Abstract
Background and Objectives
Understanding the role of vaccine hesitancy in under-vaccination or non-vaccination of childhood vaccines is important for increasing vaccine confidence and uptake.
Methods
We used data from April to June interviews in the 2018 and 2019 National Immunization Survey (NIS)-Flu (n=78,725), a nationally representative cross-sectional household cellular telephone survey. We determined the adjusted population attributable fraction (PAF) for each recommended childhood vaccine to assess the contribution of vaccine hesitancy to the observed non-vaccination level. Hesitancy is defined as being somewhat or very hesitant toward childhood vaccines. Furthermore, we assessed the PAF of non-vaccination for influenza by sociodemographic characteristics, Health and Human Services (HHS) region, and state.
Results
The proportion of non-vaccination attributed to parental vaccine hesitancy was lowest for hepatitis B (HepB) birth dose vaccine (6.5%) and highest for ≥3 dose diphtheria and tetanus toxoids, and acellular pertussis (DTaP) vaccine (31.3%). The PAF of non-vaccination on influenza non-vaccination was highest for non-Hispanic White and Black populations (15.4%), households with high educational (17.7%) and income levels (16.5%), and urban areas (16.1%).. Among states, PAF ranged from 25.4% (New Hampshire) to 7.5% (Louisiana).
Conclusions
Implementing strategies to increase vaccination confidence and uptake are important, particularly during the COVID-19 pandemic.
Keywords: vaccine hesitancy, vaccine confidence, population attributable fraction, childhood vaccines
Introduction
Several studies have shown that parental vaccine hesitancy is associated with lower vaccination coverage among children and adolescents (1–3). Vaccine hesitancy can be defined as a delay in acceptance or the refusal of vaccination despite availability of vaccination services (4–5). For many children and adolescents, parents’ hesitancy toward vaccines is a significant barrier to vaccination and has been a factor in outbreaks of vaccine-preventable diseases such as measles and pertussis (6–9). Vaccine hesitancy may contribute to parents modifying their children’s vaccination schedules by omitting or delaying vaccines (10–12).
In the United States, the Advisory Committee on Immunization Practices (ACIP) currently recommends vaccination against 17 potentially serious illnesses by the time a child reaches 12 years (13). However, by age 24 months, only 70.5% of children born in 2016 and 2017 had received the combined 7-vaccine series (diphtheria and tetanus toxoids and acellular pertussis vaccine [DTaP], poliovirus vaccine, measles-containing vaccine, Haemophilus influenza type b vaccine [Hib], hepatitis B vaccine [HepB], varicella vaccine [VAR], and pneumococcal conjugate vaccine [PCV]), which is below the Healthy People 2020 target of 80% (14–15). Furthermore, only 54.2% of adolescents were up to date with the human papillomavirus (HPV) vaccine series in 2019, well below the Healthy People 2020 target of 80% (15–16).
Factors that may contribute to lower vaccination coverage include lack of access to vaccination services, missed opportunities for vaccination during healthcare visits, and vaccine hesitancy (17). However, few studies have been able to determine whether under-vaccination or non-vaccination is due to vaccine hesitancy, barriers to access, or a combination of these or other factors. Understanding the amount of under-vaccination or non-vaccination attributable to hesitancy versus other reasons can be used to prioritize approaches to improve vaccine confidence or reduce access barriers. This study assessed the proportion of non-vaccination for influenza and other childhood vaccines attributed to parental vaccine hesitancy among a nationally representative sample of children and adolescents via estimating the population attributable fraction (PAF) across sociodemographic characteristics and states. The PAF estimates the proportion of unvaccinated in the study population that can be reduced if the vaccine hesitancy is eliminated. Understanding how much non-vaccination in the population is attributed to vaccine hesitancy is important for developing strategies for increasing the public’s confidence in vaccines.
Methods
Survey Description
The National Immunization Surveys (NIS) have been described previously (18–19). Briefly, the NIS is a family of surveys using a national, state-stratified, list-assisted random-digit-dialed cellular telephone sample of households with children in the United States. Households with children aged 19–35 months during each calendar quarter of data collection are eligible for the NIS-Child, and households with children aged 13–17 years on date of interview are eligible for the NIS-Teen. During October through June for each influenza season, households with children aged 6–18 months or 13–17 years not eligible for NIS-Child or NIS-Teen are eligible for a short child influenza module (CIM); the influenza-related questions from CIM are also included in the NIS-Child and NIS-Teen core questionnaires. Data from these three surveys are combined and referred to as the NIS-Flu. Respondents were predominantly the child’s mother (approximately 60%), while one-third were the child’s father and <10% were another family member. The response rates across the survey components ranged from 22.8%–24.4% (2018) and 23.1%–24.6% (2019). The study sample sizes were n=37,405 (2018) and n=41,320 (2019). This study was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy.*
Vaccine hesitancy questions were included in the CIM, NIS-Child, and NIS-Teen during April-June of 2018 and April-June of 2019. These questions were designed and tested by the National Center for Health Statistics and found to validly assess vaccine hesitancy (20). Vaccine hesitancy was assessed through the following question: “Overall, how hesitant about childhood shots would you consider yourself to be? Would you say not at all hesitant, not that hesitant, somewhat hesitant, or very hesitant?” The response options were “not at all hesitant,” “not that hesitant,” “somewhat hesitant,” and “very hesitant.” This question was asked as a 4-level response because people could not commit to a dichotomous response of hesitant or not hesitant during cognitive testing. Responses for “very hesitant” (6.6%) and “somewhat hesitant” (16.3%) were combined and recoded as “hesitant” and responses for “not that hesitant” (16.9%) and “not at all hesitant” (60.1%) were recoded as “not hesitant”.
