ signaling in AECs (41), we examined the effects of blocking P2Y2 receptors with the selective antagonist, AR-C 118925XX (AR-C) (46) on the IFN response. Administration of AR-C abrogated the inhibitory effect of UTP on type 1 interferon (Fig. 3C) suggesting an essential role for P2Y2 receptors in mediating UTP inhibition of the interferon response. AR-C also partially reversed ATP-mediated inhibition of type 1 interferon in submerged cultures suggesting P2Y2 receptor involvement (Supplemental Fig. 1H). Similarly, cetirizine, an antagonist of histamine H1 receptors, which are known to be expressed on AECs (47), reversed the ability of histamine to inhibit type 1 and type 3 interferon release (Fig. 3D–E). Further, in air-liquid interface cultures, AR-C, abrogated ATP-induced inhibition of IFN-β to both the basolateral and the apical surfaces (Fig. 3F–G) indicating that ATP exerts its suppression of IFN production through activation of P2Y2 receptor in ALI cultures. Collectively, these results indicate that extracellular nucleotides and histamine, acting via P2Y2 receptors and H1 receptors, respectively, inhibit TLR3-mediated IFN release.
Figure 3. P2Y2 and H1 receptors inhibit TLR3-mediated interferon release.
(A) Dose-response of the inhibition of IFN-β release by UTP and histamine. IFN-β was measured in the cell culture supernatant 20 hours following simultaneous addition of poly(I:C) (10μg/mL) and either UTP or histamine. The solid line is a four-parameter nonlinear regression fit of the Hill equation with IC50 = 0.34 μM and Hill Slope = −0.52 for UTP and IC50 = 3.4 μM and Hill Slope = −1.65 for histamine. IFN-β was undetectable without poly(I:C) and hence this value (0 pg/ml) was set to 0% and the maximal poly(I:C)-evoked response was set to 100% for the fitting procedure. Data are mean ± SEM of n = 4–18 samples from 2 independent experiments. (B) Dose-response of the inhibition of IFN-λ1/3 release by UTP and histamine. IFN-λ1/3 was measured in the cell culture supernatant 20 hours following stimulation as described in (A). The solid line is a four-parameter nonlinear regression fit of the Hill equation with IC50 = 1 μM and Hill Slope = −0.7 for UTP and IC50 = 6 μM and Hill Slope = −4.09 for histamine. IFN-λ1/3 was undetectable without poly(I:C) and this value (0 pg/ml) was set to 0% and maximal poly(I:C)-evoked response was set at 100% for the fitting procedure. Data are mean ± SEM of n = 4–18 samples from 2 independent experiments. (C) The P2Y2 antagonist, AR-C 118925XX (10μM), reverses UTP-mediated (100μM) inhibition of IFN-β release. Supernatants were collected at aan earlier (6-hour) time point as AR-C has a short half-life in culture. Data are mean ± SEM of n = 9 samples. (D and E) The H1 receptor antagonist, cetirizine (10μM), reverses histamine-mediated (100μM) inhibition of IFN-β release (D) and IFN-λ1/3 release (E). Supernatants were collected at a 20-hour time point. Data are mean ± SEM of n = 6 samples. (A-E) All experiments were performed in primary human airway epithelial cells (NHBEs) growing in submerged cultures. (F) The P2Y2 antagonist, AR-C 118925XX (10μM), reverses ATP-mediated (100μM) inhibition of IFN-β release. ALI cultures were stimulated simultaneously with 10μg/mL poly(I:C) and 100μM ATP on both apical and basolateral sides. Apical supernatant samples were collected 7 hours later and assessed for IFN-β. Data are mean ± SEM of n = 8 samples. (G) AR-C 118925XX (10μM) also reverses ATP-mediated (100μM) inhibition of IFN-λ1/3 release. This experiment was performed similarly to (H) except IFN-λ1/3 was measured in the basolateral compartment at a 24-hour time point. Data are mean ± SEM of n = 6–8 samples. *p<0.05, **p<0.01, ****p<0.0001
UTP does not inhibit TLR3 or IFN receptor signaling
The finding that UTP strongly inhibits the poly(I:C)-mediated release of IFNs from AECs led us to next investigate the underlying mechanism. We considered and excluded several possibilities. First, UTP could inhibit TLR3 signaling that occurs immediately downstream of poly(I:C) administration to impede IFN production. Activation of TLR3 drives activation of the kinase TBK1, leading to nuclear import of the transcription factors IRF3 and NF-κB (48). We therefore tested whether UTP inhibits phosphorylation of TBK1. UTP had no effect on poly(I:C)-mediated phosphorylation of TBK1 (Supplemental Fig. 2A). Further, administration of UTP did not affect the nuclear import of IRF3 or the key NF-κB subunit, p65 (Supplemental Fig. 2B). Thus, these results indicate that UTP does not directly inhibit the activation of TBK1, IRF3 or p65 downstream of TLR3 activation by poly(I:C).
Second, multiple reports suggest that IFN receptor signaling can itself drive IFN synthesis and release in a positive feedback loop (49–51). Thus, nucleotides and histamine could in theory inhibit IFN signaling downstream of the IFN receptors. A conserved kinase that drives signaling immediately downstream of both type 1 and type 3 IFN receptors is JAK1 (52). To test this hypothesis, we utilized the JAK inhibitor ruxolitinib to abrogate signaling downstream of IFN receptors and performed a time course analysis comparing IFN release in the presence of ruxolitinib with release in the presence of UTP. While ruxolitinib modestly inhibited IFN release at late time points (24 hours), there was negligible inhibition of IFN-β at 6 hours when UTP markedly inhibited IFN induction (Supplemental Fig. 2C–D). This result suggests that blunting of IFN induction by UTP is not due to inhibition of IFN receptor signaling. In a third possibility, given the faster release of IFN-β compared to IFN-λ1/3 (Supplemental Fig. 1A–B, 2C–D), we considered the possibility that IFN-λ1/3 production may depend on the initial IFN-β production from AECs which subsequently stimulates AECs to produce the type III interferon. If this is the case, UTP-induced inhibition of IFN-β may be responsible for the decrease in IFN-λ1/3 production by UTP. To test this possibility, we applied exogenous IFN-β to rescue the deficient production of IFN-β in the presence of UTP stimulation. However, exogenous IFN-β failed to reverse UTP-mediated inhibition of IFN-λ1/3 (Supplemental Fig. 2E), indicating that UTP-mediated inhibition of type 3 IFN is unlikely to be mediated by UTP-dependent inhibition of type 1 IFN. Finally, we pretreated cells with UTP and stimulated the cells with exogenous IFN-β to measure IFN receptor signaling directly. UTP did not inhibit the phosphorylation of STAT1, or the induction of STAT1 and IRF1 (Supplemental Fig. 2F), which are key steps that follow the stimulation IFN receptor signaling. Taken together, these latter results indicate that UTP does not inhibit signaling downstream of IFN receptors as a means to inhibit antiviral IFN production.
