Abstract
Background and Purpose
Aging in multiple sclerosis is associated with both disease- and age-dependent neurodegeneration. Serum metabolomic profiling of amino acids seems to be a promising method for searching for biomarkers of neurodegenerative disorders. The aim of this study was to determine the profile of nonessential amino acids in the serum of elderly patients with secondary progressive multiple sclerosis (SPMS).
Methods
We used high-performance liquid chromatography to evaluate the serum concentrations of nonessential amino acids in subjects aged >65 years: six patients with SPMS and 20 control subjects (CS).
Results
The serine and alanine levels were significantly higher in SPMS patients than in CS, whereas the concentrations of aspartic acid, arginine, and cysteine were significantly lower in SPMS patients. These observations indicate that amino acids may be involved in SPMS neurodegeneration mechanisms. There were no significant differences in the serum concentrations of the other four amino acids investigated (glutamic acid, glycine, proline, and tyrosine) between patients with SPMS and CS.
Conclusions
The preliminary results obtained in the study suggest that the metabolism of some amino acids is altered in patient with SPMS. We also conclude that amino acid profiling might be helpful in searching for putative biomarkers of central nervous system diseases. However, considering the multifactorial, heterogeneous, and complex nature of SPMS, further validation research involving larger study samples is required before applying these biomarkers in diagnostic practice.
Keywords: metabolomics, amino acid profiling, biomarkers, secondary progressive multiple sclerosis, aging
INTRODUCTION
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS) involving both inflammatory and neurodegenerative damage patterns. Most patients (85%–90%) experience initial relapsing-remitting MS (RRMS) characterized by relapses associated with the formation of new inflammatory areas in the CNS, followed by a complete or partial recovery and subsequent remission periods free from disability progression.1,2,3 The remaining 10%–15% of patients suffer from a gradual worsening of neurological deficits from the disease onset without clinical attacks, which is defined as primary progressive MS (PPMS).4,5 Within 20–25 years from the disease onset, 90% of untreated RRMS subjects experience conversion to secondary progressive MS (SPMS) in which disability accumulation is a consequence of the predominant neurodegeneration mechanisms. The mean ages at symptom onset for RRMS, PPMS, and SPMS are around 30, 40, and 49–53 years, respectively.6 However, a retrospective analysis of the disability progression of patients found that SPMS is usually not diagnosed until after a delay of several years.7,8,9 This is mainly due to the lack of universally accepted definition of SPMS and its specific biomarkers. Thus, the vast majority of elderly MS patients have SPMS and experience both disease- and age-dependent neurodegeneration processes. Unfortunately, the availability of few therapeutic options for this MS variant significantly aggravate its inconvenience in the daily lives of elderly SPMS patients.10 Therefore, there is a strong need to identify biomarkers and new treatment options for neurodegeneration in SPMS.
Serum metabolomic profiling of amino acids seems to be a promising method of searching for biomarkers of neurodegenerative disorders.11 Changes in the profile of amino acids in neurodegenerative disorders such as Parkinson’s disease as well as in the physiological process of CNS aging have been confirmed.12,13,14 Recent studies showed that measurements of a wide spectrum of proteins and their metabolites in the cerebrospinal fluid (CSF) in combination with advanced MRI methods can be useful in distinguishing the different variants of MS.15 However, routine CSF testing is performed at the beginning of the diagnostic process when most patients already have RRMS, and its reassessment after conversion to SPMS is associated with significant inconvenience, with 1) patients often refusing another lumbar puncture because of fear of post-lumbar-puncture syndrome, 2) the increasing probability of degenerative changes in the lumbar spine with age possibly resulting in iatrogenic blood contamination of the CSF and leading to false measurements especially in elderly subjects, and 3) advanced MRI methods still being reserved for clinical investigations and not being routinely used in everyday clinical practice. Therefore, the purpose of the present study was to determine the profile of nonessential amino acids in the serum of elderly SPMS patients.
METHODS
Chemicals and materials
AccQ Fluor Reagent Kit (Waters Corporation, Milford, MA, USA), amino acid standards (Waters), and internal standard α-aminobutyric acid, acetonitrile, and methanol (all Sigma Aldrich; Merck, Rahway, NJ, USA). Deionized water purified with the Direct–QUV system (Millipore, Molsheim, France) was used to produce all aqueous solutions.
Instrumentation
High-performance liquid chromatography (HPLC) was performed with a Shimadzu device combined with a diode array detector and RF-20A XS fluorescence detector (Shimadzu, Kyoto, Japan).
