Abstract
Introduction
SARS-COV-2 is associated with unexpected symptoms. Several studies in adults reported urinary frequency with COVID-19. The aim of this study is to reveal lower urinary tract symptoms associated with COVID-19 (CALUTS) in children.
Patients-methods
All children diagnosed with COVID-19 and associated multisystem inflammatory syndrome in children (MIS-C) between November 2020–June 2021 in our hospital were reviewed and asked for urinary symptoms at the time of or following their disease. The ones reporting symptoms were invited for further evaluation. Parents were inquired for their child's former bladder and bowel function, their symptoms after the diagnosis of COVID-19 or MIS-C, onset and duration of the symptoms, and their current state. They were questioned for the frequency of voiding as well as dysuria, odor, and the presence of incontinence as well as other symptoms of COVID-19. The patients who reported symptoms at the time of inquiry were followed for cessation of symptoms. The parameters age, sex, need for hospitalization and admission to ICU were also compared to the whole group to evaluate the main characteristics of patients with lower urinary tract symptoms.
Results
In total 20 patients (18/216 with acute disease and 2/36 with MIS-C) reported CALUTS (figure). Age and sex distribution were not significantly different from the patients without urinary symptoms (p = 0.777 and p = 0.141 respectively). All were otherwise healthy children with no concomitant chronic diseases other than overactive bladder in two. There were 13 girls and 7 boys. Mean age was 11 years (±5 years). Thirteen of the patients were older than 10 years; however, there were also 3 children under 5 years of age. All parents described a sudden onset of extremely increased urinary frequency and urgency lasting for weeks which disappeared gradually. Median bladder and bowel dysfunction questionnaire (BBDQ) score before COVID-19 was 2.5 (1–18) which increased to a median of 22 (15–29) at the time of the symptoms (p < 0.001). The timing of onset and duration of symptoms were variable and not associated with symptom severity (p = 0.306 and p = 0.450 respectively). Eight patients (40%) reported diarrhea. The duration of diarrhea was limited to less than one week in all.
Conclusions
Our study revealed that SARS-COV-2 can be associated with lower urinary tract symptoms also in children both during the acute phase and MIS-C. Further studies are necessary to understand the etiopathogenesis and prevalence of this unexpected aspect of COVID-19.
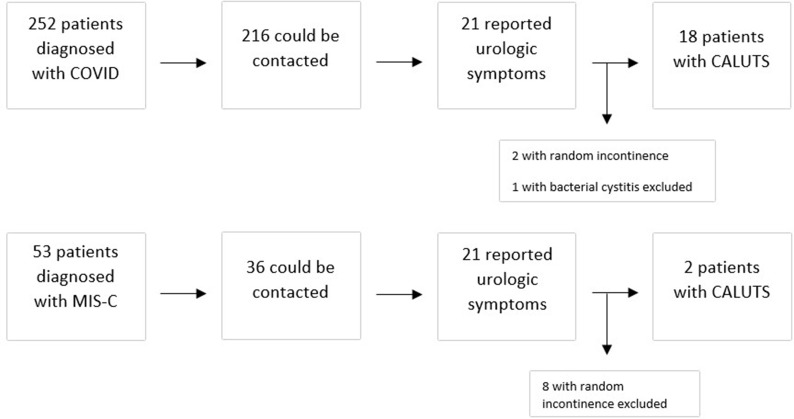
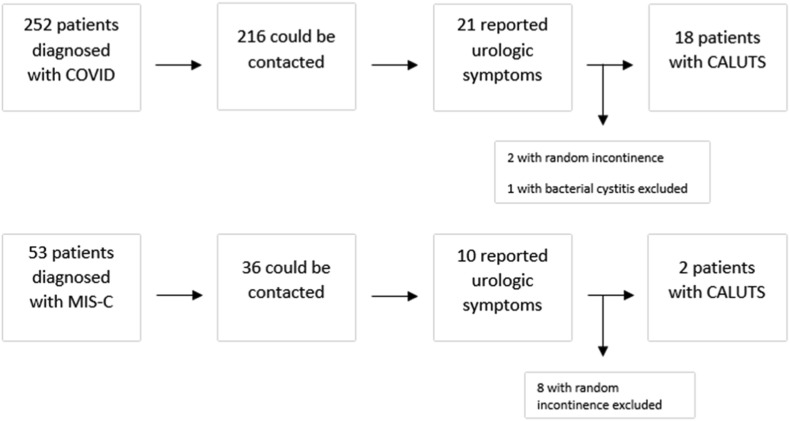
Summary Figure.