Gq-PKC signaling but not SOCE is required for UTP and histamine-mediated inhibition of IFN-β
To further probe the mechanisms of how P2Y2 and H1 receptors suppresses IFNs in AECs, we next examined the proximal signaling pathways immediately downstream of these GPCRs. Given that both P2Y2 and H1 receptors are Gq-coupled GPCRs, we asked whether blockade of Gq signaling with the selective G⍺q inhibitor, YM-254890 (53) modulates the agonist-induced suppression of IFN production. YM-254890 strongly inhibited UTP-induced Ca2+ signaling in AECs, as expected from activation of Gq activation of PLC-β isozymes downstream of P2Y2 receptor activation (Fig. 4A). Co-administration of YM-254890 along with UTP also resulted in strong reversal of UTP’s ability to inhibit release of IFN-β and IFN-λ1/3 (Fig. 4B–C). Likewise, YM-254890 strongly reversed ATP-mediated suppression of IFN-β release (Supplemental Fig. 3A–B). Thus, these results indicate that G⍺q signaling downstream of purinergic receptor activation is necessary for inhibition of IFN release.
Figure 4. Gq-PKC signaling is required for UTP and histamine-mediated inhibition of IFN-β.
(A) The selective Gq inhibitor, YM-254890 (1μM), abrogates UTP-induced [Ca2+]i elevations. [Ca2+]i was measured using Fura-2 AM as previously described (42, 96). Data are mean ± SEM of n = 27–37 cells. (B and C) The Gq inhibitor YM-254890 (1μM) reverses UTP-mediated (100μM) inhibition of IFN-β (B) and IFN-λ1/3 release (C). Supernatants were collected either 6 hours (B) or 20 hours (C) after stimulation. Data are mean ± SEM of n = 4 samples. (D) The PKC inhibitor Gö 6983 (2.5μM) partially reverses histamine- (100μM) and UTP-mediated (100μM) inhibition of IFN-β release. Supernatants were collected 20 hours after stimulation. Data are mean ± SEM of n = 6 samples. (E) The phorbol esters, PDBu (100nM) and PMA (100nM) strongly inhibit IFN-β release. Supernatants were collected 6 hours after stimulation. Data are mean ± SEM of n = 4 samples. (F) UTP (100μM), histamine (100μM), ATP (10μM), PMA (100nM) suppress poly(I:C)-induced (10μg/mL) IFNΒ1 mRNA expression. mRNA expression was normalized to the housekeeping gene RPLP0. RNA was collected 4 hours after stimulation. Data are mean ± SEM of n = 4–8 samples. (A-F) All experiments were performed in primary human airway epithelial cells (NHBEs) growing in submerged cultures. (G) Summary model of depicting suppression of type 1 IFN by P2Y2 and H1 receptors-mediated Gq-PKC signaling. *p<0.05, **p<0.01, ****p<0.0001
Given that both UTP and histamine activated Ca2+ signaling (Fig. 1A–C), we next examined whether store-operated Ca2+ entry (SOCE), the primary Ca2+ pathway in AECs (42), is involved in UTP-mediated inhibition of type 1 IFN. Pretreatment of cells with the SOCE inhibitor, BTP2, which strongly blocks Orai1-mediated SOCE (42) neither affected the induction of poly(I:C)-mediated IFN-β induction, nor the reversal of UTP-mediated inhibition (Supplemental Fig. 3C). This result indicates that Ca2+ signaling through SOCE is not involved in the generation of type I IFN by viral mimics nor in the blunting of the IFN synthesis by nucleotides.
A previous report has found that in plasmacytoid dendritic cells, PKC activation leads to suppression of IFN responses (54). Because activation of G⍺q signaling downstream of P2Y2 or H1 receptors would be expected to be linked to PKC activation, we next evaluated whether PKC has a role in the P2Y2 and H1 receptor-mediated inhibition of IFN production. We tested this hypothesis using the broad-spectrum PKC inhibitor Gö 6983. Administration of Gö 6983 partially reversed the inhibitory effects of both UTP and histamine on IFN-β release, with stronger reversal of histamine-evoked suppression of IFN-β than that evoked by UTP (Fig. 4D). Likewise, a second PKC inhibitor GF 109203X also partially reversed UTP-mediated inhibition of IFN-β release (Supplemental Fig. 3D). To examine if direct activation of PKC also inhibits IFN-β release, we examined the effects of two independent PKC activators, Phorbol 12-Myristate 13-Acetate (PMA) and Phorbol 12,13-dibutyrate (PDBu). Both PMA and PDBu strongly suppressed poly(I:C)-driven IFN-β release (Fig. 4E), consistent with the interpretation that UTP and histamine mediated inhibition of IFN-β production is mediated at least in part by PKC.
Is the underlying mechanism of inhibition of IFN-β by nucleotides and histamine due to transcriptional suppression? To address this question, we examined IFNB1 mRNA levels following poly(I:C) stimulation in the absence and presence of nucleotides or histamine. This analysis revealed that UTP, ATP, histamine, and PMA all suppressed IFNB1 mRNA four hours after poly(I:C) stimulation (Fig. 4F). The pattern of inhibition of IFNB1 mRNA was similar to the degree of inhibition of IFN-β protein detected by ELISA (Fig. 1D) with UTP showing the strongest inhibition in response to poly(I:C) stimulation and histamine and ATP eliciting highly significant, albeit somewhat lower degree of inhibition (Fig. 4F). Moreover, direct activation of PKC by PMA also resulted in strong decrease in the IFNB1 mRNA levels following poly(I:C) stimulation. Taken together, these results indicate that suppression of of poly(I:C)-driven IFN-β induction by extracellular nucleotides and histamine occurs via Gq-PKC mediated transcriptional downregulation of IFNB1 mRNA (Fig. 4G).
In contrast to type 1 IFN, the effects of PKC inhibition with Gö 6983 on IFN-λ1/3 was less clear. Here, the PKC inhibitor reversed histamine-mediated suppression but suppression by UTP remained largely intact (Supplemental Fig. 3E). These results suggest that PKC activation inhibits the transcriptional production of IFN-β while the effect on IFN-λ1/3 is likely more complex.
