Abstract
The most common etiologies of tympanic membrane perforation are infections and trauma.
Objective
The objective of the present study was to assess the healing of traumatic tympanic membrane perforation in rats.
Methods
The tympanic membrane from male Wistar rats was perforated in the anterior and posterior portions to the handle of the malleus. Five tympanic membranes were evaluated 3 days after tympanic perforation; 5 after 5 days; 5 after 7 days; 3 after 10 days; and 4 after 14 days. The tympanic membranes were submitted to histopathological evaluation after hematoxylin–eosin staining.
Results
Tympanic membrane closure occurred at about 7–10 days after injury and the healing process was complete by day 14. The proliferative activity of the outer epithelial layer was present close to the handle of the malleus and to the tympanic annulus.
Conclusion
The spontaneous healing process of the tympanic membrane starts from the outer epithelial layer, with later healing of the lamina propria and the mucosal layer.
Keywords: Tympanic membrane perforation, Wound healing, Histology
Resumo
As causas mais comuns de perfurações de membrana timpânica são infecções e trauma.
Objetivos
Avaliar o reparo cicatricial de perfurações traumáticas da membrana timpânica em ratos.
Método
A membrana timpânica de ratos Wistar machos foram perfuradas nas porções anterior e posterior ao cabo do martelo. Cinco membranas timpânicas foram avaliadas 3 dias após perfuração timpânica; 5 após 5 dias; 5 após 7 dias; 3 após 10 dias; e 4 após 14 dias. As membranas timpânicas foram submetidas à avaliação histopatológica após coloração com hematoxilina- eosina.
Resultados
O fechamento da membrana timpânica ocorreu em torno de 7 a 10 dias após perfuração traumática, e o processo de cicatrização estava completo no 14° dia. A atividade proliferativa da camada epitelial externa foi identificada próxima ao cabo do martelo e ao ânulus timpânico.
Conclusão
O processo de cicatrização espontânea da membrana timpânica se inicia com a camada epitelial externa, com posterior cicatrização da lâmina própria e da camada mucosa.
Palavras-chave: Perfuração da membrana timpânica, Cicatrização, Histologia
Introduction
The tympanic membrane (TM) is an anatomical structure that separates the outer ear from the middle ear. The TM is responsible for sound amplification and transmission through the ossicular chain to the oval window and vestibular ramp, in addition to the protection of the round window and the tympanic ramp.1
The ultrastructural anatomy of TM consists of 3 layers: the outer layer, of epithelial (ectodermal) origin; the middle layer or lamina propria, of mesodermal origin; and the inner layer, of endodermal origin, comprising the middle ear mucosa.2
The outer layer consists of keratinized stratified squamous epithelium.3, 4, 5
The middle layer or lamina propria consists of loose subepithelial connective tissue, organized dense connective tissue and submucosal loose connective tissue. Loose subepidermal and submucosal connective tissue consists of loosely arranged collagen fibers, fibroblasts, nerve fibers and capillaries. The dense connective tissue consists of more externally organized radial collagen fibers and more internally organized circular collagen fibers.2, 3, 4, 5The inner layer consists of simple columnar epithelial tissue, which is continuous with the middle ear mucosa.2, 3, 4, 5, 6
The most common etiologies of TM perforations are otitis media and trauma.7 Traumatic TM perforations have a 78.7% rate of healing.8 Factors that prevent adequate repair of TM in the absence of infection are yet to be defined.7, 8
The objective of this study is to develop the two-dimensional study of scar repair in traumatic TM perforations in rats.
Method
The experimental study was carried out with 19 male Wistar albino rats (Rattus norvegicus), weighing on average 280 g (range 270–290 g). The study followed the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA) and was approved by the Ethics Committee on Animal Experimentation (CETEA) on 08/27/2007 – protocol number for use of animals in research No. 082/2007.
Before the procedure, all animals were anesthetized with intramuscular ketamine hydrochloride (40 mg/kg) (Ketamine 50 mg/mL, Cristália Laboratory, São Paulo, Brazil) and intramuscular xylazine hydrochloride (5 mg/kg) (Dopaser 20 mg/mL, Hertape Calier, Minas Gerais, Brazil). The ears of all animals were assessed using a DFV MU-M19 otomicroscope (DFV, Rio de Janeiro, Brazil) before the procedure to rule out infection. A total of 19 animals were included in the study, equivalent to 23 bullae with normal TMs. Bullae with infection were excluded from the procedure.