Sample preparation
The preparation of serum for chromatographic analysis included deproteinization and derivatization of the sample. Serum was deproteinized using Phree Phospholipid Removal Solutions (Phenomenex, Torrance, CA, USA) with a solid-phase extraction kit. The prepared serum samples were subjected to a derivatization procedure in accordance with the Waters AccQ Tag method.
Chromatographic method
HPLC was used to measure the amino acid concentrations in samples with fluorescence detection using an AccQ Tag column (Waters). The final parameters of the chromatographic system were a 150 mm×3.9 mm chromatographic column (AccQ Tag, Waters), fluorescence detection with excitation at 250 nm and emissions measured at 395 nm, mobile phase A comprising AccQ Tag Eluent A (buffer), mobile phase B comprising acetonitrile, mobile phase C comprising water, gradient elution at a flow rate of 1 mL/min and an injection volume of 5 µL, and an analysis time of 35 minutes.
Characteristics of study participants
This study enrolled 6 patients (4 females and 2 males) aged 69.5±2.1 years (mean±SD) who consulted a neurological outpatient clinic between January and March 2019 based on a diagnosis of MS according to the 2010 McDonald criteria.16 The disease duration was 22.5±3.5 years and the patients’ disability score on the Expanded Disability Status Scale (EDSS) was 5.5±1.4.17 The clinical MS type was SPMS in all patients, as diagnosed retrospectively by confirmation of gradual and irreversible worsening of neurological deficits, progressing for at least 6 months independently of relapses and following an RRMS course.2 None of the SPMS patients had received immunomodulatory therapy, immunosuppressive therapy, or corticosteroid treatment within 6 months before obtaining blood samples. The control subjects (CS) comprised 14 females and 6 males aged 72.3±6.3 years without inflammatory disorders of the CNS and peripheral nervous system.
The study protocol was approved by the Bioethics Committee of Ludvik Rydygier Collegium Medicum (KB 135/2019), and written informed consent was obtained from all participants.
RESULTS
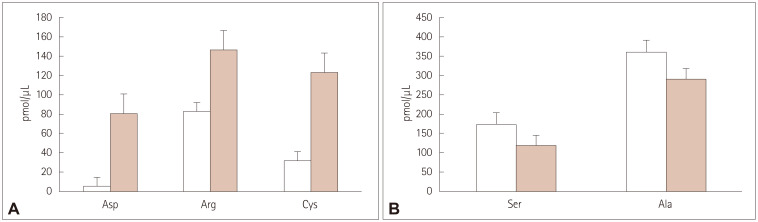
Fig. 1 present example chromatograms of the separation of the amino acid standards and the separation of amino acids in serum, respectively. The mean serum levels of aspartic acid (Asp), arginine (Arg), and cysteine (Cys) were significantly lower in the SPMS patients than in CS (Fig. 2A), whereas the mean serum concentrations of serine (Ser) and alanine (Ala) were significantly higher in SPMS patients than in CS (Fig. 2B). There were no statistically significant differences in the mean serum levels of glutamic acid (Glu), glycine (Gly), proline (Pro), and tyrosine (Tyr) between SPMS patients and CS (Fig. 3).
Fig. 1. Chromatograms showing the separation of amino acid standards (A) and the separation of amino acids in serum (B). The timing of peak elution occurred in the following order: 1, Asp; 2, Ser; 3, Glu; 4, Gly; 5, histidine; 6, NH3; 7, Arg; 8, threonine; 9, Ala; 10, Pro; 11, Cys; 12, Tyr; 13, valine; 14, methionine; 15, lysine; 16, isoleucine; 17, leucine; and 18, phenylalanine. Ala, alanine; Arg, arginine; Asp, aspartic acid; Cys, cysteine; Glu, glutamic acid; Gly, glycine; NH3, ammonia; Pro, proline; Ser, serine; Tyr, tyrosine.
Fig. 2. Serum levels of endogenous amino acids in SPMS patients (□) and in CS without disease of the nervous system ( ) for which serum concentrations were significantly lower (A) or significantly lower (B) in SPMS patients than in CS. Values are mean and SD values of three individual experiments. Ala, alanine; Arg, arginine; Asp, aspartic acid; CS, control subjects; Cys, cysteine; SD, standard deviation; Ser, serine; SPMS, secondary progressive multiple sclerosis.
