IMPORTANCE:
Pediatric ventilation liberation has limited evidence, likely resulting in wide practice variation. To inform future work, practice patterns must first be described.
OBJECTIVES:
Describe international pediatric ventilation liberation practices and regional practice variation.
DESIGN, SETTING, AND PARTICIPANTS:
International cross-sectional electronic survey. Nontrainee pediatric medical and cardiac critical care physicians.
MAIN OUTCOMES AND MEASURES:
Practices focusing on spontaneous breathing trial (SBT) eligibility, SBT practice, non-SBT extubation readiness bundle elements, and post-extubation respiratory support.
RESULTS:
Five-hundred fifty-five responses representing 47 countries were analyzed. Most respondents reported weaning followed by an SBT (86.4%). The top SBT eligibility variables reported were positive end-expiratory pressure (95%), Fio2 (93.4%), and peak inspiratory pressure (73.9%). Most reported use of standardized pressure support regardless of endotracheal tube size (40.4%) with +10 cm H2O predominating (38.6%). SBT durations included less than or equal to 30 minutes (34.8%), 31 minutes to 1 hour (39.3%), and greater than 1 hours (26%). In assigning an SBT result, top variables were respiratory rate (94%), oxygen saturation (89.3%), and subjective work of breathing (79.8%). Most reported frequent consideration of endotracheal secretion burden (81.3%), standardized pain/sedation measurement (72.8%), fluid balance (83%), and the endotracheal air leak test as a part of extubation readiness bundles. Most reported using planned high flow nasal cannula in less than or equal to 50% of extubations (83.2%). Top subpopulations supported with planned HFNC were those with chronic lung disease (67.3%), exposed to invasive ventilation greater than 14 days (66.6%), and chronic critical illness (44.9%). Most reported using planned noninvasive ventilation (NIV) following less than or equal to 20% of extubations (79.9%). Top subpopulations supported with planned NIV were those with neuromuscular disease (72.8%), chronic lung disease (66.7%), and chronic NIV use for any reason (61.6%). Regional variation was high for most practices studied.
CONCLUSION AND RELEVANCE:
International pediatric ventilation liberation practices are heterogeneous. Future study is needed to address key evidence gaps. Many practice differences were associated with respondent region, which must be considered in international study design.
Keywords: clinical pathway, extubation, mechanical ventilation, pediatrics, pediatric intensive care unit, respiratory therapy
Critically ill children are frequently supported with invasive mechanical ventilation (IMV) (1, 2). Practitioners attempt to minimize the complications by limiting IMV duration through timely ventilator liberation, while trying to avoid extubation failure, which is also associated with adverse outcomes (3–8). Clinicians often consider a battery of elements and tests to help predict extubation success. These are collectively referred to as extubation readiness testing (ERT) bundles which often include a spontaneous breathing trial (SBT). The ERT is central to the “B” component of the Society for Critical Care Medicine’s ICU Liberation Bundle (9, 10). Although pediatric controlled data are currently limited, a ventilation liberation strategy as part of a broader ICU liberation bundle may improve outcomes for critically ill children requiring IMV (11).
ERTs and SBTs have been implemented with varied strategies and success (11–16). Because there are limited controlled data and few clinical practice guidelines, there is likely wide practice variation in ventilation liberation strategies (17–20). Understanding the landscape of current practice with regards to ventilator liberation strategies is crucial to shape future research. The primary aim of this study was to describe international pediatric ventilation liberation practices at the level of individual practitioner. Areas of focus included SBT eligibility, SBT practice, non-SBT ERT bundle elements, and postextubation respiratory support. The secondary aims included the following: 1) investigating regional practice variation and 2) identifying leverage points and potential areas of equipoise for future quality improvement and clinical trials.
MATERIALS AND METHODS
Survey Development, Ethics, and Distribution
Development of this survey has been described previously (21). In summary, a pediatric ventilation liberation practice survey was developed and reviewed by national and international pediatric critical care experts. Unit-level and practitioner-level ventilation liberation practice patterns were queried guided by 15 core research questions (Supplemental Table 1, http://links.lww.com/CCX/B55) related to pediatric ventilator liberation. ERT bundle elements queried included practices relevant to SBT eligibility screening, SBT practice, non-SBT elements used in ERT bundles, and postextubation respiratory support practices. The survey was developed in English and then translated into Spanish and Portuguese. Within the user interface, respondents were not forced to provide answers making omission possible. The survey was developed and distributed using the Qualtrics platform (Qualtrics LLC, Provo, UT). Only pediatric critical care attending physician respondents were solicited. Trainees and other licensed independent providers were excluded. Respondents were asked to consider patients requiring IMV for more than 24 hours and without cyanotic heart disease. Informed consent requirement was waived following review by the institutional review board (IRB) at the University of Alabama at Birmingham (Title: International Extubation Practice Survey, Approval Date: June 8, 2021, IRB no. 300007311). Procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as most recently amended.
Participation was solicited using two e-mails separated by 2–4 weeks. Solicitations were supported by multiple international critical care organizations (21). The survey was also promoted by one of the authors on social media using a link to the survey (Twitter, San Francisco, CA). All surveys were completed between June and August 2021. The survey distribution strategy precluded calculation of number solicited and response rate.