Scheme of the study design.
Keywords: SARS-COV-2, COVID-19, Lower urinary tract symptoms, Cystitis, Urinary frequency
Introduction
Coronavirus Disease-19 (COVID-19) pandemic tumbled our lives and forced us to learn a lot about it in a short period of time. Besides respiratory symptoms, SARS-COV-2 showed a variety of unexpected manifestations [1].
COVID-19 associated urinary frequency is a phenomenon reported in adults. Several papers confirmed its presence but failed to explain its etiology. Some papers also reported dysuria and irritative symptoms [2,3]. Some of them interpreted it as a form of cystitis [3] and some of them commented on an exacerbation in the symptoms of benign prostatic hyperplasia (BPH) [4]. One paper stated higher lower urinary tract symptom (LUTS) scores in older patients [2]. None of the papers described the progress of the condition or made a satisfactory explanation about the etiopathogenesis. One systematic review suggested dysregulation of renin-angiotensin system, the inflammatory process in the prostate, the role of androgen receptors in prostate, or other metabolic disorders as possible mechanisms for exacerbation in BPH with COVID-19 [4]. Currently, there is only one case series about this topic in children [5].
The aim of this study is to reveal lower urinary tract symptoms associated with COVID-19 in children both with acute disease and associated multisystem inflammatory syndrome-children (MIS-C).
Methods
Study design and setting
This single center, retrospective study was conducted at a 665 bed, secondary care children's referral hospital with an approximately 485.000 patient admission annually. The study was approved by the regional ethical review board (protocol number: 2021/66).
Procedure and data collection
This study is based on a retrospective inquiry of urologic symptoms associated with COVID-19 in children. Study setup included phone calls to all patients diagnosed with COVID-19 and MIS-C, retrospective chart review and secondary evaluation of children whose parents report urinary symptoms.
All pediatric patients diagnosed with COVID-19 and associated MIS-C between 01.11.2020 and 31.05.2021 in our hospital were contacted. Parents of the pediatric patients with COVID-19 or MIS-C were questioned for urologic symptoms via phone calls. This phone call was performed by three pediatric specialists who were responsible for the management of COVID-19 and associated MIS-C in our hospital (A pediatrician [OE], a pediatric infectious disease specialist [AB] and a pediatric emergency care specialist [AZB]). Each parent was called 3 times at most (on the same day) and removed from the list if there was no answer. The patients reporting positive symptoms were invited with their parent(s) for further evaluation in the hospital.
Clinical data were collected from electronic medical records and patient charts. The medical charts of pediatric patients with COVID-19 or MIS-C were reviewed for age, sex, underlying diseases, the blood and urine tests performed, if the patient was hospitalized, and if they required admission to the intensive care unit (ICU).
The secondary evaluation of patients describing urinary symptoms was performed by a pediatric urologist (ST) in hospital. In this secondary evaluation, parents and patients were inquired for former bladder and bowel function using a validated questionnaire in our language (which is the translated form of the BBDQ by Drzewiecki et al.) [6,7], symptoms after the diagnosis of COVID-19, onset and duration of the symptoms, and current state. They were questioned for the frequency of voiding as well as dysuria, odor, and the presence of incontinence as well as other symptoms of COVID-19. Children and parents were not surveyed separately, the questions were answered by children primarily, parents confirmed or interfered when the child could not answer or did not recall. The patients who reported symptoms at the time of inquiry were followed for cessation of symptoms.
The parameters age, sex, need for hospitalization and admission to ICU were also compared between patients describing lower urinary tract symptoms and the whole group to evaluate the main characteristics of patients with lower urinary tract symptoms.