UTP inhibits cGAS-STING driven interferon release
In addition to TLR3, another signaling axis that can stimulate interferon production is the cGAS-STING pathway (55, 56). cGAS operates as a sensor for dsDNA in the cytosol (57–60) and upon binding to dsDNA, synthesizes the second messenger 2,3 cGAMP. 2,3 cGAMP in turn activates the transducer protein, STING, to drive IFN production (59, 61). We examined if extracellular nucleotides and histamine modulated the production of IFN production by this pathway. To first test if the STING machinery is present and functional in AECs, we introduced the STING agonist 2,3 cGAMP into AECs and compared the induction of IFN-β release to the response elicited by poly(I:C). These experiments showed that 2,3 cGAMP elicited robust induction of IFN-β release. Moreover, the time course and magnitude of release of IFN-β elicited by 2,3 cGAMP was very similar to the poly(I:C) response (Fig. 5A). Thus, human bronchial AECs express the machinery for STING and its activation leads to induction of type I interferons with kinetics comparable to the response evoked by TLR3. To examine whether UTP and histamine regulate the STING-mediated IFN induction, we introduced 2,3 cGAMP into AECs simultaneously with UTP or histamine stimulation. UTP and histamine both strongly inhibited release of IFN-β and, to a lesser degree IFN-λ1/3, induced by 2,3 cGAMP (Fig. 5B–C). Likewise, direct activation of cGAS by the 45 base-pair dsDNA termed interferon stimulatory DNA (ISD) resulted in strong upregulation of IFN-β and IFN-λ1/3 (Fig. 5D–E). UTP inhibited ISD-induction of IFN-β but had less effect on ISD-induction of IFN-λ1/3 (Fig. 5D–E), reminiscent of the lower efficacy of UTP in inhibiting IFN-λ1/3 induced by 2,3 cGAMP (Fig. 5C). Morover, the SOCE inhibitor, BTP2 also did not affect STING-mediated IFN-β production not its inhibition by UTP, indicating that, as seen before for TLR3-mediated IFN induction, SOCE does not regulate IFN-β production (Supplemental Fig. 3F). Together, these results indicate that UTP inhibits cGAS-STING-mediated interferon production, although the magnitude of the inhibition is more modest than that seen for dsRNA-TLR3 induced interferon production.
Figure 5. UTP inhibits cGAS-STING-driven interferon release.
(A) Normal human bronchial epithelial (NHBE) cells were stimulated with 10μg/mL poly(I:C) or with 10μg/mL 2,3 cGAMP, and IFN-β was measured in the supernatant at the indicated time points. Data are mean ± SEM of n = 4 samples/time point. (B and C) UTP and histamine (100 μM each) inhibit 2,3 cGAMP-induced (10μg/mL) IFN-β release (B) and IFN-λ1/3 (C) release into the supernatant (24-hour time point). Data are mean ± SEM of n = 13–19 samples. (D) UTP (100 μM) inhibits ISD-induced (1μg/mL) IFN-β release into the supernatant (24-hour time point). Data are mean ± SEM of n = 12–14 samples. (E) UTP and histamine (100μM each) do not significantly inhibit ISD-induced (1μg/mL) IFN-λ1/3 release into the supernatant (24-hour time point). Data are mean ± SEM of n = 8–10 samples. (A-E) All experiments were performed in primary human airway epithelial cells (NHBEs) growing in submerged cultures. *p<0.05, **p<0.01, ****p<0.0001
Histamine and ATP suppress respiratory virus-induced IFN release
The findings that UTP and histamine inhibit poly(I:C)-induced type I and type III IFN release led us to next consider the pathophysiological implications for this regulation in the context of cellular infection with live respiratory viruses. Influenza A virus (IAV) is widely known to drive strong IFN production from bronchial epithelial cells (62). Rates of influenza H1N1 infection in children with asthma are reportedly higher (15), raising the possibility that the elevated levels of histamine and nucleotides found in asthmatic airways may be an underlying cause of this susceptibility to infection. Similarly, rhinovirus infections are a major cause of asthma exacerbations and complications. We therefore examined the effects of extracellular nulceotides and histamine on IFN-β and IFN-λ1/3 stimulated by IAV and rhinovirus 1B. Analysis of a multiplicity of infection (MOI) dose-response with IAV indicated that IAV evokes robust synthesis and release of IFN-β from AECs into the cell supernatant, which peaked at an MOI of ~1 (Supplemental Fig. 4A). We therefore used IAV near this MOI to infect human AECs and examined the regulation of IFNs by histamine, ATP, and UTP.
Previous reports have suggested that while extracellular poly(I:C) predominantly activates TLR3 (Supplemental Fig. 1C–D), IAV activates multiple RNA sensors including TLR3 and members of the retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) (63, 64). Indeed, shRNA mediated knockdown of TLR3 only partially reversed IAV-mediated IFN release (Supplemental Fig. 4B–C) in contrast to the nearly complete inhibition of IFN production when induced by poly(I:C) (Supplemental Fig. 1C–D), indicating the involvement of other molecules in sensing IAV. To directly test if the prototypical RLR family member, RIG-I, is functional in AECs and involved in IFN release, we utilized the IAV-derived RNA species 3p-hpRNA to selectively stimulate RIG-I (65). Introduction of 3p-hpRNA into AECs triggered strong induction of IFN-β release into the supernatant (Supplemental Fig. 4D), indicating the presence of a functional RIG-I pathway in AECs that contributes to the IAV response.
To examine if nucleotides and histamine modulate this RIG-I-induced IFN release, we simultaneously transfected AECs with 3p-hpRNA and administered nucleotides or histamine. At the early time point of 6 hours, all three ligands (ATP, UTP, and histamine) suppressed RIG-I-mediated IFN-β release (Fig. 6A), with ATP showing the strongest inhibition. UTP and histamine also suppressed the induction of IFN-β release but to a lesser extent (Fig. 6A). The P2Y2 receptor antagonist, AR-C, partially reversed ATP-mediated inhibition of RIG-I-driven type 1 IFN at a 6 hour timepoint (Supplemental Fig. 4E). However, at later time points (24 hours), ATP and histamine retained their efficacy to suppress 3p-hpRNA-mediated IFN-β, while UTP was ineffective (Fig. 6B). These results indicate that ATP is highly effective at inhibiting RIG-I-induced IFN-β release stimulated by 3p-hpRNA while histamine and UTP exhibit lower efficacy. Notably, as seen earlier for poly(I:C)-induced IFN release, the SOCE inhibitor BTP2 did not affect 3p-hpRNA-mediated induction of IFN-β nor its inhibition by ATP (Supplementary Fig. 4G), indicating that SOCE does not play a role in RIG-I-mediated induction of type I IFNs nor the regulation by ATP. By contrast, RIG-I-induced IFN-β release was strongly suppressed by the PKC agonist, PMA (Supplementary Fig. 4F), indicating that PKC plays a key role in modulation of RIG-I-mediated IFN-β production.
Figure 6. Histamine and ATP suppress respiratory virus-induced IFN release.
