Abstract
Introduction
Local progression of papillary thyroid carcinoma (PTC) after failure of standard therapies may cause pain, ulceration, and bleeding. As patients are fully aware of the tumor growth, they might suffer high grade anxiety. Electrochemotherapy (ECT) is a new local palliative treatment for skin metastases of malignant melanoma or other tumors, including squamous head e neck cancer patients.
Objective
To evaluate the impact of ECT in patients with local progression of PTC.
Methods
Four patients with local progression of PTC were treated with ECT based on Bleomycin, and evaluated according to tumor response, local pain and side effects.
Results
In all cases, some grade of tumor response was observed, lasting 6, 7, 12 and 8 months, respectively. Also, reduction of local pain and anxiety was registered in all patients. Tumor infiltrated skin necrosis was the only collateral effect of the treatment. ECT induced a tumor response in all PTC patients with improvement of symptoms.
Conclusions
ECT may be an option for local palliative treatment in PTC patients with local tumor progression.
Keywords: Bleomycin, Electrochemotherapy, Head and neck cancer, Palliative therapy, Thyroid
Resumo
Introdução
A progressão local do carcinoma papilífero de tireoide (CPT) após a falha da terapia de rotina pode causar dor, ulceração e sangramento. Considerando que os pacientes estão perfeitamente cientes do crescimento tumoral, podem apresentar um alto grau de ansiedade. A eletroquimioterapia (EQT) é um novo tratamento paliativo para metástases de pele de melanoma maligno ou de outros tumores, inclusive em pacientes com carcinoma escamoso de cabeça e pescoço.
Objetivo
Avaliar o impacto da EQT em pacientes com progressão local de CPT.
Método
Quatro pacientes com progressão local de CPT foram tratados com EQT com base em bleomicina, e avaliados em relação ao grau de resposta tumoral, dor local, efeitos colaterais.
Resultados
Em todos os casos, foi observado algum grau de resposta tumoral, que perdurou por 6, 7, 12 e 8 meses, respectivamente. Da mesma forma, foi registrada diminuição da dor local e da ansiedade em todos os pacientes. Necrose cutânea na infiltração tumoral foi o único efeito colateral do tratamento. EQT induziu resposta tumoral em todos os pacientes com CPT, com melhora dos sintomas.
Conclusões
EQT pode ser uma opção para o tratamento paliativo tópico em pacientes com CPT com progressão tumoral local.
Palavras-chave: Bleomicina, Eletroquimioterapia, Câncer de cabeça e pescoço, Terapia paliativa, Tireoide
Introduction
Local relapse or regional neck metastases after failure of standard therapies of papillary thyroid carcinoma (PTC) are rare. However, patients developing local or regional tumor progression may suffer from anxiety, local pain, exudation, or bleeding.
Electrochemotherapy (ECT) is a new experimental technology based on increasing cell membrane permeability of the tumor cells (electroporation) to molecules. ECT consists of the administration of electric pulses in the tumor during perfusion of chemotherapy to facilitate drug delivery into malignant cells.1, 2 ECT is now being used as a palliative therapy for cutaneous metastases and head and neck cancer.3, 4, 5, 6, 7 A recently published meta-analysis of 915 patients studied the results of skin-directed therapy for local cutaneous metastases, including electrochemotherapy, photodynamic therapy, radiotherapy, intralesional therapy, and topical therapy.8 The histology of primary tumors was mainly melanoma and breast carcinoma, and none of them were thyroid carcinoma. ECT was more active than the other skin-directed therapies, as a 59% complete response rate was reported, and transient local pain occurring in 49% of patients that resolved within a month was the more common side effect.
The authors present the preliminary results of a pilot study of thyroid cancer patients with progression of local recurrence or neck metastases treated with ECT.
Methods
Inclusion criteria
Patients resistant to radioiodine and to anti-target agent Sorafenib with local tumor progression of PTC after primary therapy (thyroidectomy, cervical neck dissection, and adjuvant radioiodine), and after treatment of the relapse (salvage cervical surgery and radiotherapy) were included. Sorafenib resistance was considered when a 20% increase in the sum of diameters of the target lesion was observed.
Additional inclusion criteria: Eastern Cooperative Oncology Group performance status of 2 or less, life expectancy of at least three months, no active respiratory disease or serious chronic pulmonary disease, absolute white blood count above 4000 cells/μL, hemoglobin greater than 10 g/dL, platelet count above 100,000 μL, and no previous clinical history of allergy to bleomycin.
The study was approved by the Institutional Review Board. Informed consent was obtained from all patients.
Treatment
Under total anesthesia, with an inspired oxygen fraction (FiO2) of 36% or less, bleomycin, 20 mg/m2 IV was administered in a bolus. Between eight and 28 min later, electric pulses of 100 μs and 1000 V were administered to the target tumor by an electrode (model N-30-HG; IGEA S.r.l. – Carpi, Italy), powered by a commercial pulse generator for electroporation treatments (CLINIPORATOR; IGEA S.r.l. – Carpi, Italy). Tumor tissue was treated homogeneously, covering the entire target volume.