Outcomes, Data Definitions, and Unique Respondent Identification
The primary outcome was international pediatric ventilation liberation practices at the level of individual practitioner with a focus on SBT eligibility, SBT practice, non-SBT ERT bundle elements, and postextubation respiratory support. The secondary outcome was regional practice variation. A protocol was defined as a mutually agreed upon and documented clinical pathway or approach that standardizes work for the majority of patients in the PICU. Extubation failure was defined as replacement of an endotracheal tube for any reason other than a planned procedure within 48 hours of a planned extubation attempt. A large academic ICU was defined as having more than 1,000 annual admissions and at least one type of physician trainee on the prescriber team. Noninvasive ventilation (NIV) included both continuous and bilevel positive airway pressure. High-flow nasal cannula (HFNC) was considered separate from NIV.
Responses without answers to nondemographic questions were excluded followed by identification of high-risk potential duplicate responses. This was determined by comparing identical responses with all of the following: hospital name, hospital city, hospital country, length of clinical experience, percent clinical practice time, PICU type, and division chief/medical director status. Where identified, the response with the fewest answers was excluded unless responses were equal and then the second response was excluded. Remaining responses were classified as unique practitioner responses.
Statistical Analysis
Descriptive analyses included medians with interquartile ranges and frequency distributions. Comparisons employed the chi-square, Fisher exact, Mann-Whitney U, or Student’s t tests as appropriate. As the focus study was descriptive analysis and hypothesis generation, no control for multiple comparisons in univariate analyses occurred. Questions without answers or with “don’t know/not sure” selected were excluded as missing data where applicable. Responses to each variable presented are reported to provide context for percentages. A p value of less than 0.05 was used to indicate statistical significance. All analyses used SPSS (Version 25; IBM, Armonk, NY).
RESULTS
Survey Respondents
There were 709 responses with 151 excluded for only demographic questions answered and three excluded due to high-risk of potential duplicate response. Therefore, 555 responses were included for analysis with 83.6% of respondents completing the entire survey (Supplemental Fig. 1, http://links.lww.com/CCX/B55). Descriptive characteristics for all respondents with stratification by region are shown in Table 1. Among the 47 different countries represented, most responses were from the United States (23.6%), Brazil (17.3%), and Argentina (8.6%). Most respondents reported more than 5 years of clinical experience (78.4%) and did not practice in a large academic ICU (77%). In the cohort, 29.5% self-identified as either the unit medical director or division chief.
TABLE 1.
Descriptive Statistics for All Individual Practitioner Respondents and Stratified by Region
| Variables | All Respondents | South America, Central America, and Mexico | United States and Canada | Europe | Asia | Australia and New Zealand | Middle East, Africa, and Caribbean |
|---|---|---|---|---|---|---|---|
| Total responses, n (%) | 555 (100) | 251 (45.2) | 140 (25.2) | 89 (16) | 41 (7.4) | 11 (2) | 23 (4.1) |
| Duration of clinical practice, %, yr | |||||||
| ≤ 5 | 21.6 | 18.7 | 24.3 | 21.3 | 29.3 | 18.2 | 26.1 |
| 6–10 | 22.3 | 23.1 | 25 | 12.4 | 24.4 | 9.1 | 39.1 |
| 11–15 | 19.5 | 19.9 | 18.6 | 21.3 | 17.1 | 18.2 | 17.4 |
