Abstract
The exudate of germinated spores of B. cereus IFO 13597 in 0.15 M KCl–50 mM potassium phosphate (pH 7.0) contained a spore-lytic enzyme which has substrate specificity for fragmented spore cortex from wild-type organisms (cortical-fragment-lytic enzyme [CFLE]), in addition to a previously characterized germination-specific hydrolase which acts on intact spore cortex (spore cortex-lytic enzyme [SCLE]) (R. Moriyama, S. Kudoh, S. Miyata, S. Nonobe, A. Hattori, and S. Makino, J. Bacteriol. 178:5330–5332, 1996). CFLE was not capable of degrading isolated cortical fragments from spores of Bacillus subtilis ADD1, which lacks muramic acid δ-lactam. This suggests that CFLE cooperates with SCLE in cortex hydrolysis during germination. CFLE was purified in an active form and identified as a 48-kDa protein which functions as an N-acetylglucosaminidase. Immunochemical studies suggested that the mature enzyme is localized on a rather peripheral region of the dormant spore, probably the exterior of the cortex layer. A gene encoding the enzyme, sleL, was cloned in Escherichia coli, and the nucleotide sequence was determined. The gene encodes a protein of 430 amino acids with a deduced molecular weight of 48,136. The N-terminal region contains a repeated motif common to several peptidoglycan binding proteins. Inspection of the data banks showed no similarity of CFLE with N-acetylglucosaminidases found so far, suggesting that CFLE is a novel type of N-acetylglucosaminidase. The B. subtilis genome sequence contains genes, yaaH and ydhD, which encode putative proteins showing similarity to SleL.
The bacterial endospore cortex is responsible for the maintenance of spore dormancy and heat resistance. The cortex peptidoglycan has a unique spore-specific structure which allows it to fulfill its role, and cortex hydrolysis during spore germination is essential to allow spore outgrowth and the formation of a new vegetative cell (9, 21). The enzymes involved in these hydrolytic reactions have been identified from spores of several species.
A germination-specific cortex-lytic enzyme (GSLE) from Bacillus megaterium spores (8) and a spore cortex-lytic enzyme (SCLE) from Bacillus cereus spores (16, 24), a mature form of SleB, were shown to hydrolyze intact spore peptidoglycan. A counterpart of B. cereus SCLE was also found to exist in Bacillus subtilis spores (23), though attempts to solubilize it were unsuccessful. The B. subtilis SCLE was involved in the response of spores to l-alanine-stimulated germination, and spores lacking this enzyme were unable to complete germination but showed limited cortex degradation (23). This suggests the contribution of a cortex-lytic enzyme(s) in addition to SCLE in the germination of B. cereus and B. subtilis spores. Indeed, analysis of muropeptide dynamics during germination of B. megaterium and B. subtilis spores unequivocally revealed that multiple enzymes are implicated in cortex degradation (2, 4). In Clostridium perfringens S40 spores, a cortical fragment-lytic enzyme (CFLE) which attacks disrupted cortex but not intact spores cooperates with SCLE in cortex hydrolysis during germination (6, 19, 20). These results led us to investigate the enzyme(s) other than SCLE responsible for cortex degradation in B. cereus spores. This report deals with the purification and the characterization of a CFLE from the germination exudate of B. cereus IFO 13597 spores. This enzyme functions as an N-acetylglucosaminidase, and the gene, named sleL, has been cloned. The specificity of the enzyme indicates that it acts only in spore germination, as the enzyme hydrolyzes peptidogtycan containing muramic acid δ-lactam, a substrate recognition determinant for cortex-lytic enzymes (28).
MATERIALS AND METHODS
Bacterial strains and plasmids.
Spores and vegetative cells of B. cereus IFO 13597 were used as the source of CFLE and chromosomal DNA, respectively. B. cereus IFO 13597, B. subtilis 168 AJ12866, B. subtilis ADD1 (a B. subtilis mutant with an insertionally inactivated cwlD gene), and C. perfringens S40 were cultured as described by Makino et al. (16), Moriyama et al. (23), Sekiguchi et al. (32), and Miyata et al. (19), respectively. Escherichia coli XL1-Blue (Stratagene Cloning System, La Jolla, Calif.) was used as a host for screening the genomic library of B. cereus DNA. Plasmids pUC118 (Novagen, Inc., Madison, Wis.), pBluescript II KS(+) (Stratagene), and pGEM T-vector (Promega Co., Madison, Wis.) were used as cloning vectors. E. coli was routinely grown at 37°C in 2× YT medium with ampicillin added to 100 μg per ml for plasmid-carrying strains (30).
Preparation of spores, decoated spores, isolated cortical fragments, and isolated vegetative cell walls and disruption of spores and vegetative cells.
Spores and decoated spores of B. cereus, C. perfringens, and B. subtilis 168 and B. subtilis ADD1 were prepared by methods described previously (references 16, 19, and 23, respectively).
Decoated spores (1 g [wet weight]) from each organism were disrupted at 0 to 4°C with a bead beater in a 20-ml tube containing 10 ml of 50 mM Tris-HCl (pH 8.0) and 10 g of glass beads (diameter, 0.1 mm). The disrupted spores were recovered by centrifugation (13,000 × g for 5 min at 4°C) and heated at 90 to 95°C for 1 h in 1 M NaCl–50 mM Tris-HCl (pH 8.0) containing 2% sodium dodecyl sulfate (SDS) and 1% 2-mercaptoethanol. After being washed extensively with 50 mM Tris-HCl (pH 8.0), the precipitate was treated with tosylamide phenylethyl chloromethyl ketone (TPCK)-trypsin (Sigma-Aldrich, Tokyo, Japan) (0.1 mg/ml in 20 mM Tris-HCl [pH 8.0] containing 10 mM CaCl2) at 37°C for 16 h. The trypsin-treated cortical fragments were heated at 90 to 95°C for 15 min in 20 mM Tris-HCl (pH 8.0) containing 1% SDS. The cortical fragments were then washed in 20 mM Tris-HCl (pH 8.0) until no trace of SDS could be detected in the wash supernatant fluid (11) and used as substrate for CFLE. Dormant spores and vegetative cells were also disrupted with a bead beater containing glass beads in 0.15 M KCl–50 mM potassium phosphate (pH 7.0) as described above, and the supernatants obtained by centrifugation (13,000 × g for 5 min at 4°C) were used as extracts.