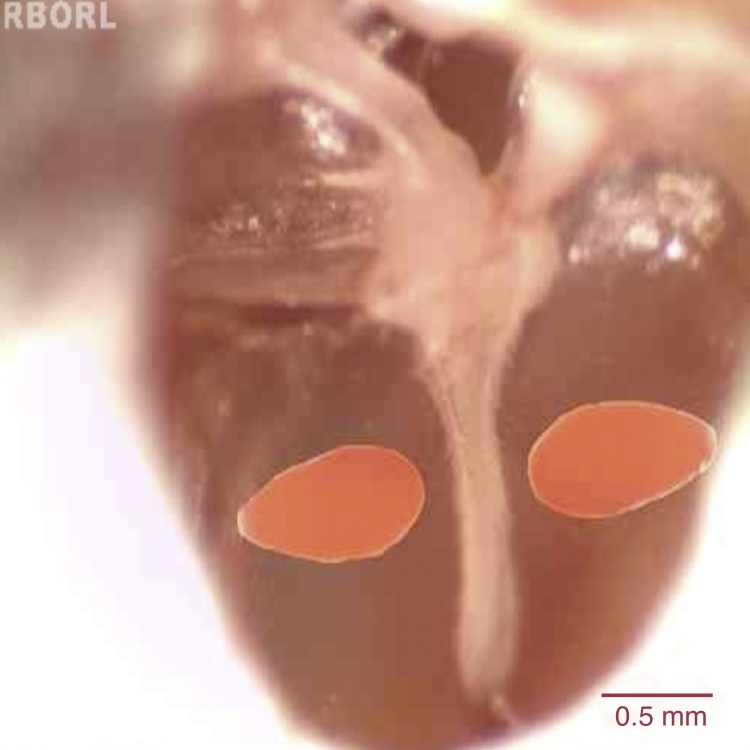
Traumatic perforation of the tympanic membrane was performed with a 30 mm × 0.8 mm BD needle (Becton Dickinson, New Jersey, USA) anterior and posterior to the malleus handle, in the pars tensa region of the TM (Fig. 1). For histological evaluation, 3 animals were euthanized 3 days after the perforation (5 bullae), 4 animals after 5 days (5 bullae), 5 animals after 7 days (5 bullae), 3 animals after 10 days (3 bullae) and 3 animals after 14 days (4 bullae). One animal (1 bulla) with intact TM was assessed as control. The animals were euthanized with an intraperitoneal injection of an overdose of thiopental (Thionembutal, Abbot, São Paulo, Brazil).
Figure 1.
Schematic representation of tympanic perforation in the pars tensa region of the rat TM region, anteriorly and posteriorly to the malleus handle.
The bullae were removed from the animals and fixed for 24 h in 10% formalin (Merck) diluted in phosphate buffer solution and then decalcified in an aqueous solution of 4.13 g EDTA (Merck) and 0.55 g NaOH (Merck) for approximately 50 days. After decalcification, the samples were dehydrated in ethanol, xylene and embedded in paraffin. Histological sections of 6 μm thickness were performed with Leica Jung RM2065 microtome (Leica Microsystems GmbH, Wetzlar, Germany). Sections were oriented using the malleus handle as a reference. To allow histological evaluation, the blocks were worn until they reached approximately the same perpendicular level relative to the malleus handle. Samples were stained with hematoxylin and eosin.
Histological evaluation was performed with an Olympus BX50 microscope (Olympus America, Inc., Pennsylvania, USA), and digital high-resolution images were acquired with a Spot RT3 camera (Diagnostic Instruments, Inc. Michigan, USA). The thicknesses of the outer epithelial layer, lamina propria and mucosa were measured using the Image-Pro-Plus® program, release 7.0 (Media Cybernetics Inc., MD, USA). In TM perforations, thickness measurements were performed at a distance of 50–100 μm from the border of the perforation in order to evaluate the healing activity near the lesion.9 In the intact TMs, this assessment was standardized at a distance of 450–550 μm from the malleus handle, a region that corresponds to the site of the previously performed TM perforation.
Results
Intact tympanic membrane – control
The thickness of the TM was approximately 9.6 μm, having as reference the distance of 500 μm from the malleus handle. At a distance of 250 μm from the malleus handle, the TM thickness was 15.8 μm, and at 250 μm from the tympanic annulus, its thickness was 26.6 μm (Table 1).
Table 1.