| 16–20 | 13.2 | 13.9 | 8.6 | 15.7 | 17.1 | 36.4 | 4.3 |
| > 20 | 23.4 | 24.3 | 23.6 | 29.2 | 12.2 | 18.2 | 13 |
| Clinical care percent time, %a | |||||||
| < 25 | 3.6 | 2.4 | 5.7 | 2.2 | 4.9 | 0 | 8.7 |
| 25–49 | 14.6 | 10 | 23.6 | 11.2 | 19.5 | 18.2 | 13 |
| 50–74 | 25.8 | 23.1 | 33.6 | 18 | 34.1 | 54.5 | 8.7 |
| 75–100 | 56 | 64.5 | 37.1 | 68.5 | 41.5 | 27.3 | 69.6 |
| PICU type, %b | |||||||
| General medical/surgical | 43.8 | 46.6 | 49.3 | 43.8 | 26.8 | 9.1 | 26.1 |
| Mixed medical/surgical/cardiac | 44.7 | 40.2 | 40 | 47.2 | 58.5 | 90.9 | 65.2 |
| Medical only | 7.2 | 9.6 | 5 | 4.5 | 12.2 | 0 | 0 |
| Cardiac only | 3.8 | 2.8 | 5.7 | 4.5 | 2.4 | 0 | 4.3 |
| Other | 0.5 | 0.8 | 0 | 0 | 0 | 0 | 4.3 |
| Practice in large academic PICU, %a | 84.7 | 77.7 | 83.6 | 96.6 | 95.1 | 90.9 | 100 |
| 23 | 5.1 | 68.4 | 12.8 | 12.8 | 20 | 0 | |
| Median maximum patient capacity (IQR)a | 15 (10–21) | 10 (8–16) | 23 (18–32) | 15 (10–20) | 12 (8–18) | 22 (13–27) | 15 (8–24) |
| Median physician to patient ratio (IQR)a | 96.9 | 98.4 | 97.9 | 96.7 | 100 | 100 | 73.9 |
| 1:8 (1:6–1:12) | 1:7 (1:5–1:10) | 1:12 (1:10–1:15) | 1:8 (1:5–1:13) | 1:7 (1:4–1:10) | 1:12 (1:6–1:15) | 1:11 (1:7–1:16) | |
| Prescriber team composition, % | |||||||
| General pediatric traineesa | 73.3 | 59.4 | 88.6 | 83.1 | 78 | 81.8 | 82.6 |
| Pediatric critical care traineesa | 59.6 | 47.8 | 77.9 | 70.8 | 46.3 | 9 | 47.8 |
| Nonphysician licensed independent providersa | 54.2 | 63.3 | 80.7 | 25.8 | 12.2 | 0 | 4.3 |
| None of the above | 8.3 | 10 | 3.6 | 9 | 14.6 | 9.1 | 4.3 |
| Dedicated respiratory therapista | 63.6 | 71.3 | 86.4 | 40.4 | 22 | 9.1 | 30.4 |
| Average annual PICU admissions, %a | 84.7 | 77.7 | 83.6 | 96.6 | 95.1 | 90.9 | 100 |
| < 500 | 44.3 | 67.7 | 6.8 | 37.2 | 53.8 | 20 | 56.5 |
| 500–1,000 | 30.6 | 24.6 | 23.1 | 48.8 | 33.3 | 50 | 39.1 |
| 1,001–2,000 | 20.4 | 7.2 | 53.8 | 12.8 | 12.8 | 30 | 0 |
| > 2,000 | 4.7 | 0.5 | 16.2 | 1.2 | 0 | 0 | 4.3 |
| Median invasive ventilation admits, % (IQR)a | 71.9 | 71.7 | 58.6 | 82 | 85.4 | 81.8 | 87 |
| 35 (23-55) | 40 (21.3-57) | 25 (20–33) | 50 (30-60) | 50 (30-60) | 30 (20-45.5) | 40 (31-59.5) | |
| PICU noninvasive support resource, % | |||||||
| High-flow nasal cannulaa | 87.4 | 76.9 | 100 | 97.8 | 82.9 | 100 | 87 |
| Noninvasive positive pressurea | 96.4 | 95.6 | 100 | 98.9 | 90.2 | 100 | 82.6 |
| None of the above | 0.9 | 1.2 | 0 | 0 | 2.4 | 0 | 4.3 |
IQR = interquartile range.
Where response rates were not 100% for each column variable, rates are shown (as a percentage of total responses in row 2) in italics (%). Statistical comparisons at the region level were insignificant unless annotated in column 1 as follows:
ap < 0.001,
bp = 0.008.
Weaning Strategy and SBT Eligibility
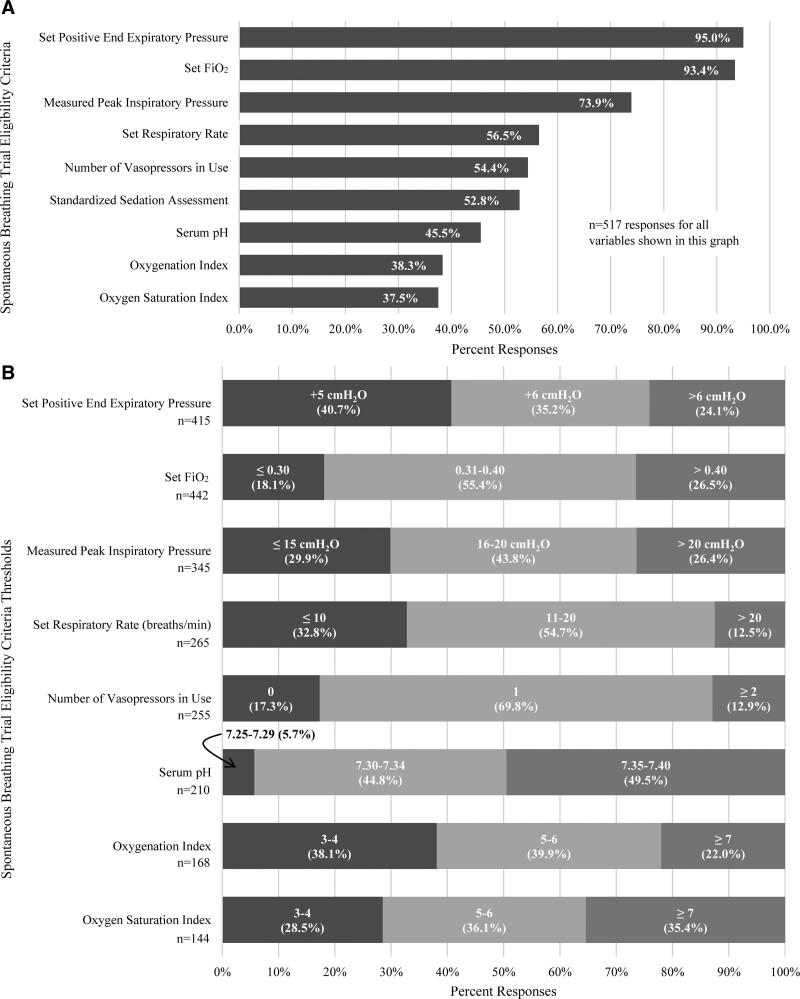
Most respondents (86.4%) reported weaning followed by an either a protocolized or nonprotocolized SBT compared with 13.5% weaning based on clinical impression without an SBT. A protocolized SBT approach was most prevalent in the South/Central America/Mexico as well as the Middle East/Africa/Caribbean regions (Supplemental Table 2, http://links.lww.com/CCX/B55). The most common self-reported SBT eligibility variables included positive end-expiratory pressure (PEEP; 95%), Fio2 (93.4%), and peak inspiratory pressure (73.9%) (Fig. 1). The most common eligibility thresholds were PEEP less than or equal to 6 (75.9%), Fio2 less than or equal to 40% (73.5%), and peak inspiratory pressure less than or equal to 20 (73.7%). There were statistically significant regional differences in the prevalence of each eligibility variable except for PEEP and vasopressor support (Supplemental Fig. 2, http://links.lww.com/CCX/B55).
