Abstract
Background and Purpose
Intracranial vertebrobasilar atherosclerotic stenosis (IVBAS) is a major cause of posterior circulation stroke. Some patients suffer from stroke recurrence despite receiving medical treatment. This study aimed to determine the prognostic value of a new score for the posterior communicating artery and the P1 segment of the posterior cerebral artery (PCoA-P1) for predicting stroke recurrence in IVBAS.
Methods
We retrospectively enrolled patients with severe IVBAS (70%–99%). According to the number of stroke recurrences, patients were divided into no-recurrence, single-recurrence, and multiple-recurrences groups. We developed a new 5-point grading scale, with the PCoA-P1 score ranging from 0 to 4 based on magnetic resonance angiography, in which primary collaterals were dichotomized into good (2–4 points) and poor (0 or 1 point). Stroke recurrences after the index stroke were recorded. Patients who did not experience stroke recurrence were compared with those who experienced single or multiple stroke recurrences.
Results
From January 2012 to December 2019, 176 patients were enrolled, of which 116 (65.9%) had no stroke recurrence, 35 (19.9%) had a single stroke recurrence, and 25 (14.2%) had multiple stroke recurrences. Patients with single stroke recurrence (odds ratio [OR]=4.134, 95% confidence interval [CI]=1.822–9.380, p=0.001) and multiple stroke recurrences (OR=6.894, 95% CI=2.489–19.092, p<0.001) were more likely to have poor primary collaterals than those with no stroke recurrence.
Conclusions
The new PCoA-P1 score appears to provide improve predictions of stroke recurrence in patients with IVBAS.
Keywords: posterior cerebral artery, ischemic stroke, magnetic resonance angiography, collateral circulation
INTRODUCTION
Approximately 35% of posterior circulation ischemic strokes are caused by intracranial vertebrobasilar atherosclerotic stenosis (IVBAS).1 Despite receiving medical treatment, the rate of stroke recurrence in patients with IVBAS ranges from 24.6% to 38.7%.2,3,4 This makes it necessary to identify high-risk patients, especially those with multiple stroke recurrences, for whom aggressive medical therapy or elective intervention might fundamentally improve their management.
Many factors have been reported to be associated with stroke recurrence in patients with IVBAS, including a chief complaint of dysphagia, repeated transient ischemic attacks within 3 months before the stroke, ≥70% stenosis in the responsible artery, multisector infarcts, and not being on antithrombotic treatment at discharge.5 However, these factors are insufficient for accurately predicting stroke recurrence. Collateral circulation dramatically alters the subsequent stroke risk in intracranial atherosclerosis.6 Patients with impaired collateral flow in the anterior circulation have a particularly high risk of stroke recurrence even when they receive medical treatment.7 Few studies have investigated the association between stroke recurrence and collateral circulation in patients with IVBAS, and there is no practicable scoring system for evaluating the primary collaterals.
Given the understudied nature of the above-mentioned association, we examined the relationship between stroke recurrence and collateral circulation in patients with IVBAS using a newly designed score for the posterior communicating artery (PCoA) and the P1 segment of the posterior cerebral artery (P1). The aim of our study was to determine the prognostic value of this new magnetic resonance angiography (MRA)-based grading system focusing on the primary collateral pathways.
METHODS
Study population
This study was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University. We used prospectively curated clinical and radiological databases to retrospectively identify consecutive ischemic stroke patients with intracranial atherosclerosis stenosis from 2012 to 2019. Eligible patients had experienced posterior circulation ischemic stroke due to severe IVBAS (70%–99%). Newly developed ischemic stroke was diagnosed by diffusion-weighted imaging (DWI) within 7 days after symptom onset. The degree of stenosis was measured according to the Warfarin-Aspirin for Symptomatic Intracranial Disease (WASID) trial.8 Patients with Moyamoya disease, vasculitis, dissection, atrial fibrillation, or extracranial tandem lesions were excluded. Patients diagnosed with transient ischemic attack and those for whom radiological images were unavailable were also excluded.