) for which serum concentrations were significantly lower (A) or significantly lower (B) in SPMS patients than in CS. Values are mean and SD values of three individual experiments. Ala, alanine; Arg, arginine; Asp, aspartic acid; CS, control subjects; Cys, cysteine; SD, standard deviation; Ser, serine; SPMS, secondary progressive multiple sclerosis.
Fig. 3. Serum levels of endogenous amino acids in SPMS patients (□) and CS ( ) for which serumconcentrations did not differ significantly between the groups. Values are mean and SD values of three individual experiments. CS, control subjects; Glu, glutamic acid; Gly, glycine; Pro, proline; SD, standard deviation; SPMS, secondary progressive multiple sclerosis; Tyr, tyrosine.
) for which serumconcentrations did not differ significantly between the groups. Values are mean and SD values of three individual experiments. CS, control subjects; Glu, glutamic acid; Gly, glycine; Pro, proline; SD, standard deviation; SPMS, secondary progressive multiple sclerosis; Tyr, tyrosine.
DISCUSSION
Neurodegeneration is a major contributor to disability progression in MS and remains the dominant process underlying the secondary progressive phase of the disease.18 Unfortunately, SPMS patients, especially those older than 65 years, also experience age-related neurodegeneration processes. Therefore, identifying SPMS-specific neurodegeneration markers may be crucial for the early recognition of this MS variant and the implementation of appropriate treatment. We found that the serum levels of five amino acids differed significantly between SPMS patients older than 65 years and CS. The serum concentrations of Ala and Ser were higher in SPMS patients, while circulating levels of Asp, Arg, and Cys were higher in CS. On the other hand, there were no significant differences in serum concentrations of the other four amino acids (Glu, Gly, Pro, and Tyr) between patients with SPMS and CS. The potential causes of changes in serum amino acid concentrations in neurodegenerative CNS disorders include the death of oligodendrocytes and neurons, oxidative stress, and mitochondrial dysfunction.14 Adachi et al.12 compared the serum concentrations of amino acids between severely frail and nonfrail subjects older than 65 years, and found that glutamine and Tyr concentrations were higher and lower, respectively, in the severity frail subgroup. In our study, significant differences in serum levels between SPMS patients and CS were found for 5 other nonessential amino acids, which may indicate the distinctness of neurodegeneration in SPMS from brain aging processes. The role of Ser as a possible MS biomarker was suggested by Poser et al.19 as early as 1974, who observed that CSF protein Ser residue was strongly correlated with the CSF IgG, and that the serum/CSF protein Ser ratio appeared to be correlated most strongly with the clinical severity of the disease (the most active cases with the lowest ratio) . It was already found in some studies that the levels of asparagine, glutamate, and N-acetyl-aspartate (NAA) were higher in plasma samples from patients with MS due to activation of the Asp biosynthesis pathway. This phenomenon was confirmed by Kasakin et al.,20 however their results of an increase in Asp concentration in their MS group and an increase in the glutamate concentration were not statistically significant. It should be emphasized that Asp, Glu, and other amino acids are related to the amino acid superpathway, and that their metabolism is connected to the tricarboxylic acid cycle and oxaloacetate and α-ketoglutarate intermediates; furthermore, Asp is a precursor of NAA.
Sarchielli et al.21 found that CSF glutamate levels were significantly higher in SPMS patients with an increase of 1 or more points on the EDSS during the previous 6 months than in stable SPMS patients and CS. Those authors did not find any difference in CSF glutamate levels between SPMS patients without disability progression and CS, or any difference in the CSF aspartate concentration between SPMS patients and CS.21 It is not possible to compare these results with those from our cohort due to the amino acids concentrations being assessed in different body compartments. Gawwam and Sharquie22 not only confirmed elevated concentrations of serum glutamate in MS patients, but also suggested that it should be measured during different activity phases of MS (acute attack, remission, and relapse) or even in close relatives of patients suffering from MS in order to determine if they are susceptible to developing this disease in the future. However, those authors suggested that further investigations are needed. Haghikia et al.23 found no differences in serum levels of Arg, homoarginine, nitrate, and nitrite between MS patients and subjects with other neurological diseases. Arg is a precursor of nitric oxide (NO), whose excessive production causes nitroxidative stress and contributes to neurodegeneration in MS. Thus, the plasma concentration of Arg may affect NO levels in the circulation and the CNS.24 The plasma NO concentration and the levels of its metabolites in the CSF are elevated in active forms of MS. However, the levels of NO metabolites in the CSF can be lower in advanced disease, and there is a lack of data on this relationship in serum.25 At the same time, there are few data on the plasma concentrations of other nonessential amino acids (Ser, Ala, Cys, Gly, Pro, and Tyr) in SPMS patients.