Spore-lytic enzyme assay.
Peptidoglycan-degrading enzymes were assayed by measuring the decrease in optical density at 600 nm (OD600) of suspensions of decoated spores and/or isolated peptidoglycan in a cell with a 1-mm light path at 32°C with a Jasco UV spectrophotometer (Japan Spectroscopic Co., Tokyo, Japan), as described previously (16). One unit of activity was defined as a decrease in OD600 of 0.100 per min.
Purification of CFLE from germination exudate.
B. cereus spores, which were washed with 50 mM potassium phosphate (pH 7.0) containing 2 M urea to remove spore surface-bound subtilisin-like protease (25), were heated at 75°C for 30 min in deionized water. The spores (5 g [packed weight]) were then germinated at 32°C for 1 h in 100 ml of 0.15 M KCl–50 mM potassium phosphate (pH 7.0) containing 10 mM l-alanine and 4 mM adenosine; the germination exudate contained activities which digest decoated spores and cortical fragments. After centrifugation (8,000 × g for 10 min at 4°C), the germination exudate was dialyzed at 4°C for 20 h against 2 liters of 60 mM potassium phosphate (pH 8.0) containing 1 mM sodium thioglycollate (buffer A). The dialyzed fluid was applied to an SP-Sephadex C25 column (2.2 by 15 cm; Pharmacia, Uppsala, Sweden) which had been equilibrated with buffer A at 4°C, and adsorbed materials were eluted at 4°C with a 200-ml linear gradient of up to 0.4 M KCl in buffer A. A CFLE was recovered in fractions eluted at a KCl concentration of 0.15 to 0.2 M. The fractions were dialyzed against 40 mM potassium phosphate (pH 7.0) and then applied to a hydroxyapatite column (2.2 by 10 cm; Wako Pure Chemicals, Osaka, Japan) which had been equilibrated with 40 mM potassium phosphate (pH 7.0). CFLE was eluted in 0.2 M potassium phosphate with a 160-ml linear gradient of 40 mM to 0.4 M of potassium phosphate (pH 7.0). Active fractions were concentrated under low-pressure centrifugation and further purified by a Superose 12 gel exclusion column (1.5 by 25 cm; Pharmacia) in 0.15 M KCl–50 mM potassium phosphate (pH 7.0).
Preparation of antiserum and immunoprecipitation.
Preparation of mouse anti-CFLE antiserum and immunoprecipitation of the extract from dormant spores with the antiserum were carried out as described previously (6).
Mode of action of CFLE.
Lyophilized cortical fragments from B. subtilis and C. perfringens spores (8.0 mg each) were suspended in 2.5 ml of 0.1 M potassium phosphate (pH 6.0), and the suspension was treated with the purified enzyme (3.0 U; 25 μl) at 32°C, monitoring the change in OD600. Aliquots (300 μl) were centrifuged (8,000 × g for 10 min at 4°C) at appropriate time intervals, and samples of the supernatant fluid were assayed for the appearance of amino groups with trinitrobenzenesulfonate as described by Fields (7) and for reducing groups by the method of Thompson and Shockman (34). A control experiment was performed using enzyme boiled for 10 min.
B. subtilis cortical fragments (5.0 mg) were digested with purified CFLE (3.0 U; 25 μl) in 0.5 ml of 0.1 M potassium phosphate (pH 6.0) for 24 h at 30°C. The suspension was centrifuged, and the supernatant (0.1 ml) was applied to a reverse-phase high-pressure liquid chromatography (HPLC) column to recover muropeptides. An M&S PACK C18 column from M&S Instruments, Osaka, Japan (4.6 by 150 mm; particle size, 5 μm) was used, and the column was submerged in a water bath and maintained at 52°C. Elution was carried out at a flow rate of 0.5 ml/min with a linear gradient initiated 5 min after injection in 100% buffer B and an increase from 0 to 67% for buffer C in 120 min. The elution buffers were as follows: B, 0.1% trifluoroacetic acid (pH 1.5); C, 0.1% trifluoroacetic acid in 20% acetonitrile (pH 1.5). The eluted compounds were detected by monitoring the absorbance at 205 nm, and muropeptides were collected individually at the detector outlet. Two major peak fractions were subjected to amino acid analysis.
The HPLC-purified muropeptides were lyophilized and dissolved in 0.1 ml of deionized water. After 50 μl of the solutions was mixed with an equal volume of 0.2 M sodium borate (pH 10.0), the muropeptides were reduced with sodium borohydride (0.16 mg/0.1 ml) for 8 min at room temperature with vigorous vortexing. The reduced and nonreduced materials were dried in vacuo, washed three times with methanol, and hydrolyzed with 6 N HCl for 4, 8, and 16 h at 105°C. The hydrolysate was dissolved in 0.1 M sodium citrate (pH 2.2) and analyzed for amino acid and amino sugar compositions on an amino acid analyzer (model JLC-500L; JEOL, Ltd., Tokyo, Japan).
Cloning of the sleL gene.