Clinical information assessment
Demographic and clinical information was collected, including sex, age, risk factors, and National Institutes of Health Stroke Scale (NIHSS) score at enrollment, as was whether any antithrombotic agents were taken before or after the index stroke. Stroke recurrence was defined as relapse or aggravation on the basis of the previous symptom, extension of a previous infarction, or new infarction in the territory of vertebrobasilar artery. According to the number of stroke recurrences, patients were divided into the no-recurrence, single-recurrence, and multiple-recurrences groups. The time interval from the index stroke to enrollment was also recorded. Hypertension was defined as a history of hypertension or taking any hypotensive drug. Diabetes mellitus was defined as a history of diabetes mellitus, taking any hypoglycemic agent, or glycosylated hemoglobin ≥7%. Hyperlipidemia was defined as a history of hyperlipidemia, or receiving lipid-lowering treatment. Coronary artery disease was defined as a history of myocardial infarction or angina pectoris. Smoking was defined as current or previous smoking.9,10
Magnetic resonance imaging analysis
Imaging sequences were performed, including T1-weighted imaging, T2-weighted imaging, DWI, and three-dimensional time-of-flight (3D-TOF) MRA. The stroke mechanisms, lesion location, PCoA-P1 score, and vertebral artery (VA) anatomic patterns were evaluated. All images were interpreted by two of the authors (a neurosurgeon and a neurologist) on a consensus basis. To reduce bias, each author first independently reviewed all the data, and then they conferred to reach an agreement. If they did not reach agreement, a third author was consulted to decide the results. The evaluated parameters are described in detail below.
Based on the infarcts seen on DWI distributed in the distal territories of the culprit artery, stroke mechanisms were divided into artery-to-artery (A-A) embolism, hypoperfusion, perforator, and mixed mechanism:11 1) A-A embolism, multiple infarcts located in the cerebellum and/or cortex; 2) hypoperfusion, infarcts located in a border zone; 3) perforator, single or multiple infarcts located in the brainstem; 4) mixed mechanism, coexistence of two or more of the above mechanisms.
Lesion location was divided into the intracranial VA and basilar artery (BA) based on MRA imaging.
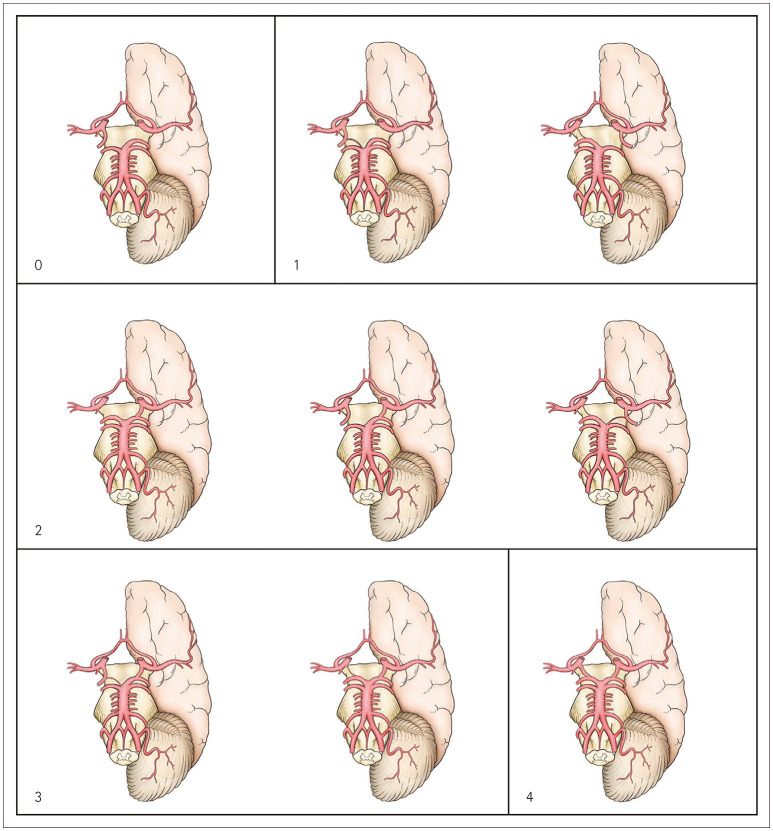
The PCoA-P1 score was determined as follows from 3D-TOF MRA observations as depicted in Fig. 1: 1) 0 points: absence of a bilateral anterior-to-posterior circulation connection; 2) 1 point: presence of a unilateral PCoA-to-P1 connection, but with PCoA or P1 hypoplasia; 3) 2 points: presence of a unilateral normal PCoA-to-P1 connection or a bilateral PCoA-to-P1 connection, but with either PCoA or P1 hypoplasia; 4) 3 points: presence of a bilateral PCoA-to-P1 connection, but with one side normal combined with contralateral PCoA or P1 hypoplasia; 5) 4 points: presence of bilateral connections between a normal PCoA and P1. Primary collaterals was dichotomized into good (2–4 points) and poor (0 or 1 point).