The above-mentioned evidence indicates that there are several promising candidate biomarkers suitable for clinical application in MS. However, some results remain inconclusive. For example, most reported studies show that L-glutamine is decreased in the CSF or serum,26 while Poddighe et al.27 reported that the plasma level of L-glutamine was higher in patients with MS than in healthy controls. It was particularly interesting that the HPLC analyses of CSF conducted by Reinke et al.28 found no significant differences in amino acid levels between MS patients and controls, what indicates the need for further extensive research in this field. Considering the relatively small number of participants in our study, which is its main limitation, future research should assess the serum levels of nonessential amino acids in larger cohorts of SPMS patients.
In conclusion, we found that Ser and Ala levels were significantly higher in SPMS patients than in CS, whereas the concentrations of Asp, Arg, and Cys were significantly lower in SPMS patients. The preliminary results of this study suggest that the metabolism of some amino acids is altered in patients with SPMS, and that amino acids are involved in SPMS neurodegeneration mechanisms and hence that they could also act as potential biomarkers for diagnosing SPMS patients. Therefore, amino acid profiling might be helpful in searching for putative biomarkers of CNS diseases, what is an interesting research direction for future studies.
Footnotes
- Conceptualization: Łukasz Rzepiński, Piotr Kośliński.
- Data curation: Piotr Kośliński, Marcin Koba.
- Formal analysis: Marcin Koba.
- Funding acquisition: Marcin Koba.
- Investigation: Łukasz Rzepiński, Piotr Kośliński.
- Methodology: Piotr Kośliński.
- Project administration: Łukasz Rzepiński, Piotr Kośliński.
- Resources: Łukasz Rzepiński, Piotr Kośliński.
- Software: Marcin Koba, Marcin Gackowski.
- Supervision: Zdzisław Maciejek.
- Validation: Marcin Koba, Piotr Kośliński.
- Visualization: Marcin Gackowski, Piotr Kośliński, Marcin Koba.
- Writing—original draft: Łukasz Rzepiński, Marcin Gackowski, Piotr Kośliński.
- Writing—review & editing: Marcin Gackowski, Łukasz Rzepiński, Piotr Kośliński.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available due to the nature of this research, where participants of this study did not agree for their data to be shared publicly, but are available from the corresponding author on reasonable request.
References
- 1.Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129(Pt 3):606–616. doi: 10.1093/brain/awl007. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 3.Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol. 2018;25:215–237. doi: 10.1111/ene.13536. [DOI] [PubMed] [Google Scholar]
- 4.Cottrell DA, Kremenchutzky M, Rice GP, Koopman WJ, Hader W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain. 1999;122(Pt 4):625–639. doi: 10.1093/brain/122.4.625. [DOI] [PubMed] [Google Scholar]
- 5.Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–912. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- 6.Tremlett H, Zhao Y. Primary and secondary progressive MS have a similar age at onset of progression-NO. Mult Scler. 2017;23:640–642. doi: 10.1177/1352458516684559. [DOI] [PubMed] [Google Scholar]
- 7.Lorscheider J, Buzzard K, Jokubaitis V, Spelman T, Havrdova E, Horakova D, et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139(Pt 9):2395–2405. doi: 10.1093/brain/aww173. [DOI] [PubMed] [Google Scholar]
- 8.Rzepiński Ł, Zawadka-Kunikowska M, Maciejek Z, Newton JL, Zalewski P. Early clinical features, time to secondary progression, and disability milestones in polish multiple sclerosis patients. Medicina (Kaunas) 2019;55:232. doi: 10.3390/medicina55060232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz Sand I, Krieger S, Farrell C, Miller AE. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult Scler. 2014;20:1654–1657. doi: 10.1177/1352458514521517. [DOI] [PubMed] [Google Scholar]
- 10.Dumitrescu L, Constantinescu CS, Tanasescu R. Siponimod for the treatment of secondary progressive multiple sclerosis. Expert Opin Pharmacother. 2019;20:143–150. doi: 10.1080/14656566.2018.1551363. [DOI] [PubMed] [Google Scholar]