Definitions
-
•
The patient with COVID-19 was defined as the person whose diagnosis was confirmed with positive polymerase chain reaction (PCR) test for SARS-CoV-2.
-
•
The patient with MIS-C was defined according to the WHO criteria [8].
Statistical analysis
The statistical analyses were performed using IBM SPSS Package Version 20 (Armonk, NY, USA: IBM Corp.). Descriptive statistics were used to summarize the baseline patient characteristics. Pearson Chi-square, Wilcoxon, Mann–Whitney U, ANOVA tests were used where indicated and noted throughout the text for each analysis. Distribution of normality for numeric data was tested using Kolmogorov–Smirnov test. A p-value below 0.05 was considered statistically significant.
Results
There were 252 patients diagnosed with COVID-19, 216 (86%) could be contacted and 21 (10%) of them reported urologic symptoms. There were 53 patients diagnosed with MIS-C associated with COVID-19, 36 (68%) could be contacted, 10 (27%) of them reported urologic symptoms. The median interval between the phone call and the initial diagnosis of COVID-19 or MIS-C was 5 (1–7) months. All 31 patients who confirmed presence of any urinary symptom during the phone call were evaluated by the pediatric urologist on the following week after the call.
Two of the 21 patients in acute COVID-29 group reported intermittent random urinary incontinence without any other symptoms including increased frequency, so they were not considered as COVID-19 associated LUTS. One patient had a concomitant culture proven bacterial urinary tract infection, and symptoms not typical like the others, therefore excluded. Eighteen patients (8%) described a typical course of increased voiding frequency with a sudden onset and gradual recovery (Fig. 1 ). Eight of the ten patients in the MIS-C group described random intermittent incontinence with no associated symptoms, so excluded. Two of them (5%) described a course of urinary frequency similar to the patients with acute COVID-19 infection (Fig. 1). In total, 20 patients were confirmed to describe a similar pattern of urinary symptoms termed by the authors as COVID-19 associated LUTS (CALUTS).
Fig. 1.
Scheme of the study design.
A summary of the study group is depicted in Table 1 . All were otherwise healthy children with no concomitant chronic diseases other than detrusor overactivity in two. There were 13 girls and 7 boys. Mean age was 11 years (±5 years). Thirteen of the patients were older than 10 years; however, there were also 3 children under 5 years of age.
Table 1.
Clinical characteristics of patients with COVID associated lower urinary tract symptoms.
| Patient # | Age (years) | Sex | Acute/MIS-C | Onset of CALUTS (weeks) | Duration of CALUTS (months) | Pre-COVID BBDQ | Per-CALUTS BBDQ | Per-CALUTS Incontinence | Other symptoms |
Hospitalization | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Respa | GISb | ||||||||||
| 1 | 6 | M | MIS-C | 4 | 6 | 1 | 26 | + | + | + | – | + (+ICU) |
| 2 | 16 | M | MIS-C | 0 | 3 | 1 | 16 | – | + | – | – | + |
| 3 | 14 | M | Acute | 0 | 3 | 1 | 15 | – | + | – | + | – |
| 4 | 16 | F | Acute | 2 | 1 | 3 | 15 | – | – | + | – | – |
| 5 | 17 | M | Acute | 0 | 1 | 1 | 15 | – | + | – | – | – |
| 6 | 11 | F | Acute | 1 | 1 | 3 | 23 | + | – | + | – | – |
| 7 | 11 | F | Acute | 1 | 3 | 1 | 23 | + | + | + | – | – |
| 8 | 12 | F | Acute | 2 | 3 | 1 | 24 | + | + | – | – | – |
| 9 | 11 | F | Acute | 0 | 6 | 1 | 15 | – | – | + | – | – |
| 10 | 12 | M | Acute | 0 | 3 | 3 | 24 | + | + | – | + | – |
| 11 | 5 | F | Acute | 11 | 1 | 18 | 23 | + | + | + | – | + |
| 12 | 15 | F | Acute | 1 | 3 | 2 | 15 | – | + | + | + | – |
| 13 | 17 | M | Acute | 0 | 1 | 6 | 18 | – | + | – | – | – |
| 14 | 14 | F | Acute | 0 | 1 | 3 | 15 | – | + | + | + | – |
| 15 | 4 | F | Acute | 0 | 3 | 1 | 21 | + | + | + | + | + |
| 16 | 6 | F | Acute | 0 | 1 | 5 | 24 | + | + | – | – | – |
| 17 | 17 | F | Acute | 2 | 3 | 2 | 28 | + | + | – | + | – |
| 18 | 4 | M | Acute | 4 | 3 | 5 | 17 | + | + | + | – | – |
| 19 | 6 | F | Acute | 1 | 3 | 14 | 29 | + | + | + | + | + |
| 20 | 4 | F | Acute | 1 | 3 | 3 | 28 | + | + | + | + | + |
Resp: respiratory symptoms.