Fig. 1. Schematics for explaining the score for the posterior communicating artery (PCoA) and the P1 segment of the posterior cerebral artery (P1) as determined from three-dimensional time-of-flight magnetic resonance angiography: 0 points, absence of a bilateral anterior-to-posterior circulation connection; 1 point, presence of a unilateral PCoA-to-P1 connection, but with PCoA or P1 hypoplasia; 2 points, presence of a unilateral normal PCoA-to-P1 connection or a bilateral PCoA-to-P1 connection, but with either PCoA or P1 hypoplasia; 3 points, presence of a bilateral PCoA-to-P1 connection, but with one side normal combined with contralateral PCoA or P1 hypoplasia; and 4 points, presence of bilateral connections between a normal PCoA and P1.
VA anatomic patterns were divided into unilateral VA (contralateral VA absent), bilateral VA (the two VAs of equal size), and dominant VA (one VA dominant and hypoplasia in the contralateral VA). Hypoplasia was defined as in a previous study.12
Statistical analysis
We performed statistical analysis using SPSS software (version 20.0, IBM Corp., Armonk, NY, USA). Categorical data are expressed as frequencies and percentages. Normally distributed continuous data are expressed as mean±standard-deviation values, while nonnormally distributed continuous data are expressed as median and interquartile range (IQR) values. The characteristics of patients with no recurrence, single recurrence, and multiple recurrences were compared. The χ2 test was used for comparisons of categorical variables. ANOVA was used for comparisons of continuous variables conforming to a normal distribution, while the other continuous variables were compared using the Kruskal-Wallis test. Multivariable analysis was performed while adjusting for sex and age. A probability value of p<0.05 was considered to indicate a statistically significant difference.
RESULTS
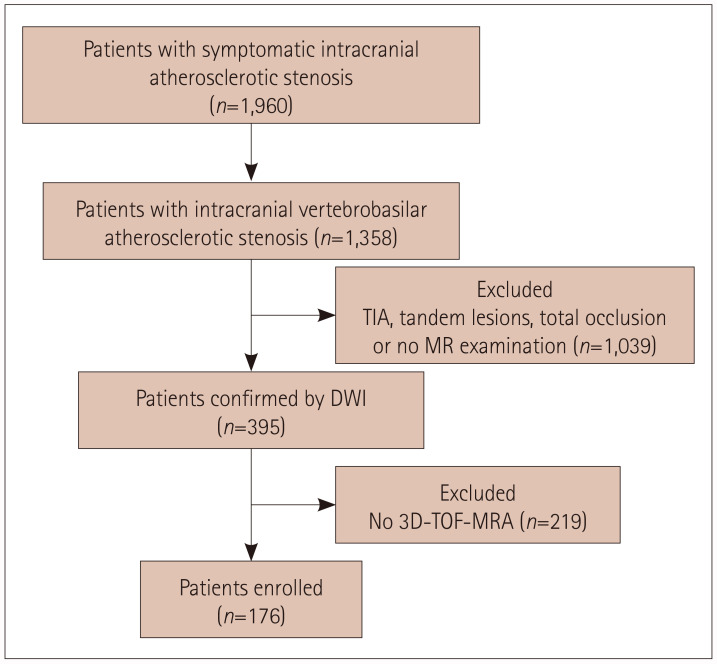
We rescreened 1,960 patients with symptomatic intracranial atherosclerotic stenosis, and 176 patients who met the inclusion criteria were finally enrolled (Fig. 2). These patients comprised 116 (65.9%) with no recurrence, 35 (19.9%) with a single recurrence, and 25 (14.2%) with multiple recurrences. The patients were aged 58.9±9.6 years, and most of them were male (81.8%). Hypertension was present in 145 (82.4%) patients, diabetes mellitus in 77 (43.8%), hyperlipidemia in 47 (26.7%), smoking in 106 (60.2%), and coronary heart disease in 22 (12.5%). The median NIHSS score at enrollment was 0.5 (IQR=0–2). Thirty-three (18.8%) and 