Abstract
Aim: No flow-limiting dissection after drug-coated balloon (DCB) treatment for femoropopliteal (FP) lesions is considered as one of the endpoints, but it has not investigated the difference between each vessel dissection. This study aimed to clarify whether there is a difference between no dissection and type C dissection without flow-limiting dissection for 3 months by peak systolic velocity ratio (PSVR) based on duplex ultrasonography.
Methods: Between February 2020 and April 2021, 44 consecutive de novo FP diseases that underwent endovascular therapy (EVT) with DCB were enrolled in this study. 65.9% of the patients had intermittent claudication, and mean lesion lengths were 194±107 mm. The chronic total occlusion was 38.6%. After DCB treatment, vessel dissection pattern was categorized by angiography. The minimum lumen area (MLA) identified by intravascular ultrasound was serially evaluated with PSVRs at 1 day, 1 month, and 3 months after EVT.
Result: All lesions were treated with DCB without provisional stents. The vessel dissection pattern after DCB treatment showed that types D, E, and F were not observed, 9% were no dissection, 27% were type A, 32% were type B, and 32% were type C. In all cases, the PSVR values of MLA site were less than 2.6 at 3 months, and there were no significant differences between no dissection and type C dissection.
Conclusion: Up to dissection pattern “C” is considered acceptable as one of the endpoints to determine the need for provisional stenting after DCB treatment.
Keywords: Drug-coated balloon, Vessel dissection, Provisional stenting, Peak systolic velocity ratio, Intravascular ultrasound
Introduction
The indication of endovascular treatment (EVT) using drug-coated balloon (DCB), which combines balloon dilatation with local delivery of an antiproliferative drug, has gained widespread acceptance for femoropopliteal (FP) disease. Its primary patency estimates at 1 year range between 73.5% and 91.1%. However, provisional stenting after suboptimal DCB treatment is still necessary in 7.3% to 40%, mainly due to flow-limiting dissection or residual stenosis (>50%) 1 - 5) . Currently, insurance coverage for provisional stenting after suboptimal DCB treatment has not been approved in Japan. In addition, the guidelines for the appropriate use of DCB recommend that dissection should be reduced to up to type C and residual stenosis should be less than 50% during the predilation phase 6) . On the other hand, there are no clear endpoint criteria to avoid provisional stenting after DCB treatment.
Peak systolic velocity ratio (PSVR) based on duplex ultrasonography (DUS) has been widely used as a noninvasive tool in the identification of restenosis after EVT of FP disease 7) . In single FP stenosis, a PSVR value of 2.6 or higher correlates with angiographically significant stenosis of 50% or more; therefore, a PSVR value more than 2.6 is considered appropriate as a significant hemodynamic index after DCB treatment for FP lesion 8 , 9) .
Aim
This study aimed to clarify whether there is a difference between no dissection and type C dissection without flow-limiting dissection for 3 months by PSVR based on DUS.
Methods
Study Design and Patient Population
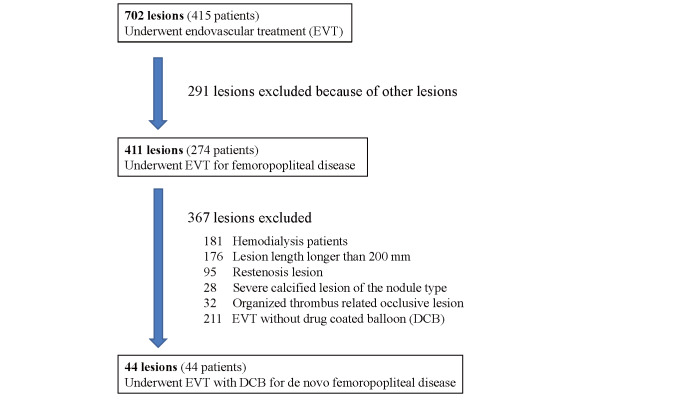
EVT for FP disease was performed in 411 lesions, 274 patients from February 2020 to April 2021 at Morinomiya Hospital. We prospectively enrolled the patients who had successfully undergone EVT with DCBs for de novo FP lesions under the intravascular ultrasound (IVUS) guidance. Patients with symptoms of peripheral arterial disease were screened using physical and noninvasive examinations, including ankle-brachial index (ABI), DUS, and imaging studies (computed tomography, magnetic resonance imaging, or angiography). Lesion lengths longer than 200 mm, in stent restenosis and/or reocclusion, and severe calcified lesion of the nodule type were excluded because those lesions were not recommended to use guidelines for the proper use of DCB in Japan. Hemodialysis patients were excluded because they would be discharged the day after EVT and therefore would not be able to undergo duplex testing for the study protocol. Long occlusive lesions including fresh or organized thrombus were also excluded because they were not expected to benefit from DCB. Finally, 44 lesions, 44 patients that underwent EVT with DCBs were evaluated ( Fig.1 ) . Informed consent was obtained from each patient, and the institutional ethics committee reviewed the protocol and approved this analysis.