Parents were also asked about their child’s vaccination coverage. Influenza vaccination status was assessed for all children aged 6 months to 17 years with the questions: “Since July 1, 2017 [or 2018] has [child] had a flu vaccination? There are two types of flu vaccinations. One is a shot and the other is a spray, mist or drop in the nose.” Vaccines that were assessed for children aged 19 to 35 months were DTaP ≥3 and ≥4 doses, poliovirus ≥3 doses, measles, mumps, and rubella vaccine (MMR) ≥1 dose, Hib primary series, Hib full series (≥3 or ≥4 doses, depending on product type), VAR ≥1 dose, PCV ≥3 and ≥4 doses, hepatitis A (HepA) ≥1 and ≥2 doses, rotavirus ≥3 doses, and combined 7-vaccine series. The combined 7-vaccine series (4:3:1:3:3:1:4) includes ≥4 doses of DTaP; ≥3 doses of poliovirus vaccine; ≥1 dose of measles-containing vaccine; ≥3 or ≥4 doses (depending upon product type) of Hib vaccine; ≥3 doses of HepB vaccine; ≥1 dose of VAR vaccine; and ≥4 doses of PCV vaccine. Vaccines that were assessed for adolescents aged 13 to 17 years were the HPV vaccine (≥1 dose, not distinguishing between 9-, 4-, or 2-valent HPV vaccines), meningococcal conjugate (MenACWY) (≥1 dose of serogroups A, C, W, and Y meningococcal conjugate vaccine or meningococcal vaccine of unknown type), and tetanus-diphtheria toxoids (Td) or tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) (≥1 dose of Td or Tdap).
Sociodemographic characteristics were based on respondent report and included the child’s age and race/ethnicity, mother’s educational level, urban-rural residence, Health and Human Services (HHS) region, annual household income, and city and zip code of the child’s residence. Metropolitan Statistical Area (MSA) status (MSA principal city, MSA non-principal city, and non-MSA) was determined based on the city and county of the household’s residence (21). Non-MSA areas include urban populations not located within an MSA as well as rural areas. HHS regions were categorized from 1 to 10 based on the 10 HHS regions (22). Annual household income was categorized as below or at the federal poverty level, above the poverty level but ≤$75,000 per year, above $75,000 per year, or not reported (23). Children were classified as below poverty level if their total family income was less than the federal poverty level specified for the applicable family size and number of children aged <18 years. All others were classified as at or above the poverty level.
Statistical Methods
Data from April to June interviews in the 2018 and 2019 NIS-Flu were analyzed (n=78,725). The proportion of hesitant parents among non-vaccinated children, and the proportion of non-vaccinated children among hesitant and non-hesitant parents were assessed for each vaccine. The adjusted PAF was calculated for each vaccine to assess the potential contribution of vaccine hesitancy to the observed non-vaccination level. PAF was calculated using the formula: p (rr-1) / rr, where p is the proportion of hesitant individuals among the not-vaccinated group of individuals and rr denotes the model-based relative risk comparing the proportion of those who are not vaccinated among the hesitant group with the proportion of those who are not vaccinated among the non-hesitant group (24). The rr is obtained using a log-binomial regression model with not vaccinated as the outcome measure and vaccine hesitancy as one of the covariates in the model. This method properly accounts for survey design features in estimating the variances and covariances of the estimates in the model. For the influenza vaccine, the rr is adjusted for age, race/ethnicity, mother’s educational level, MSA status, HHS region, and household poverty level; for all other vaccines, the rr is adjusted for race/ethnicity, mother’s educational level, MSA status, HHS region, and household poverty level. Because the influenza vaccination question was asked of all children aged 6 months to 17 years and had enough sample size for stratified analyses, we assessed PAF for the influenza vaccine overall and by select sociodemographic characteristics: child’s age, child’s race/ethnicity, mother’s educational level, MSA status, and household poverty level. The proportion of hesitant parents among non-vaccinated children, the proportion of non-vaccinated children among hesitant and non-hesitant parents, and the PAF of non-vaccination attributed to hesitancy were assessed by HHS region and state.