Gastrointestinal symptoms.
Mean BBDQ score of patients during the symptoms was 20.70 (±5.07). Total BBDQ scores had a positive correlation with age. Preschool children (age 0–6) had higher scores than older children (24 vs 18.92 respectively, p = 0.029, Independent Samples t = test). Answers to questions are depicted in Table 2 . The answer to the question of frequency (question #3) was more than 8/day in all. On the other hand, none described pain during voiding, straining, difficulty to start voiding or an interruption in voiding (questions 6, 9 and 10).
Table 2.
Bladder Bowel Dysfunction Questionnaire (BBDQ) consists of 13 questions about urinary symptoms in the form of Likert scale (0–4) and one question about ease in answering. Answers to the 13 questions about symptoms are grouped for each clinical aspect and total score for each group is given. The columns depicting the answers to questions are as follows: daytime incontinence (questions #1–2), urinary frequency (question #3), urgency symptoms (questions #4–5), voiding problems (#6, 9, 10), nighttime symptoms (questions #7–8), constipation and encopresis (questions #11–13). (F: Female, M: Male).
| Patient# | Sex | Age | Daytime incontinence | Frequency | Urgency symptoms | Nighttime symptoms | Voiding symptoms | Constipation& encopresis | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 6 | 5 | 4 | 8 | 8 | 0 | 1 | 26 |
| 2 | M | 16 | 0 | 4 | 8 | 3 | 0 | 1 | 16 |
| 3 | M | 14 | 0 | 4 | 7 | 3 | 0 | 1 | 15 |
| 4 | F | 16 | 0 | 4 | 6 | 4 | 0 | 1 | 15 |
| 5 | M | 17 | 0 | 4 | 7 | 3 | 0 | 1 | 15 |
| 6 | F | 11 | 6 | 4 | 5 | 7 | 0 | 1 | 23 |
| 7 | F | 11 | 2 | 4 | 7 | 8 | 0 | 2 | 23 |
| 8 | F | 12 | 3 | 4 | 7 | 8 | 0 | 2 | 24 |
| 9 | F | 11 | 0 | 4 | 6 | 4 | 0 | 1 | 15 |
| 10 | M | 12 | 6 | 4 | 7 | 6 | 0 | 1 | 24 |
| 11 | F | 5 | 8 | 4 | 7 | 3 | 0 | 1 | 23 |
| 12 | F | 15 | 0 | 4 | 5 | 3 | 0 | 3 | 15 |
| 13 | M | 17 | 0 | 4 | 7 | 3 | 0 | 4 | 18 |
| 14 | F | 14 | 0 | 4 | 5 | 3 | 0 | 3 | 15 |
| 15 | F | 4 | 4 | 4 | 6 | 6 | 0 | 1 | 21 |
| 16 | F | 6 | 0 | 4 | 8 | 6 | 0 | 6 | 24 |
| 17 | F | 17 | 7 | 4 | 7 | 6 | 0 | 4 | 28 |
| 18 | M | 4 | 4 | 4 | 6 | 2 | 0 | 1 | 17 |
| 19 | F | 6 | 7 | 4 | 8 | 8 | 0 | 2 | 29 |
| 20 | F | 4 | 7 | 4 | 8 | 8 | 0 | 1 | 28 |
The course of the symptoms was similar in all patients with sudden onset of extremely increased frequency (more than twice an hour), continuation of symptoms for a while, and then gradual recovery with time. All parents reported that it was most intense during the week it started. However, timing of onset and duration of symptoms showed some differences. Nine patients (45%) had increased voiding frequency at the time of COVID-19 diagnosis (starting with other symptoms of COVID-19), 6 (30%) said it started after a week, 3 (15%) after two weeks, and 2 (10%) said after a month. There was no relation between BBDQ scores and the timing of onset (p = 0.306, Kruskal–Wallis). Duration of intense symptoms was one month in 7 (35%), three months in 11 (55%), and 6 months in 2 (%10). There was no relation between BBDQ scores and the duration of symptoms (p = 0.450, Kruskal–Wallis).