Mean thickness of the epithelial, lamina propria and mucosal layers; and total thickness of the tympanic membrane (TM) throughout the study on days 0 (intact TM), 3, 5, 7, 10 and 14.
| Time of euthanization (days) | Epithelial layer, μm (%) | Lamina propria layer, μm (%) | Mucosal layer, μm (%) | Total, μm (100%) |
|---|---|---|---|---|
| Intact TMa | 1.8 (18.7%) | 6.3 (65.6%) | 1.5 (15.6%) | 9.6 |
| Intact TMb | 2.6 (16.4%) | 11.9 (75.3%) | 1.3 (8.2%) | 15.8 |
| Intact TMc | 2.3 (8.6%) | 22.3 (83.8%) | 2 (7.5%) | 26.6 |
| 3 daysa | 18.9 (48.4%) | 14.3 (36.7%) | 5.8 (14.9%) | 39.0 |
| 5 daysa | 27.1 (37.9%) | 36.2 (50.7%) | 8.1 (11.3%) | 71.4 |
| 7 daysa | 37.5 (42.1%) | 46.1 (51.7%) | 5.5 (6.1%) | 89.1 |
| 10 daysa | 11.2 (29.9%) | 23.3 (62.1%) | 3 (8.0%) | 37.5 |
| 14 daysa | 5.7 (27.9%) | 12.5 (61.3%) | 2.2 (10.8%) | 20.4 |
In the intact TMs, the thickness was measured at a distance of 450–550 μm from the malleus handle. In perforated TMs, measurements of thickness were performed at a distance of between 50 and 100 μm from the border of the perforation.
The thickness measurements were performed at a distance of 250 μm from the malleus handle in animals with intact TMs.
The thickness measurements were performed at a distance of 250 μm from the tympanic annulus in animals with intact TMs.
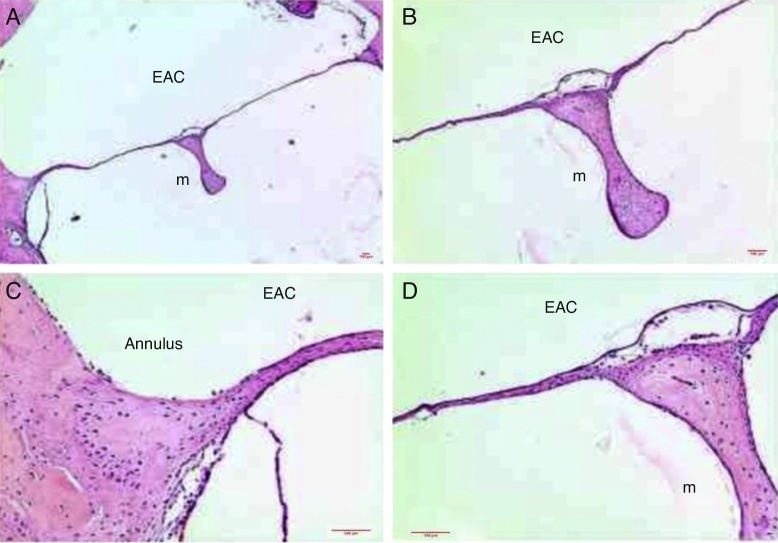
The stratified squamous epithelial tissue showed from 1 to 2 rows of flattened epithelial cells throughout the length of the TM (Fig. 2). The thickness of the epithelial layer was 1.8 μm at a distance of 500 μm from the malleus handle (Table 1).
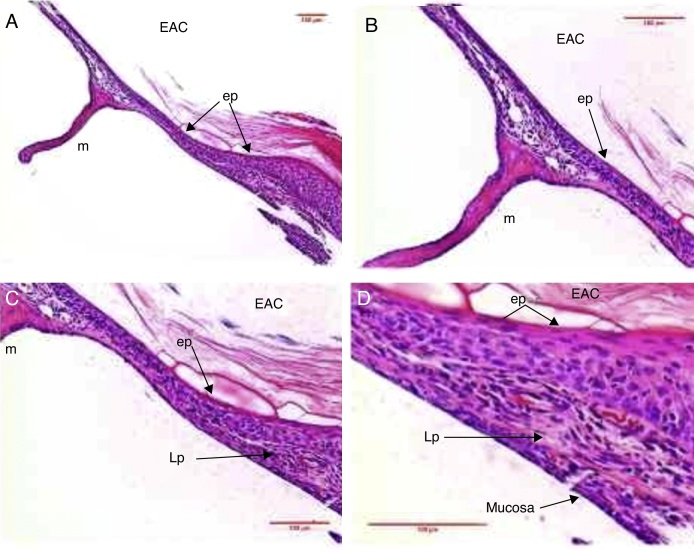
Figure 2.
Histological section images of rat TM, stained with hematoxylin–eosin (HE), showing intact TM. EAC, external auditory canal; m, malleus handle; annulus, tympanic annulus. Image (A) shows a magnification of 40×; (B) 100× and (C and D) 200×.
This connective tissue present in its middle layer was organized, with occasional flattened nucleus cells, probably fibroblasts, and organized collagen fibers, with little or no response of inflammatory cells (Fig. 2).
The collagen fibers were connected to the malleus handle, and in the tympanic annulus region, such fibers formed the fibrocartilaginous ring of the tympanic annulus. Few capillaries without edema or vascular proliferation were located near the malleus handle and were not found in the intermediate portions between the annulus and malleus handle. The lamina propria thickness was 6.3 μm (Table 1).