Analyses were weighted to population totals and adjusted for households having multiple telephone lines, unit non-response, and non-coverage of non-cellular-telephone households. Estimates, along with 95% confidence intervals (CIs), were calculated using SAS-callable SUDAAN (Research Triangle Institute, Research Triangle Park, NC, version 11.0.1) to account for the complex survey design. The PAF confidence intervals were based on a method described in Vaish and Khavjou and used in previous studies (2, 25).
Results
Over 23% of children in this sample were less than 4 years, 47% were 5 to 12 years, and 30% were 13-17 years (Table 1). The majority were non-Hispanic (NH) White (51%), followed by Hispanic (25%), NH Black (14%), and NH other/multiple races (10%). Most children had mothers with a college degree (45%), lived in a suburban area (MSA non-principal city) (61%), and resided in households with annual incomes of >$75,000 (42%). The majority of adults were not hesitant toward childhood vaccines (60% were not at all hesitant and 17% were not that hesitant), while 16% were somewhat hesitant and 7% were very hesitant.
Table 1.
Sociodemographic characteristics of children and adolescents, National Immunization Surveys, April-June interviews, 2018–2019
| Overall | |
|---|---|
| % (95% CI) | |
| Child’s age | |
| 6–23 months | 6.2 (5.9-6.4) |
| 2–4 years | 17.1 (16.7-17.6) |
| 5–12 years | 46.9 (46.3-47.5) |
| 13–17 years | 29.8 (29.2-30.4) |
| Child’s race/ethnicity | |
| Hispanic | 25.0 (24.4-25.7) |
| Non-Hispanic White | 51.0 (50.3-51.7) |
| Non-Hispanic Black | 13.8 (13.2-14.3) |
| Non-Hispanic other/multiple races | 10.2 (9.9-10.6) |
| Mother’s educational level | |
| High school education or less | 29.6 (29.0-30.3) |
| Some college | 25.1 (24.5-25.7) |
| College graduate | 45.3 (44.6-46.0) |
| MSA status | |
| MSA, principal city | 26.8 (26.1-27.4) |
| MSA, non-principal city | 61.0 (60.3-61.7) |
| Non-MSA | 12.2 (11.9-12.6) |
| Poverty level | |
| Above poverty, >$75,000 | 42.2 (41.5-42.9) |
| Above poverty, ≤$75,000 | 28.8 (28.1-29.4) |
| At or below poverty level | 17.0 (16.4-17.6) |
| Unknown | 12.1 (11.6-12.5) |
| Parental hesitancy toward vaccines | |
| Not at all hesitant | 60.1 (59.4-60.8) |
| Not that hesitant | 16.9 (16.4-17.5) |
| Somewhat hesitant | 16.3 (15.8-16.8) |
| Very hesitant | 6.6 (6.3-7.0) |
Parental vaccine hesitancy and the PAF of non-vaccination attributed to parental vaccine hesitancy (i.e., the estimated contribution of vaccine hesitancy to the non-vaccination level) on each child and adolescent vaccines is presented in Table 2. Parental hesitancy among non-vaccinated children ranged from 26.4% for ≥1 dose HPV vaccine to 47.1% for ≥3 dose DTaP vaccine (Table 2). For all vaccines, the proportion of unvaccinated children was higher among hesitant parents than non-hesitant parents, with the largest difference (26.6%) seen for ≥1 dose influenza vaccine. The PAF ranged from 6.5% for HepB birth dose vaccine to 31.3% for ≥3 dose DTaP vaccine.
Table 2.
Population attributable fraction (PAF)* of non-vaccination attributed to parental vaccine hesitancy for recommended child and adolescent vaccines, National Immunization Surveys, April-June interviews, 2018–2019
| % Hesitant among Non-Vaccinated (95% CI) | % Not Vaccinated Among Hesitant (95% CI) | % Not Vaccinated Among Non-Hesitant (95% CI) | PAF (95% CI) † | |
|---|---|---|---|---|
| All children (6 months–17 years) | ||||
| Influenza ≥1 dose | 34.5 (33.4-35.6) | 60.9 (59.5-62.4) | 34.3 (33.5-35.0) | 14.9 (13.9-16.0) |
| Childhood vaccines (among children 19–35 months) ‡ | ||||
| DTaP ≥3 doses | 47.1 (37.8-56.6) | 11.2 (8.5-14.6) | 3.9 (2.9-5.1) | 31.3 (20.2-44.9) |
| DTaP ≥4 doses | 41.6 (33.2-50.5) | 29.9 (23.1-37.8) | 13.0 (11.1-15.0) | 21.8 (13.9-32.7) |