Fig.1.
Participant flowchart
EVT Procedures
After the initial diagnostic angiography of the lower limb, we scheduled EVT when the lesions had diameter stenosis of more than 75% or occlusion. All quantitative vascular angiography (QVA) analyses were performed using commercially available software (QAngio XA 7.3, Medis Medical Imaging Systems BV, Leiden, The Netherlands). A 4.5-Fr or 6-Fr guiding sheath (Medikit, Parent plus, Japan) was inserted into the common femoral artery via a contralateral or ipsilateral antegrade approach according to the operator’s discretion. After insertion of guiding sheath, unfractionated heparin (100 units/kg) was injected into the artery, and additional heparin was administered to maintain an active clotting time of >200 s during the procedure. EVT was performed with a radiopaque ruler set from the edge of the femoral head as a landmark. After a 0.014-in. guidewire was advanced down to the lesion, predilation was performed with an optimally sized balloon (vessel/balloon ratio, 1:1) with IVUS guidance. After predilation, patients were treated with same size DCB, any one of the Lutonix (C.R. Bard, Murray Hill, NJ, USA), IN.PACT Admiral (Medtronic Vascular, Santa Clara, CA, USA), or Ranger (Boston Scientific, Marlborough, MA, USA) according to the operator’s decision. Finally, a final angiogram was performed to allow for the assessment of thrombolysis in myocardial infarction (TIMI) grade, residual stenosis, and vessel dissection pattern. Antiplatelet therapy (aspirin 100 mg/day and clopidogrel 75 mg/day) was prescribed for at least 1 week prior to EVT and continued during the follow-up period after DCB treatment in all cases.
IVUS Analysis
IVUS was done in all cases with a commercially available IVUS console (s5TM Imaging System; Volcano, Rancho Cordova, CA, USA) and a phased-array 20-MHz IVUS catheter (Eagle Eye Gold; Volcano). IVUS images were recorded before and after EVT pulled back manually through the entire lesion. The proximal and distal reference segments were selected as the most normal-looking cross sections. After DCB treatment, a minimum lumen cross-sectional area (MLA) was measured at the site with the smallest lumen area by IVUS. The MLA site was accurately identified by the distance from the bifurcation to the MLA site so that the MLA site could be measured at the same site on each follow-up examination.
Angiographic Analysis
QVA data was obtained before and immediately after DCB treatment. The severity of vessel dissection pattern after final angiogram was assessed through the consensus of two experienced vascular interventionalists with a single anteroposterior view. If several dissection patterns were evident in the treated lesion, the worst was used for the analysis. Six grades of dissection (A–F) were categorized according to the previous report 6) .
Dus Follow-Up
Clinical evaluations were made at 1 day, 1 month, and 3 months after EVT. DUS employing a commercially available machine should be performed using Aplio 400 US system (Toshiba Medical Systems, Tochigi, Japan). All DUS examinations were performed by an experienced dedicated sonographer who participated in this study. All patients were examined in a supine position using a duplex scanner with a 7.5-MHz transducer. The segment of MLA after DCB treatment can be visualized using a combined B-mode and color Doppler ultrasound. The Doppler signal is acquired at an angle of 60 degrees or as small as possible, and velocity spectra are recorded proximal to and at the site of MLA. Doppler spectral analysis can determine the highest PSV (PSV at the MLA) and the PSV in the area adjacent to the normal-looking segment (PSV proximal). PSVR can be calculated by the following formula: PSV at the MLA/PSV proximal. Restenosis was defined as PSVR ≥ 2.6 on DUS at rest 8 , 9) .