Purified CFLE (30 μg) was digested with TPCK-trypsin (2.5 μg) for 16 h at 30°C in 0.1 ml of 5 mM Tris-HCl (pH 8.0) containing 10 mM CaCl2 and applied to an octyldecyl silane-2PU column (4.6 by 250 mm; particle size, 5 μm; Mitsubishi Chemical Co., Tokyo, Japan). Peptides were eluted from the column with a linear gradient of 5 to 95% acetonitrile in the presence of 0.05% trifluoroacetic acid. Five peak fractions were collected, and the amino-terminal sequences of the peptides were determined. Based on the N-terminal sequence of purified CFLE and one of the peptides (peptide C [see Fig. 5]), two oligonucleotide primers, N1 [5′-ATGAT(A/C/T)CA(A/G)AT(A/C/T)GT(A/C/G/T)AC(A/C/G/T)GT-3′] and CR [5′-GC(A/C/G/T)GT(C/T)TG(A/C/G/T)A(A/G)(A/G/T)AT(A/G)TT(A/C/G/T)GT-3′], were synthesized. The PCR was performed with B. cereus chromosomal DNA, and a PCR product obtained (617 bp) was analyzed. EcoRI-digested fragments of chromosomal DNA were ligated into the EcoRI site of pUC118 to produce templates for single-specific-primer PCR. Based on the sequence of the 617-bp PCR product, two oligonucleotide primers, SleLF1 (5′-CCAGCTGAAACGCTCGTTGT-3′) and SleLR1 (5′-GTGCCATCTCTTTTTGCCTC-3′), were synthesized. The single-specific-primer-PCR method as described by Shyamala and Ames (33) was performed with the ligated mixture described above by use of the pairs M13 universal primer (5′-TTTCACACAGGAAACAGCTATGAC-3′) and SleLF1, and M13 universal primer and SleLR1. Products of 2.3 and 3.5 kbp, respectively, were subcloned into the pGEM T vector. Nucleotide sequences containing the sleL gene from the 2.3- and 3.5-kbp products were analyzed for at least three subclones obtained from each PCR product, and their determined sequences were consistent with those of the products made with the same primers. Finally, PCR was performed against chromosomal DNA from B. cereus using two oligonucleotide primers, SleLF4 (5′-GCGAAGTGGGCTCACAGTATCTACC-3′) and SleLR2 (5′-CGCTGAAAAGTTACAAGAAATGGTGC-3′), to generate the PCR product containing the sleL gene, and the product was analyzed.
FIG. 5.
Nucleotide sequence of B. cereus chromosomal DNA between two oligonucleotide primers, SleLF4 and SleLR2, and deduced amino acid sequences. The deduced amino acid sequences of orf1 (nucleotides 1 to 210), sleL (nucleotides 371 to 1660), and orf3 (nucleotides 1695 to 1860) are given below the nucleotide sequence. The numbers of the nucleotides and amino acids are shown on the right. A candidate for a putative ribosome-binding site (RBS) is indicated by shading. The asterisk indicates a termination codon. The bold-underlined amino acid sequence shows the N-terminal sequence of CFLE (20 residues), and the thin-underlined sequences show peptides A, B, C, D, and E derived from the purified enzyme by digestion with trypsin. An inverted repeat is indicated by arrows.
Nucleotide sequencing and analysis.
Nucleotide sequencing was performed by the dideoxynucleotide chain termination method of Sanger et al. (31), using the ABI PRISM genetic analyzer (Perkin-Elmer, Applied Biosystems, Foster City, Calif.). The nucleotide and amino acid sequence analysis and sequence comparison with DNAs and proteins registered in the databases (GenBank, EMBL, PIR, and SWISS-PROT) were performed with MACDNASIS software (Hitachi Software Engineering, Tokyo, Japan).
Other procedures.
Protein concentrations were determined by the methods of Lowry et al. (15) and/or Groves et al. (10), with bovine serum albumin as the standard. SDS-polyacrylamide gel electrophoresis (12.5% polyacrylamide) and immunoblotting were carried out as described elsewhere (6). Analyses of N-terminal amino acid sequences were carried out on a protein sequencer (model 477A/120A; Applied Biosystems).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession number AB029921.
RESULTS
Purification of CFLE.
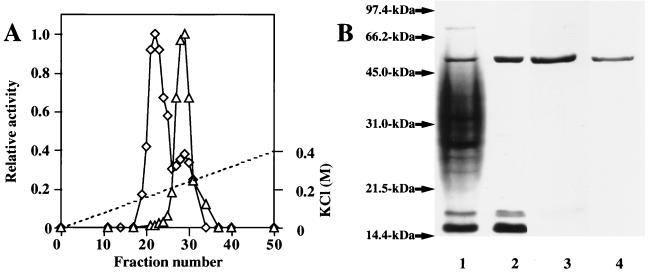
In addition to SCLE activity acting on decoated spore cortex described previously (16), CFLE activity hydrolyzing disrupted spore cortex was detected in the germination exudate of B. cereus spores. These activities were separated by SP-Sephadex C25 column chromatography (Fig. 1A). This demonstrates the presence of cortex-lytic enzymes differing in the recognition of substrate morphology. Neither enzyme is synthesized de novo during germination, as shown by the release of activity during germination in the presence of chloramphenicol.
FIG. 1.
Chromatography of CFLE on an SP-Sephadex C25 column and SDS-gel electrophoretic profiles showing purification of the enzyme. (A) Germination exudate (100 ml from 5 g of packed spores) was dialyzed against 60 mM potassium phosphate (pH 8.0) containing 1 mM sodium thioglycollate and put on an SP-Sephadex C25 column. Proteins were eluted with a linear gradient of KCl. Fractions (4 ml each) were collected, and the cortex-lytic activity of each fraction was measured by using cortical fragments (◊) and decoated spores (▵). The broken line shows the molarity of KCl. (B) The CFLE was purified as described in Table 1 and analyzed by 0.1% SDS–12.5% polyacrylamide gel electrophoresis. Approximately 3 to 30 μg of protein was electrophoresed and Coomassie blue stained. Standard proteins were run with samples in each gel, and the time of electrophoresis for lane 3 was not the same as those for other lanes. Lanes: 1, germination exudate; 2, fractions containing CFLE activity eluted from an SP-Sephadex C25 column; 3, fractions containing CFLE activity eluted from a hydroxyapatite column; 4, Superose 12 column-purified enzyme. The standard proteins run were rabbit muscle phosphorylase b (97.4 kDa), bovine serum albumin (66.2 kDa), ovalbumin (45.0 kDa), bovine carbonic anhydrase (31.0 kDa), soybean trypsin inhibitor (21.5 kDa), and egg white lysozyme (14.4 kDa).