Main symptoms were frequency and urgency. Some parents also described intermittent urinary incontinence while others were continent despite nocturia (Table 1). Details for each patient are depicted in Table 1. None of the parents described pain, dysuria, or bad odor. Eight patients (40%) reported diarrhea. The duration of diarrhea was limited to less than one week in all. The others did not describe any differences in stool frequency or consistency. Median BBDQ score before COVID-19 was 2.5 (1–18) which increased to a median of 22 (15–29) at the time of the symptoms (p < 0.001, Wilcoxon). All patients with symptoms at the time of inquiry were followed and returned to their baseline toilet habits at a maximum duration of six months (Table 1). Urine tests at the time of study was normal in all patients.
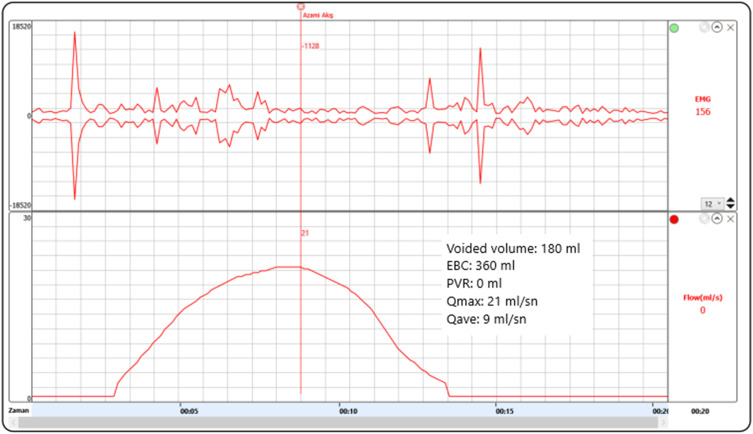
All parents confirmed that they reported their child's symptoms to their physician which were overlooked in most. Eight patients had a urine test during symptoms, all were normal. Three patients were referred to pediatric urologist for ongoing symptoms (after a month in two sisters [patient #19 and 20] and after three months in patient #1). One patient (patient #9) with ongoing symptoms was discovered with this study with no former referral. In total, four patients were seen by the pediatric urologist when they still had symptoms. Uroflowmetry with EMG was normal besides a reduced amount in these four patients (Fig. 2 ). None had post-voiding residuals. Two-days voiding diary showed increased frequency without polyuria. They all reported that frequency was worse when the symptoms first started and that it was getting better. These four children reported no other symptoms of discomfort or a feeling of incomplete emptying. Oxybutynin was given to these four patients (by mother empirically as described below to two sisters and by the prescription of the pediatric urologist who is one of the authors in other two) and it changed nothing.
Fig. 2.
Uroflowmetry of patient #9 four months after the start of symptoms. Symptoms lasted 6 months in this 11 years old girl with only mild respiratory symptoms of COVID-19 (EBC: expected bladder capacity for age, Qmax: maximum flow rate, Qave: average flow rate).