Definition
The successful EVT was defined as TIMI grade 3 blood flow and % residual diameter stenosis of less than 50% with QVA analysis. A PSVR value more than 2.6 or occlusion was defined as restenosis by DUS follow-up. The target lesion revascularization (TLR) was performed when the PSVR was 2.6 or higher with recurrence of symptoms.
Study Endpoints
The primary endpoint of this study was the serial change of PSVRs based on DUS at 1 day, 1 month, and 3 months. The secondary endpoint was the difference in PSVR between each vessel dissection for 3-month follow-up.
Statistical Analysis
The continuous variables were expressed as the mean±standard deviation. The categorical variables were expressed as numbers with percentage, if not otherwise mentioned. The continuous variables were compared using the t test or Mann–Whitney U test. The categorical variables were assessed using the chi-square or Fisher exact test. A P value <0.05 was considered statistically significant. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Shimotsuke, Tochigi, Japan), a graphical user interface for R (version 3.6.1; The R Foundation for Statistical Computing Platform, Vienna, Austria).
Results
Baseline Characteristics
The patient and lesion characteristics are summarized in Table 1 ; 65.9% of patients had intermittent claudication, and 34.1% had chronic limb-threatening ischemia. Diabetes was present as notable comorbidities in 68.2%.
Table 1. Patient and lesion characteristics.
| Patient Characteristics | |
| Age, y | 74±9 |
| Male gender | 30 (68.2) |
| Risk factors | |
| Hypertension | 35 (79.6) |
| Hyperlipidemia | 26 (59.1) |
| Diabetes mellitus | 30 (68.2) |
| Renal insufficiency (eGFR <30mL/min/1.73m2) | 7 (16.7) |
| Current smoking | 34 (81.0) |
| Rutherford grade (2/3/4/5) | 11/18/4/11 (25.0/40.9/9.1/25.0) |
| Lesion Characteristics | |
| Mean lesion length, mm | 194±107 |
| Chronic total occlusion | 17 (38.6) |
| Mean occlusion length, mm | 35±71 |
| % Diameter stenosis | 90.2±13.9 |
| Proximal reference vessel diameter, mm | 6.3±0.8 |
| Distal reference vessel diameter, mm | 6.0±1.1 |
| PACSS grade (0/1/2/3/4) | 23/7/6/2/6 (52.3/15.9/13.6/4.6/13.6) |
| Target lesion | |
| SFA | 27 (61) |
| SFA involving popliteal artery | 13 (30) |
| Popliteal artery | 4 (9) |
Data are presented as mean±SD or number (percentage).
Abbreviations: eGFR, estimated glomerular filtration rate. PACSS, Proposed Peripheral Artery Calcium Scoring System. SFA, superficial femoral artery.
The mean lesion and occlusion length were 194±107 mm and 35±71 mm. Chronic total occlusion (CTO) was 38.6%. The distribution of lesions was 61% in superficial femoral artery (SFA), 30% in SFA involving popliteal artery lesion, and 9% in popliteal artery lesion.
Procedural Results
The procedural results are shown in Table 2 . All target lesions were treated with DCB without provisional stents. The mean residual diameter stenosis of final angiogram after DCB treatment was 26.1%±13.6%. MLA based on IVUS after DCB treatment was 12.7±4.0 mm2 (range, 5.7 to 25.1 mm2). The vessel dissection pattern after DCB treatment based on final angiogram showed that types D, E, and F were not observed, 9% were no dissection, 27% were type A, 32% were type B, and 32% were type C. The rates of IN.PACT, Lutonix, and Ranger were 54%, 30%, and 16%, respectively.
Table 2. Procedural results.
| Mean DCB number | 1.36±0.49 |
| Mean DCB diameter, mm | 5.6±0.7 |
| Mean DCB length, mm | 134±37 |
| DCB inflation time, sec | 185±13 |
| Final TIMI grade | 3±0 |
| % Residual diameter stenosis | 26.1±13.6 |
| DCB type (IN.PACT/Lutonix/Ranger) | 24/13/7 (54/30/16) |
| Mean MLA, mm2 | 12.7±4.0 |
| Final dissection type | |
| Type 0, A, B, C | 4/12/14/14 (9/27/32/32) |
| Type D, E, F | 0/0/0 (0/0/0) |
| Provisional stenting | 0 (0) |
Data are presented as mean±SD or number (percentage).
Abbreviation: DCB, drug coated balloon. TIMI, thrombolysis in myocardial infarction trial. MLA, minimum lumen cross-sectional area.
