Abstract
Ear infection is one of the common diseases occurring throughout the world. Different etiological agents are responsible for ear infections.
Aim
To assess the antimicrobial potential of Terminalia arjuna leaves and bark extracts against Staphylococcus aureus, Acinetobacter sp., Proteus mirabilis, Escherchia coli, Pseudomonas aeruginosa and Candida albicans, pathogens causing ear infections and their comparison with locally available ear drops.
Materials and Methods
Methanol, ethanol, acetone, aqueous (hot and cold) extracts from the leaves and bark of T. arjuna were tested for their antimicrobial activity.
Results
Of the three organic solvents evaluated, acetonic leaf extract was found to be best against S. aureus. Organic bark extract showed almost equal inhibition of all tested Gram negative bacteria except P. aeruginosa. However, aqueous extract of T. arjuna bark exhibited good activity against S. aureus. All the extracts were unable to exhibit any antifungal activity.
Conclusion
Organic extract obtained from the T. arjuna bark and leaves may be used to treat the bacterial ear pathogens especially S. aureus, which has shown greater inhibition zones than the herbal drops, however, we still need more detailed studies as in vivo testing and pharmacokinetics properties for their therapeutic utility in treating ear infections.
Keywords: antimicrobial activity, ear pathogen, plant extract
INTRODUCTION
Ear infection is more common in children than adults, approximately 75% of children experience at least three or more ear infections during the first three years of life1. Ear infection is mainly caused by bacterial (Pseudomonas aeruginosa, Staphylococcus aureus, S. epidermidis, Streptococcus pneumoniae, Escherichia coli, Proteus mirabilis) and fungal pathogens (Aspergillus niger, A. fumigatus, A. flavus, Candida albicans)2, 3, 4, 5.
The clinical efficacy of many existing chemotherapeutic agents is being threatened by the emergence of multidrug-resistant pathogens. Bacteria naturally develop resistance to antimicrobial drugs. In recent years, however, the overuse and misuse of antibiotics has caused a growing number of staphylococcus bacteria to evolve into disease causing “superbugs” resistant to drugs like methicillin, vancomycin6, 7, 8. The increasing failure of chemotherapeutics and antibiotic resistance exhibited by pathogenic microbial infectious agents has led to the screening of several plants for their potential antimicrobial activity and development of new antimicrobials by drug companies7, 9. Herbs are staging a comeback and herbal 'renaissance' is happening all over the globe and according to WHO, 80% of the world's population relies on plant-based traditional medicines for their primary healthcare needs10, 11, 12.
Nature has bestowed India with rich flora that is widely distributed. Herbal medicines have been used for the treatment and cure of various diseases in the Indian traditional practiced methods such as Ayurveda, Unani and Siddha13, 14. Terminalia arjuna Wight & Arn., commonly called arjuna (family Combretaceae), is a deciduous and evergreen tree distributed throughout India and also found in Burma, Srilanka and Mauritius, growing up to a height of 60 to 90 feet. Leaves of arjuna are simple, oblong or elliptic with pale and dark green upper surface and pale brown lower surface. Flowers are bisexual, sessile and white arranged in short axillary spikes or in terminal pannicule. The bark is smooth, pinkish-grey from outside and flakes off in large, curved and rather flat pieces15, 16. The bark and leaves of this plant have been used in indigenous system of medicine for curing different diseases, the bark in the treatment for angina (hritshool),expectorant, antidysentric, purgative, laxative, leucoderma, anaemia, hyperhidrosis, asthama, tumors and other cardiovascular disorders16, 17 and leaves as a remedy for the treatment of ear ache18. The bark also possesses good anticancer, antiviral and antimicrobial activities19, 20.
Plants have limitless ability to synthesize secondary metabolites such as tannins, terpenoids, alkaloids, flavonoids, glycosides and phenols which have been found to have antimicrobial properties11, 21, 22. The leaves and bark of T. arjuna contain glycosides having cardio protective effect, flavanoids having antioxidant, anti-inflamatory, antimicrobial (luteolin), anti cancerous and lipid lowering effects, tannins responsible for astringent, wound healing, antioxidant, anti cancer, anti viral and antimicrobial activity. In addition to these phytocompounds bark also contains triterpenoids responsible mainly for cardio protective and antibacterial (arjunic acid, arjungenin and, arjunetin) effect15, 16, 23.