| Poliovirus ≥3 doses | 46.7 (37.1-56.6) | 13.0 (10.1-16.6) | 4.6 (3.3-6.2) | 30.5 (18.7-45.6) |
| MMR ≥1 dose | 43.3 (34.2-52.8) | 14.8 (11.1-19.4) | 6.0 (4.7-7.6) | 24.6 (14.2-39.2) |
| Hib Primary series | 46.8 (35.7-58.3) | 14.6 (10.9-19.3) | 5.1 (3.8-6.9) | 30.8 (18.4-46.8) |
| Hib Full series | 40.8 (32.8-49.4) | 32.7 (25.7-40.6) | 14.6 (12.7-16.8) | 21.0 (13.5-31.1) |
| HepB Birth dose | 27.6 (22.8-33.0) | 29.6 (24.1-35.8) | 23.9 (21.5-26.5) | 6.5 (2.6-15.4) |
| HepB ≥3 doses | 42.5 (34.9-50.5) | 13.0 (10.1-16.5) | 5.4 (4.4-6.7) | 26.0 (17.0-37.5) |
| VAR (≥1 dose) | 42.3 (33.7-51.4) | 15.3 (11.6-19.9) | 6.4 (5.1-8.1) | 10.4 (4.5-22.4) |
| PCV ≥3 doses | 44.0 (34.8-53.5) | 13.5 (10.5-17.2) | 5.3 (4.0-7.1) | 27.2 (16.3-41.8) |
| PCV ≥4 doses | 41.2 (33.1-49.9) | 30.5 (23.6-38.3) | 13.4 (11.7-15.4) | 21.7 (13.9-32.3) |
| HepA ≥1 dose | 37.9 (31.1-45.2) | 19.4 (15.2-24.3) | 9.8 (8.2-11.6) | 18.1 (10.4-29.6) |
| HepA ≥2 doses (by 35 months) | 29.7 (26.0-33.7) | 47.7 (40.9-54.6) | 34.8 (32.3-37.4) | 7.2 (3.9-13.0) |
| Rotavirus (by age 8 months) | 36.8 (30.4-43.7) | 40.0 (32.9-47.5) | 21.2 (19.1-23.4) | 15.7 (10.2-23.5) |
| Combined 7-vaccine series§ | 35.7 (29.7-42.3) | 41.4 (34.4-48.8) | 22.9 (20.8-25.3) | 14.8 (9.6-22.2) |
| Adolescent vaccines (among adolescents 13–17 years) | ||||
| HPV vaccine ≥1 dose | 26.4 (23.5-29.6) | 46.7 (42.3-51.2) | 27.7 (25.6-29.8) | 10.8 (8.1-14.2)§ |
| MenACWY ≥1 dose | 27.3 (22.7-32.4) | 18.4 (15.2-22.0) | 10.4 (9.1-11.9) | 11.3 (6.8-18.1)§ |
| Td/Tdap ≥1 dose | 29.0 (23.1-35.8) | 14.3 (11.6-17.5) | 7.5 (6.0-9.2) | 13.3 (7.4-22.7)§ |
Abbreviations: CI = confidence interval; DTaP = diphtheria and tetanus toxoids, and acellular pertussis vaccine; HepA = hepatitis A vaccine; HepB = hepatitis B vaccine; Hib = Haemophilus influenzae type b conjugate vaccine; MMR = measles, mumps, and rubella vaccine; PCV = pneumococcal conjugate vaccine; VAR = varicella vaccine; HPV = human papillomavirus vaccine; MenACWY = meningococcal conjugate vaccine; Td = tetanus-diphtheria toxoids vaccine; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine.
PAF = p (rr-1)/rr, where p is the proportion of hesitant individuals among the not vaccinated group of individuals and rr denotes the relative risk comparing the proportion of those who are not vaccinated among the hesitant group with the proportion of those who are not vaccinated among the non-hesitant group.
For influenza vaccine, model was adjusted for child’s age, child’s race/ethnicity, mother’s educational level, MSA status, and poverty level. For all other vaccines, model was adjusted for child’s race/ethnicity, mother’s educational level, MSA status, and poverty level.
Includes vaccinations received by age 24 months (before the day the child turns 24 months), except for the HepB birth dose, rotavirus vaccination, and ≥2 HepA doses by 35 months.
The combined 7-vaccine series (4:3:1:3:3:1:4) includes ≥4 doses of DTaP, ≥3 doses of poliovirus vaccine, ≥1 dose of measles-containing vaccine, the full series of Hib (≥3 or ≥4 doses, depending on product type), ≥3 doses of HepB, ≥1 dose of varicella vaccine, and ≥4 doses of PCV.
The PAF of non-vaccination by select sociodemographic characteristics is detailed in Table 3. The PAF of non-vaccination attributed to hesitancy was lower among children aged 13–17 years (10.0%) compared with children 6–23 months (23.8%) (Table 3). The PAF ranged from 15.4% for NH White populations to 12.4% for Hispanic populations. PAF was higher among mothers with higher versus lower educational level, ranging from 10.7% among mothers with a high school education or less to 17.7% for mothers with a college degree or higher. PAF was highest for households with incomes >$75,000 (16.5%) and lowest for households at or below poverty level (9.8%).
Table 3.