Response criteria
Radiological evaluation was performed at 0, 6, and 12 weeks after ECT, according to Response Evaluation Criteria in Solid Tumor (RECIST) criteria (version 1.1).9 Symptom evaluation of pain and anxiety was performed with the Edmonton Symptom Assessment System before treatment and 12 weeks after ECT. The patients subjectively scored the intensity of these symptoms on a visual analogical scale (VAS) between 0 (absence) and 10 (maximum intensity). Toxicity and adverse events related to ECT were also evaluated at 6, 12, and 24 h after the procedure.
During the follow-up period, the patients were evaluated at least every three months or when disease progression was observed.
Results
Four consecutive patients, one male and three females, were enrolled in the study. The age ranged between 51 and 60 years. The target tumor was a large cervical mass in three patients and skin metastases (less than 2 cm) in one.
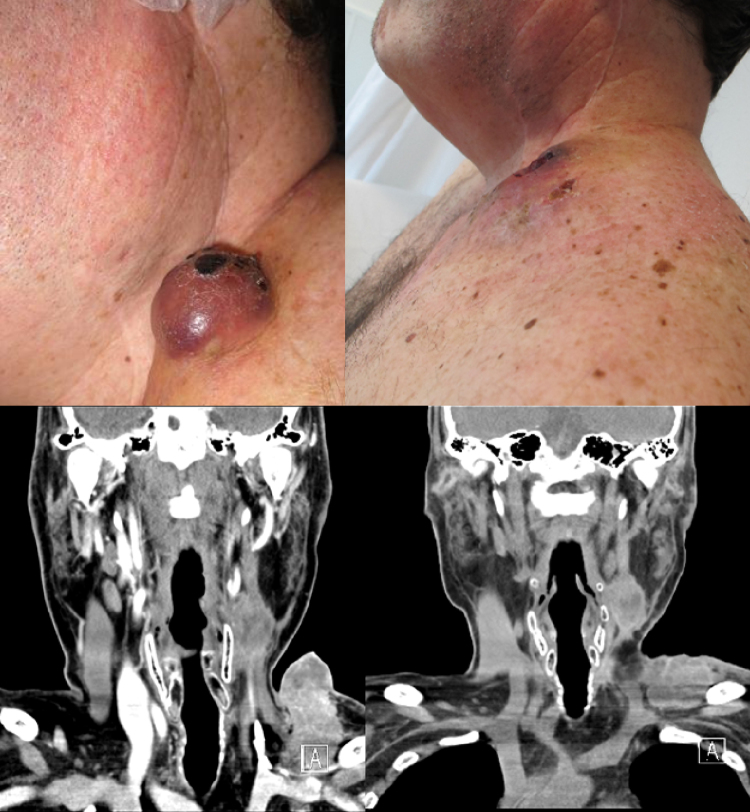
A clinical response was observed in all four patients within the first three weeks after ECT (Fig. 1). These findings were consistent with the radiological evaluation, showing partial response (PR) in two patients and two cases of disease stabilization with lower tumor burden, but not reaching the PR criteria as defined by RECIST.
Figure 1.
Case 1: External aspect and CT-scan of a lower left cervical mass prior (left images) and after four weeks (right images) of the electrochemotherapy.
The targeted lesions’ size decreased over the next three months and remained without progression during six to 12 months after the procedure. Three of the patients died because of visceral metastases, and there was no disease progression in the targeted lesions treated with ECT. In one case, the patient died 18 months after ECT due to local and metastatic disease progression.
The symptom evaluation showed a moderate decrease in the reported median numerical scale for anxiety (10–7) and mild reduction in pain (7–6). No severe adverse events were observed in the next 24 h after ECT, apart from mild local pain controlled with non-opioid analgesia. The changes in the targeted lesion included painless skin necrosis.
Discussion
This is the first report to assess the clinical efficacy of ECT as a palliative treatment for PTC. The response was evaluated using RECIST version 1.1.9 There were some difficulties in the interpretation of the RECIST criteria. Firstly, some increase in the size of the lesion could be found, especially in the first few weeks, attributed to the local inflammatory reaction after ECT. In the two cases reported as “stable disease”, tumor response was observed in the central part of the tumor, but this is not evaluated in the RECIST criteria.