A wide variety of medicinal plants used traditionally have not yet been systematically investigated against various microbial pathogens24. Literature search reveals the conducting of a few studies on antibacterial and antifungal activity of various parts of this plant, such as bark23, 25, 26, leaves, root and fruit15, 27, 28. However, antimicrobial studies against pathogens causing ear infection are lacking. The present study was carried out to validate the antimicrobial potential of T. arjuna leaves and bark against the bacterial and fungal ear pathogens isolated from the local patients of Kurukshetra and their comparison with locally available ear drops with a view of searching a novel extract as a remedy for treating ear infections.
MATERIAL AND METHODS
Plant collection
The bark and leaves of Terminalia arjuna were collected from the trees alongside the roads of University of Kurukshetra, Haryana. The taxonomic identity of this plant was confirmed by Dr. B.D. Vashishta of Botany Department, Kurukshetra University, Kurukshetra.
Extraction of plant material
The samples were carefully washed under running tap water followed by sterile distilled water and air dried at room temperature (40°C) for 4-5 days and then homogenized to a fine powder using a sterilized mixer grinder and stored in air tight bottles. Four different solvents namely ethanol, methanol, acetone and aqueous (hot and cold) were used for extraction. A 10 g amount of homogenized bark and leaves were separately soaked in conical flasks each containing100 ml of acetone, ethanol, methanol (95%) and sterile distilled water. Also the same amount (i.e. 10 g) of homogenized bark and leaves were immersed separately in 100 ml of hot sterile distilled water in conical flasks and allowed to stand for 30 min on a waterbath with occasional shaking followed by keeping all the flasks on rotary shaker at 200 rpm for 24 h29, 30, 31. Each preparation was filtered through a sterilized Whatman No. 1 filter paper and finally concentrated to dryness under vacuum at 40°C using rotaevaporator. The dried extract thus obtained was sterilized by overnight UV-irradiation and checked for sterility on nutrient agar plates and stored at 4°C in labeled sterile bottles until further use14, 32.
Test microorganisms
Five bacteria namely Staphylococcus aureus (HM626197)* (Gram-positive), Acinetobacter sp. (HM626198), Proteus mirabilis (HM626199), Escherchia coli (HM626200), Pseudomonas aeruginosa (HM626201) (Gram-negative) and one yeast, Candida albicans used in this study, were isolated from the patients having ear infection from the local ENT clinics of Kurukshetra. Bacterial strains were identified on the basis of staining, biochemical and molecular characteristics (16S rRNA sequencing)33 and yeast on the basis of staining, morphological and cultural characteristics5, 34, 35. The bacterial isolates were subcultured on Nutrient agar and yeast on Malt yeast agar and incubated aerobically at 37oC. The media were procured from Hi Media Laboratory Pvt. Ltd., Bombay, India.
Ear drops
Three commonly prescribed ear drops by otolaryngologist, two allopathic Ciplox (antibacterial), Candid (antifungal), and a herbal ear drop Bilwa Tel (antimicrobial), used in this study, were procured from the local market of Kurukshetra.