The mucosal layer had one row of flattened cells arranged as simple columnar tissue and exhibited continuity with the middle ear mucosa. The mucosal layer thickness was 1.5 μm (Fig. 2) (Table 1).
Tympanic membrane 3 days after traumatic perforation
The mean thickness of TM was approximately 39 μm (Table 1).
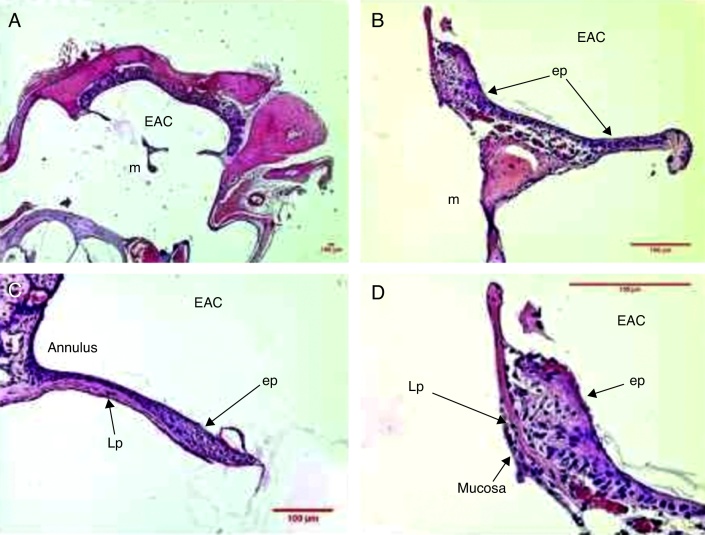
Three days after the traumatic perforation, there was a more proliferative and hyperplastic epithelial layer, with approximately three to four rows of epithelial cells, both near the malleus handle and the tympanic annulus (Fig. 3). The mean epithelial layer thickness was 18.9 μm (Table 1).
Figure 3.
Histological section images of rat TM 3 days after traumatic perforation, stained with HE. EAC, external auditory canal; m, malleus handle; ep, epithelial layer; Lp, lamina propria; mucosa, mucosal layer. Image (A) shows a magnification of 20×; (B and C) 200× and (D) 400×.
The middle layer of the TM showed the presence of cells with basophilic nucleus, compatible with fibroblasts. These occasional disorganized fibroblasts did not overcome the limits of the ruptured collagen fibers in the TM perforation. There was edema in the loose subepithelial connective and submucosal tissue near the malleus handle and in the tympanic annulus.
There was a predominance of inflammation with recruitment of polymorphonuclear cells located in the perivascular portion, and loose subepithelial and submucosal connective tissue. Blood vessels with plethora or turgescence were present, close to the malleus handle and the tympanic annulus region (Fig. 3). The mean lamina propria thickness was 14.3 μm (Table 1).
Hyperplasia was observed in the mucosal tissue near the region of the perforation borders. A row of mucosal tissue with hyperplastic cells and a mean thickness of 5.8 μm (Table 1) was identified.
Tympanic membrane 5 days after traumatic perforation
Five days after the perforation, the mean thickness of the TM was about 71.4 μm (Table 1).
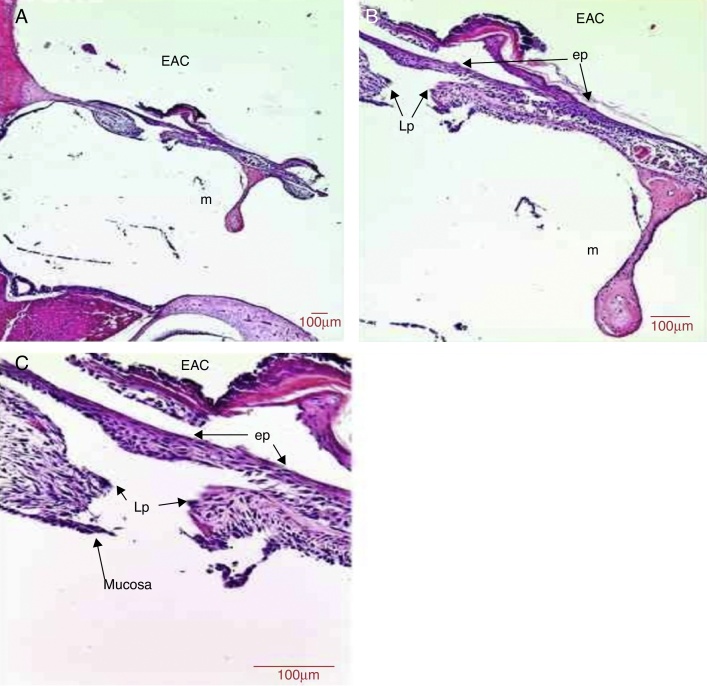
The epithelial layer showed hyperplasia in the region next to the malleus handle and the annulus region. The TM showed areas with three to five rows of epithelial tissue (Fig. 4). There was formation of an epithelial bridge that advanced toward the perforation closure in some cases (Fig. 4). The perforation closure started at the epithelial layer. The mean thickness of the epithelial layer was 27.1 μm (Table 1).