The steps in the purification procedure and the yields obtained for CFLE are shown in Table 1. The enzyme, which was finally purified by gel exclusion chromatography, showed a single band in SDS-polyacrylamide gel electrophoresis with an apparent molecular mass of 48 kDa (Fig. 1B, lane 4). A 189-fold purification was achieved, with a final yield of enzyme activity of 14.9%. The N-terminal amino acid sequence of the enzyme was determined to be MIQIVTVRSGDSVYSLASKY (20 residues) by Edman degradation.
TABLE 1.
Purification of CFLE of B. cereusa
| Procedure | Total protein (mg) | Total activity (U) | Sp act (U/mg of protein) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Germination exudate | 39.3 | 1,133 | 28.8 | 100 | 1 |
| SP-Sephadex C25 column | 0.53 | 542 | 1,022.6 | 47.8 | 35.5 |
| Hydroxyapatite | 0.11 | 339 | 3,081.8 | 29.9 | 107.0 |
| Superose 12 | 0.031 | 169 | 5,451.6 | 14.9 | 189.3 |
B. cereus spores (5 g [packed weight]) were germinated as described in Materials and Methods. After centrifugation (8,000 × g for 10 min at 4°C), the supernatant was used as a germination exudate.
Digestion of CFLE with trypsin provided five main products, and their N-terminal amino acid sequences were as follows: peptide A, GNNYYVQPGDSLY; peptide B, EQGIGGISYWK; peptide C, FITNILQTAQK; peptide D, YDFTAQAPHFNY; and peptide E, IGLPFPQNWR.
Substrate specificity and mode of action of CFLE.
Purified CFLE was examined for its potency to degrade a variety of peptidoglycan substrates (Table 2). The enzyme did not show lytic activity against decoated spores which possess the morphology of intact spores. The enzyme hydrolyzed isolated cortical fragments from spores of wild-type organisms (B. cereus, B. subtilis, and C. perfringens) which have muramic acid δ-lactam as the peptidoglycan constituent. However, it was unable to digest the lactam residue-lacking isolated peptidoglycans from spores of mutant B. subtilis ADD1 and vegetative cell walls of wild-type organisms (B. cereus and B. subtilis). Since the muramic acid δ-lactam residue of spore peptidoglycan likely functions as a substrate recognition determinant for cortex-lytic enzymes (28), the CFLE appears to be specific for broken spore peptidoglycan in vivo.
TABLE 2.
Substrate specificity of CFLEa
| Substrate | Lytic activity (% of control) |
|---|---|
| Normal B. cereus IFO 13597 spores | <1 |
| Isolated B. cereus IFO 13597 spore cortical fragments | 100 |
| Isolated B. subtilis 168 AJ12866 spore cortical fragments | 92 |
| Isolated C. perfringens S40 spore cortical fragments | 85 |
| Isolated B. subtilis ADD1 spore cortical fragments | <5 |
| Decoated B. cereus IFO 13597 spores | <3 |
| Decoated B. subtilis 168 AJ12866 spores | <3 |
| Decoated C. perfringens S40 spores | <3 |
| Decoated B. subtilis ADD1 spores | <3 |
| Isolated B. cereus IFO 13597 cell walls | <1 |
| Isolated B. subtilis 168 AJ12866 cell walls | <1 |
Lytic activity was measured by the decrease in OD600. The control value (0.05 U for isolated B. cereus IFO 13597 spore cortical fragment) was taken as 100%. The substrates tested here, except for normal B. cereus IFO 13597 spore, were digested by egg white lysozyme.
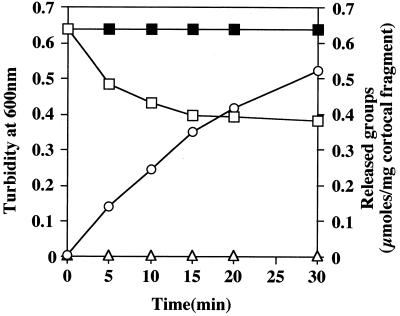
The appearance of new free amino groups and reducing sugars during enzymatic hydrolysis of cortical fragments by the purified CFLE was monitored (Fig. 2). After a 30-min treatment with the enzyme, the OD600 of the suspension had been reduced by 40%. An equivalent amount of the enzyme boiled for 10 min before use had no effect on the suspension as measured by loss of OD600. Concomitant with the decrease in OD600, there was a marked increase in reducing sugars. In contrast, over this time period, no free amino group appeared. The results suggest a cleavage of the cortex polysaccharide by the enzyme.
FIG. 2.
Release of reducing groups during digestion of cortical fragments with CFLE. Cortical fragments of C. perfringens spores (8.0 mg) were suspended in 2.5 ml of 0.1 M potassium phosphate (pH 6.0). CFLE (3.0 U; 25 μl) was added to the suspension, and the digestion was performed at 32°C. Aliquots were taken at the indicated times for determination of the turbidity at 600 nm in a cell of 1 mm light path (□), the release of reducing sugars (○), and the release of amino groups (▵). No decrease in turbidity was seen when heat-denatured enzyme was used (■). The same results were obtained when B. subtilis spore cortical fragments were used as a substrate.
The bond specificity of the enzyme was further studied by using fragmented cortex from B. subtilis spores, whose peptidoglycan structure has been explored (3, 27). The fragments were digested with the purified CFLE, and the resulting soluble muropeptides were separated by reversed phase HPLC. The two major fractions, A and B, which were eluted at the retention times of 41 and 46 min, respectively, were collected (20.2 and 27.7% of total products, respectively). Half of each fraction was reduced with sodium borohydride. The nonreduced and reduced materials were hydrolyzed with 6 N HCl, and the amino acid and amino sugar compositions were analyzed (Table 3). Nonreduced samples from both fractions were shown to contain glucosamine and muramic acid in a molar ratio of 1:1. After reduction, the glucosamine content was reduced by about 50% and was replaced by glucosaminitol, whereas the amounts of muramic acid, alanine, glutamic acid and diaminopimelic acid in fraction A and muramic acid and alanine in fraction B remained unchanged. Thus, the enzyme is shown to be an N-acetylglucosaminidase.