Screening for antimicrobial activity
The acetone, methanol, ethanol, hot and cold aqueous T. arjuna leaves and bark extracts were used for evaluation of the antimicrobial activity by the agar well diffusion method36, 37. In this method, pure isolate of each microbe was subcultured on the agar media plates at 370C for 24 h. One plate of each microorganism was taken and a minimum of four colonies were touched with a sterile loop and transferred into normal saline (0.85%) under aseptic conditions. Density of each microbial suspension was adjusted equal to that of 106 cfu/ml (standardized by 0.5McFarland standard) and used as the inoculum for performing agar well diffusion assay. One hundred microlitre (100μl) of inoculum of each test organism was spread onto the agar plates so as to achieve a confluent growth. The agar plates were allowed to dry and wells of 8mm were made with a sterile borer in the inoculated agar plates and the lower portion of each well was sealed with a little specific molten agar medium. The dried extracts were reconstituted in 20% dimethylsulphoxide (DMSO) for the bioassay analysis38. A 100μl volume of each extract was propelled directly into the wells (in triplicates) of the inoculated agar plates for each test organism. The plates were allowed to stand for 1hr for diffusion of the extract into the agar and incubated at 37OC for 24h.32, 39. Sterile DMSO (20%) served as the negative control and ciplox (for bacteria), candid (for fungi) and Bilwa tel (antimicrobial) ear drop served as the positive control. The antimicrobial activity, indicated by an inhibition zone surrounding the well containing the extract, was recorded if the zone of inhibition was greater than 8mm40. The experiments were performed in triplicates and the mean values of the diameter of inhibition zones with ± standard deviation were calculated.
Determination of minimum inhibitory concentration (MIC)
MIC for each test organism was determined by following the modified agar well diffusion method. A twofold serial dilution of each extract was prepared by first reconstituting the dried extract (100 mg/ml) in 20% DMSO followed by dilution in sterile distilled water (1:1) to achieve a decreasing concentration range of 50mg/ml to 0.39mg/ml. A 100 μl volume of each dilution was introduced into wells (in triplicate) in the agar plates already seeded with 100μl of standardized inoculum (106 cfu/ml) of the test microbial strain. All test plates were incubated aerobically at 37oC for 24 hrs and observed for the inhibition zones. MIC, taken as the lowest concentration of the test extract that completely inhibited the growth of the microbe, showed by a clear zone of inhibition (>8mm), was recorded for each test organism32, 37, 41, 42.
Statistical analysis
All data were presented as mean ± SD (Standard deviation). Results were statistically evaluated using Dennett's T-test. P value less than 0.01 were considered significant.
RESULTS
The antibacterial activity of T. arjuna bark and leaves extracts on the agar plates varied in different solvents. Of the ten extracts screened for antifungal activity, none of the extract showed the production of inhibition zone against Candida albicans thus lacking antifungal activity. Positive controls produced significantly sized inhibition zones against the tested bacteria and yeast, however, negative control produced no observable inhibitory effect against any of the test organism (Table 1).
Table 1.
Antimicrobial activity of Terminalia arjuna leaves and bark extract on ear pathogens determined by agar well diffusion method.
| Arjuna plant | Diameter of growth of inhibition zones (mm) |
|||||
|---|---|---|---|---|---|---|
| Staphylococcus aureus | Pseudomonas aeruginosa | Proteus mirabilis | Escherchia coli | Acitenobacter Sp. | Candida albicans | |
| Leaves extracts (mg/ml) | ||||||
| Methanol | 25.6*a ±0.57b | 16.3±0.57 | 24.6±0.57 | - | 15±0 | - |
| Ethanol | 26.3±0.57 | 15.6±0.57 | 26±0 | - | 15.3±0.57 | - |
| Acetone | 28±0 | 16±0 | 27.6±0.57 | - | 16.6±0.57 | - |
| Hot aqueous | - | - | - | - | - | - |
| Cold aqueous | - | - | - | - | - | - |
| Bark extracts (mg/ml) | ||||||
| Methanol | 23.6±0.57 | - | 15±0 | 15.6±0.57 | 17±0 | - |
| Ethanol | 26±0 | - | 14.6±0.57 | 15±0 | 16.3±0.57 | - |
| Acetone | 23.3±0.57 | - | 16.3±0.57 | 15.6±0.57 | 17.6±0.57 | - |
| Hot aqueous | 27.6±0 .57 | - | - | - | - | - |
| Cold aqueous | 26.3±0.57 | - | - | - | - | - |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 |
| Ciplox ear drop | 56.3±0.57 | 34±0 | 46.3±0.57 | 36±0 | 32.6±0.57 | nt |
| Bilva tel ear drop | 13.6±0.57 | - | 11.6±0.57 | - | - | - |
| Candid ear drop | nt | nt | nt | nt | nt | 21.3±0.57 |
No activity, nt = not tested.