Figure 4.
Histological section images of rat TM 5 days after traumatic perforation, stained with HE. EAC, external auditory canal; m, malleus handle; ep, epithelial layer; Lp, lamina propria; mucosa, mucosal layer. Image (A) shows a magnification of 40×; (B) 100× and (C) 400×.
In the middle layer, we observed an increase in the thickness of the TM in the region adjacent to the perforation due to basophilic nucleus cells, probably fibroblasts. Clusters of red blood cells, indicating blood capillaries were present in the connective tissue of the TM (Fig. 4). The middle layer thickness was 36.2 μm (Table 1).
There was hyperplasia of mucosal tissue with two to three rows of mucosal cells (Fig. 4). The thickness of the mucosal layer was 8.1 μm (Table 1).
Tympanic membrane 7 days after traumatic perforation
Seven days after the perforation, the repaired TM had a mean thickness of 89.1 μm (Table 1).
The epithelial layer of the TM showed hyperplasia, with three to five rows throughout the TM length. There was a more marked epithelial reaction in the TM portion midway between the annulus and malleus handle, at the place where the perforation was performed (Fig. 5). The mean thickness of the epithelial layer was 37.5 μm (Table 1).
Figure 5.
Histological section images of rat TM 7 days after traumatic perforation, stained with HE. EAC, external auditory canal; m, malleus handle; ep, epithelial layer; Lp, lamina propria; mucosa, mucosal layer. Image (A) shows a magnification of 100×; (B and C) 200× and (D) 400×.
In the middle layer, there was a great predominance of basophilic nucleus cells, probably fibroblasts. The lamina propria filled the perforation site in some of the animals. Clusters of red blood cells were present, probably indicating blood capillaries. The greater proliferative reaction, as well as the thickest region of the TM, occurred midway between the annulus and the malleus handle (Fig. 5). The mean thickness of the lamina propria was 46.1 μm (Table 1).
The mucosal layer showed a row of simple hyperplasic columnar cells in most of the tympanic membranes (Fig. 5). The mean thickness of the mucosal layer was 5.5 μm (Table 1).
Tympanic membrane 10 days after traumatic perforation
Ten days after the perforation, the repaired tympanic membrane showed thickness of 37.5 μm (Table 1).
Epithelial tissue was identified with up to two rows of cells in most of the TM (Fig. 6). The thickness of the epithelial layer was 11.2 μm (Table 1).
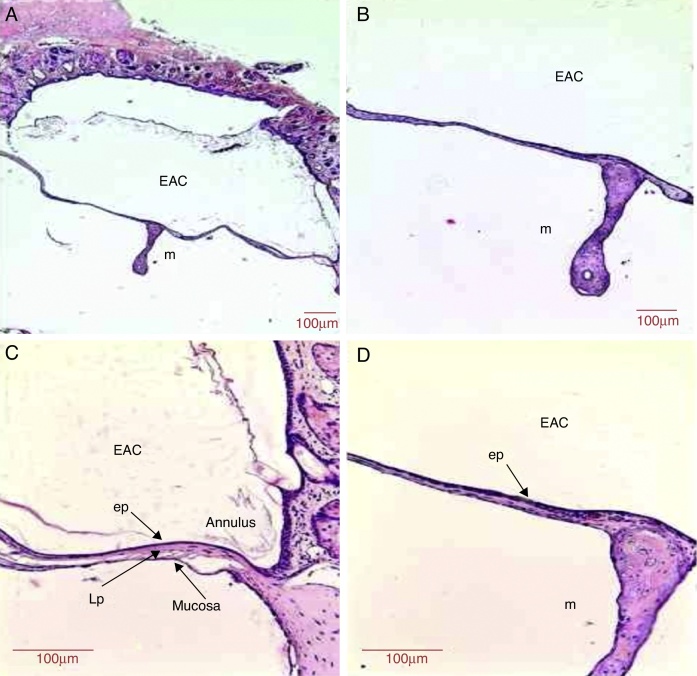
Figure 6.
Histological section images of rat TM 10 days after traumatic perforation, stained with HE. EAC, external auditory canal; m, malleus handle; ep, epithelial layer; Lp, lamina propria; annulus, tympanic annulus; mucosa, mucosal layer. Image (A) shows a magnification of 40×; (B) 100× and (C and D) 200×.