Adjusted population attributable fraction (PAF)* of non-vaccination attributed to parental vaccine hesitancy for ≥1 dose influenza vaccination by select sociodemographic characteristics, National Immunization Surveys, April-June interviews, 2018–2019
| % Hesitant among Non-Vaccinated (95% CI) | % Not Vaccinated Among Hesitant (95% CI) | % Not Vaccinated Among Non-Hesitant (95% CI) | PAF† (95% CI) | |
|---|---|---|---|---|
| Child’s age | ||||
| 6–23 months (reference) | 43.6 (39.0-48.3) | 46.5 (41.7-51.3) | 19.7 (17.7-21.9) | 23.8 (18.9-29.6) |
| 2–4 years | 42.9 (40.0-45.7) | 57.6 (54.3-60.8) | 25.3 (23.9-26.8) | 23.0 (20.1-26.2) |
| 5–12 years | 35.8 (34.3-37.4) | 59.7 (57.6-61.7) | 32.5 (31.5-33.6) | 15.6 (14.1-17.3) |
| 13–17 years | 28.9 (27.2-30.6) | 68.9 (66.4-71.4) | 44.5 (43.0-45.9) | 10.0 (8.7-11.5) |
| Child’s race/ethnicity | ||||
| Hispanic (reference) | 32.9 (30.4-35.5) | 52.7 (49.4-55.9) | 33.4 (31.6-35.2) | 12.4 (10.2-15.1) |
| Non-Hispanic White | 32.7 (31.3-34.1) | 68.0 (66.1-69.8) | 34.3 (33.4-35.2) | 15.4 (14.3-16.6) |
| Non-Hispanic Black | 43.6 (40.4-46.9) | 58.2 (54.7-61.5) | 38.1 (35.6-40.6) | 15.4 (12.3-19.1) |
| Non-Hispanic other/multiple races | 34.2 (31.2-37.3) | 56.6 (52.6-60.4) | 31.9 (29.7-34.2) | 14.1 (11.3-17.3) |
| Mother’s educational level | ||||
| High school education or less (reference) | 34.8 (32.8-36.9) | 53.8 (51.3-56.3) | 37.9 (36.4-39.5) | 10.7 (8.8-12.9) |
| Some college | 35.9 (33.9-38.0) | 64.4 (61.7-66.9) | 40.0 (38.3-41.6) | 14.0 (12.2-15.9) |
| College graduate | 33.3 (31.7-35.1) | 65.1 (62.8-67.2) | 29.4 (28.3-30.4) | 17.7 (16.2-19.3) |
| MSA status | ||||
| MSA, principal city (reference) | 35.9 (33.4-38.3) | 56.1 (53.1-59.0) | 30.7 (29.1-32.3) | 16.1 (13.7-18.7) |
| MSA, non-principal city | 33.3 (31.9-34.8) | 62.1 (60.2-63.9) | 34.9 (34.0-35.9) | 14.3 (13.0-15.7) |
| Non-MSA | 37.6 (35.3-39.9) | 65.7 (62.5-68.7) | 38.8 (37.0-40.6) | 15.7 (13.7-18.0) |
| Poverty level | ||||
| Above poverty, >$75,000 (reference) | 31.6 (29.9-33.4) | 65.1 (62.7-67.5) | 29.7 (28.7-30.8) | 16.5 (15.0-18.1) |
| Above poverty, ≤$75,000 | 35.9 (34.1-37.8) | 64.5 (62.0-66.8) | 39.5 (38.0-41.0) | 14.2 (12.6-15.9) |
| At or below poverty level | 35.3 (32.5-38.2) | 50.5 (47.1-53.9) | 36.3 (34.1-38.5) | 9.8 (7.3-13.1) |
| Unknown | 38.9 (35.8-42.1) | 58.5 (54.8-62.1) | 37.1 (34.8-39.5) | 14.9 (12.1-18.2) |
PAF = p (rr-1)/rr, where p is the proportion of hesitant individuals among the not vaccinated group of individuals and rr denotes the relative risk comparing the proportion of those who are not vaccinated among the hesitant group with the proportion of those who are not vaccinated among the non-hesitant group.
Model adjusted for child’s age, child’s race/ethnicity, mother’s educational level, MSA status, and poverty level.
The adjusted PAF of non-vaccination among children aged 6 months to 17 years by state is presented in Table 4. PAF was highest in HHS regions 1 (21.0%; Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont) and 2 (21.9%; New Jersey, New York). Similarly, influenza vaccination coverage was highest in these regions (HHS regions 1 and 2: 72.7% and 67.8%, respectively). PAF was lowest in region 6 (11.8%; Arkansas, Louisiana, New Mexico, Oklahoma, and Texas) (Table 4). Among states, PAF ranged from 25.4% (New Hampshire) to 7.5% (Louisiana). For all states, the proportion of children who were not vaccinated for influenza was higher among hesitant parents than among non-hesitant parents.
Table 4.