Values, including diameter of the well (8mm), are means of three replicates
± Standard deviation. The data were analyzed by one way ANOVA followed by Dunnett's t test.
p<0.01 compared to positive control.
A perusal of the data in Table 1 reveals that all the six organic solvent extracts of both leaves and bark possessed antibacterial activity against the four bacterial pathogens, of the five tested pathogens. The acetonic leaf extract was found to be most effective against Staphylococcus aureus (28mm) (Figure 1) followed by Proteus mirabilis (27.6mm), Acinetobacter sp (16.6mm) and Pseudomonas aeruginosa (16mm). The inhibiton zones produced by the methanolic and ethanolic extracts against S. aureus and P. mirabilis ranged between 24.6mm and 26.3 mm. S. aureus and P. mirabilis were found to be most sensitive pathogens which survived upto 1.56 mg/ml and 3.12 mg/ ml (Table 2), thus having an MIC of 3.12 mg/ml and 6.25 mg/ml, respectively. The inhibition zone produced by the organic leaf extracts against P. aeruginosa and Acitenobacter sp. ranged between 15mm and 16.6mm. P. aeruginosa and Acitenobacter sp. were found to be comparatively less sensitive as they survived up to 25mg/ml (methanolic and ethanolic extracts) and 12.5mg/ml (acetonic extract) thus having an MIC of 50 mg/ml and 25 mg/ml, respectively. No antibacterial activity was observed in aqueous and organic leaf extracts against E. coli.
Figure 1.
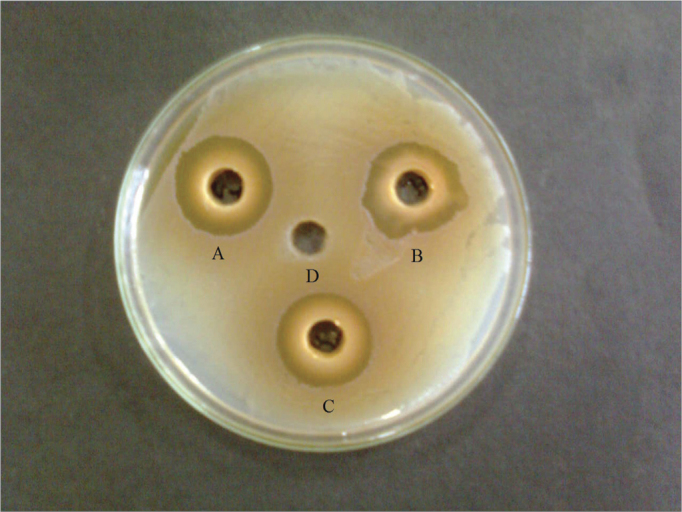
Zone of antibacterial inhibition of T. arjuna leaves organic extracts against S. aureus by acetonic (A), methanolic (B), ethanolic extract (C), and negative control DMSO (D).
Table 2.
MIC of Terminalia arjuna leaves and bark extract on ear pathogens determined by modified agar well diffusion method.