169 (96.0%) patients were taking antithrombotic agents before and after the index stroke, respectively. The median time interval from the index stroke to enrollment was 35.9 days (IQR=25.4–63.1 days). A-A embolism was found in 99 (56.3%) patients, followed by perforator in 40 (22.7%) and mixed mechanism in 37 (21.0%), while hypoperfusion was not found. The lesion was located in the BA for 83 (47.2%) patients and in the intracranial VA for 93 (52.8%) patients. The baseline patient features are listed in Table 1.
Fig. 2. Flowchart of study enrollment. DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; TIA, transient ischemic attack; 3D-TOF, three-dimensional time-of-flight.
Table 1. Comparison of characteristics in the no-recurrence, single-recurrence, and multiple-recurrences groups.
| Variable | No recurrence (n=116) | Single recurrence (n=35) | Multiple recurrences (n=25) | p | |
|---|---|---|---|---|---|
| Sex, male | 95 (81.9) | 29 (82.9) | 20 (80.0) | 0.960 | |
| Age (yr) | 59.7±9.5 | 56.7±10.0 | 58.6±9.2 | 0.255 | |
| Hypertension | 96 (82.8) | 27 (77.1) | 22 (88.0) | 0.544 | |
| Diabetes mellitus | 53 (45.7) | 13 (37.1) | 11 (44.0) | 0.671 | |
| Hyperlipidemia | 31 (26.7) | 7 (20.0) | 9 (36.0) | 0.385 | |
| Coronary artery disease | 15 (12.9) | 5 (14.3) | 2 (8.0) | 0.726 | |
| Smoking | 68 (58.6) | 21 (60.0) | 17 (68.0) | 0.685 | |
| NIHSS score at enrollment | 0.0 (0.0–1.0) | 2.0 (1.0–3.0) | 1.0 (0.0–3.0) | <0.001 | |
| Taking any antithrombotic agents before the index stroke | 20 (17.2) | 6 (17.1) | 7 (28.0) | 0.441 | |
| Taking any antithrombotic agents after the index stroke | 112 (96.6) | 34 (97.1) | 23 (92.0) | 0.594 | |
| Time interval from index stroke to enrollment (day) | 35.7 (23.7–61.2) | 40.7 (27.0–67.0) | 31.6 (25.7–63.2) | 0.586 | |
| Index stroke mechanism | 0.990 | ||||
| Artery-to-artery embolism | 64 (55.2) | 21 (60.0) | 14 (56.0) | ||
| Perforator | 27 (23.3) | 7 (20.0) | 6 (24.0) | ||
| Mixed | 25 (21.6) | 7 (20.0) | 5 (20.0) | ||
| Hypoperfusion | 0 (0) | 0 (0) | 0 (0) | ||
| Lesion location | 0.078 | ||||
| BA | 48 (41.4) | 19 (54.3) | 16 (64.0) | ||
| VA | 68 (58.6) | 16 (45.7) | 9 (36.0) | ||
| PCoA-P1 score | <0.001 | ||||
| 2–4 | 77 (66.4) | 12 (34.3) | 6 (24.0) | ||
| 0 or 1 | 39 (33.6) | 23 (65.7) | 19 (76.0) | ||
| VA anatomic pattern | 0.562 | ||||
| Unilateral | 27 (23.3) | 9 (25.7) | 7 (28.0) | ||
| Hypoplasia | 50 (43.1) | 18 (51.4) | 8 (32.0) | ||
| Bilateral | 39 (33.6) | 8 (22.9) | 10 (40.0) | ||
Data are mean±standard deviation, n (%), or median (interquartile range) values.