Figure 1.
Self-reported use of selected criteria for spontaneous trial eligibility: A, Frequency each variable was selected by respondents; B, specific cut-off values self-reported by respondents (note: threshold values are reported within each bar alongside percent responses. Only respondents that selected a given variable were asked to indicate a specific cut-off value. Respondents were not forced to provide a specific cut-off value). Total responses are reported below the column label for each variable.
SBT Practice
Among those that reported using an SBT with any frequency, most used PEEP with a standardized pressure support (PS) regardless of endotracheal tube size (40.4%). The PS levels most commonly reported were +10 (38.6%), +5 (22.9%), and +6 cm H2O (12.1%; range +5 to +15 cm H2O). The second most common approach was PEEP with PS based on endotracheal tube size (39.8%). PEEP with no PS and T-piece were selected by 8.9% and 8.7%, respectively. Reported SBT durations were as follows: less than or equal to 30 minutes (34.8%), 31 minutes to 1 hour (39.3%), and greater than 1 hours (26%). SBT approaches using any amount of PS were associated with an SBT duration greater than 1 hour compared with those using T-piece or Continuous Positive Airway Pressure (CPAP) with no PS (odds ratio, 2.1; 95% CI 1.1–3.9; p = 0.021). Although SBT with any PS was most prevalent in all regions, the Asia and South/Central America/Mexico regions had a comparatively higher reported use of T-piece or no PS during SBTs (Supplemental Table 2, http://links.lww.com/CCX/B55).
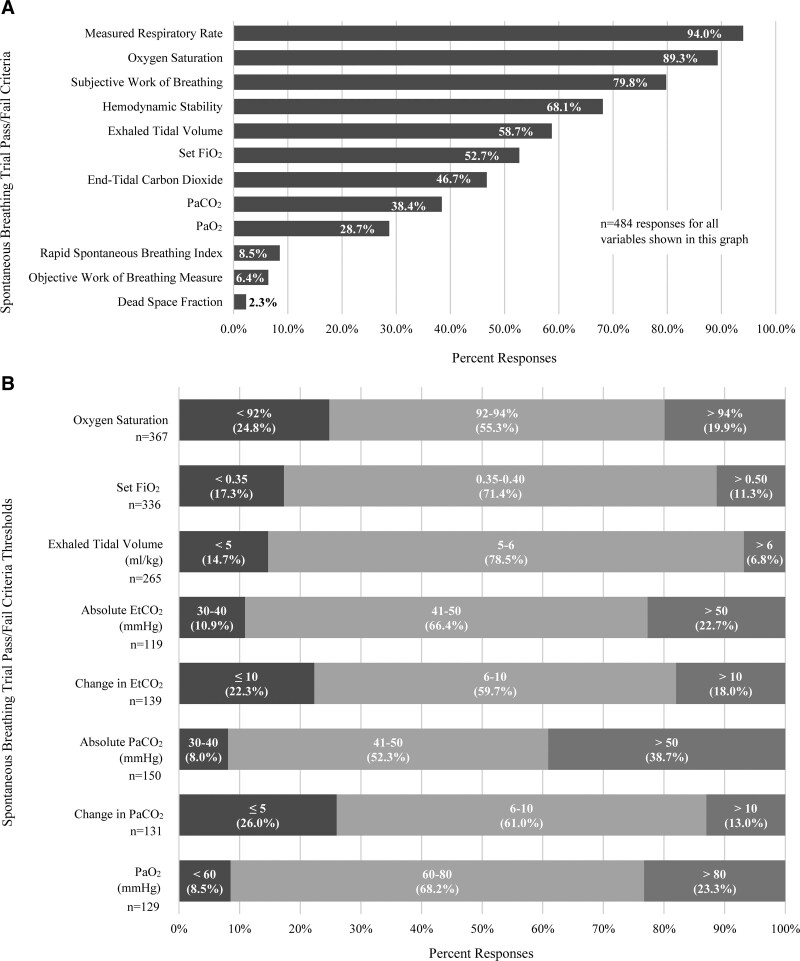
In assigning an SBT result, the most prevalent self-reported variables were respiratory rate (94%), oxygen saturation (89.3%), and subjective work of breathing (79.8%) (Fig. 2). Among those that reported using respiratory rate, 40.9% used a percent change from pre-SBT, values and 40.1% used any consistent values above age specific norms. Most endorsed targeting oxygen saturations greater than or equal to 92% (75.2%) with a set Fio2 less than or equal to 50% (88.7%) (Fig. 2). Respiratory rate was the most commonly reported variable used for all regions except in Australia/New Zealand (Supplemental Fig. 3, http://links.lww.com/CCX/B55).