| Arjuna plant | MIC (mg/ml) |
||||
|---|---|---|---|---|---|
| Staphylococcus aureus | Pseudomonas aeruginosa | Proteus mirabilis | Escherchia coli | Acitenobacter Sp. | |
| Leaves extracts | |||||
| Methanol | 6.25 | 50 | 6.25 | nt | 50 |
| Ethanol | 6.25 | 50 | 6.25 | nt | 50 |
| Acetone | 3.12 | 25 | 3.12 | nt | 25 |
| Bark extracts | |||||
| Methanol | 3.12 | nt | 50 | 25 | 25 |
| Ethanol | 1.56 | nt | 50 | 50 | 25 |
| Acetone | 3.12 | nt | 25 | 50 | 25 |
| Hot aqueous | 1.56 | nt | nt | nt | nt |
| Cold aqueous | 1.56 | nt | nt | nt | nt |
nt = not tested
Among the tested bark extracts, all the five solvent extracts (organic and aqueous) showed antibacterial activity against S. aureus with highest zone of inhibition in hot aqueous extract (27.6mm) followed by cold aqueous extract (26.3mm) (Figure 2), ethanol extract (26mm), methanol and acetone extracts (23mm). S. aureus was again found to be the most sensitive pathogen which survived upto lowest concentration i.e.0.78 mg/ml in hot water, cold water and ethanol extracts (Table 2), thus having an MIC of 1.56 mg/ml followed by the methanolic and acetonic extracts 3mg/ml. The inhibition zones produced by the three organic solvents against Acitenobacter sp. ranged between 16.3mm and 17mm. Acitenobacter sp. was comparatively less sensitive as it survived upto 12.5 mg/ml, thus having an MIC of 25mg/ml. The zone of inhibition produced by the organic solvent bark extracts against P. mirabilis and Escherchia coli was almost equal and ranged between 14.6mm and 16.3mm. P. mirabilis survived up to 12.5mg/ml (acetone) and 25mg/ml (methanol and ethanol), thus having an MIC of 25mg/ml and 50 mg/ml respectively while E. coli survived upto 25mg/ml (ethanol and acetone) and 12.5mg/ml (methanol) thus having an MIC of 50 mg/ml and 25 mg/ml, respectively. Bark extracts, both organic and aqueous, however, lacked antibacterial activity against P. aeruginosa. All the obtained results are statistically significant as they showed (p < 0.001) as compared with control.
Figure 2.
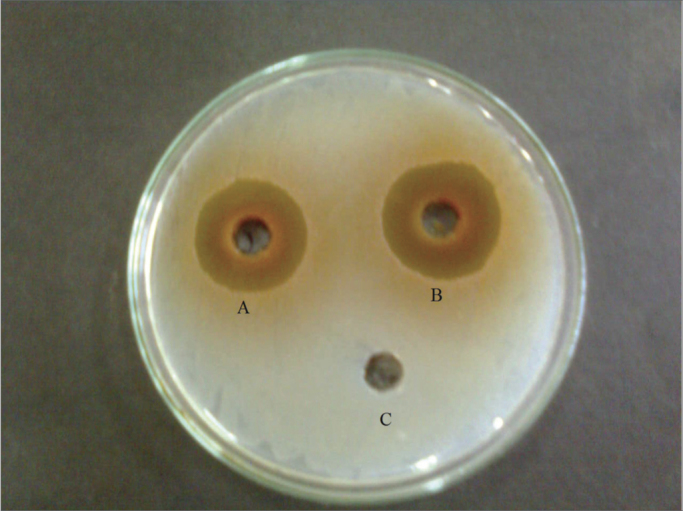
Zone of antibacterial inhibition of T. arjuna bark aqueous extracts against S. aureus by hot aqueous (A), cold aqueous extract (B), and negative control DMSO (C).
DISCUSSION
The organic extracts of T. arjuna leaves possessed good activity against the four bacterial ear pathogens namely Staphylococcus aureus, Pseudomonas aeruginosa, Proteus mirabilis, Acitenobacter sp. and did not exhibit any activity against E. coli. Interestingly, the bark extract also showed good bioactivity against the four bacterial ear pathogens, however, P. aeruginosa was not found sensitive to the bark extracts. The antifungal activity against C. albicans was totally absent in the both organic and aqueous T. arjuna leaf and bark extracts. In earlier study, the root extracts of this plant had been shown to possess activity against Aspergillus niger and Candida albican27.
The aqueous leaves and bark extracts of T. arjuna lacked antifungal activity against C. albicans and antibacterial activity against the tested bacteria except a Gram positive bacterium, S. aureus that was found sensitive both to the hot and cold aqueous bark extracts. The limited spectrum of antimicrobial activity in the aqueous extracts may be due to three reasons: firstly the polarity of antibacterial compounds make them more readily extracted by organic solvents as compared to aqueous extract; secondly active compound may be present in insufficient amount in the crude extract to show activity with the dose level employed; and thirdly if the active principle is present in high quantities, there could be other constituents present in the extract exerting antagonistic effects of the bioactive compounds43.