Subacute Changes
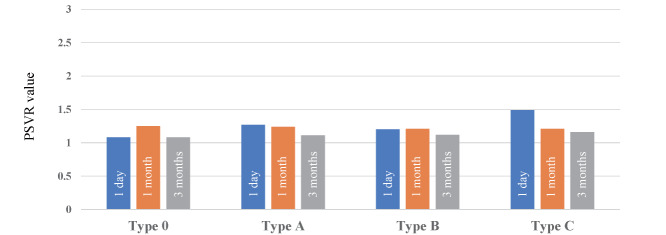
Table 3 shows the PSVR values of MLA site at 1 day, 1 month, and 3 months after DCB treatment (1.28±0.59, 1.22±0.31, 1.14±0.25, respectively). In all cases, the PSVR value were less than 2.6 at 3 months, and there were no significant difference in PSVR values between no dissection and type C dissection; rather, the improvement in PSVR values at 3 months tended to be stronger in type C dissection than the others ( Fig.2 ) . In addition, the average of PSVRs at 1 day, 1 month, and 3 months for each DCB type were shown in Table 4 . There were no significant differences of PSVRs between each DCB at any phase.
Table 3. Subacute changes.
| Serial PSVRs change | |
| PSVR at 1 day | 1.28±0.59 |
| PSVR at 1 month | 1.22±0.31 |
| PSVR at 3 months | 1.14±0.25 |
| Serial ABIs change | |
| Before | 0.62±0.18 |
| 1 month | 0.89±0.13 |
| 3 months | 0.90±0.15 |
| Adversary Event | |
| Subacute occlusion | 0 (0) |
| Primary patency | 44 (100) |
| TLR within 3 months | 0 (0) |
Data are presented as mean±SD or number (percentage). Abbreviations: PSVR, peak systolic velocity ratio. ABI, ankle brachial index. TLR, target lesion revascularization.
Fig.2.
Serial change of PSVR value at MLA site according to vessel dissection pattern after DCB treatment
Table 4. Difference in DCB type and PSVR in subacute phase.
| PSVR | DCB type | P value | ||
|---|---|---|---|---|
| Lutonix | IN.PACT | Ranger | ||
| 1 day | 1.14±0.21 | 1.19±0.52 | 1.15±0.24 | 0.932 |
| 1 month | 1.26±0.21 | 1.21±0.37 | 1.13±0.34 | 0.765 |
| 3 months | 1.06±0.23 | 1.18±0.26 | 1.25±0.07 | 0.351 |
Data are presented as mean±SD or number. Abbreviations: DCB, drug coated balloon. PSVR, peak systolic velocity ratio.
Adverse Events
There were no perioperative complication such as death, major amputation, and TLR within 3 months in all patients.
Representative Case
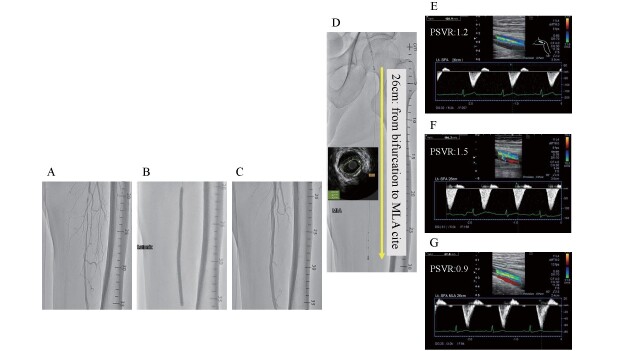
A 70-year-old male was admitted to our hospital for intermittent claudication of his left calf. The angiography showed a severe stenosis with a lesion length of 10 cm in the middle of the FP ( Fig.3-A ) . The diseased lesion was dilated at nominal pressure of 8 atm with 5.0×150 mm Lutonix DCB after predilatation using 5.0-mm conventional balloon according to IVUS measurement ( Fig.3-B ) . The final angiogram showed that residual stenosis was 40% with dissection type B ( Fig.3-C ) . The MLA identified by IVUS was 9.0 mm2 and was found to be located 26 cm from the bifurcation ( Fig.3-D ) . The PSVR value of the MLA site was 1.2, 1.5, and 0.9 at the 1 day, 1 month, and 3 months after EVT, respectively ( Fig.3-E, F, G ) .
Fig.3. Representative case.