Patients with CALUTS were also evaluated for the presence of other system symptoms. Both patients with MIS-C had cardiac involvement and fever. One of them had respiratory distress, neither described gastrointestinal symptoms. Eight of the patients with acute disease described gastrointestinal symptoms (mostly abdominal pain and diarrhea). Present symptoms for each patient are depicted in Table 1. There was no relation with the presence of gastrointestinal symptoms and BBDQ scores (21.9 vs. 19.9) (p = 0.413, Independent samples t-test).
Two patients had abnormal BBDQ scores before COVID-19. Both had a former diagnosis of overactive bladder. One had no symptoms with anticholinergics and described no effect of oxybutynin during CALUTS. The other did not use anticholinergics despite being prescribed, started it during the symptoms with no success either.
The two patients with MIS-C and four of the patients with acute disease required hospitalization. BBDQ scores were higher for children who required hospitalization (24 vs. 19); however, difference was statistically insignificant (p = 0.069). One of the two children with MIS-C required ICU, the other did not.
We evaluated if age and sex distribution was different from the whole group in patients with CALUTS. Mean age was 11 years for patients with CALUTS and 11.3 years for patients without symptoms (p = 0.777, Independent samples t-test). The ratio of girls seemed higher in the group of patients with CALUTS (65% vs 47%), but the difference was statistically insignificant (p = 0.141, Pearson Chi-square).
We also evaluated if admission to hospital and ICU differed from the whole group in patients with CALUTS. Six of the 20 children with CALUTS (30%) and 42 of the 232 children with no symptoms (18%) required hospital admission (p = 0.194, Pearson Chi-square). One of the 20 children (5%) with CALUTS and 28 of the 232 children with no symptoms (12%) required ICU admission (p = 0.342, Pearson Chi-square).
We also observed some incidents that might shed light to the etiology.
-
•
The two patients with MIS-C did not have urinary symptoms during the acute phase of the disease.
-
•
One of the patients had increased urinary frequency when she was diagnosed with COVID-19. She was hospitalized with MIS-C one month later, her symptoms continued for three months also during the MIS-C period, and then ceased gradually.
-
•
Two of the children in our study group were sisters. The older one had a diagnosis of overactive bladder suppressed with oxybutynin. The younger left diapers at age 2 and had no urinary symptoms. They both had a mild COVID-19. After a week, frequent voiding started the same day for both. The mother being familiar with detrusor overactivity started oxybutynin to the younger and increased the dose for the older after seeing normal urine tests. It didn't work. The symptoms lasted 3 months for both and then gradually ceased. The younger returned to normal and the older ‘to her normal’ at about the same time.
Discussion
Increased urinary frequency with COVID-19 was first reported in seven men by Mumm et al. [9]. They interpreted these findings as a form of viral cystitis. Later, several papers in adults focused on this topic with some variations in study design and various conclusions about the etiology.
There is only one case series regarding LUTS in children associated with COVID-19. This paper involves three children aged 14–17 years who presented with urinary retention and required clean intermittent catheterization after a median interval of three months. The authors performed urodynamics and showed a-contractile detrusor in two patients and hypo-contractile detrusor in one patient) [5]. Our study did not involve invasive urodynamics but had some discordant findings. None of the children in our study described difficulty in voiding, straining or intermittent voiding. The four children who had uroflowmetries in our series had normal flow patterns with no residuals. It is difficult to make conclusions as both studies involve a limited number of patients; however, this might be secondary to different clinical presentations of CALUTS or that none of the patients in our study were evaluated at the time of initiation of symptoms.
Urgency and frequency are in fact nonspecific symptoms hard to comment on without other findings; however, all our patients had an identical course. All parents described a sudden onset of extremely increased urinary frequency (more than twice an hour) and urgency lasting for weeks which disappeared gradually. Another interesting finding was the variation in the timing of onset and duration of symptoms in every patient. Despite the identical pattern, onset and duration was variable in each patient with no relation between intensity of symptoms and duration or timing of onset.
Tian et al. showed urine shedding of SARS-COV-2 in 38 of the 52 COVID patients [10]. Viral load in urine might be an explanation for the symptoms. On the other hand, there was no hematuria, dysuria, pain, or bad odor in any patient in our study. All performed urine tests were also normal. This is not a typical course for any form of cystitis. We also demonstrated symptoms in two patients with MIS-C which is a phase without active viral replication. Also, these two patients had no symptoms during acute phase. We therefore sought for an explanation other than cystitis for the etiology.