Of the three organic extracts of leaves screened, the acetonic extract has been found to have a better antibacterial activity than the corresponding ethanolic and methanolic extracts (Table 1) thus substituting the findings of earlier workers11, 37, 44, 45 who rated acetone as the best solvent. The antibacterial activity exhibited by T. arjuna leaves and bark extracts against the bacterial ear pathogens might be due to the presence of secondary metabolites such as arjunic acid, arjungenin, arjunetin and luteolin which have been reported to be antimicrobial in nature15, 16, 23.
This study reveals that both the leaves and bark organic extracts of T. arjuna have broad spectrum antibacterial activity against the ear pathogens as visualized by the formation of inhibition zones of both Gram positive and Gram negative bacteria. Interestingly, all the organic extracts of this plant showed much more potent activity against the tested ear bacteria than that of standard herbal ear drop (Bilwa tel) thus having a great potential to be developed as a herbal ear drop to control the bacterial ear infections.
CONCLUSIONS
Our results allow us to conclude that, all the tested T. arjuna extracts (in any of the tested preparation-acetone, ethanol or methanol) have shown good activity against both the Gram positive and Gram negative ear pathogens, the inhibition being higher in Gram positive bacteria than the Gram negative bacteria. This probably explains the use of this plant by the indigenous people against a number of infections since generations. As of now, little work has been done on the antimicrobial activity and plausible medicinal applications of the phytochemical compounds and hence extensive investigations are needed such as in vivo studies on this plant necessary to determine toxicity of the active constituents, their side effects, pharmacokinetics properties to exploit the bioactive principles, for therapeutic utility in treating the ear infections. The antibacterial activities can be enhanced if the active components are purified and adequate dosage determined for proper administration. At last, the need of the hour is the development of an effective phytocompound into an exploitable herbal product, which is devoid of side effects and drug resistance problem.
Footnotes
Paper submitted to the BJORL-SGP (Publishing Management System – Brazilian Journal of Otorhinolaryngology) on September 8, 2010; and accepted on February 20, 2011. cod. 7312
Nucleotide sequence of all the five bacteria have been submitted to GenBank database, which have provided GenBank accession number, HM626197-HM626201.
REFERENCES
- 1.Gates GA. Consideration of Otitis Media treatment. Otolaryngol Head Neck Surg. 1996;114(4):525–530. doi: 10.1016/S0194-59989670243-7. [DOI] [PubMed] [Google Scholar]
- 2.Roland PS, Stroman DW. Microbiology of acute otitis externa. La-rygoscope. 2002;112(7 Pt 1):166–177. doi: 10.1097/00005537-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld RM, Brown L, Cannon RC, Dolor JR, Ganiats GT, Hannley M, et al. Clinical practice guideline: Acute otitis externa. American Acad Otolaryngology-Head and Neck Surgery Foundation. 2006;134(4 Suppl):s4–s23. doi: 10.1016/S0194-5998(06)00266-X. [DOI] [PubMed] [Google Scholar]
- 4.Dhingra PL. Diseases of Ear, Nose and Throat. 4th. Reed Elsevier India. Private Limited; New Delhi: 2007. [Google Scholar]