The lamina propria showed an apparent reduction in the number of fibroblasts in all regions of TM. Such occasional fibroblasts showed a more organized and aggregated distribution. There was a trend of decreased blood capillaries (Fig. 6). The mean thickness of the lamina propria was 23.3 μm (Table 1).
The mucosal layer showed one row of flattened mucosal cells in most of the TM (Fig. 6). The thickness of the mucosal layer was 3 μm (Table 1).
Tympanic membrane 14 days after traumatic perforation
Fourteen days after the perforation, the repaired TM had a thickness of 20.4 μm (Table 1).
The epithelial tissue showed up to two rows of flattened epithelial cells throughout the length of the TM (Fig. 7). The mean thickness of the epithelial layer was 5.7 μm (Table 1).
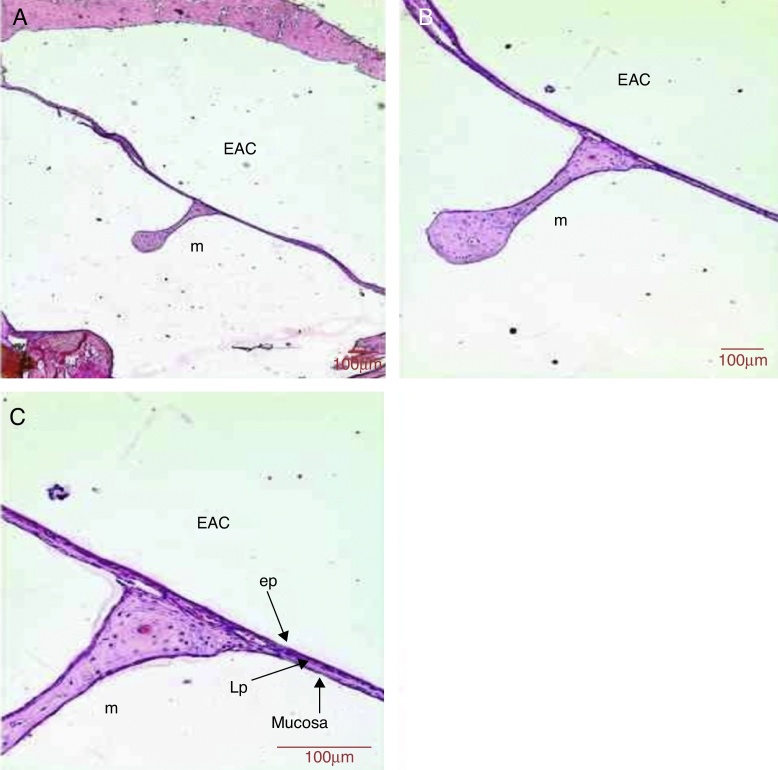
Figure 7.
Histological section images of rat TM 14 days after traumatic perforation, stained with HE. EAC, external auditory canal; m, malleus handle; ep, epithelial layer; LP, lamina propria; mucosa, mucosal layer. Image (A) shows a magnification of 40×; (B) 100× and (C) 200×.
In the middle layer, basophilic nucleus cells, probably fibroblasts were present, but possibly at smaller amounts when compared to the shorter periods (Fig. 7). The mean thickness of the lamina propria was 12.5 μm (Table 1).
The mucosal layer showed one row of flattened cells throughout the length of the TM (Fig. 7). The mean thickness of the mucosal layer was 2.2 μm (Table 1).
Closure of the tympanic perforation
The closure of the tympanic membrane occurred around 7–10 days after traumatic perforation, and the healing process was complete on the 14th day.
Discussion
After the perforation, the healing process of the TM is typically described as occurring in three distinct phases, but temporally overlapping: inflammatory, proliferative and remodeling.3, 4, 5, 6, 7
In experimental skin studies, the inflammatory phase begins immediately after tissue injury and lasts for 4–6 days.10, 11 This phase consists of a disarray of blood vessels with increased vascular permeability, leakage of serum proteins, platelets and coagulation factors. After 5 or 6 h of tissue injury, polymorphonuclear neutrophils are recruited to the wound, while monocytes are recruited after 48–96 h.11
The proliferative phase is classically characterized by epithelial proliferation; proliferation of fibroblasts with collagen deposition; and by angiogenesis, with granulation tissue formation.11 The proliferative phase is usually present from day 4 to day 14 in an experimental study of skin.11
On the 3rd day after the traumatic perforation, a more proliferative, hyperplastic epithelial layer, with approximately 3–4 rows of epithelial cells, was observed in the TM, both near the malleus handle and close to the tympanic annulus (Fig. 3). After 5–7 days, the epithelial mitotic activity intensified, with portions of 5 rows of epithelial cells (Figure 4, Figure 5). There was a tendency for epithelial bridge formation that advanced in the direction of the tympanic perforation closure.