Lamb et al. found increased urine cytokines in patients with COVID-19 and suggested that ongoing symptoms after the acute course can be the result of a chronic inflammatory condition [3]. This seems to be a better explanation considering symptoms also in patients with MIS-C. We thought a worse prognosis in patients with more symptoms will support this theory. Current studies also support this. In the study by Tian et al., patients with urine shedding had worse renal function and clinical outcome [10]. They attributed this relation to a higher viral load or presence of a new or preexisting endothelial damage in those patients. Bladder function was not a parameter evaluated in their study. Karabulut et al. suggested worse progress in patients with more LUTS symptoms [11]. In our study, BBDQ scores were higher for children who required hospitalization, and hospitalization rate was higher in patients with CALUTS, but the difference was statistically insignificant for both. On the contrary, ICU admission rate was lower in patients with CALUTS. This might be due to sample size; however, our study failed to show a relation between disease severity and intensity of bladder symptoms.
Another explanation for the etiology could be autonomic dysfunction. Autonomic dysfunction was reported to be prevalent in patients after COVID-19 and was attributed to either virus infection or immune response on the autonomic nervous system. Stella et al. evaluated autonomic dysfunction symptom score in post-COVID patients to question this theory and revealed higher scores in patients with neurological symptoms. Urinary domain score was also higher in these patients [12]. Progression of the symptoms in our patients resembled a form of autonomic neurogenic dysfunction. Some of our patients also had diarrhea which is in fact a common finding in COVID. However, alteration in stool frequency and consistency was not a consistent finding in our patients. We unfortunately had no further data evaluating autonomic dysfunction in our patient population.
Androgen receptors were hypothesized to be essential for priming of the viral spike protein [13]. Some papers in the subject attributed increased urinary frequency to an exacerbation in BPH and pointed this relation with androgen receptor for etiology [4,11,14]. The epidemiologic data about COVID-19 in general also revealed a gender selection with worse disease in men [15]. There were more girls with CALUTS in our study despite the balanced ratio in the whole COVID group. Girl predilection in our study might have been due to sample size bias or inclusion of both hospitalized and non-hospitalized patients. Even in that case, our study revealed evidence against BPH and androgen receptor theories for CALUTS.
A study by Werion et al. reported renal proximal tubular dysfunction in patients with COVID which was independent of pre-existing comorbidities, glomerular proteinuria, nephrotoxic medications, or viral load [16]. We did not specifically search for renal tubular dysfunction; however, urine tests performed were normal in all patients with normal density and no overt proteinuria.
The frequency of patients with symptoms was higher (12%) in the study by Mumm et al. [9] than ours. Karabulut et al. [11] found worse clinical outcome in patients with more LUTS symptoms. The lower frequency in our study can be due to the difference in sample size, that our patients are all children or that all patients in Mumm's study were hospitalized. The design of other published studies does not allow any further comment about the prevalence of CALUT among patients with COVID-19.
Can et al. found higher LUTS scores in older patients [2]. Our study showed higher BBDQ scores and more frequent incontinence in younger children. It's hard to comment as the study group is small and data was retrospective, but this probably better control of continence ensured by going to toilet more frequently in older children.
The major limitation of our study was the retrospective inquiry of symptoms from parents which makes our study prone to recall bias. Nonetheless, urinary frequency was so intense (more than twice in an hour) that there was probably no problem in its reporting. Other possible milder symptoms that were recorded as absent (i.e., dysuria, bad odor) might actually be overlooked. We also did not have urine tests performed from all patients at the time of symptoms to exclude bacterial cystitis in all; however, disease course was identical in all and atypical for bacterial cystitis.
Conclusion
Coronavirus disease not only effected our lives hugely as a pandemic, but also changed clinical practice in every medical profession due to wide spectrum of symptoms COV-SARS-2 causes. Our study revealed an interesting pattern of severe but temporary lower urinary tract symptoms associated with COVID in children. Future studies with larger sample size will provide more data and evidence on the etiology of CALUTS in children. Further follow-up of these children is necessary to evaluate for symptom recurrence.