Adjusted population attributable fraction (PAF)* of non-vaccination attributed to parental vaccine hesitancy for ≥1 dose influenza vaccination among children 6 months – 17 years by health and human services (HHS) region and state, National Immunization Survey-Flu, April-June interviews, 2018–2019
| % Hesitant among Non-Vaccinated (95% CI) | % Not Vaccinated Among Hesitant (95% CI) | % Not Vaccinated Among Non-Hesitant (95% CI) | PAF (95% CI) | Influenza Vaccination Coverage (%, 95% CI) | |
|---|---|---|---|---|---|
| National | 34.5 (33.4-35.6) | 60.9 (59.5-62.4) | 34.3 (33.5-35.0) | 14.9 (13.9-16.0) | 59.7(59.0-60.4) |
| HHS Region 1 | 36.1 (32.4-39.8) | 51.4 (46.5-56.2) | 21.4 (19.6-23.3) | 21.0 (17.5-25.1) | 72.7 (70.9-74.5) |
| Connecticut | 33.5 (26.8-40.9) | 47.1 (38.5-55.8) | 24.3 (20.6-28.4) | 16.2 (10.2-24.8) | 71.1 (67.3-74.5) |
| Maine | 44.9 (38.7-51.3) | 73.2 (66.5-79.1) | 32.5 (28.7-36.5) | 25.0 (19.5-31.4) | 57.0 (53.2-60.8) |
| Massachusetts | 33.9 (26.5-42.1) | 43.9 (34.6-53.6) | 17.0 (14.2-20.1) | 20.8 (13.9-30.0) | 78.2 (75.0-81.2) |
| New Hampshire | 39.1 (31.6- 47.2) | 67.9 (58.5-76.0) | 23.9 (20.0 -28.2) | 25.4 (18.6-33.5) | 68.0 (63.7-71.9) |
| Rhode Island | 40.3 (32.6- 48.4) | 44.1 (36.0-52.4) | 18.3 (15.0 -22.1) | 23.6 (16.0-33.4) | 76.2 (72.6-79.5) |
| Vermont | 31.4 (23.3 -40.9) | 69.0 (57.7-78.4) | 35.1 (29.1-41.7) | 15.4 (9.2-24.6) | 58.2 (52.6-63.7) |
| HHS Region 2 | 41.1 (36.9-45.2) | 53.9 (49.2-58.6) | 25.2 (23.0-27.4) | 21.9 (17.8-26.6) | 67.8 (65.6-69.9) |
| New Jersey | 37.9 (32.1-44.2) | 53.8 (46.4-61.1) | 25.1 (21.9-28.6) | 18.6 (13.1-25.7) | 68.5 (65.3-71.6) |
| New York | 42.5 (37.3-47.9) | 53.9 (48.0-59.7) | 25.2 (22.5-28.2) | 22.6 (17.5-28.7) | 67.4 (64.6-70.1) |
| HHS Region 3 | 32.8 (29.8-35.8) | 55.3 (51.3-59.4) | 28.8 (26.9-30.6) | 15.8 (13.1-18.9) | 66.1 (64.3-67.8) |
| Delaware | 31.5 (25.6-38.1) | 49.6 (40.9-58.3) | 30.2 (25.7-35.1) | 12.3 (7.2-20.4) | 65.5 (61.4-69.5) |
| District of Columbia | 35.0 (27.6-43.2) | 50.4 (40.8-59.8) | 24.6 (21.2-28.4) | 17.9 (11.4-26.9) | 71.4 (67.0-75.4) |
| Maryland | 36.5 (30.4-43.1) | 50.9 (42.7-59.1) | 23.9 (20.7 -27.4) | 19.4 (13.6-26.9) | 70.5 (67.2-73.6) |
| Pennsylvania | 35.8 (30.9-41.1) | 59.1 (52.6-65.2) | 27.6 (24.7-30.8) | 19.0 (14.6-24.5) | 66.1 (63.2-68.8) |
| Virginia | 25.9 (20.3-32.5) | 51.4 (41.9-60.8) | 31.7 (27.9-35.9) | 9.9 (5.6-17.0) | 65.0 (61.2-68.6) |
| West Virginia | 34.3 (29.5- 39.5) | 65.6 (58.5-72.0) | 39.6 (35.7-43.7) | 13.6 (9.6-18.8) | 54.1 (50.6-57.5) |
| HHS Region 4 | 36.8 (34.6-39.1) | 63.7 (60.9-66.6) | 38.3 (36.6-40.0) | 14.7 (12.6-17.0) | 55.3 (53.8-56.8) |
| Alabama | 37.2 (32.3-42.4) | 63.7 (56.7-70.2) | 36.6 (32.9-40.4) | 15.8 (11.6-21.3) | 56.8 (53.5-60.1) |
| Florida | 36.7 (31.7- 42.0) | 69.0 (62.2-75.0) | 44.1 (39.8-48.5) | 13.2 (9.2-18.6) | 49.3 (45.6-53.0) |
| Georgia | 36.6 (32.0- 41.5) | 62.6 (56.1-68.7) | 41.3 (37.6-45.1) | 12.5 (8.6-17.8) | 53.3 (50.0-56.5) |