Figure 2.
Self-reported use of selected criteria for spontaneous trial pass or fail: A, Frequency each variable was selected by respondents; B, specific cut-off values self-reported by respondents (note: threshold values are reported within each bar alongside percent responses. Only respondents that selected a given variable were asked to indicate a specific cut-off value. Respondents were not forced to provide a specific cut-off value). Total responses are reported below the column label for each variable. Etco2 = end-tidal carbon dioxide (EtCO2).
Other Components of ERT and Practice
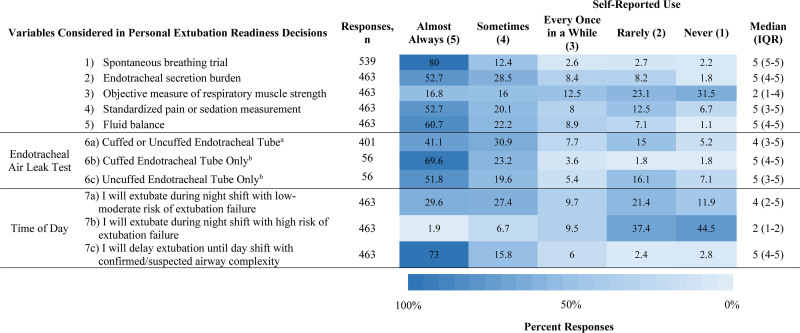
In addition to an SBT, most reported “almost always” or “sometimes” using endotracheal secretion burden (81.3%), standardized pain/sedation measurement (72.8%), fluid balance (83%), and the endotracheal air leak test (see below) as a part of ERT (Fig. 3). Only SBT use demonstrated significant regional variation (Supplemental Fig. 4, http://links.lww.com/CCX/B55). Most reported identical practice regardless of if the endotracheal tube was cuffed or uncuffed (87.6%) with 73% of that group “almost always” or “sometimes” using the endotracheal leak test. Regarding corticosteroid prescription to prevent postextubation upper airway obstruction, 62.7% prescribed them only for self-perceived high-risk groups. Based on self-perceived risk of extubation failure, respondents were more willing to extubate low-moderate risk patients at night when compared with those at high risk (Fig. 3). However, 31.3% of all respondents “rarely” or “never” extubate low-moderate risk patients at night.
Figure 3.
Heat map depicting selected components of extubation readiness testing and responses regarding inclusion in personal practice. aIncluding only respondents who reported managing cuffed and uncuffed endotracheal tubes the same. bIncluding only respondents who reported different management based on endotracheal tube type. IQR = interquartile range.
Postextubation Respiratory Support Practice
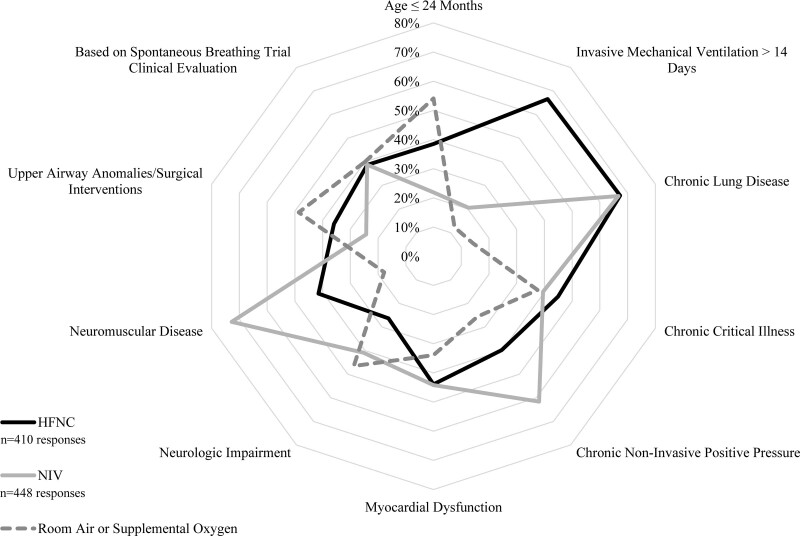
Most respondents (83.2%) reported using planned HFNC in less than or equal to 50% of extubations. The most common patient subpopulations supported with planned HFNC were those with chronic lung disease (67.3%), exposed to IMV greater than 14 days (66.6%), and chronic critical illness (44.9%) (Fig. 4). Respondents most commonly endorsed planned NIV use following less than or equal to 20% of planned extubations (79.9%). The most common patient subpopulations supported with planned NIV were those with neuromuscular disease (72.8%), chronic lung disease (66.7%), and chronic NIV use for any reason (61.6%) (Fig. 4). The most common patient subpopulations extubated to lower levels of planned respiratory support (room air or supplemental oxygen) were those less than or equal to 24 months old (54.1%), with upper airway anomalies/surgical interventions (48.6%) and with neurologic impairment (46.5%) (Fig. 4). There was no statistically significant difference in regional practice regarding postextubation respiratory support (Supplemental Table 2, http://links.lww.com/CCX/B55).