BA, basilar artery; NIHSS, National Institutes of Health Stroke Scale; PCoA, posterior communicating artery; P1, P1 segment of the posterior cerebral artery; VA, vertebral artery.
PCoA-P1 score
The 176 patients comprised 38 (21.6%), 43 (24.4%), 76 (43.2%), 15 (8.5%), and 4 (2.3%) with PCoA-P1 scores of 0, 1, 2, 3, and 4 points, respectively, and hence 95 and 81 patients were classified as having good and poor primary collaterals, respectively (Figs. 3 and 4). Two patients with normal PCoA and P1 connections received 0 points because of the presence of retrograde blood flow from the posterior to the anterior circulation due to occlusion of the internal carotid artery.
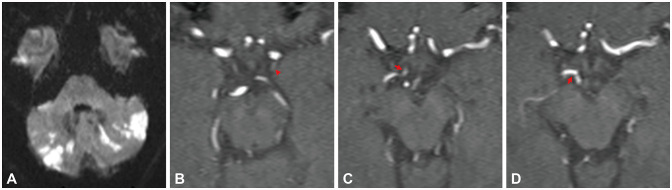
Fig. 3. A male adult presented with dizziness and blurred vision with a 6-day history. DWI demonstrated the infarcts distributed in the bilateral cerebellum (A). The index stroke mechanism was classified as artery-to-artery embolism. The patient had a history of hypertension. 3D-TOF MRA showed that the PCoA was hypoplastic (arrowhead) (B) and the connection from the right PCoA to the right P1 was of equal size (arrows) (C and D). The PCoA-P1 score was 3 points. DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; PCoA, posterior communicating artery; P1, P1 segment of the posterior cerebral artery; 3D-TOF, three-dimensional time-of-flight.
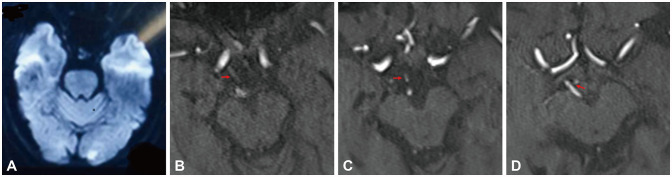
Fig. 4. A female adult presented with dizziness with a 1-day history. DWI demonstrated infarcts distributed in the left occipital lobe (A). The index stroke mechanism was classified as artery-to-artery embolism. She had a history of hyperlipidemia, hypertension, and diabetes mellitus. 3D-TOF MRA showed that the PCoA-to-P1 connection was present on the right side while the PCoA was hypoplastic, and there was no connection on the left side (arrows) (B-D). The PCoA-P1 score was 1 point. The patient experienced two strokes during the 35-day period following the initial onset. DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; PCoA, posterior communicating artery; P1, P1 segment of the posterior cerebral artery; 3D-TOF, three-dimensional time-of-flight.
Comparison of no recurrence, single recurrence, and multiple recurrences
There was no significant difference among the three groups in sex, age, the taking of any antithrombotic agents before and after the index stroke, time intervals from the index stroke to enrollment, lesion location, or VA anatomic patterns. Patients with no recurrence had a lower NIHSS score (median=0.0, IQR=0.0–1.0, p<0.001). There was an increasing trend in the prevalence of BA involvement from the no-recurrence group to the single-recurrence and multiple-recurrences groups (41.4%, 54.3%, and 64.0%, respectively), and also in poor primary collaterals (33.6%, 65.7%, and 76.0%, respectively).