This proliferative activity of the outer epithelial layer of TM has been described as occurring within the first hours after tissue injury.3, 12, 13 In the literature, there are reports of the existence of epithelial proliferative centers near the malleus handle and the tympanic annulus region.9, 14, 15 However, one study that described only one center of epithelial proliferation near the malleus handle was found.3
In experimental models of skin wound healing, the remodeling phase was described as starting after 8 days of tissue injury and persisting for a few months. The main molecular events occur in the extracellular matrix of the lamina propria.11
In TM, epithelial remodeling was observed from day 10, which remained until the 14th day. The outer epithelial layer became thinner, with approximately 2 rows of epithelial cells. In the middle layer, fibroblasts were observed in increasingly smaller amounts, showing a flattened shape. The tendency was the reduction in the number of capillaries along the tympanic membrane. The mucosal layer also became thinner, with one row of flattened cells throughout the length of the TM.
Due to the morphological characteristics of the TM, the squamous epithelium of the TM outer layer would be initially responsible for the perforation closure, with the formation of an epithelial bridge over the lesion and only subsequently there would be the restoration of the fibrous tissue of the middle layer and mucosal layer tissue (Fig. 4).7
The epithelial layer was the first to close the TM, which does not make the events in the other layers less important. At the moment when most cell proliferation and migration occurred, the epithelial layer showed higher metabolic activity, requiring greater supply of oxygen and nutrients.16 Therefore, increased vascularization and angiogenesis, both occurring in the lamina propria, played an important role in providing molecular supply required for the healing events to satisfactorily develop in the TM.17, 18, 19
The lamina propria is responsible for the fibroelastic characteristics of the TM, such as vibration capacity for sound transmission and middle ear protection. In some cases, the newly formed TM can heal with only two layers, one outer epithelial and one inner mucosal layer, as it has been described in experimental studies.7
However, it is possible that such neomembranes do not have the ideal characteristics of sound transmission20 or the fibroelastic structural characteristics which enable withstanding air pressure variations, such as barotrauma or tubal dysfunction due to its weaker than usual structure, caused by deficient formation of the fibrous layer.8 Kristensen pointed to the possibility of atelectasis or retraction pouches in atrophic neomembranes due to fibrous layer deficiency.
To prevent the occurrence of atrophic membranes due to lamina propria deficiency, treatments that increase fibroblast activity and collagen production may be interesting to provide better organization of the lamina propria. In tympanoplasty surgery, whether with autografts such as temporalis muscle fascia or tragal perichondrium, or even with the use of allografts, such as AlloDerm®,21 the purpose of these procedures is to restore the TM and the grafts are used to repair the fibrous layer, participating in the formation of the new extracellular matrix.
A previous study reported that the inner mucosal layer contributed little to the healing process of the TM.17 On the other hand, for Weinberger, Hawke and Gotlieb,22 the medial mucosal layer of TM would be the first of the structures to undergo perforation closure. It was not possible, through the methodology employed in this study, to define the role of the mucosal layer in the healing process of the TM. Moreover, even though it is a layer with a less complex histological architecture than the others, it is important to remember that healing events in the three TM layers must be harmoniously organized, so that proper TM integrity can be restored.
Conclusion
Evaluating the normal healing process of the TM in rats, we concluded that the spontaneous healing process is initiated by the TM outer epithelial layer, with subsequent closure of the lamina propria and mucosal layer.
Funding
This study was supported by FAPESP.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Please cite this article as: Araújo MM. Murashima AA, Alves VM, Jamur MC, Hyppolito MA. Spontaneous healing of the tympanic membrane after traumatic perforation in rats. Braz J Otorhinolaryngol 2014;80:330–8.
Institution: Department of Ophthalmology, Otorhinolaryngology and Head and Neck Surgery, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, São Paulo, SP, Brazil.