(A) ATP (100μM) strongly inhibits 3p-hpRNA-induced (10ng/mL) IFN-β release into the supernatant while UTP (100μM) and histamine (100μM) elicit more moderate suppression. Supernatants were collected at a 6-hour time point following cell stimulation with the RIG-I agonist. The absolute concentrations of IFN-β ranged from 12.9–321 pg/mL. Data are mean ± SEM of n = 12 samples from two independent experiments. (B) ATP (100μM) and histamine (100μM) inhibit 3p-hpRNA-induced (10ng/mL) IFN-β release into the supernatant. Supernatants were collected at a 24-hour time point. The absolute concentrations of IFN-β ranged from 156–1552 pg/mL. Data are mean ± SEM of n = 13–30 samples. (C and D) Histamine (100μM) inhibits IAV-induced (MOI 1) IFN-β (C) and IFN-λ1/3 (D) release into the supernatant. Supernatants were collected at a 24-hour time point. Concentrations of IFN-β ranged from 11–19 pg/mL. The absolute concentrations of IFN-λ1/3 ranged from 94–258 pg/mL. Data are mean ± SEM of n = 6 samples from two independent experiments. (E and F) ATP (100μM) inhibits IAV-induced (MOI 0.5) IFN-β (E) and IFN-λ1/3 (F) release into the supernatant. Cell supernatants were collected at a 24-hour time point. Concentrations of IFN-β ranged from 2–81 pg/mL. Concentrations of IFN-λ1/3 ranged from 68–836 pg/mL. Data are mean ± SEM of n = 8 samples from two independent experiments. (G) ATP (100μM) inhibits RV1B-induced (MOI 10) IFNΒ1 mRNA expression. IFNΒ1 mRNA elevels were normalized to the housekeeping gene RPLP0. RNA was collected 24 hours following infection with rhinovirus. Data are mean ± SEM of n = 5–8 samples from two independent experiments. (A-G) All experiments were performed in primary human airway epithelial cells (NHBEs) growing in submerged cultures. (H) A model summarizing how the three Gq agonists we examined (histamine, UTP, and ATP) modulate type 1 and 3 IFN production depending on the upstream PRR that stimulates IFN: poly(I:C) drives TLR3-mediated IFN release while 3p-hpRNA drives RIG-I-mediated IFN release. IAV and likely RV activate both TLR3 and RIG-I pathways to induce IFN release. Histamine dampens IFN release from both pathways. UTP strongly inhibits poly(I:C)-mediated IFN release but only inhibits early release of RIG-I-induced IFN. ATP shows modest inhibition of poly(I:C)-mediated type 1 IFN but powerfully suppresses RIG-I-, IAV-, and RV1B-induced IFN production. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
We next turned our attention to the ability of nucleotides and histamine to regulate IFN responses induced by the live viruses. To address this question, we infected cells with IAV and simultaneously applied either nucleotides or histamine. IAV-mediated production of IFNs requires viral replication so we focused on the 24-hour time point, a time by which considerable replication is predicted to have occurred. This analysis revealed that histamine reduced both type 1 and type 3 IFN release but UTP was without effect (Fig. 6C–D). This pattern is consistent with the modulation seen with 3p-hpRNA (Fig. 6B) suggesting that IAV infection more closely resembles the RIG-I-induced IFN response than TLR3 response. Likewise, administration of ATP resulted in strong suppression of the IAV-mediated type 1 and type 3 IFN release (Fig. 6E–F). Notably, inhibition of IFN-β and IFN-λ1/3 by ATP was considerably stronger than the inhibition elicited by histamine. IAV infection and ATP, histamine, and UTP did not significantly affect cytotoxicity or cellular proliferation/metabolism (Supplemental Fig. 4H–I), indicating that the attenuation of IFN production by histamine and ATP is not due to a generalized decrease in cell health. Interestingly, adenosine (but not the synthetic adenosine receptor agonist NECA), also significantly suppressed IAV-induced IFN release (Supplemental Fig. 4J–L) suggesting the involvement of an adenosine metabolite rather than a classical adenosine receptor. In agreement with this finding, ARL 67156, an inhibitor of the ecto-ATPase that catalyzes the hydrolysis of extracellular ATP to ADP and inorganic phosphate, also strongly reversed ATP-mediated inhibition of RIG-I-mediated type 1 IFN induction (Supplemental Fig. 4L). This result indicates that ATP and subsequent ATP metabolites may be responsible for the ATP-mediated suppression of RIG-I and IAV induced IFN release.
Rhinoviruses, a family of viruses that cause the common cold, are another important cause of asthma exacerbation (4). We infected airway cells with rhinovirus strain 1B (RV1B) and measured the regulation of IFN induction by nucleotides and histamine. In response to RV1B infection, IFN-β protein levels in the supernatant were too low to be accurately quantifed by ELISA, indicating that AECs produces far less type I IFNs with RV1B compared to IAV. To circumvent this issue, we therefore examined IFNB1 mRNA levels via qPCR in AECs infected with RV1B. These experiments showed that RV1B caused strong upregulation of IFNB1 mRNA in AECs 24 hours following infection. The addition of ATP caused strong suppression of RV1B-induced IFNB1 mRNA. Histamine showed more modest inhibition (Fig. 6G). Thus, these results indicate that histamine and particularly ATP are powerful suppressors of RIG-I-, IAV-, and RV1B-induced IFN production from AECs and suggest that these agonists may in part be responsible for the well-described suppression of IFN responses in asthmatics.
Discussion
Type 1 and 3 interferons are key components of the innate immune system necessary for early detection and inhibition of viral infections. In addition to recruiting innate immune cells such as macrophages and neutrophils to mediate killing of microbes and infected cells, IFNs also stimulate adaptive immune responses, particularly through their ability to stimulate dendritic cell-mediated T-cell activation and antibody responses (66, 67). Pediatric and adult asthmatic populations show impaired IFN production, leaving them susceptible to more serious viral infections (16) and virus-induced asthma exacerbations (4, 12–14). However, the mechanisms contributing to IFN deficiency in asthmatic AECs are not well understood. Previous work has established that histamine and nucleotides are elevated in asthmatic airways (25, 26, 32, 68). Here we demonstrate that histamine and nucleotides suppress the stimulus-evoked release of type 1 and type 3 IFNs from AECs via H1 and P2Y2 receptor signaling through a mechanism requiring Gq signaling and partly involving PKC. Attenuation of antiviral IFN induction by nucleotides and histamine is also seen following mucociliary differentiation of primary epithelial cells at an air-liquid interface (ALI), and following infection of AECs with live influenza A virus and rhinovirus 1B. Together, these results reveal a novel mode of modulation of IFN production from airway epithelia and help advance our understanding of regulatory signaling interactions among innate immune processes in the airways.
Following viral infection, several reports have shown that asthmatic patients display more severe and prolonged lower respiratory tract symptoms than healthy controls (16) leading to asthma exacerbations (4, 12–14). Further, higher loads of viral RNA have been measured in asthmatic tissues and cells following rhinovirus infection (13, 18, 19). These clinical observations have been attributed to impaired type I and 3 IFN production by asthmatic airways (13, 17–21). IFN deficiency is also linked worsening of asthma exacerbations (69–73). In line with this explanation, nebulized IFN-β treatment following cold symptoms reportedly offers protection against worsening lung symptoms in asthmatics with poorly controlled disease (74). Likewise, inhaled IFN-β has been shown to improve outcomes of infection with SARS-CoV-2 (75). These observations indicate that IFN responses in asthmatics during respiratory tract infections are important for the preservation of lung function and prevention of exacerbations. Our present finding that histamine and nucleotides, which are elevated in the airways following allergen exposure (25, 26, 32), strongly inhibit type 1 and 3 IFN release from AECs provides an important mechanistic explanation for the deficient IFN responses in asthmatics.