| Kentucky | 31.3 (24.6-39.0) | 49.2 (39.3-59.1) | 31.8 (27.0-37.0) | 11.1 (5.6-20.6) | 64.0 (59.4-68.3) |
| Mississippi | 39.0 (33.3-45.0) | 66.4 (59.2-73.0) | 43.9 (39.4-48.5) | 13.2 (8.7-19.5) | 49.2 (45.2-53.2) |
| North Carolina | 39.1 (33.7-44.7) | 63.3 (56.5-69.7) | 31.1 (27.6-34.9) | 19.9 (15.0-25.8) | 61.1 (57.7-64.4) |
| South Carolina | 33.0 (28.1-38.4) | 56.3 (49.1- 63.2) | 38.0 (34.1-42.0) | 10.7 (6.7-16.8) | 57.6 (54.2-61.0) |
| Tennessee | 31.0 (28.1-34.1) | 63.4 (55.0-71.1) | 32.3 (28.2-36.7) | 19.1 (13.5-26.3) | 60.4 (56.4-64.3) |
| HHS Region 5 | 34.2 (39.1-36.4) | 63.5 (60.5-66.6) | 36.7 (35.0-38.3) | 14.5 (12.5-16.6) | 57.1 (55.6-58.6) |
| Illinois | 35.9 (32.0-39.9) | 64.3 (58.8-69.4) | 35.8 (33.1-38.7) | 15.9 (12.5-19.9) | 57.4 (54.9-60.0) |
| Indiana | 34.9 (29.5-40.6) | 63.1 (55.2-70.4) | 38.9 (34.8-43.3) | 13.4 (9.0-19.4) | 55.4 (51.6-59.1) |
| Michigan | 37.2 (31.8-43.0) | 68.1 (60.6-74.7) | 40.9 (36.5-45.4) | 14.9 (10.5-20.7) | 51.8 (48.0-55.7) |
| Minnesota | 32.2 (24.8-40.5) | 59.0 (47.4-69.7) | 30.0 (25.4 -34.9) | 15.8 (9.8-24.5) | 64.6 (60.0-69.0) |
| Ohio | 31.2 (26.3-36.6) | 60.7 (53.0 -67.9) | 37.0 (33.2-41.0) | 12.2 (8.2-17.7) | 57.7 (54.2-61.2) |
| Wisconsin | 31.1 (25.8-36.9) | 62.3 (54.3-69.7) | 35.5 (31.5-39.7) | 13.4 (9.1-19.2) | 58.5 (54.8-62.2) |
| HHS Region 6 | 31.6 (29.3-33.8) | 58.2 (55.1-61.3) | 36.5 (34.8-38.2) | 11.8 (9.9-14.0) | 58.7 (57.2-60.2) |
| Arkansas | 34.3 (28.2-41.1) | 58.0 (49.6-66.0) | 28.2 (24.6-32.0) | 17.6 (12.3-24.6) | 65.8 (62.1-69.3) |
| Louisiana | 30.0 (25.0- 35.4) | 52.9 (45.6-60.1) | 39.7 (35.4-44.1) | 7.5 (3.8-14.3) | 56.8 (53.1-60.5) |
| New Mexico | 37.5 (31.5-43.8) | 51.3 (43.6-58.9) | 27.5 (23.9-31.4) | 17.4 (11.8-24.9) | 66.7 (63.2-70.1) |
| Oklahoma | 33.7 (29.0-38.6) | 66.3 (59.0-72.9) | 41.6 (37.7-45.5) | 12.6 (8.9-17.5) | 52.5 (49.1-55.9) |
| Texas | 31.0 (28.1-34.1) | 58.5 (54.2-62.6) | 36.8 (34.6-39.0) | 11.5 (9.0-14.5) | 58.6 (56.6-60.5) |
| HHS Region 7 | 32.9 (29.3-36.4) | 66.0 (61.0-70.9) | 33.7 (31.3-36.1) | 16.1 (13.2-19.5) | 60.0 (57.8-62.2) |
| Iowa | 34.9 (27.9-42.6) | 59.9 (49.5-69.4) | 31.9 (27.6-36.5) | 16.3 (10.5-24.4) | 61.9 (57.5-66.1) |
| Kansas | 32.0 (24.9-40.1) | 69.3 (58.9-78.0) | 30.8 (26.2-35.9) | 17.7 (12.0-25.4) | 62.9 (58.1-67.4) |
| Missouri | 31.4 (26.2-37.2) | 69.5 (60.9-76.9) | 38.5 (34.4-42.7) | 14.0 (9.9-19.5) | 55.4 (51.5-59.1) |
| Nebraska | 36.3 (28.5-44.9) | 61.6 (48.6 -73.0) | 26.8 (22.8-31.1) | 20.5 (13.7-29.6) | 66.7 (62.2-70.8) |
| HHS Region 8 | 31.8 (28.7-34.9) | 65.4 (60.8-70.0) | 35.3 (33.0-37.7) | 14.6 (12.0-17.7) | 58.6 (56.5-60.7) |
| Colorado | 36.4 (30.4-42.9) | 58.8 (50.3-66.7) | 28.0 (24.3-32.0) | 19.1 (13.6-26.1) | 65.5 (61.8-69.0) |
| Montana | 39.9 (33.6- 46.6) | 76.4 (68.6-82.8) | 36.9 (32.2-41.9) | 20.6 (15.4-27.1) | 53.6 (49.1-58.0) |
| North Dakota | 37.1 (28.5-46.5) | 72.0 (61.0-81.0) | 26.4 (22.4-30.9) | 23.5 (16.5-32.4) | 65.2 (60.4-69.7) |