- 5.Aneja KR, Sharma C, Joshi R. Fungal infection of the ear: A common problem in the north eastern part of Haryana. Int J Ped Otorhinolaryngol. 2010;74(6):604–607. doi: 10.1016/j.ijporl.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Bandow JE, Brotz H, Leichert LIO, Labischinski H, Hecker M. Prote-omic approach to understanding antibiotic action. Antimicrob Agents Chemother. 2003;47(3):948–955. doi: 10.1128/AAC.47.3.948-955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anonymous. Fighting superbugs. CQ Researcher. 2007;17(29):673–696. [Google Scholar]
- 8.Aneja KR, Sharma C, Joshi R. In: Antimicrobial Resistance: From Emerging Threat to Reality. Lawrence R, Gulati AK, Abraham G, editors. Narosa Publishing House; New Delhi: 2009. Antimicrobial susceptibility pattern of common human pathogenic bacteria and yeasts against commercially available drugs; pp. 84–90. [Google Scholar]
- 9.Scazzocchio F, Cometa MF, Tomassini L, Palmery M. Antibacterial activity of Hydratis canadensis extract and its major isolated alkaloids. Planta Med. 2001;67(6):561–564. doi: 10.1055/s-2001-16493. [DOI] [PubMed] [Google Scholar]
- 10.Clark AM. Natural products as a resource for new drugs. Pharmacy Research. 1996;13(8):1133–1144. doi: 10.1023/a:1016091631721. [DOI] [PubMed] [Google Scholar]
- 11.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . WHO Policy Perspectives on Medicine. World Health Organization; Geneva: 2002. Traditional Medicine: Growing needs and potential; pp. 1–6. [Google Scholar]
- 13.Dahanukar SA, Kulkarni RA, Rege NN. Pharmacology of medicinal plants and natural products. Indian J Pharmacol. 2000;32(4):S81–S118. [Google Scholar]
- 14.Kumar VP, Chauhan NS, Padh H, Rajani M. Search for antibacterial and antifungal agents from selected Indian medicinal plants. J Ethnopharmacol. 2006;107(2):182–188. doi: 10.1016/j.jep.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Dwivedi S. Terminalia arjuna Wight & Arn.-A useful drug for cardiovascular disorders. J Ethnopharmacol. 2007;114(2):114–129. doi: 10.1016/j.jep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Jain S, Yadav PP, Gill V, Vasudeva N, Singla N. Terminalia arjuna a sacred medicinal plant; phytochemical and pharmacological profile. Phytochem Rev. 2009;8(2):491–502. [Google Scholar]
- 17.Udupa KN. Scope of use of Terminalia arjuna in ischaemic heart disease. Ann Natl Acad Indian Med. 1986;1:54. [Google Scholar]
- 18.Khory RN. The Bombay material medica and their therapeutics. Renina's Union Press; 1867. [Google Scholar]
- 19.Tripathi VK, Singh B. Terminalia arjuna- its present status (a review) Orient J Chem. 1996;12(1):1–16. [Google Scholar]
- 20.Cooper EL. CAM, eCAM, Bioprospecting: the 21st century pyramid. Evid Based Compliment Alternat Med. 2005;2(2):125–127. doi: 10.1093/ecam/neh094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sher A. Antimicrobial activity of natural products from medicinal plants. Gomal J Med Sci. 2009;7(1):72–78. [Google Scholar]
- 22.Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J Med Plants Res. 2010;4(2):104–111. [Google Scholar]
- 23.Singh DV, Gupta MM, Kumar TRS, Saikia D, Khanuja SPS. Antibacterial principles from the bark of Terminalia arjuna. Curr Sci. 2008;94(1):27–29. [Google Scholar]
- 24.Farrukh A, Iqbal A. Broad spectrum antibacterial and antifungal properties of certain traditionally used Indian medicinal plants. World J Microbiol Biotechnol. 2003;19(6):653–657. [Google Scholar]
- 25.Aggarwal RR, Dutt S. Chemistry, pharmacology and therapeutic actions of Terminalia arjuna. Proc Nat Acad Sci. 1936;6:305. [Google Scholar]
- 26.Perumalsamy R, Ignacimuthu S, Sen A. Screening of 34 Indian Medicinal plants for antibacterial properties. J Ethnopharmacol. 1998;62(2):173–182. doi: 10.1016/s0378-8741(98)00057-9. [DOI] [PubMed] [Google Scholar]