It is known that IFN production can be regulated in several ways. In macrophages, CCL2 has been shown to dampen IFN-α release (50). In plasmacytoid dendritic cells (pDCs), ligands such as histamine and nucleotides have been shown to inhibit IFN-α release (54, 76). In AECs, EGFR activation can decrease IFN-λ production thereby enhancing viral infection (77, 78). The cytokines IL-4, IL-13, and IL-17 have also been shown to dampen IFN production from AECs (17, 49, 79). Cigarette smoke can also dampen AEC IFN production (80–82). Interestingly, allergens such as aspergillus, alternaria, and house dust mite, have been shown to inhibit IFN production from AECs (37, 83, 84) and this reduction is proposed to drive a shift in the airway milieu towards a Th2 phenotype upon exposure to the allergens (37, 85). Mechanistically, both aspergillus and alternaria have been shown to activate proteinase-activated receptor 2 (PAR2) on AECs and signaling from this receptor limits the release of Th1 chemokines and IFNs (36, 37). Here we show that suppression of IFN release by histamine H1 receptors and nucleotide P2Y2 receptors requires PKC activation and occurs in ALI cultures and in response to influenza virus infection. These results indicate that activation of Gq-coupled GPCRs in AECs is a conserved mechanism for dampening IFN type I and III release. Interestingly, we found that although Gq signaling was essential for attenuation of IFN production, there was no effect of blocking SOCE on either the induction of IFN nor its inhibition by nucleotides. Thus, SOCE does not seem to regulate the production of antiviral IFNs in airway epithelia.
In ALI differentiated primary cells, we found that ATP, UTP, histamine, and SLIGKV all effectively inhibited the release of both type 1 and type 3 IFNs to the basolateral compartment (Fig. 2D,F). However, on the apical side, whereas ATP and UTP inhibited release of IFN-β, histamine and the PAR2 peptide were without effect (Fig. 2E). This polarity correlates well with the polarized localization of the respective receptors. P2Y2 receptors are expressed on both apical and basolateral surfaces of differentiated cultures while both H1 and PAR2 receptors typically reside exclusively on basolateral surfaces (38, 86–89).
Regulation of IFN release by histamine and nucleotides was partially dependent upon the stimulus evoking IFN production. We found that histamine consistently inhibited IFN-β release, regardless of whether the stimulus was a TLR3 activator (poly(I:C)), a RIG-I activator (3p-hpRNA), or IAV (Fig. 6A–D, 6H). However, while UTP strongly inhibited TLR3-mediated IFN release, it was a weaker inhibitor of both RIG-I- and IAV-induced IFN release, only inhibiting type 1 IFN release at early time points (Fig. 6A–D, 6H). On the other hand, ATP was a weaker inhibitor of TLR3-mediated IFN induction but a strong suppressor of RIG-I-, RV1B-, and IAV-driven IFN production (Fig. 6A–B, 6E–H). Adenosine also suppressed IAV-induced IFN production (Supplemental Fig. 4J–K). Based on these results, we propose that ATP activates two distinct pathways to suppress type IFN production, the first mediated by adenosine or a closely related metabolite, and the second mediated by P2Y2 receptors. By contrast, because UTP would be expected to activate only the P2Y2 class of receptors on AECs (and does not produce adenosine metabolites) it is insufficient to inhibit respiratory virus-induced IFN as powerfully as ATP. It is noteworthy in this context that ATP-mediated suppression of IFN production by RIG-I activation more closely resembles the pattern seen with IAV rather than those seen with the TLR3 activator. One report has suggested that in contrast to pathogenic bacteria, commensal bacteria evoke TLR3-induced type I IFN elicits anti-inflammatory and protective immune responses, which protects against infection and colitis (90). Collectively, these results suggest caution must be exercised in interpreting IFN responses induced by viral mimics such as poly(I:C) alone without confirmation using live respiratory viruses or bacteria.
Histamine is a classic allergic mediator in the context of Th2 inflammation (25). By contrast, IFNs are known to inhibit Th2 bias (69–71). Because IAV and rhinoviruses directly stimulate mast cells to release histamine among many other mediators, (94, 95), our finding that histamine inhibits IFN release from AECs raises the possibility that histamine may reinforce Th2 inflammation in the airways. In line with this scenario, pediatric atopic patients often progress from atopic dermatitis (AD) towards asthma later in childhood via a process termed the “atopic march” (91). Similarly, ATP released by infected airway cells during viral infections (28–31) and at sites of inflammation (26, 32, 68) may exacerbate allergic lung inflammation (26, 32) by suppressing IFN production. There are reports that treatment of atopic infants with an H1 receptor antagonist decreases the likelihood of the subsequent development of asthma (92, 93). These studies and our current results suggest that selective blockade of P2 and H1 receptors may mitigate allergic airway inflammation by restoring IFN levels in the lung airways.
Supplementary Material
Key Points:
ATP and histamine suppress virus-mediated IFN release from airway epithelial cells.
Inhibition of IFN release by ATP/histamine occurs through Gq/PKC signaling.
P2Y2 and H1 receptor blockade reverses ATP/histamine inhibition of IFN release.
Acknowledgments
This work used resources of the Northwestern University Structural Biology Facility in the Robert H Lurie Comprehensive Cancer Center. We would like to thank members of the Prakriya laboratory for helpful discussions. We would like to thank Laura Dada of the Sznajder Lab for assistance related to the influenza A virus experiments. Models of an air-liquid interface were created using BioRender.com.
Funding
This research was funded by 5T32AI007476 and F31HL151170 to TSK and NIH grant R01 HL149385 to MP. The Northwestern University Structural Biology Facility was supported by NCI CCSG P30 CA060553.
Footnotes
Competing interests
N/A.
Data availability
All data generated or analyzed during this study are included in the manuscript and the supporting files.