TABLE 3.
Muropeptide analysisa
| Peak | Materials | Composition (M)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mur | MurOH | Glc | GlcOH | Glu | Ala | Dpm | ||
| A | Nonreduced | 1.00 | 0.00 | 0.99 | 0.00 | 0.54 | 1.01 | 0.53 |
| Reduced | 1.00 | 0.00 | 0.53 | 0.48 | 0.51 | 0.98 | 0.50 | |
| B | Nonreduced | 1.00 | 0.00 | 0.99 | 0.00 | <0.01 | 0.50 | <0.01 |
| Reduced | 1.00 | 0.00 | 0.52 | 0.50 | <0.01 | 0.52 | <0.01 | |
Cortical fragments from B. subtilis (∼1 mg) were digested with CFLE, and the resulting muropeptides were separated by HPLC as described in Materials and Methods. The major peak fractions, A and B, were collected. Their nonreduced and reduced materials were analyzed for amino acids and amino sugars. These values are normalized, with the result for muramic acid set at 1, and are presented in a molar basis. Mur, muramic acid; MurOH, muramitol; Glc, glucosamine; GlcOH, glucosaminitol; Glu, glutamic acid; Ala, alanine; Dpm, diaminopimelic acid.
Effects of pH, temperature, salt concentration, and chemicals on activity.
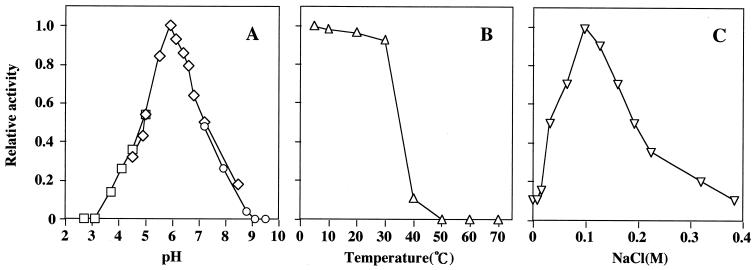
The enzyme was active over a pH range of 3.0 to 9.0, with an optimum at pH 6.0 (Fig. 3A). Heat treatment of the isolated enzyme at 40°C for 30 min led to a loss of activity (∼90%) (Fig. 3B), while the enzyme in the dormant spore resisted heat treatment at 75°C for 30 min. The enzyme activity depended on the NaCl concentration of the medium, with maximum activity at 0.1 M and a rapid decrease at both lower and higher salt concentrations (Fig. 3C). The same was true with KCl and CaCl2. The addition of dipicolinic acid (DPA; 10 mM), which is released with Ca2+ from the spore core at an early stage of germination (12), to 0.1 M NaCl (or KCl)–5 mM Tris-HCl (pH 7.6) had no effect on the CFLE activity. However, the activity observed in 50 mM CaCl2–5 mM Tris-HCl (pH 7.6) was reduced to 70% by the addition of DPA (10 mM). This may be due to a change in ionic strength based on a formation of chelate complex between DPA and Ca2+. However, it is not obvious how DPA and Ca2+ ion might affect activities of cortex-lytic enzymes in vivo.
FIG. 3.
Effects of pH, temperature, and NaCl concentration on the activity of CFLE. The CFLE activity is shown relative to the maximum activity. (A) Enzyme (5 μl with 0.08 U) in 5 mM Tris-HCl (pH 7.6) was mixed with 135 μl of 0.1 M buffer solutions to obtain the indicated pH. After incubation at 32°C for 10 min, 5 μl of C. perfringens cortical fragments was added and the enzyme activity was measured. The following buffers were used: CH3COOH-CH3COONa (□), Na2HPO4-NaH2PO4 (◊), and NaHCO3-Na2CO3 (○). (B) Enzyme (5 μl with 0.08 U) in 5 mM Tris-HCl (pH 7.6) was mixed with 135 μl of 5 mM Tris-HCl (pH 7.6) containing 0.1 M NaCl and incubated for 30 min at the indicated temperature. Then 5 μl of cortical fragments was added, and the residual activity was assayed at 32°C. (C) Enzyme (5 μl with 0.08 U) in 5 mM Tris-HCl (pH 7.6) was mixed with 135 μl of 5 mM Tris-HCl (pH 7.6) containing the desired concentration of NaCl. After incubation at 32°C for 10 min, 5 μl of cortical fragments was added and the enzyme activity was measured. Similar results were obtained when KCl was replaced with NaCl.
Although p-nitrophenyl-N-acetyl-β-d-glucosaminide and p- nitrophenyl-tetra-N-acetyl-β-chitotetraoside (synthetic substrates of hexosaminidase and egg white lysozyme, respectively) were not hydrolyzed by CFLE, their structural similarity to the spore peptidoglycan caused partial inhibition of the enzyme (21 and 30% at 1 mM, respectively). ZnCl2 inactivated the enzyme, but no inhibitory effect was observed with MgCl2, MnCl2, or HgCl2 (1 mM each), as well as CaCl2. Enzyme inhibitors, such as diisopropylfluorophosphate, sodium thioglycollate, N-ethylmaleimide, and EDTA (1 mM each), had no effect on CFLE activity. However, the enzyme was completely inhibited by 1 mM diethylpyrocarbonate, and the addition of hydroxylamine (0.2 M; incubation at 25°C for 2 h) restored activity (55%) which had been lost with 1 mM diethylpyrocarbonate.
Detection of CFLE in spore fractions.