- 27.Chouksey BK, Srivastava SK. Antifungal agent from Terminalia arjuna. Indian J Chem. 2001;40B:354–356. [Google Scholar]
- 28.Ramya S, Kalaivani T, Rajasekaran C, Jepachanderamohan P, Alaguc-hamy N, Kalayasundaram M, et al. Antimicrobial activity of aqueous extracts of bark, root, leaves and fruits of Terminalia arjuna Wright & Arn. Ethanobotanical leaflets. 2008;12:1192–1197. [Google Scholar]
- 29.Akueshi CO, Kadiri CO, Akueshi EU, Agina SE, Ngurukwem B. Antimicrobial potential of Hyptis sauvedena Poit (Lamisceae) Nigeria J Bot. 2002;15(1):37–41. [Google Scholar]
- 30.Ogundiya MO, Okunade MB, Kolapo AL. Antimicrobial activities of some Nigerian Chewing Sticks. Ethnobotanical leaflets. 2006;10:265–271. [Google Scholar]
- 31.Preethi R, Devanathan VV, Loganathan M. Antimicrobial and antioxidant efficacy of some medicinal plants against food borne pathogens. Advances Biol Res. 2010;4(2):122–125. [Google Scholar]
- 32.Okeke MI, Iroegbu CU, Eze EN, Okoli AS. Esimone, CO. Evaluation of extracts of the roots of Landolphia owerrience for antibacterial activity. J Ethnopharmacol. 2001;78(2-3):119–127. doi: 10.1016/s0378-8741(01)00307-5. [DOI] [PubMed] [Google Scholar]
- 33.Lawongsa P, Boonkerd N, Wongkaew S, Gara FO, Teaumroong N. Molecular and phenotypic characterization of potential plant growth-promoting Pseudomonas from rice and maize rhizospheres. World J Microbiol Biotechnol. 2008 doi: 10.1007/s11274-008-9685-7. [DOI] [Google Scholar]
- 34.Aneja KR. Experiments in Microbiology, Plant Pathology and Biotechnology. 4th. New Age International Publishers; New Delhi: 2003. [Google Scholar]
- 35.Cappuccino JG, Sherman N. Microbiology Lab Manual. 7th. Benjamin Cummings Publishing Company; USA: 2008. [Google Scholar]
- 36.Ahmad I, Beg AZ. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol. 2001;74(2):113–123. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 37.Aneja KR, Joshi R, Sharma C. Antimicrobial activity of Dalchini (Cinnamomum zeylanicum bark) extracts on some dental caries pathogens. J Phar Res. 2009;2(9):1370–1372. [Google Scholar]
- 38.Rajasekaran C, Meignanam E, Vijayakumar V, Kalaivani T, Ramya S, Premkumar N, et al. Investigation on antibacterial activities of leaf extracts of Azadirachta indica A. Juss (Meliaceae) a traditional medicinal plant of India. Ethnabotanical Leaflets. 2008;12:1213–1217. [Google Scholar]
- 39.Rios JL, Recio MC, Villar A. Screening methods for natural products with antibacterial activity: a review of the literature. J Ethnopharmacol. 1980;23(2-3):127–149. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 40.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J App Microbiol. 1999;86(6):985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 41.Thongson C, Davidson PM, Mahakarnchanakul W, Weiss J. Antimicrobial activity of ultra sound- assisted solvent - extracted spices. Lett Appl Microbiol. 2004;39(5):401–406. doi: 10.1111/j.1472-765X.2004.01605.x. [DOI] [PubMed] [Google Scholar]
- 42.Nkere CK, Iroegbu CU. Antibacterial screening of the root, seeds and stem bark extracts of Picralima nitida. Afr J Biotechnol. 2005;4(6):522–526. [Google Scholar]
- 43.Sangetha SN, Zuraini Z, Sasidharan S, Suryani S. Antimicrobial activities of Cassia surattensis and Cassia fistula. J Mol Bio Biotech. 2008;1(1):1–4. [Google Scholar]
- 44.Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol. 1998;60(1):1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 45.Nair R, Kalariya T, Sumitra C. Antibacterial activity of some selected Indian medicinal plants. Turk J Biol. 2005;29(1):41–47. [Google Scholar]