References
- 1.Wullstein H. Theory and practice of tympanoplasty. Laryngoscope. 1956;66:1076–1095. doi: 10.1288/00005537-195608000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Lim D.J. Tympanic membrane. Electron microscopic observation. I: pars tensa. Acta Otolaryngol Stock. 1968;66:181–198. doi: 10.3109/00016486809126286. [DOI] [PubMed] [Google Scholar]
- 3.Santa Maria P.L., Redmond S.L., Atlas M.D., Ghassemifar R. Histology of the healing tympanic membrane following perforation in rats. Laryngoscope. 2010;120:2061–2070. doi: 10.1002/lary.20998. [DOI] [PubMed] [Google Scholar]
- 4.Stenfeldt K., Johansson C., Hellstrom S. The collagen structure of the tympanic membrane. Arch Otolaryngol Head Neck Surg. 2006;132:293–298. doi: 10.1001/archotol.132.3.293. [DOI] [PubMed] [Google Scholar]
- 5.Wenig B.M., Michaels L. In: Histology for pathologists. 3rd ed. Mills S.E., editor. Lippincott Williams & Wilkins; Philadelphia: 2007. The ear and temporal bone; pp. 372–400. [Google Scholar]
- 6.Lim D.J. Structure and function of the tympanic membrane: a review. Acta Othorhinolaryngol Belg. 1995;49:101–115. [PubMed] [Google Scholar]
- 7.Gladstone H.B., Jackler R.K., Varav K. Tympanic membrane wound healing – an overview. Otolaryngol Clin North Am. 1995;28:913–932. [PubMed] [Google Scholar]
- 8.Kristensen S. Spontaneous healing of traumatic tympanic membrane perforations. J Laryngol Otol. 1992;106:1037–1050. doi: 10.1017/s0022215100121723. [DOI] [PubMed] [Google Scholar]
- 9.Güneri E.A., Tekin S., Yilmaz O., Özkara E., Erdag T.K., Ikiz A.O., et al. The effects of hyaluronic acid, epidermal growth factor, and mitomycin in an experimental model of acute traumatic tympanic membrane perforation. Otol Neurotol. 2003;24:371–376. doi: 10.1097/00129492-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Koopmann C.F. Cutaneous wound healing. Otolaryngol Clin North Am. 1995;28:835–845. [PubMed] [Google Scholar]
- 11.Witte M.B., Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–528. doi: 10.1016/s0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 12.Koba R. Epidermal cell migration and healing of the tympanic membrane: an immunohistochemical study of cell proliferation using bromodeoxyuridine labelling. Ann Otol Rhinol Laryngol. 1995;104:218–225. doi: 10.1177/000348949510400307. [DOI] [PubMed] [Google Scholar]
- 13.Spratley J., Hellstrom S., Eriksson P.O., Pais-Clemente M. Early structural tympanic membrane reactions to myringotomy: a study in an acute otitis media model. Acta Otolaryngol Stock. 2002;122:479–487. doi: 10.1080/00016480260092264. [DOI] [PubMed] [Google Scholar]
- 14.Koba R., Yagi M., Tabe H., Kawabata I. Kinetic analysis of cell proliferation using bromodeoxyuridine labeling in situ detection of dying cells in the tympanic membrane and middle ear cholesteatoma. Arch Histol Cytol. 1996;59:339–346. doi: 10.1679/aohc.59.339. [DOI] [PubMed] [Google Scholar]
- 15.Somers T.H., Houben V., Goovaerts G., Govaerts P.J., Offeciers E.E. Histology of the perforated tympanic membrane and its muco-epithelial junction. Clin Otolaryngol Allied Sci. 1997;22:162–166. doi: 10.1046/j.1365-2273.1997.00006.x. [DOI] [PubMed] [Google Scholar]
- 16.Giles B. Wound healing in spontaneous perforation or myringotomy and middle ear reconstruction. Ear Nose Throat J. 2007;86:30–32. [Google Scholar]
- 17.Johnson A.P., Smallman L.A., Kent S.E. The mechanism of healing of tympanic membrane perforations: a two-dimensional histological study in guinea pigs. Acta Otolaryngol Stockh. 1990;109:406–415. doi: 10.3109/00016489009125162. [DOI] [PubMed] [Google Scholar]
- 18.Makino K., Amatsu M. Epithelial migration on the tympanic membrane and external canal. Arch Otorhinolaryngol. 1986;243:39–42. doi: 10.1007/BF00457906. [DOI] [PubMed] [Google Scholar]
- 19.Masutani H., Nakai Y., Sugita M., Ohashi K., Moriguchi M., Matsunaga K. Microvasculature of the tympanic membrane. Acta Otolaryngol Suppl Stockh. 1991;486:99–104. doi: 10.3109/00016489109134988. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor K.N., Tam M., Blevins N.H., Puria S. Tympanic membrane collagen fibers: a key to high frequency sound conduction. Laryngoscope. 2008;118:483–490. doi: 10.1097/MLG.0b013e31815b0d9f. [DOI] [PubMed] [Google Scholar]
- 21.Lai P., Propst E.J., Papsin B.C. Lateral graft type 1 tympanoplasty using Alloderm for tympanic membrane reconstruction in children. Int J Ped Otorhinolaryngol. 2006;70:1423–1429. doi: 10.1016/j.ijporl.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Weinberger J.M., Hawke M., Gotlieb A.I. Repair of the wounded guinea pig tympanic membrane: organization of filamentous actin and spatial cellular reorganization. J Otolaryngol. 1988;17:352–358. [PubMed] [Google Scholar]