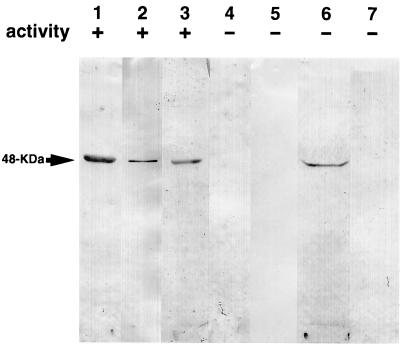
An anti-CFLE antiserum was used to detect CFLE-related proteins in spore fractions separated by SDS-polyacrylamide gel electrophoresis to locate CFLE in the dormant spore. The antiserum recognized only a 48-kDa polypeptide in the germination exudate (Fig. 4, lane 2), which has the same electrophoretic mobility as purified CFLE (Fig. 4, lane 1). The extract made from disrupted dormant spores in 0.15 M KCl–50 mM potassium phosphate (pH 7.0) exhibited CFLE activity and contained a 48-kDa protein which cross-reacted with the antiserum (Fig. 4, lane 3). The extract which had been immunoprecipitated with the anti-CFLE serum lost enzymatic activity, in parallel with the disappearance of the 48-kDa protein (Fig. 4, lane 4). These results indicate that the 48-kDa protein in the extract is CFLE, which is present in an active form in the dormant spore. No CFLE-related protein was detected in vegetative cells collected from a stationary-phase culture (Fig. 4, lane 5).
FIG. 4.
Immunological detection of CFLE-related proteins in dormant spores and vegetative cells. Spores and vegetative cells were disrupted in and extracted with 0.15 M KCl–50 mM potassium phosphate (pH 7.0), and the coat fraction was recovered as described in the text. The germination exudate, the spore extracts, and the coat fraction were subjected to SDS-polyacrylamide gel electrophoresis followed by immunoblotting. Before electrophoresis, all the samples were dialyzed against 10 mM Tris-HCl (pH 8.0) containing 0.2% SDS and 5 mM 2-mercaptoethanol, and approximately 3 to 30 μg of protein was loaded on the gel. Lanes: 1, purified CFLE; 2, germination exudate; 3, extract made from disrupted dormant spores; 4, the same extract as in lane 3 but pretreated with anti-CFLE serum; 5, extract made from disrupted vegetative cells; 6, coat fraction; 7, extract made from disrupted decoated spores. Prior to electrophoresis, the CFLE activity of the samples was examined. Symbols: +, positive hydrolytic activity; −, no hydrolytic activity.
B. cereus spores were treated with 0.1 M sodium borate (pH 10.0) containing 1% SDS and 1% 2-mercaptoethanol for 8 h at 40°C (16), and the released coat fraction was separated from decoated spores by centrifugation. CFLE was detected in the coat fraction (Fig. 4, lane 6) but not in the extract of disrupted decoated spores (Fig. 4, lane 7). This suggests a rather peripheral location of the enzyme in the dormant spore.
Nucleotide and predicted amino acid sequences of the sleL gene.
The nucleotide sequence for the SleLF4-SleLR2 region of B. cereus chromosomal DNA is shown in Fig. 5. The nucleotide sequence, consisting of 1,860 bp, had three open reading frames. The B. cereus sleL gene (nucleotides 371 to 1660) was present between the C-terminally truncated orf1 and the N-terminally truncated orf3, which encode unknown proteins. The sleL gene starts at an ATG initiation codon (nucleotides 371 to 373), which is preceded by a potential ribosome-binding site (GAAAGGAG; nucleotides 356 to 363), and terminates at a TGA stop codon (nucleotides 1661 to 1663). An inverted repeated sequence (nucleotides 1652 to 1664 and 1669 to 1681) was found near the stop codon.
The sleL gene encoded a highly basic (pI 9.7) polypeptide of 430 amino acid residues with a molecular mass of 48,136 Da, which was similar to that estimated from SDS-polyacrylamide gel electrophoresis of purified CFLE. A stretch of 20 residues determined to be the N-terminal amino acid sequence of CFLE was found in the sequence predicted from the nucleotide sequence starting at an initiation codon. Five tryptic peptide sequences obtained from a purified preparation of CFLE were found. The CFLE lacks cysteine residues and has a repeated sequence, VX2GDSX1YX5YX8KX1NX1LX5LX1VGQX1LX1V (residues 7 to 47 and 56 to 96; Xn represents an alignment of any amino acid X consisting of n residues), which has been found in a series of cell wall-lytic enzymes and may be a cell wall-binding motif (13, 17).
DISCUSSION
In this study, we identified a novel spore-lytic enzyme, CFLE, in addition to a known spore-lytic enzyme, SCLE (16, 24), in the exudate of germinated spores of B. cereus. They differ from each other in the recognition of the morphology of substrates; CFLE requires disrupted spore peptidoglycans for its activity, and SCLE preferentially hydrolyzes intact spore peptidoglycan, although it has minimal activity on cortical fragments (the degradation rate is <5% of that for decoated spores [24]). However, the enzymes possess common characteristics as follows: (i) they recognize muramic acid δ-lactam residues specific to spore peptidoglycans, which serve as a major determinant for germination-specific spore-lytic enzymes (28); (ii) they are extracted from spores by treatment with reduced alkaline solution containing SDS, a procedure to strip the spore coat (16), suggesting a close localization of CFLE and SCLE in the dormant spore, possibly on the exterior of the cortex layer (22); and (iii) the sensitivities of CFLE activity to temperature, pH, and ionic strength (Fig. 4) are similar to those of SCLE (16), suggesting that in vivo the activities function in the same environment. These findings suggest that in B. cereus spores, both SCLE and CFLE are germination-specific spore peptidoglycan hydrolases which cooperatively function during germination and that peptidoglycan degradation during germination consists of a cascade of hydrolytic reactions controlled by the morphologies of substrates. This cascade might be common to cortex hydrolysis during germination of B. megaterium and B. subtilis spores, as suggested by the presence of SleB and a putative protein homologous to B. cereus CFLE in B. subtilis (see below) and by a similar pattern of enzyme activities involved in cortex hydrolysis in B. megaterium and B. subtilis (2, 4).