Conflicts of interest
None.
Funding
None.
References
- 1.Peiris S., Mesa H., Aysola A., Manivel J., Toledo J., Borges-Sa M., et al. Pathological findings in organs and tissues of patients with COVID-19: a systematic review. PLoS One. 2021;16:1–18. doi: 10.1371/journal.pone.0250708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Can O., Erkoç M., Ozer M., Karakanli M.U., Otunctemur A. The effect of COVID-19 on lower urinary tract symptoms in elderly men. Int J Clin Pract. 2021;75:2–5. doi: 10.1111/ijcp.14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb L.E., Dhar N., Timar R., Wills M., Dhar S., Chancellor M.B. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC) Med Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haghpanah A., Masjedi F., Salehipour M., Hosseinpour A., Roozbeh J., Dehghani A. Is COVID-19 a risk factor for progression of benign prostatic hyperplasia and exacerbation of its related symptoms?: a systematic review. Prostate Cancer Prostatic Dis. 2021;25(1):27–38. doi: 10.1038/s41391-021-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvi I., Dönmez M.İ., Ziylan O., Oktar T. Urodynamically proven lower urinary tract dysfunction in children after COVID-19: a case series. LUTS Low Urin Tract Symptoms. 2022:17–20. doi: 10.1111/luts.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaya Narter F., Tarhan F., Narter K.F., Sabuncu K., Alay Eser R., Akin Y., et al. Reliability and validity of the bladder and bowel dysfunction questionnaire among Turkish children. Turk J Med Sci. 2017;47:1765–1769. doi: 10.3906/sag-1601-122. [DOI] [PubMed] [Google Scholar]
- 7.Drzewiecki B.A., Thomas J.C., Pope J.C., Adams M.C., Brock J.W., Tanaka S.T. Use of validated bladder/bowel dysfunction questionnaire in the clinical pediatric urology setting. J Urol. 2012;188:1578–1583. doi: 10.1016/j.juro.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 8.WHO Scientif Brief . 2020. Multi system inflammatory syndrome in children and adolescents temporally related to COVID-19. WHO/2019-NCoV/Sci_Brief/Multisystem_Syndrome_Children/20201. [DOI] [Google Scholar]
- 9.Mumm J.N., Osterman A., Ruzicka M., Stihl C., Vilsmaier T., Munker D., et al. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does SARS-CoV-2 cause viral cystitis? Eur Urol. 2020;78:624–628. doi: 10.1016/j.eururo.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian M., Zhang L., Zhu K., Shen B., Wang G., Song Y., et al. Urinary SARS-CoV-2 RNA is an indicator for the progression and prognosis of COVID-19 disease. Diagnostics. 2021;11:2089. doi: 10.21203/rs.3.rs-203728/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karabulut I., Cinislioglu A.E., Cinislioglu N., Yilmazel F.K., Utlu M., Alay H., et al. The effect of the presence of lower urinary system symptoms on the prognosis of COVID-19 : preliminary results of a prospective study. Urol Int. 2020;104:853–858. doi: 10.1159/000511787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buoite Stella A., Furlanis G., Frezza N.A., Valentinotti R., Ajcevic M., Manganotti P. Autonomic dysfunction in post-COVID patients with and without neurological symptoms: a prospective multidomain observational study. J Neurol. 2021;269(2):587–596. doi: 10.1007/s00415-021-10735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wambier C.G., Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020;83:308–309. doi: 10.1016/j.jaad.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elaimeri A., Alemairy A.A. 2021. Effect of COVID 19 on lower urinary track symptoms in patients with benign prostatic hyperplasia introduction; pp. 1–8.Https//DoiOrg/1021203/Rs3Rs-514550/v1 Licens [Google Scholar]
- 15.Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werion A., Blekhir L., Perrot M., Schmit G., Aydin S., Chen Z., et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98:1296–1307. doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]