- 11.Socha E, Koba M, Kośliński P. Amino acid profiling as a method of discovering biomarkers for diagnosis of neurodegenerative diseases. Amino Acids. 2019;51:367–371. doi: 10.1007/s00726-019-02705-6. [DOI] [PubMed] [Google Scholar]
- 12.Adachi Y, Ono N, Imaizumi A, Muramatsu T, Andou T, Shimodaira Y, et al. Plasma amino acid profile in severely frail elderly patients in Japan. Int J Gerontol. 2018;12:290–293. [Google Scholar]
- 13.Corso G, Cristofano A, Sapere N, la Marca G, Angiolillo A, Vitale M, et al. Serum amino acid profiles in normal subjects and in patients with or at risk of Alzheimer dementia. Dement Geriatr Cogn Dis Extra. 2017;7:143–159. doi: 10.1159/000466688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figura M, Kuśmierska K, Bucior E, Szlufik S, Koziorowski D, Jamrozik Z, et al. Serum amino acid profile in patients with Parkinson’s disease. PLoS One. 2018;13:e0191670. doi: 10.1371/journal.pone.0191670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman S, Khoonsari PE, Tolf A, Steinmetz J, Zetterberg H, Åkerfeldt T, et al. Integration of magnetic resonance imaging and protein and metabolite CSF measurements to enable early diagnosis of secondary progressive multiple sclerosis. Theranostics. 2018;8:4477–4490. doi: 10.7150/thno.26249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 18.Bermel RA. Unravelling neurodegeneration in multiple sclerosis. Lancet Neurol. 2017;16:764–766. doi: 10.1016/S1474-4422(17)30302-2. [DOI] [PubMed] [Google Scholar]
- 19.Poser CM, Sylwester DL, Ho B. Amino acid residues of serum and CSF protein in multiple sclerosis. Trans Am Neurol Assoc. 1974;99:235–237. [PubMed] [Google Scholar]
- 20.Kasakin MF, Rogachev AD, Predtechenskaya EV, Zaigraev VJ, Koval VV, Pokrovsky AG. Changes in amino acid and acylcarnitine plasma profiles for distinguishing patients with multiple sclerosis from healthy controls. Mult Scler Int. 2020;2020:9010937. doi: 10.1155/2020/9010937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarchielli P, Greco L, Floridi A, Floridi A, Gallai V. Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch Neurol. 2003;60:1082–1088. doi: 10.1001/archneur.60.8.1082. [DOI] [PubMed] [Google Scholar]
- 22.Al Gawwam G, Sharquie IK. Serum glutamate is a predictor for the diagnosis of multiple sclerosis. ScientificWorldJournal. 2017;2017:9320802. doi: 10.1155/2017/9320802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haghikia A, Kayacelebi AA, Beckmann B, Hanff E, Gold R, Haghikia A, et al. Serum and cerebrospinal fluid concentrations of homoarginine, arginine, asymmetric and symmetric dimethylarginine, nitrite and nitrate in patients with multiple sclerosis and neuromyelitis optica. Amino Acids. 2015;47:1837–1845. doi: 10.1007/s00726-015-2015-0. [DOI] [PubMed] [Google Scholar]
- 24.Virarkar M, Alappat L, Bradford PG, Awad AB. L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit Rev Food Sci Nutr. 2013;53:1157–1167. doi: 10.1080/10408398.2011.573885. [DOI] [PubMed] [Google Scholar]
- 25.Rejdak K, Eikelenboom MJ, Petzold A, Thompson EJ, Stelmasiak Z, Lazeron RH, et al. CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology. 2004;63:1439–1445. doi: 10.1212/01.wnl.0000142043.32578.5d. [DOI] [PubMed] [Google Scholar]
- 26.Porter L, Shoushtarizadeh A, Jelinek GA, Brown CR, Lim CK, de Livera AM, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poddighe S, Murgia F, Lorefice L, Liggi S, Cocco E, Marrosu MG, et al. Metabolomic analysis identifies altered metabolic pathways in multiple sclerosis. Int J Biochem Cell Biol. 2017;93:148–155. doi: 10.1016/j.biocel.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Reinke SN, Broadhurst DL, Sykes BD, Baker GB, Catz I, Warren KG, et al. Metabolomic profiling in multiple sclerosis: insights into biomarkers and pathogenesis. Mult Scler. 2014;20:1396–1400. doi: 10.1177/1352458513516528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are not publicly available due to the nature of this research, where participants of this study did not agree for their data to be shared publicly, but are available from the corresponding author on reasonable request.