Multivariable analysis adjusting for sex and age revealed that patients with multiple stroke recurrences were more likely to have BA involvement (odds ratio [OR]=2.851, 95% confidence interval [CI]=1.105–7.358, p=0.030). The prevalence of poor primary collaterals was higher in both single recurrence (OR=4.134, 95% CI=1.822–9.380, p=0.001) and multiple recurrences (OR=6.894, 95% CI=2.489–19.092, p<0.001) (Table 2).
Table 2. Results from multivariable analysis of characteristics associated with stroke recurrence.
| Variable | No recurrence | Single recurrence | Multiple recurrences | ||
|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p | Adjusted OR (95% CI) | p | ||
| Sex, male | Reference | 1.054 (0.358–3.101) | 0.924 | 0.997 (0.306–3.242) | 0.996 |
| Age, years | Reference | 0.964 (0.924–1.006) | 0.095 | 0.984 (0.936–1.034) | 0.521 |
| Lesion located in BA | Reference | 1.823 (0.816–4.070) | 0.143 | 2.851 (1.105–7.358) | 0.030 |
| PCoA-P1 score=0 or 1 | Reference | 4.134 (1.822–9.380) | 0.001 | 6.894 (2.489–19.092) | <0.001 |
BA, basilar artery; CI, confidence interval; OR, odds ratio; PCoA-P1, posterior communicating artery and the P1 segment of the posterior cerebral artery.
DISCUSSION
We have proposed an MRA-based primary collateral score called the PCoA-P1 score for quantifying the collateral status of patients with IVBAS. Patients with poor primary collaterals (0 or 1 point) were more likely to have multiple stroke recurrences. The goal is for the PCoA-P1 score to serve as a prognostic tool for screening high-risk patients who may benefit from aggressive medical therapy or endovascular treatment.
The collateral status was an independent predictor of stroke recurrence in patients with intracranial atherosclerotic stenosis.6 A distal flow status of symptomatic vertebrobasilar atherosclerotic stenosis was associated with a higher risk of subsequent stroke.13 Furthermore, it was difficult to define the hemodynamics in patients with posterior circulation ischemic stroke due to IVBAS, because of the challenge in determining the presence of border-zone infarction. However, hemodynamics factors may play a role in stroke occurrence by coexisting with other stroke mechanisms.14 The PCoA is the most important primary collateral between the anterior and posterior circulations, and so it was selected for predicting stroke recurrence in the present study. The results showed that the new PCoA-P1 score may be both practical and effective in evaluating the collateral status in patients with IVBAS, since poor primary collaterals were more common among the patients in this study with single stroke recurrence and multiple stroke recurrences than in those without recurrence.
Several scoring systems have been proposed for evaluating the collateral status of the posterior circulation. For example, the Basilar Artery on Computed Tomography Angiography15 and Posterior Circulation Collateral Score16 scoring systems are based on computed tomography angiography. However, these scoring systems focus on patients with acute BA occlusion rather than IVBAS. Scoring systems for BA stenosis have been proposed, but they have been used to predict functional outcomes rather than stroke recurrence,17,18 and there has been no scoring system for predicting stroke recurrence in patients with IVBAS.
The PCoA-to-P1 channel is the primary collateral circulation for patients with IVBAS and is more important than other collateral routes, including the anterior spinal arteries and leptomeningeal and dura arteriolar anastomoses with cortical vessels. The proposed scoring system considers not only the existence of a PCoA-to-P1 connection but also the sizes of the PCoA and P1. This study found that smaller PCoA and P1 were associated with lower PCoA-P1 scores in patients with IVBAS, indicating a higher risk of multiple stroke recurrences. One advantage of the new scoring system is that MRA is a routine, noninvasive examination; however, its predictive value needs to be confirmed by prospective studies in the future.