A: Baseline angiogram. B: Lesion dilatation with 5.0×150 mm Lutonix DCB. C: Final angiogram. D: Identification and localization of MLA by IVUS. E: PSVR at 1 day. F: PSVR at 1 month. G: PSVR at 3 months.
Discussion
This is the first study to evaluate the difference of vessel dissection after DCB treatment in the subacute phase using PSVR based on DUS. The main finding of this study was that there were no significant difference in PSVR of MLA site for 3 months after DCB treatment between no dissection and type C dissection.
Mechanism of Vessel Dissection
Based on our experience using IVUS and endoscopy during EVT for various FP lesions, we believe that most of dissection types E and F occur in CTO lesions that contain a large amount of organizing thrombus and that type E occurs when the recoil of the organizing thrombus after balloon dilation does not reach the total occlusion and type F occurs when the recoil completely occludes the lesion. Type D dissection represented by spirally dissection is mainly caused by messy wire manipulation or loop wire technique for long segment CTO lesions 10 , 11) . On the other hand, the dissection that occurs after balloon dilatation for intimal thickness type lesions is either no dissection or between types A, B, and C. Therefore, in this study, we selected only lesions consisting of intimal thickness type lesion, excluding CTOs associated with organizing thrombus and long segment CTOs, and finally none of the cases were severe flow-limiting dissection.
Natural Course of Dissected Lesion After DCB Treatment
In this study, the MLA of the dissection site after DCB treatment was observed for up to 3 months because the healing period of dissection was about 3 months from experience of our follow-up angiography at 3 months after DCB treatment for FP lesions. In the coronary artery field, Hui et al. reported that almost dissections (93.9%) were completely healed and there was no newly developed dissection at 6-month angiography. Furthermore, they also concluded that the presence of a dissection following successful DCB treatment of a de novo coronary lesion may not be associated with an increased risk of late lumen loss or target vessel failure 12 , 13) . In the present study, all cases up to dissection C kept the patency without adverse events for 3 months. This suggests that the patient, lesion background, and selected final device may have a significant impact on patency following 3 months.
Serial PSVR Evaluation of MLA Site
Recently, PSVRs based on DUS have been widely used as a noninvasive tool in place of an invasive angiographic evaluation in identification of restenosis after EVT for FP disease 7) . Macharzina et al. reported that, in single FP stenosis not multisegmented lesion, a PSVR value of 2.6 or higher correlates with angiographically significant stenosis of 50% or more; therefore, a PSVR value more than 2.6 is considered appropriate as a significant hemodynamic index in the subacute phase after DCB treatment 8 , 9) . In the present study, PSVR value of 2.6 was used as the cutoff value for the patency, because even if restenosis had occurred, it was not expected to be a multisegmented lesion in the short-term period.
DCB Selection
It has been reported that there is a difference in the patency of the chronic phase between available DCBs 1 - 3) . In the present study, three DCBs were selected according to operator’s decision because the observation period was short (3 months) and the effect on patency in each DCB was expected to be minimal.
Study Limitation
This study has several limitations, including the small sample size, single-center study. The main limitation of the present study was that grading for vessel dissection was evaluated by noncore laboratory and single operator assessment. The kind of lesions selected were intimal thickness type lesion, excluding CTOs associated with organizing thrombus and long segment CTOs. In the present study, the transition of PSVR of type D, E, and F dissection was not examined. Further studies are needed to determine which parameters influence early events after DCB treatment. This study evaluated PSVR values of subacute phase between each dissection and did not evaluate differences of chronic phase.
Conclusion
Up to dissection pattern “C” is considered acceptable as one of the endpoints to determine the need for provisional stenting after DCB treatment.
Acknowledgements
Support from institutional sources only.
Conflict of Interest
The Authors declare that there is no conflict of interest.