Atrih et al. (2, 4) indicated the involvement of three enzyme activities in spore cortex hydrolysis, based on structural analysis of spore peptidoglycan and its dynamics during germination of B. megaterium and B. subtilis spores. These enzymes are a lytic transglycosylase, an N-acetylglucosaminidase, and an activity generating the subtle modification suggested to be an epimerization of muramic acid. The present study confirmed the existence of N-acetylglucosaminidase in B. cereus spores; this enzyme is suggested to function in a late stage of a cascade of cortex hydrolytic reaction during germination, as it cleaves only broken spore peptidoglycans. It is most probable that GSLE in B. megaterium and/or SCLE in B. cereus and B. subtilis, which have specificity for intact spores, contributes to the initial step of cortex hydrolysis, allowing dissolution of cortex structure. These enzymes were suggested to be amidases (8, 16, 24). However, as mentioned above, an amidase activity has not been detected from the muropeptide analysis of germinated spores of B. megaterium and B. subtilis in the form of amidase products, although the possibility that the activity occurs has not been excluded (2, 4). Indeed, Atrih et al. have questioned the reliability of the classical methods for identification of lytic enzyme activities (2) using decoated spores as a substrate (8, 16). On the other hand, a lytic transglycosylase has not yet been identified from B. megaterium and B. subtilis spores. Identification of a lytic transglycosylase and reexamination of the hydrolytic bond specificity of GSLE and SCLE are needed for further understanding of the role of lytic enzymes in cortex hydrolysis during the germination of spores of Bacillus species.
Comparison of the putative amino acid sequence of B. cereus CFLE with the N-terminal amino acid sequence of purified enzyme indicates that the enzyme is produced in a mature form. The enzyme is probably present in an active form in the dormant spore, as suggested by the presence of activity in the extract made from disrupted dormant spores (Fig. 4). Furthermore, the enzyme did not act on intact cortex. Thus, expression of a CFLE activity which does not need activation must be regulated by the requirement for disrupted cortex as a substrate for hydrolysis. This is also the case for C. perfringens CFLE (6).
A computer search for sequence similarity with B. cereus CFLE revealed no similarity with N-acetylglucosaminidases found so far, including LytD, a cell wall N-acetylglucosaminidase of B. subtilis (29), indicating that the B. cereus CFLE is a novel type of N-acetylglucosaminidase. However, the B. subtilis genome sequence revealed the presence of two genes, yaaH and ydhD (14), which encode putative proteins showing high identity to B. cereus CFLE (SleL), as shown in Fig. 6. Besides the presence of the proposed cell wall-binding motif in the N-terminal region, both YaaH and YdhD showed significant similarity to the C-terminal region of B. cereus CFLE, and YaaH has been indicated to be a component of the system involved in the l-alanine-stimulated germination of B. subtilis spores (13). A B. cereus CFLE was inhibited with diethylpyrocarbonate, which reacts with histidine residues (18). The involvement of histidine in the catalytic activity of N-acetylglucosaminidase has been indicated in the enzymes from B. subtilis (LytD) (29) and Bacillus sp. strain NCIM 5120 (1). Among 4 His residues of B. cereus CFLE, His-379 in the C-terminal region is conserved in YaaH and YdhD. The possible involvement of YaaH and YdhD in cortex hydrolysis during spore germination and of the histidine residue in the catalytic activity of CFLE are currently being investigated.
FIG. 6.
Comparison of the amino acid sequence of B. cereus CFLE (SleL) with those of YaaH and YdhD of B. subtilis. The amino acid sequences are numbered on the left. Identical amino acids in two or three proteins are shown by a black background. Dashes are introduced to obtain a better alignment of the sequences.
In addition to SleL, the nucleotide sequence for a SleLF4-SleLR2 region of B. cereus chromosomal DNA encoded two unknown proteins, the C-terminally truncated Orf1 (70 amino acid residues) and the N-terminally truncated Orf3 (55 amino acid residues), counterparts for which are found in B. subtilis; the former has homology with the N-terminal 70 amino acid residues of a putative protein, YneP (identity, 51.7%) (14), and the latter has homology with the C-terminal 55 amino acid residues of a minor, small acid-soluble protein, Tlp (identity, 48.1%) (5, 14). In contrast to the nucleotide sequence of the B. cereus genome, in which sleL is intercalated in an opposite direction between orf1 and orf3, no open reading frame is found in the region between the tlp and the yneP genes of the B. subtilis genome. This observation is in agreement with a recent finding that the genome organization is not conserved between B. cereus and B. subtilis (26).
ACKNOWLEDGMENTS
We thank R. Moriyama for his valuable comments during the course of this experiment.