This study found that more lesions were located in the intracranial VA than in the BA in patients with posterior circulation ischemic stroke due to IVBAS (52.8% vs. 47.2%). A similar result was observed in the New England Medical Center Posterior Circulation Registry.19 However, patients with multiple stroke recurrences were more likely to demonstrate BA involvement in the present study (OR=2.851, 95% CI 1.105–7.358, p=0.030). To our knowledge, this is the first report of an association between multiple recurrences and BA involvement.
This study found that atherosclerotic risk factors were not related to stroke recurrence, which is consistent with several previous reports.5 The stroke mechanism was not associated with stroke recurrence in patients with IVBAS. A previous study found that the mixed mechanism of A-A embolism plus hypoperfusion was associated with stroke recurrence in patients with intracranial atherosclerotic stenosis.20 The difference in the study populations—patients with ≥70% IVBAS in the present study vs. patients with intracranial atherosclerotic stenosis—may explain the difference.
This study found no difference between taking antithrombotic agents before or after the index stroke, or in the time intervals from index stroke to enrollment among the three groups. The WASID study also suggested that taking antithrombotic agents at the time of the qualifying event was not associated with stroke recurrence.21 Furthermore, the present study found that the NIHSS score was higher in patients with stroke recurrence. These high-risk patients with multiple stroke recurrences who were refractory to medical management may benefit from endovascular treatment.
The present study did not include patients with total occlusion. Posterior circulation ischemic strokes are more commonly caused by stenotic lesions than by total occlusion. In a Taiwan Stroke Registry study, 3.8% cases of ischemic stroke or transient ischemic attack were due to vertebrobasilar total occlusion. Furthermore, the severity was greater in patients with symptomatic total occlusion than in those with a stenotic lesion. Compared with patients with 0%–49% vertebrobasilar artery stenosis, the risks of recurrent stroke (hazard ratio [HR]=1.94, 95% CI=1.03–3.64) and death (HR=2.10, 95% CI=1.46–3.04) were significantly higher among patients with vertebrobasilar artery occlusion. No difference in the rate of recurrent ischemic stroke or death was observed between patients with 50%–99% and 0%–49% stenosis of the vertebrobasilar artery.22
This study had some limitations. First, 3D-TOF MRA might not reveal the actual blood flow in certain circumstances. Second, the study had a single-center, retrospective design, and so enrollment elective bias was probably present. Third, there may have been bias in the timing of stroke recurrence and the occurrence of stroke events. Therefore, the conclusions drawn from this study need to be confirmed in future prospective studies.
In conclusion, this study developed a new MRA-based scoring system for evaluating the primary collaterals in IVBAS. The new PCoA-P1 score may be useful for predicting stroke recurrence. These patients may benefit from early identification and aggressive treatment.
Acknowledgements
We thank all of the patients and health-care providers who participated in this study.
Footnotes
- Conceptualization: Yongjun Wang, Zhongrong Miao, Ning Ma.
- Data curation: Long Yan, Ying Yu, Zhikai Hou, Min Wan, Weilun Fu.
- Formal analysis: Long Yan.
- Funding acquisition: Xin Lou.
- Investigation: Long Yan.
- Methodology: Ning Ma, Xin Lou.
- Supervision: Yongjun Wang, Zhongrong Miao.
- Writing—original draft: Long Yan.