References
- 1.Hodinka RL 2016. Respiratory RNA Viruses. Microbiol Spectr 4. [DOI] [PubMed] [Google Scholar]
- 2.Monto AS, and Fukuda K. 2020. Lessons From Influenza Pandemics of the Last 100 Years. Clin Infect Dis 70: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alshammary AF, and Al-Sulaiman AM. 2021. The journey of SARS-CoV-2 in human hosts: a review of immune responses, immunosuppression, and their consequences. Virulence 12: 1771–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs SE, Lamson DM, St George K, and Walsh TJ. 2013. Human rhinoviruses. Clin Microbiol Rev 26: 135–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herold S, Becker C, Ridge KM, and Budinger GR. 2015. Influenza virus-induced lung injury: pathogenesis and implications for treatment. Eur Respir J 45: 1463–1478. [DOI] [PubMed] [Google Scholar]
- 6.Lazear HM, Nice TJ, and Diamond MS. 2015. Interferon-lambda: Immune Functions at Barrier Surfaces and Beyond. Immunity 43: 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambrecht BN, and Hammad H. 2012. The airway epithelium in asthma. Nat Med 18: 684–692. [DOI] [PubMed] [Google Scholar]
- 8.Levy DE, Marie IJ, and Durbin JE. 2011. Induction and function of type I and III interferon in response to viral infection. Curr Opin Virol 1: 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaacs A, and Lindenmann J. 1957. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 147: 258–267. [PubMed] [Google Scholar]
- 10.Ioannidis I, Ye F, McNally B, Willette M, and Flano E. 2013. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J Virol 87: 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meager A, Visvalingam K, Dilger P, Bryan D, and Wadhwa M. 2005. Biological activity of interleukins-28 and −29: comparison with type I interferons. Cytokine 31: 109–118. [DOI] [PubMed] [Google Scholar]
- 12.Jartti T, Bonnelykke K, Elenius V, and Feleszko W. 2020. Role of viruses in asthma. Semin Immunopathol 42: 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, and Johnston SL. 2006. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 12: 1023–1026. [DOI] [PubMed] [Google Scholar]
- 14.Busse WW, Lemanske RF Jr., and Gern JE. 2010. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 376: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloepfer KM, Olenec JP, Lee WM, Liu G, Vrtis RF, Roberg KA, Evans MD, Gangnon RE, Lemanske RF Jr., and Gern JE. 2012. Increased H1N1 infection rate in children with asthma. Am J Respir Crit Care Med 185: 1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, and Johnston SL. 2002. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 359: 831–834. [DOI] [PubMed] [Google Scholar]
- 17.Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, Stanciu LA, Gnesini G, Pastore A, Spanevello A, Morelli P, Johnston SL, Caramori G, and Papi A. 2015. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 70: 910–920. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Message SD, Mallia P, Kebadze T, Contoli M, Ward CK, Barnathan ES, Mascelli MA, Kon OM, Papi A, Stanciu LA, Edwards MR, Jeffery PK, and Johnston SL. 2019. Bronchial mucosal IFN-alpha/beta and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol 143: 114–125 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, Calabrese F, Caramori G, Ballarin A, Snijders D, Barbato A, Saetta M, and Papi A. 2012. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. J Allergy Clin Immunol 130: 1307–1314. [DOI] [PubMed] [Google Scholar]
- 20.Gielen V, Sykes A, Zhu J, Chan B, Macintyre J, Regamey N, Kieninger E, Gupta A, Shoemark A, Bossley C, Davies J, Saglani S, Walker P, Nicholson SE, Dalpke AH, Kon OM, Bush A, Johnston SL, and Edwards MR. 2015. Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J Allergy Clin Immunol 136: 177–188 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, and Davies DE. 2005. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 201: 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Valero J, Olloquequi J, Montes JF, Rodriguez E, Martin-Satue M, Texido L, and Ferrer Sancho J. 2019. Deficient pulmonary IFN-beta expression in COPD patients. PLoS One 14: e0217803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singanayagam A, Loo SL, Calderazzo M, Finney LJ, Trujillo Torralbo MB, Bakhsoliani E, Girkin J, Veerati P, Pathinayake PS, Nichol KS, Reid A, Footitt J, Wark PAB, Grainge CL, Johnston SL, Bartlett NW, and Mallia P. 2019. Antiviral immunity is impaired in COPD patients with frequent exacerbations. Am J Physiol Lung Cell Mol Physiol 317: L893–L903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel DA, You Y, Huang G, Byers DE, Kim HJ, Agapov E, Moore ML, Peebles RS Jr., Castro M, Sumino K, Shifren A, Brody SL, and Holtzman MJ. 2014. Interferon response and respiratory virus control are preserved in bronchial epithelial cells in asthma. J Allergy Clin Immunol 134: 1402–1412 e1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akdis CA, and Blaser K. 2003. Histamine in the immune regulation of allergic inflammation. J Allergy Clin Immunol 112: 15–22. [DOI] [PubMed] [Google Scholar]
- 26.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC Jr., and Lambrecht BN. 2007. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13: 913–919. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi K, and Ogasawara M. 2019. The Role of Histamine in the Pathophysiology of Asthma and the Clinical Efficacy of Antihistamines in Asthma Therapy. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, and Boucher RC. 2011. Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol 45: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shishikura Y, Koarai A, Aizawa H, Yamaya M, Sugiura H, Watanabe M, Hashimoto Y, Numakura T, Makiguti T, Abe K, Yamada M, Kikuchi T, Hoshikawa Y, Okada Y, and Ichinose M. 2016. Extracellular ATP is involved in dsRNA-induced MUC5AC production via P2Y2R in human airway epithelium. Respir Res 17: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson SK, Morice AH, and Sadofsky LR. 2020. Rhinovirus-16 increases ATP release in A549 cells without concomitant increase in production. ERJ Open Res 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamiya Y, Fujisawa T, Katsumata M, Yasui H, Suzuki Y, Karayama M, Hozumi H, Furuhashi K, Enomoto N, Nakamura Y, Inui N, Setou M, Ito M, Suzuki T, Ikegami K, and Suda T. 2020. Influenza A virus enhances ciliary activity and mucociliary clearance via TLR3 in airway epithelium. Respir Res 21: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F, Su X, Huang G, Xin XF, Cao EH, Shi Y, and Song Y. 2017. Adenosine Triphosphate Promotes Allergen-Induced Airway Inflammation and Th17 Cell Polarization in Neutrophilic Asthma. Journal of immunology research 2017: 5358647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carey RM, Freund JR, Hariri BM, Adappa ND, Palmer JN, and Lee RJ. 2020. Polarization of protease-activated receptor 2 (PAR-2) signaling is altered during airway epithelial remodeling and deciliation. J Biol Chem 295: 6721–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang WC, Nelson C, and Parekh AB. 2006. Ca2+ influx through CRAC channels activates cytosolic phospholipase A2, leukotriene C4 secretion, and expression of c-fos through ERK-dependent and -independent pathways in mast cells. FASEB J 20: 2381–2383. [DOI] [PubMed] [Google Scholar]