Patients reported a moderate improvement in the two most frequent tumor-derived symptoms: local pain and anxiety. The treatment was well tolerated, and as the administered dose of bleomycin was below the maximum tolerated dose, the procedure could have been repeated. None of the patients had the toxicities frequently associated to treatments with bleomycin.10
PTC is commonly considered resistant to bleomycin because its unavailability to diffuse through the cell membrane. ECT induces electroporation of the cell membrane, facilitating the entrance of bleomycin into the cell from the plasma.2, 11, 12 Once there, bleomycin appears to act strongly against cell growth, inducing cell apoptosis that can be observed up to several weeks after the procedure.13
The present results showed that ECT may be a valid option as palliative local treatment in PTC patients with local relapse or skin metastases. The number of patients initially recruited was small, but that could be explained since papillary thyroid cancer is a highly curable disease, and because patients with progressive disease are rare and all the cases were from a unique institution. Moreover, other systemic chemotherapy agents are currently under investigation for such patients.
It appears that this procedure may have an antitumoral effect, particularly in lesions smaller than 3 cm.5 Another meta-analysis published on 413 patients with cutaneous and subcutaneous tumors indicated that ECT had significantly (p < 0.001) higher effectiveness (by more than 50%) than bleomycin or cisplatin alone and that ECT was more effective in sarcoma than in melanoma or carcinoma patients. No patients with thyroid carcinoma were reported.14 Further clinical trials with more patients will clarify the role of this procedure.
Also, it should be determined whether a larger electrode would enable reaching the tumor more extensively, potentially leading to improved results.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgement
This study was supported by a research project grant (PI09/90664) from the Spanish Ministry for Science and Innovation and by a research project grant (EC10-067) from the Spanish Ministry of Health, Department of Pharmacy and Health Products.
Footnotes
Please cite this article as: Grau JJ, Caballero M, Langdon C, Bernal-Sprekelsen M, Blanch JL. Electrochemotherapy as palliative treatment in patients with thyroid papillary carcinoma. Braz J Otorhinolaryngol. 2016;82:285–8.
References
- 1.Miklavčič D., Mali B., Kos B., Heller R., Serša G. Electrochemotherapy: from the drawing board into medical practice. Biomed Eng Online. 2014;13:29. doi: 10.1186/1475-925X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J.O., Collins C.G., Aarons S., Tangney M., Whelan M., O’Reily S., et al. Electrochemotherapy: aspects of preclinical development and early clinical experience. Ann Surg. 2007;245:469–479. doi: 10.1097/01.sla.0000250419.36053.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gargiulo M., Papa A., Capasso P., Moio M., Cubicciotti E., Parascandolo S. Electrochemotherapy for non-melanoma head and neck cancers: clinical outcomes in 25 patients. Ann Surg. 2012;255:1158–1164. doi: 10.1097/SLA.0b013e31824f68b2. [DOI] [PubMed] [Google Scholar]
- 4.Landstrom F.J., Nilsson C.O., Crafoord S., Reizenstein J.A., Adamsson G.B., Lofgren L.A. Electroporation therapy of skin cancer in the head and neck area. Dermatol Surg. 2010;36:1245–1250. doi: 10.1111/j.1524-4725.2010.01617.x. [DOI] [PubMed] [Google Scholar]
- 5.Mali B., Miklavcic D., Campana L.G., Cemazar M., Sersa G., Snoj M., et al. Tumor size and effectiveness of electrochemotherapy. Radiol Oncol. 2013;47:32–41. doi: 10.2478/raon-2013-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mir L.M., Orlowski S., Belehradek J., Jr., Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27:68–72. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 7.Reinhold U. Electrochemotherapy for primary skin cancer and skin metastasis related to other malignancies. Anticancer Drugs. 2011;22:711–718. doi: 10.1097/CAD.0b013e32834618da. [DOI] [PubMed] [Google Scholar]
- 8.Spratt D.E., Gordon Spratt E.A., Wu S., DeRosa A., Lee N.Y., Lacouture M.E., et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32:3144–3155. doi: 10.1200/JCO.2014.55.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Albiol S., Grau J.J., Pereira A., Reguart N., Gascon P. Epidemic hemolytic-uremic syndrome related to bleomycin. Haematologica. 2001;86:E10. [PubMed] [Google Scholar]
- 11.Mir L.M., Orlowski S. Mechanisms of electrochemotherapy. Adv Drug Deliv Rev. 1999;35:107–118. doi: 10.1016/s0169-409x(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 12.M Mir L.M., Orlowski S. The basis of electrochemotherapy. Methods Mol Med. 2000;37:99–117. doi: 10.1385/1-59259-080-2:99. [DOI] [PubMed] [Google Scholar]
- 13.Mir L.M., Tounekti O., Orlowski S. Bleomycin: revival of an old drug. Gen Pharmacol. 1996;27:745–748. doi: 10.1016/0306-3623(95)02101-9. [DOI] [PubMed] [Google Scholar]
- 14.Mali B., Jarm T., Snoj M., Sersa G., Miklavcic D. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur J Surg Oncol. 2013;39:4–16. doi: 10.1016/j.ejso.2012.08.016. [DOI] [PubMed] [Google Scholar]