Figure 4.
Self-reported practice patterns regarding subpopulations most likely to receive planned postextubation support with high-flow nasal cannula (HFNC), noninvasive ventilation (NIV), or neither modality. Respondents were asked to select all subpopulations for which they are most likely to routinely support with HFNC or NIV separately. Where a single respondent did not select either for a given population, most likely extubation to room air or supplemental oxygen was presumed. Axis represents percentage selected by respondents.
Opinions and Perspectives
Practitioner opinions and perspectives regarding selected topics relevant to pediatric ventilation liberation are shown in Supplemental Figure 5 (http://links.lww.com/CCX/B55). A majority agreed that children requiring IMV for more than 24 hours should have protocolized screening for SBT eligibility (70.8%), have protocolized ERT (76.7%), and have an SBT included in the ERT bundle (77.3%). Meanwhile, most respondents “neither agree nor disagree” that NIV is superior to HFNC for children extubated to planned respiratory support (44.3%). Most disagreed that unplanned escalation to NIV should be considered extubation failure (66%).
DISCUSSION
This international cross-sectional survey of 555 pediatric intensivists across 47 countries demonstrated heterogeneous pediatric ventilation liberation practices. Practice variation specifically relating to ERT eligibility determination, use of PS during an SBT, SBT pass criteria, SBT duration, and postextubation respiratory support represented specific areas where available evidence can be leveraged in multicenter quality improvement implementation studies. Further, some areas of relative equipoise signaled an opportunity for pragmatic randomized control clinical trials to address key evidence gaps. Practices were often associated with region, an association that is likely multifactorial including resource differences and limitations, cultural differences, and center-specific biases. These differences may indicate the need for differential approaches to clinical trials and quality improvement work relating to pediatric ventilation liberation within each region described.
Weaning IMV begins with practitioner suspicion that the primary pathologic process is improving and the patient is nearing extubation candidacy (22). This period of diagnostic triggering, based predominately on practitioner intuition, represents a potential for delay leading to unnecessarily prolonged IMV. Multidisciplinary collaboration at this point, enhanced by protocols, may lead to goal-directed weaning and shorter duration of IMV and lower extubation failure rates (11, 13, 23). Protocols foster a shared mental model that may mitigate the negative influence of the complex PICU environment where attention may be triaged away from the weaning patient (14). Protocol use was generally supported by practitioners in this survey. In contrast, previously reported unit level data from this survey demonstrated generally low adoption of ventilation liberation protocols internationally (21). The ERT eligibility variables and thresholds reported in this study provide a starting point for an ERT screening protocol. Selection of screening variables and corresponding thresholds with self-reported use above 50% likely represents a feasible starting point. Such variables have excellent external validity as they are widely available and only require the patient-ventilator circuit. Prospective implementation and monitoring of sensitivity for extubation readiness must inform data-driven threshold adjustments to improve positive predictive value.
The highly prevalent use of PS during SBTs contrasts with physiologic evidence that PS use underestimates postextubation work of breathing (24–29). Roughly the same percentage of practitioners reported using standardized PS compared with PS inversely related to endotracheal tube diameter. Therefore, although PS use is highly prevalent, there is relative equipoise about what PS is ideal. Lower PS may more accurately predict postextubation respiratory work. However, extubation decisions are triggered partly by the clinical impression of SBT tolerance. Both measured respiratory rate and subjective work of breathing were among the top self-reported factors considered for SBT passage. PS would improve both metrics for most patients. Therefore, it is plausible to hypothesize that a moderate level of PS during an SBT may encourage earlier extubation without sacrificing positive predictive value for extubation success. This could negatively impact postextubation support approaches leading to increased use of rescue therapy. A minority indicated that planned use of HFNC or NIV changed based on clinical evaluation during an SBT. Therefore, it is unlikely that such an SBT approach would alter planned postextubation support.
An SBT aims to predict readiness to accept the respiratory workload with SBT duration used to predict stamina. SBT duration also represents an evidence gap as well as an area of significant practice variation. Responses fell into three roughly equivalent groups; less than or equal to 30 minutes, 31 minutes to 1 hour, and greater than 1 hour. Unsurprisingly, SBT duration and level of support (PS representing higher support) during an SBT were directly correlated. It is plausible that practitioners use shorter SBT durations with modalities such as T-piece due to perceived patient comfort and sensitivity for postextubation work of breathing. Secondarily, PS may positively influence stamina leading to a more delayed SBT failure in marginal patients. This could necessitate a longer duration to preserve predictive utility for extubation outcome. However, there is no evidence to support a specific approach in the pediatric population. This is also debated in the adult population with evidence suggesting equivalence between 30-minute and 2-hour SBT durations (30, 31). A pragmatic evaluation of which SBT support mode and duration combination appropriately balances IMV duration and extubation failure is needed in pediatric ventilation liberation.