- 35.Kato A, Favoreto S Jr., Avila PC, and Schleimer RP. 2007. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 179: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nhu QM, Shirey K, Teijaro JR, Farber DL, Netzel-Arnett S, Antalis TM, Fasano A, and Vogel SN. 2010. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol 3: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Homma T, Kato A, Bhushan B, Norton JE, Suh LA, Carter RG, Gupta DS, and Schleimer RP. 2016. Role of Aspergillus fumigatus in Triggering Protease-Activated Receptor-2 in Airway Epithelial Cells and Skewing the Cells toward a T-helper 2 Bias. Am J Respir Cell Mol Biol 54: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon DB, Workman AD, Kohanski MA, Carey RM, Freund JR, Hariri BM, Chen B, Doghramji LJ, Adappa ND, Palmer JN, Kennedy DW, and Lee RJ. 2018. Protease-activated receptor 2 activates airway apical membrane chloride permeability and increases ciliary beating. FASEB J 32: 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noah TL, Paradiso AM, Madden MC, McKinnon KP, and Devlin RB. 1991. The response of a human bronchial epithelial cell line to histamine: intracellular calcium changes and extracellular release of inflammatory mediators. Am J Respir Cell Mol Biol 5: 484–492. [DOI] [PubMed] [Google Scholar]
- 40.Lazarowski ER, and Boucher RC. 2009. Purinergic receptors in airway epithelia. Curr Opin Pharmacol 9: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kountz TS, Jairaman A, Kountz CD, Stauderman KA, Schleimer RP, and Prakriya M. 2021. Differential Regulation of ATP- and UTP-Evoked Prostaglandin E2 and IL-6 Production from Human Airway Epithelial Cells. J Immunol. 207(5):1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jairaman A, Yamashita M, Schleimer RP, and Prakriya M. 2015. Store-Operated Ca2+ Release-Activated Ca2+ Channels Regulate PAR2-Activated Ca2+ Signaling and Cytokine Production in Airway Epithelial Cells. J Immunol 195: 2122–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma W, Korngreen A, Weil S, Cohen EB, Priel A, Kuzin L, and Silberberg SD. 2006. Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J Physiol 571: 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theatre E, Bours V, and Oury C. 2009. A P2X ion channel-triggered NF-kappaB pathway enhances TNF-alpha-induced IL-8 expression in airway epithelial cells. Am J Respir Cell Mol Biol 41: 705–713. [DOI] [PubMed] [Google Scholar]
- 45.von Kugelgen I, and Hoffmann K. 2016. Pharmacology and structure of P2Y receptors. Neuropharmacology 104: 50–61. [DOI] [PubMed] [Google Scholar]
- 46.Kemp PA, Sugar RA, and Jackson AD. 2004. Nucleotide-mediated mucin secretion from differentiated human bronchial epithelial cells. Am J Respir Cell Mol Biol 31: 446–455. [DOI] [PubMed] [Google Scholar]
- 47.Muller T, Myrtek D, Bayer H, Sorichter S, Schneider K, Zissel G, Norgauer J, and Idzko M. 2006. Functional characterization of histamine receptor subtypes in a human bronchial epithelial cell line. Int J Mol Med 18: 925–931. [PubMed] [Google Scholar]
- 48.Chow KT, Gale M Jr., and Loo YM. 2018. RIG-I and Other RNA Sensors in Antiviral Immunity. Annu Rev Immunol 36: 667–694. [DOI] [PubMed] [Google Scholar]
- 49.Niwa M, Fujisawa T, Mori K, Yamanaka K, Yasui H, Suzuki Y, Karayama M, Hozumi H, Furuhashi K, Enomoto N, Nakamura Y, Inui N, Suzuki T, Maekawa M, and Suda T. 2018. IL-17A Attenuates IFN-lambda Expression by Inducing Suppressor of Cytokine Signaling Expression in Airway Epithelium. J Immunol 201: 2392–2402. [DOI] [PubMed] [Google Scholar]
- 50.Williams DW, Askew LC, Jones E, and Clements JE. 2019. CCR2 Signaling Selectively Regulates IFN-alpha: Role of beta-Arrestin 2 in IFNAR1 Internalization. J Immunol 202: 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teijaro JR, Studer S, Leaf N, Kiosses WB, Nguyen N, Matsuki K, Negishi H, Taniguchi T, Oldstone MB, and Rosen H. 2016. S1PR1-mediated IFNAR1 degradation modulates plasmacytoid dendritic cell interferon-alpha autoamplification. Proc Natl Acad Sci U S A 113: 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivashkiv LB, and Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol 14: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K, and Kobori M. 2004. A novel Galphaq/11-selective inhibitor. J Biol Chem 279: 47438–47445. [DOI] [PubMed] [Google Scholar]
- 54.Shin A, Toy T, Rothenfusser S, Robson N, Vorac J, Dauer M, Stuplich M, Endres S, Cebon J, Maraskovsky E, and Schnurr M. 2008. P2Y receptor signaling regulates phenotype and IFN-alpha secretion of human plasmacytoid dendritic cells. Blood 111: 3062–3069. [DOI] [PubMed] [Google Scholar]
- 55.Ishikawa H, and Barber GN. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishikawa H, Ma Z, and Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461: 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, and Chen ZJ. 2013. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339: 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun L, Wu J, Du F, Chen X, and Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339: 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao P, Ascano M, Wu Y, Barchet W, Gaffney BL, Zillinger T, Serganov AA, Liu Y, Jones RA, Hartmann G, Tuschl T, and Patel DJ. 2013. Cyclic [G(2’,5’)pA(3’,5’)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 153: 1094–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Shu C, Yi G, Chaton CT, Shelton CL, Diao J, Zuo X, Kao CC, Herr AB, and Li P. 2013. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity 39: 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diner EJ, Burdette DL, Wilson SC, Monroe KM, Kellenberger CA, Hyodo M, Hayakawa Y, Hammond MC, and Vance RE. 2013. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep 3: 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brazee PL, Morales-Nebreda L, Magnani ND, Garcia JG, Misharin AV, Ridge KM, Budinger GRS, Iwai K, Dada LA, and Sznajder JI. 2020. Linear ubiquitin assembly complex regulates lung epithelial-driven responses during influenza infection. J Clin Invest 130: 1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu W, Zhang W, Duggan ES, Booth JL, Zou MH, and Metcalf JP. 2015. RIG-I and TLR3 are both required for maximum interferon induction by influenza virus in human lung alveolar epithelial cells. Virology 482: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, and Si-Tahar M. 2007. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol 178: 3368–3372. [DOI] [PubMed] [Google Scholar]
- 65.Liu G, Park HS, Pyo HM, Liu Q, and Zhou Y. 2015. Influenza A Virus Panhandle Structure Is Directly Involved in RIG-I Activation and Interferon Induction. J Virol 89: 6067–6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, and Tough DF. 2001. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14: 461–470. [DOI] [PubMed] [Google Scholar]