- Writing—review & editing: Kaijiang Kang, Ning Ma.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Labropoulos N, Nandivada P, Bekelis K. Stroke of the posterior cerebral circulation. Int Angiol. 2011;30:105–114. [PubMed] [Google Scholar]
- 2.Gulli G, Khan S, Markus HS. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke. 2009;40:2732–2737. doi: 10.1161/STROKEAHA.109.553859. [DOI] [PubMed] [Google Scholar]
- 3.Gulli G, Marquardt L, Rothwell PM, Markus HS. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis: pooled data analysis from prospective studies. Stroke. 2013;44:598–604. doi: 10.1161/STROKEAHA.112.669929. [DOI] [PubMed] [Google Scholar]
- 4.Mazighi M, Tanasescu R, Ducrocq X, Vicaut E, Bracard S, Houdart E, et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. 2006;66:1187–1191. doi: 10.1212/01.wnl.0000208404.94585.b2. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Wang Y, Zhao X, Liu L, Wang C, Pu Y, et al. Prediction of recurrent stroke or transient ischemic attack after noncardiogenic posterior circulation ischemic stroke. Stroke. 2017;48:1835–1841. doi: 10.1161/STROKEAHA.116.016285. [DOI] [PubMed] [Google Scholar]
- 6.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–974. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. 2019;50:143–147. doi: 10.1161/STROKEAHA.118.020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45:663–669. doi: 10.1161/STROKEAHA.113.003508. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Li M, Lyu J, Hou Z, He J, Mo D, et al. Different risk factors in identical features of intracranial atherosclerosis plaques in the posterior and anterior circulation in high-resolution MRI. Ther Adv Neurol Disord. 2020;13:1756286420909991. doi: 10.1177/1756286420909991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong KS, Caplan LR, Kim JS. Stroke mechanisms. Front Neurol Neurosci. 2016;40:58–71. doi: 10.1159/000448302. [DOI] [PubMed] [Google Scholar]
- 12.Gaigalaite V, Dementaviciene J, Vilimas A, Kalibatiene D. Association between the posterior part of the circle of Willis and the vertebral artery hypoplasia. PLoS One. 2019;14:e0213226. doi: 10.1371/journal.pone.0213226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin-Hanjani S, Pandey DK, Rose-Finnell L, Du X, Richardson D, Thulborn KR, et al. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol. 2016;73:178–185. doi: 10.1001/jamaneurol.2015.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 15.Alemseged F, Shah DG, Diomedi M, Sallustio F, Bivard A, Sharma G, et al. The Basilar Artery on Computed Tomography Angiography Prognostic Score for basilar artery occlusion. Stroke. 2017;48:631–637. doi: 10.1161/STROKEAHA.116.015492. [DOI] [PubMed] [Google Scholar]
- 16.van der Hoeven EJ, McVerry F, Vos JA, Algra A, Puetz V, Kappelle LJ, et al. Collateral flow predicts outcome after basilar artery occlusion: the posterior circulation collateral score. Int J Stroke. 2016;11:768–775. doi: 10.1177/1747493016641951. [DOI] [PubMed] [Google Scholar]
- 17.Samaniego EA, Shaban A, Ortega-Gutierrez S, Roa JA, Hasan DM, Derdeyn C, et al. Stroke mechanisms and outcomes of isolated symptomatic basilar artery stenosis. Stroke Vasc Neurol. 2019;4:189–197. doi: 10.1136/svn-2019-000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee WJ, Jung KH, Ryu YJ, Kim JM, Lee ST, Chu K, et al. Utility of digital subtraction angiography-based collateral evaluation in medically treated acute symptomatic basilar artery stenosis. Eur J Neurol. 2017;24:1148–1155. doi: 10.1111/ene.13351. [DOI] [PubMed] [Google Scholar]
- 19.Caplan LR, Wityk RJ, Glass TA, Tapia J, Pazdera L, Chang HM, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389–398. doi: 10.1002/ana.20204. [DOI] [PubMed] [Google Scholar]
- 20.Feng X, Chan KL, Lan L, Abrigo J, Liu J, Fang H, et al. Stroke mechanisms in symptomatic intracranial atherosclerotic disease: classification and clinical implications. Stroke. 2019;50:2692–2699. doi: 10.1161/STROKEAHA.119.025732. [DOI] [PubMed] [Google Scholar]
- 21.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi AI, Qureshi MH, Lien LM, Lee JT, Jeng JS, Hu CJ, et al. One-year risk of recurrent stroke and death associated with vertebrobasilar artery stenosis and occlusion in a cohort of 10,515 patients. Cerebrovasc Dis. 2019;47:40–47. doi: 10.1159/000495418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.