Pediatric HFNC use is increasing due to ease of use and better patient comfort relative to NIV (32, 33). A recent randomized controlled trial failed to demonstrate noninferiority of HFNC to CPAP for duration of postextubation respiratory support (34). This study had a preponderance of infants, which highlights potential differences in risk benefit profiles for CPAP versus HFNC as a function of age. Interestingly, most cases of NIV/HFNC after extubation were planned, although subgroup analysis did not identify major differences in the benefits of CPAP over HFNC between planned and unplanned use. This remains an important area for future investigation, given the clinical preference for HFNC due to patient comfort, with evidence from controlled trials that CPAP may be superior. Hence, identifying the patients at highest risk of extubation failure who may benefit from postextubation respiratory support is an important goal.
A key strength of this study is the sample size and regional representation. This study has several limitations. First, despite efforts to accurately identify unique respondents and eliminate duplicate responses, complete accuracy cannot be assured. Second, these data represent self-reported practices introducing recall bias. Third, selection bias of respondents may exist despite an effort for a broad distribution strategy. Finally, there are many unmeasured confounders particularly related to illness severity and case mix that may impacted the self-reported practice patterns. As a future direction, these results can be further evaluated in contrast with the upcoming ventilation liberation guidelines (R13HD102137). Where practices dramatically differ from the guidelines, multicenter quality improvement can be undertaken.
CONCLUSIONS
International pediatric ventilation liberation practices are heterogeneous. Multicenter quality improvement and clinical trials are needed to address key evidence gaps as well as limit unwarranted practice variation. These key areas included SBT eligibility determination, use of PS during an SBT, SBT duration, SBT pass criteria, and postextubation respiratory support. Many practice differences were associated with respondent region, which must be considered in future study.
ACKNOWLEDGMENTS
We would like to acknowledge the following organizations for their assistance in survey solicitation and support: Australia and New Zealand Intensive Care Society, Brazilian Research Network in Pediatric Intensive Care, European Society of Pediatric and Neonatal Intensive Care, Groupe Francophone de Réanimation et d’Urgences Pédiatricques, Latin American Pediatric Collaborative, Pediatric Acute and Critical Care Medicine Asian Network, Pediatric Acute Lung Injury and Sepsis Investigators, Sociedad Latino Americana de Cuidados Intensivos Pediatricos, and the World Federation of Pediatric Intensive and Critical Care Societies. We would also like to acknowledge Priya Prabhakaran, MD, for her mentorship as well as feedback on the survey and final article.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Khemani and Abu-Sultaneh disclose using National Institutes of Health (NIH) funding to cover publication costs (1 R13 HD102137-01; principal investigator: Drs. Khemani and Abu-Sultaneh; NIH/National Institute of Child Health and Human Development (NICHD)/National Heart, Lung, and Blood Institute (NHLBI)).
This work was performed at University of Alabama at Birmingham.
REFERENCES
- 1.Khemani RG, Markovitz BP, Curely MAQ. Epidemiologic factors of mechanically ventilated PICU patients in the United States. Pediatr Crit Care Med 2007; 8:A39 [Google Scholar]
- 2.Heneghan JA, Reeder RW, Dean JM, et al. : Characteristics and outcomes of critical illness in children with feeding and respiratory technology dependence. Pediatr Crit Care Med 2019; 20:417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivera R, Tibballs J: Complications of endotracheal intubation and mechanical ventilation in infants and children. Crit Care Med 1992; 20:193–199 [DOI] [PubMed] [Google Scholar]
- 4.Glau CL, Conlon TW, Himebauch AS, et al. : Progressive diaphragm atrophy in pediatric acute respiratory failure. Pediatr Crit Care Med 2018; 19:406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RW, Ng KWP, Dietz AR, et al. : Muscle atrophy in mechanically-ventilated critically ill children. PLoS One 2018; 13:e0207720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurachek SC, Newth CJ, Quasney MW, et al. : Extubation failure in pediatric intensive care: A multiple-center study of risk factors and outcomes. Crit Care Med 2003; 31:2657–2664 [DOI] [PubMed] [Google Scholar]
- 7.Farias JA, Retta A, Alía I, et al. : A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med 2001; 27:1649–1654 [DOI] [PubMed] [Google Scholar]
- 8.Silva-Cruz AL, Velarde-Jacay K, Carreazo NY, et al. : Risk factors for extubation failure in the intensive care unit. Rev Bras Ter Intensiva 2018; 30:294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pun BT, Balas MC, Barnes-Daly MA, et al. : Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019; 47:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choong K, Abu-Sultaneh S: Applying the ICU liberation bundle to critically ill children. In: Critical Connections: The Complete News Source for Critical Care Professionals. Mount Prospect, IL, Society of Critical Care Medicine, 2020, Vol. 1, pp 2–3 [Google Scholar]