- 67.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, and Tough DF. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol 4: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 68.Esther CR Jr., Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Ribeiro CM, Moore CG, Davis SD, and Boucher RC. 2008. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J 31: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pritchard AL, Carroll ML, Burel JG, White OJ, Phipps S, and Upham JW. 2012. Innate IFNs and plasmacytoid dendritic cells constrain Th2 cytokine responses to rhinovirus: a regulatory mechanism with relevance to asthma. J Immunol 188: 5898–5905. [DOI] [PubMed] [Google Scholar]
- 70.Duerr CU, McCarthy CD, Mindt BC, Rubio M, Meli AP, Pothlichet J, Eva MM, Gauchat JF, Qureshi ST, Mazer BD, Mossman KL, Malo D, Gamero AM, Vidal SM, King IL, Sarfati M, and Fritz JH. 2016. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 17: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swieter M, Ghali WA, Rimmer C, and Befus D. 1989. Interferon-alpha/beta inhibits IgE-dependent histamine release from rat mast cells. Immunology 66: 606–610. [PMC free article] [PubMed] [Google Scholar]
- 72.Edwards MR, Strong K, Cameron A, Walton RP, Jackson DJ, and Johnston SL. 2017. Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness. J Allergy Clin Immunol 140: 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rich HE, Antos D, Melton NR, Alcorn JF, and Manni ML. 2020. Insights Into Type I and III Interferons in Asthma and Exacerbations. Frontiers in immunology 11: 574027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Djukanovic R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, Niven R, Singh D, Reddel HK, Davies DE, Marsden R, Boxall C, Dudley S, Plagnol V, Holgate ST, Monk P, and Group IS. 2014. The effect of inhaled IFN-beta on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med 190: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, Gabbay FJ, Davies DE, Holgate ST, Ho LP, Clark T, Djukanovic R, Wilkinson TMA, and Inhaled Interferon Beta C-SG. 2021. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir Med 9: 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazzoni A, Leifer CA, Mullen GE, Kennedy MN, Klinman DM, and Segal DM. 2003. Cutting edge: histamine inhibits IFN-alpha release from plasmacytoid dendritic cells. J Immunol 170: 2269–2273. [DOI] [PubMed] [Google Scholar]
- 77.Kalinowski A, Galen BT, Ueki IF, Sun Y, Mulenos A, Osafo-Addo A, Clark B, Joerns J, Liu W, Nadel JA, Dela Cruz CS, and Koff JL. 2018. Respiratory syncytial virus activates epidermal growth factor receptor to suppress interferon regulatory factor 1-dependent interferon-lambda and antiviral defense in airway epithelium. Mucosal Immunol 11: 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ueki IF, Min-Oo G, Kalinowski A, Ballon-Landa E, Lanier LL, Nadel JA, and Koff JL. 2013. Respiratory virus-induced EGFR activation suppresses IRF1-dependent interferon lambda and antiviral defense in airway epithelium. J Exp Med 210: 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moriwaki A, Matsumoto K, Matsunaga Y, Fukuyama S, Matsumoto T, Kan-o K, Noda N, Asai Y, Nakanishi Y, and Inoue H. 2011. IL-13 suppresses double-stranded RNA-induced IFN-lambda production in lung cells. Biochem Biophys Res Commun 404: 922–927. [DOI] [PubMed] [Google Scholar]
- 80.Bauer CM, Dewitte-Orr SJ, Hornby KR, Zavitz CC, Lichty BD, Stampfli MR, and Mossman KL. 2008. Cigarette smoke suppresses type I interferon-mediated antiviral immunity in lung fibroblast and epithelial cells. J Interferon Cytokine Res 28: 167–179. [DOI] [PubMed] [Google Scholar]
- 81.Eddleston J, Lee RU, Doerner AM, Herschbach J, and Zuraw BL. 2011. Cigarette smoke decreases innate responses of epithelial cells to rhinovirus infection. Am J Respir Cell Mol Biol 44: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Proud D, Hudy MH, Wiehler S, Zaheer RS, Amin MA, Pelikan JB, Tacon CE, Tonsaker TO, Walker BL, Kooi C, Traves SL, and Leigh R. 2012. Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PLoS One 7: e40762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu L, Lee B, Zhao F, Zhou X, Chin V, Ling SC, and Chen Y. 2014. Modulation of airway epithelial antiviral immunity by fungal exposure. Am J Respir Cell Mol Biol 50: 1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akbarshahi H, Menzel M, Ramu S, Mahmutovic Persson I, Bjermer L, and Uller L. 2018. House dust mite impairs antiviral response in asthma exacerbation models through its effects on TLR3. Allergy 73: 1053–1063. [DOI] [PubMed] [Google Scholar]
- 85.Bhushan B, Homma T, Norton JE, Sha Q, Siebert J, Gupta DS, Schroeder JW Jr., and Schleimer RP. 2015. Suppression of epithelial signal transducer and activator of transcription 1 activation by extracts of Aspergillus fumigatus. Am J Respir Cell Mol Biol 53: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ribeiro CM, Paradiso AM, Carew MA, Shears SB, and Boucher RC. 2005. Cystic fibrosis airway epithelial Ca2+ i signaling: the mechanism for the larger agonist-mediated Ca2+ i signals in human cystic fibrosis airway epithelia. J Biol Chem 280: 10202–10209. [DOI] [PubMed] [Google Scholar]
- 87.Paradiso AM, Mason SJ, Lazarowski ER, and Boucher RC. 1995. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature 377: 643–646. [DOI] [PubMed] [Google Scholar]
- 88.Paradiso AM, Ribeiro CM, and Boucher RC. 2001. Polarized signaling via purinoceptors in normal and cystic fibrosis airway epithelia. J Gen Physiol 117: 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clarke LL, Paradiso AM, and Boucher RC. 1992. Histamine-induced Cl- secretion in human nasal epithelium: responses of apical and basolateral membranes. Am J Physiol 263: C1190–1199. [DOI] [PubMed] [Google Scholar]
- 90.Kawashima T, Kosaka A, Yan H, Guo Z, Uchiyama R, Fukui R, Kaneko D, Kumagai Y, You DJ, Carreras J, Uematsu S, Jang MH, Takeuchi O, Kaisho T, Akira S, Miyake K, Tsutsui H, Saito T, Nishimura I, and Tsuji NM. 2013. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-beta. Immunity 38: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 91.Paller AS, Spergel JM, Mina-Osorio P, and Irvine AD. 2019. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J Allergy Clin Immunol 143: 46–55. [DOI] [PubMed] [Google Scholar]
- 92.Iikura Y, Naspitz CK, Mikawa H, Talaricoficho S, Baba M, Sole D, and Nishima S. 1992. Prevention of asthma by ketotifen in infants with atopic dermatitis. Ann Allergy 68: 233–236. [PubMed] [Google Scholar]
- 93.Carlsen KH 2004. Therapeutic strategies for allergic airways diseases. Paediatr Respir Rev 5: 45–51. [DOI] [PubMed] [Google Scholar]
- 94.Graham AC, Hilmer KM, Zickovich JM, and Obar JJ. 2013. Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling. J Immunol 190: 4676–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee IH, Kim HS, and Seo SH. 2017. Porcine mast cells infected with H1N1 influenza virus release histamine and inflammatory cytokines and chemokines. Arch Virol 162: 1067–1071. [DOI] [PubMed] [Google Scholar]
- 96.Jairaman A, Maguire CH, Schleimer RP, and Prakriya M. 2016. Allergens stimulate store-operated calcium entry and cytokine production in airway epithelial cells. Sci Rep 6: 32311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the manuscript and the supporting files.