References
- 1).Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR; IN.PACT SFA Trial Investigators: Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation, 2015; 131: 495-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Müller-Hülsbeck S, Nehler MR, Benenati JF, Scheinert D; LEVANT 2 Investigators: Trial of a Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease. N Engl J Med, 2015; 373: 145-153 [DOI] [PubMed] [Google Scholar]
- 3).Sachar R, Soga Y, Ansari M, Kozuki A, Lopez L, Brodmann M, Schroë H, Ramanath V, Diaz-Cartelle J, Zeller T: One Year Results from the RANGER II SFA Randomized Trial of the Ranger Drug-coated Balloon. JACC Cardiovasc Interv, 2021; 14: 1123-1133 [DOI] [PubMed] [Google Scholar]
- 4).Schmidt A, Piorkowski M, Görner H, Steiner S, Bausback Y, Scheinert S, Banning-Eichenseer U, Staab H, Branzan D, Varcoe RL, Scheinert D: Drug-Coated Balloons for Complex Femoropopliteal Lesions: 2-Year Results of a Real-World Registry. JACC Cardiovasc Interv, 2016; 9: 715-724 [DOI] [PubMed] [Google Scholar]
- 5).Fanelli F, Cannavale A, Citone M, Santoni M, Gazzetti M, Falcone GM, Miele V: Provisional Stenting Using the Zilver PTX Drug-Eluting Stent After Drug-Coated Balloon Angioplasty: Initial Experience From the Double Drug Dose "3D" Study. J Endovasc Ther, 2020; 27: 34-41 [DOI] [PubMed] [Google Scholar]
- 6).Fujihara M, Takahara M, Sasaki S, Nanto K, Utsunomiya M, Iida O, Yokoi Y: Angiographic Dissection Patterns and Patency Outcomes After Balloon Angioplasty for Superficial Femoral Artery Disease. J Endovasc Ther, 2017; 24: 367-375 [DOI] [PubMed] [Google Scholar]
- 7).Ranke C, Creutzig A, Alexander K: Duplex scanning of the peripheral arteries: correlation of the peak velocity ratio with angiographic diameter reduction. Ultrasound Med Biol, 1992; 18: 433-440 [DOI] [PubMed] [Google Scholar]
- 8).Macharzina RR, Schmid SF, Beschorner U, Noory E, Rastan A, Vach W, Schwarzwälder U, Sixt S, Bürgelin K, Neumann FJ, Zeller T: Duplex ultrasound assessment of native stenoses in the superficial femoral and popliteal arteries: a comparative study examining the influence of multisegment lesions. J Endovasc Ther, 2015; 22: 254-260 [DOI] [PubMed] [Google Scholar]
- 9).Kawarada O, Hozawa K, Zen K, Huang HL, Kim SH, Choi D, Park K, Kato K, Kato T, Tsubakimoto Y, Ichihashi S, Fujimura N, Higashimori A, Sato T, Yan BP, Pang SY, Wongwanit C, Leong YP, Chua B, George RK, Chen IC, Lee JK, Hsu CH, Pua U, Iwata Y, Miki K, Okada K, Obara H: Peak systolic velocity ratio derived from quantitative vessel analysis for restenosis after femoropopliteal intervention: a multidisciplinary review from Endovascular Asia. Cardiovasc Interv Ther, 2020; 35: 52-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Kawasaki D, Iida O, Fukunaga M, Kato M, Ohkubo N: Wire Passages of 0.035-inch Looped Wire Technique for Femoropopliteal Long Total Occlusions. J Atheroscler Thromb, 2015; 22: 1071-1079 [DOI] [PubMed] [Google Scholar]
- 11).Mori S, Hirano K, Ito Y, Yamawaki M, Araki M, Kobayashi N, Takimura H, Sakamoto Y, Tsutsumi M, Takama T, Honda Y, Tokuda T, Makino K, Shirai S: Clinical Outcomes of the Intraluminal Approach for Long Occlusive Femoropopliteal Lesions Assessed by Intravascular Ultrasound. J Atheroscler Thromb, 2017; 24: 477-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Hui L, Shin ES, Jun EJ, Bhak Y, Garg S, Kim TH, Sohn CB, Choi BJ, Kun L, Yuan SL, Zhi W, Hao J, Zhentao S, Qiang T: Impact of Dissection after Drug-Coated Balloon Treatment of De Novo Coronary Lesions: Angiographic and Clinical Outcomes. Yonsei Med J, 2020; 61: 1004-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Soga Y, Fujihara M, Tomoi Y, Iida O, Ishihara T, Kawasaki D, Ando K: One-Year Late Lumen Loss between A Polymer-Coated Paclitaxel-Eluting Stent (Eluvia) and a Polymer-Free Paclitaxel-Coated Stent (Zilver PTX) for Femoropopliteal Disease. J Atheroscler Thromb, 2020; 27: 164-171 [DOI] [PMC free article] [PubMed] [Google Scholar]