- 11.Blackwood B, Tume LN, Morris KP, et al. ; SANDWICH Collaborators: Effect of a sedation and ventilator liberation protocol vs usual care on duration of invasive mechanical ventilation in pediatric intensive care units: A randomized clinical trial. JAMA 2021; 326:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foronda FK, Troster EJ, Farias JA, et al. : The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: A randomized controlled trial. Crit Care Med 2011; 39:2526–2533 [DOI] [PubMed] [Google Scholar]
- 13.Abu-Sultaneh S, Hole AJ, Tori AJ, et al. : An interprofessional quality improvement initiative to standardize pediatric extubation readiness assessment. Pediatr Crit Care Med 2017; 18:e463–e471 [DOI] [PubMed] [Google Scholar]
- 14.Loberger JM, Jones RM, Prabhakaran P: A respiratory therapist-driven pathway improves timeliness of extubation readiness assessment in a single PICU. Pediatr Crit Care Med 2020; 21:e513–e521 [DOI] [PubMed] [Google Scholar]
- 15.Randolph AG, Wypij D, Venkataraman ST, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: A randomized controlled trial. JAMA 2002; 288:2561–2568 [DOI] [PubMed] [Google Scholar]
- 16.Krawiec C, Carl D, Stetter C, et al. : Challenges with implementation of a respiratory therapist-driven protocol of spontaneous breathing trials in the pediatric ICU. Respir Care 2017; 62:1233–1240 [DOI] [PubMed] [Google Scholar]
- 17.Newth CJ, Hotz JC, Khemani RG: Ventilator liberation in the pediatric ICU. Respir Care 2020; 65:1601–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newth CJ, Venkataraman S, Willson DF, et al. ; Eunice Shriver Kennedy National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med 2009; 10:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mhanna MJ, Anderson IM, Iyer NP, et al. : The use of extubation readiness parameters: A survey of pediatric critical care physicians. Respir Care 2014; 59:334–339 [DOI] [PubMed] [Google Scholar]
- 20.Krasinkiewicz JM, Friedman ML, Slaven JE, et al. : Extubation readiness practices and barriers to extubation in pediatric subjects. Respir Care 2021; 66:582–590 [DOI] [PubMed] [Google Scholar]
- 21.Loberger JM, Campbell CM, Colleti J, Jr, et al. : Ventilation liberation practices among 380 international PICUs. Crit Care Explor 2022; 4:e0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobin MJ, Jubran A: Weaning from mechanical ventilation. In: Principles and Practice of Mechanical Ventilation. Third Edition. Tobin MJ. (Ed). New York, NY, McGraw Hill, 2013. pp 1307–1351 [Google Scholar]
- 23.Rose L, Blackwood B, Egerod I, et al. : Decisional responsibility for mechanical ventilation and weaning: An international survey. Crit Care 2011; 15:R295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bock KR, Silver P, Rom M, et al. : Reduction in tracheal lumen due to endotracheal intubation and its calculated clinical significance. Chest 2000; 118:468–472 [DOI] [PubMed] [Google Scholar]
- 25.Willis BC, Graham AS, Yoon E, et al. : Pressure-rate products and phase angles in children on minimal support ventilation and after extubation. Intensive Care Med 2005; 31:1700–1705 [DOI] [PubMed] [Google Scholar]
- 26.Keidan I, Fine GF, Kagawa T, et al. : Work of breathing during spontaneous ventilation in anesthetized children: A comparative study among the face mask, laryngeal mask airway and endotracheal tube. Anesth Analg 2000; 91:1381–1388 [DOI] [PubMed] [Google Scholar]
- 27.Argent AC, Newth CJ, Klein M: The mechanics of breathing in children with acute severe croup. Intensive Care Med 2008; 34:324–332 [DOI] [PubMed] [Google Scholar]
- 28.Ferguson LP, Walsh BK, Munhall D, et al. : A spontaneous breathing trial with pressure support overestimates readiness for extubation in children. Pediatr Crit Care Med 2011; 12:e330–e335 [DOI] [PubMed] [Google Scholar]
- 29.Khemani RG, Hotz J, Morzov R, et al. : Pediatric extubation readiness tests should not use pressure support. Intensive Care Med 2016; 42:1214–1222 [DOI] [PubMed] [Google Scholar]
- 30.Perren A, Domenighetti G, Mauri S, et al. : Protocol-directed weaning from mechanical ventilation: Clinical outcome in patients randomized for a 30-min or 120-min trial with pressure support ventilation. Intensive Care Med 2002; 28:1058–1063 [DOI] [PubMed] [Google Scholar]
- 31.Esteban A, Alía I, Gordo F, et al. : Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med 1997; 156:459–465 [DOI] [PubMed] [Google Scholar]
- 32.Coletti KD, Bagdure DN, Walker LK, et al. : High-flow nasal cannula utilization in pediatric critical care. Respir Care 2017; 62:1023–1029 [DOI] [PubMed] [Google Scholar]
- 33.Ramnarayan P, Schibler A: Glass half empty or half full? The story of high-flow nasal cannula therapy in critically ill children. Intensive Care Med 2017; 43:246–249 [DOI] [PubMed] [Google Scholar]
- 34.Ramnarayan P, Richards-Belle A, Drikite L, et al. ; FIRST-ABC Step-Up RCT Investigators and the Paediatric Critical Care Society Study Group: Effect of high-flow nasal cannula therapy vs continuous positive airway pressure therapy on liberation from respiratory support in acutely ill children admitted to pediatric critical care units: A randomized clinical trial. JAMA 2022; 328:162–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.