This work was supported in part by a Grant-in-Aid for Scientific Research (10660081) from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Amutha B, Khire J M, Khan M S. Active site characterization of the exo-N-acetyl-β-d-glucosaminidase from thermotolerant Bacillus sp. NCIM 5120: involvement of tryptophan, histidine and carboxylate residues in catalytic activity. Biochim Biophys Acta. 1999;1427:121–132. doi: 10.1016/s0304-4165(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 2.Atrih A, Bacher G, Körner R, Allmaier G, Foster S J. Structural analysis of Bacillus megaterium KM spore peptidoglycan and its dynamics during germination. Microbiology. 1999;145:1033–1041. doi: 10.1099/13500872-145-5-1033. [DOI] [PubMed] [Google Scholar]
- 3.Atrih A, Zöllner P, Allmaier G, Williamson M P, Foster S J. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol. 1996;178:6173–6183. doi: 10.1128/jb.178.21.6173-6183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atrih A, Zöllner P, Allmaier G, Williamson M P, Foster S J. Peptidoglycan structural dynamics during germination of Bacillus subtilis 168 endospores. J Bacteriol. 1998;180:4603–4612. doi: 10.1128/jb.180.17.4603-4612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagyan I, Setlow B, Setlow P. New small, acid-soluble proteins unique to spores of Bacillus subtilis: identification of the coding genes and regulation and function of two of these genes. J Bacteriol. 1998;180:6704–6712. doi: 10.1128/jb.180.24.6704-6712.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Miyata S, Makino S, Moriyama R. Molecular characterization of a germination-specific muramidase from Clostridium perfringens S40 spores and nucleotide sequence of the corresponding gene. J Bacteriol. 1997;179:3181–3187. doi: 10.1128/jb.179.10.3181-3187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields R. The rapid determination of amino groups with TNBS. Methods Enzymol. 1972;25:464–468. doi: 10.1016/S0076-6879(72)25042-X. [DOI] [PubMed] [Google Scholar]
- 8.Foster S J, Johnstone K. Purification and properties of a germination-specific cortex-lytic enzyme from spores of Bacillus megaterium KM. Biochem J. 1987;242:573–579. doi: 10.1042/bj2420573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster S J, Johnstone K. Pulling the triggers: the mechanism of bacterial spore germination. Mol Microbiol. 1990;4:137–141. doi: 10.1111/j.1365-2958.1990.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 10.Groves W E, Davis F C, Jr, Sells B H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968;22:195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi K. A rapid determination of sodium dodecyl sulfate with methylene blue. Anal Biochem. 1975;67:503–506. doi: 10.1016/0003-2697(75)90324-3. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone K, Stewart G S A B, Scott I R, Ellar D J. Zinc release and the sequence of biochemical events during triggering of Bacillus megaterium KM spore germination. Biochem J. 1982;208:407–411. doi: 10.1042/bj2080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama T, Takamatsu H, Asai K, Kobayashi K, Ogasawara N, Watabe K. The Bacillus subtilis yaaH gene is transcribed by SigE RNA polymerase during sporulation, and its product is involved in germination of spores. J Bacteriol. 1999;181:4584–4591. doi: 10.1128/jb.181.15.4584-4591.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunst F, Ogasawara N, Moszer I, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:436–442. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 15.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Makino S, Ito N, Inoue T, Miyata S, Moriyama R. A spore-lytic enzyme released from Bacillus cereus spores during germination. Microbiology. 1994;140:1403–1410. doi: 10.1099/00221287-140-6-1403. [DOI] [PubMed] [Google Scholar]
- 17.Margot P, Wahlen M, Gholamhuseinian A, Piggot P, Karamata D. The lytE gene of Bacillus subtilis 168 encodes a cell wall hydrolase. J Bacteriol. 1998;180:749–752. doi: 10.1128/jb.180.3.749-752.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miles E W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- 19.Miyata S, Moriyama R, Miyahara N, Makino S. A gene (sleC) encoding a spore cortex-lytic enzyme from Clostridium perfringens S40 spores; cloning, sequence analysis and molecular characterization. Microbiology. 1995;141:2643–2650. doi: 10.1099/13500872-141-10-2643. [DOI] [PubMed] [Google Scholar]
- 20.Miyata S, Moriyama R, Sugimoto K, Makino S. Purification and partial characterization of a spore cortex-lytic enzyme of Clostridium perfringens S40 spores. Biosci Biotechnol Biochem. 1995;59:514–515. doi: 10.1271/bbb.59.514. [DOI] [PubMed] [Google Scholar]
- 21.Moir A, Smith D A. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama R, Fukuoka H, Miyata S, Kudoh S, Hattori A, Kozuka S, Yasuda Y, Tochikubo K, Makino S. Expression of a germination-specific amidase, SleB, of bacilli in the forespore compartment of sporulating cells and its localization on the exterior side of the cortex in dormant spores. J Bacteriol. 1999;181:2373–2378. doi: 10.1128/jb.181.8.2373-2378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriyama R, Hattori A, Miyata S, Kudoh S, Makino S. A gene (sleB) encoding a spore cortex-lytic enzyme from Bacillus subtilis and response of the enzyme to l-alanine-mediated germination. J Bacteriol. 1996;178:6059–6063. doi: 10.1128/jb.178.20.6059-6063.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriyama R, Kudoh S, Miyata S, Nonobe S, Hattori A, Makino S. A germination-specific spore cortex-lytic enzyme from Bacillus cereus spores: cloning and sequencing of the gene and molecular characterization of the enzyme. J Bacteriol. 1996;178:5330–5332. doi: 10.1128/jb.178.17.5330-5332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriyama R, Sugimoto K, Zhang H, Inoue T, Makino S. A cysteine-dependent serine protease associated with the dormant spores of Bacillus cereus: purification of the protein and cloning of the corresponding gene. Biosci Biotechnol Biochem. 1998;62:268–274. doi: 10.1271/bbb.62.268. [DOI] [PubMed] [Google Scholar]
- 26.Okstad O A, Hegna I, Lindbak T, Rishovd A L, Kolsto A B. Genome organization is not conserved between Bacillus cereus and Bacillus subtilis. Microbiology. 1999;145:621–631. doi: 10.1099/13500872-145-3-621. [DOI] [PubMed] [Google Scholar]
- 27.Popham D L, Helin J, Costello C, Setlow P. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J Bacteriol. 1996;178:6451–6458. doi: 10.1128/jb.178.22.6451-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popham D L, Helin J, Costello C, Setlow P. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA. 1996;93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rashid M H, Mori M, Sekiguchi J. Glucosaminidase of Bacillus subtilis: cloning, regulation, primary structure and biochemical characterization. Microbiology. 1995;141:2391–2404. doi: 10.1099/13500872-141-10-2391. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 31.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekiguchi J, Akeo K, Yamamoto H, Khasanov F K, Alonso J C, Kuroda A. Nucleotide sequence and regulation of a new putative cell wall hydrolase gene, cwlD, which affects germination in Bacillus subtilis. J Bacteriol. 1995;177:5582–5589. doi: 10.1128/jb.177.19.5582-5589.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shyamala Y, Ames G F. Recombinant DNA. Methods Enzymol. 1993;217:436–446. doi: 10.1016/0076-6879(93)17082-g. [DOI] [PubMed] [Google Scholar]
- 34.Thompson J S, Shockman G D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1969;22:260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]