| South Dakota | 32.9 (25.9-40.7) | 61.1 (50.1-71.2) | 29.3 (25.2-33.7) | 17.1 (11.3-25.1) | 65.0 (60.7-69.0) |
| Utah | 24.7 (20.2-29.9) | 70.2 (61.5-77.7) | 45.2 (40.8-49.7) | 8.8 (5.7-13.3) | 50.4 (46.4-54.4) |
| Wyoming | 32.1 (25.8-39.1) | 76.7 (68.8- 83.1) | 51.6 (46.7-56.5) | 10.5 (6.7-16.0) | 42.3 (37.9-46.8) |
| HHS Region 9 | 32.9 (28.6-37.2) | 61.9 (56.2-67.6) | 36.3 (33.2-39.4) | 13.6 (10.2-17.7) | 58.1 (55.2-60.8) |
| Arizona | 35.5 (30.0-41.4) | 70.7 (63.1-77.3) | 40.7 (36.5-45.1) | 15.0 (10.7-20.7) | 52.0 (48.1-55.9) |
| California | 31.9 (26.6-37.7) | 60.2 (52.6-67.3) | 35.3 (31.6-39.3) | 14.9 (10.4-21.0) | 59.4 (55.9-62.9) |
| Hawaii | 43.0 (35.2-51.2) | 61.9 (54.5-68.7) | 29.8 (23.9 - 36.5) | 22.3 (14.8-32.1) | 61.1 (56.1-65.9) |
| Nevada | 34.6 (28.5-41.3) | 62.2 (49.5-73.5) | 42.6 (37.1-48.2) | 10.9 (5.7-20.0) | 52.8 (47.9-57.8) |
| HHS Region 10 | 37.1 (33.4-40.7) | 66.6 (61.6-71.6) | 33.9 (31.4-36.4) | 18.2 (15.0-22.0) | 58.7 (56.4-61.0) |
| Alaska | 33.5 (27.8-39.7) | 71.1 (63.4-77.8) | 43.7 (38.7-48.8) | 12.9 (8.6-18.9) | 50.7 (46.3-55.0) |
| Idaho | 38.1 (31.4- 45.3) | 71.0 (60.9-79.3) | 42.6 (37.7-47.6) | 15.2 (10.1-22.3) | 49.9 (45.3-54.6) |
| Oregon | 42.6 (36.0-49.5) | 70.9 (60.9-79.3) | 32.7 (28.2-37.6) | 23.0 (17.0-30.3) | 58.1 (53.6-62.4) |
| Washington | 33.9 (28.3-40.1) | 61.6 (53.3-69.3) | 31.3 (27.6-35.2) | 16.7 (11.8-23.0) | 62.3 (58.7-65.7) |
PAF = p (rr-1)/rr), where p is the proportion of hesitant individuals among the not vaccinated group of individuals and rr denotes the relative risk comparing the proportion of those who are not vaccinated among the hesitant group with the proportion of those who are not vaccinated among the non-hesitant group.
Discussion
PAF of non-vaccination attributed to hesitancy ranged from 7% to 31% for the vaccines examined in the study. PAF of non-vaccination for influenza vaccine due to hesitancy was highest among parents of younger children (less than 2 years) suggesting that about 24% of the prevalence of non-vaccination could be reduced if vaccine hesitancy is eliminated from this subgroup. The PAF of non-vaccination on hesitancy was highest for NH White and Black populations (15.4%), households with high educational (17.7%) and income levels (16.5%), urban areas (16.1%), and HHS regions 1 and 2 (21.0% and 21.9%, respectively), suggesting that efforts to increase confidence in vaccines among these groups would be most helpful in reducing non-vaccination for influenza due to hesitancy.
Vaccine hesitancy among non-vaccinated children is also higher in the Northeast and several other states across the U.S. Previous studies have shown that states with the highest hesitancy (New Hampshire, Maine, Rhode Island, New Jersey, New York) tended to have higher influenza coverage (ranging from 60.6% to 77.5%) than the national average of 58.1% (1). While these states have higher vaccination coverage than the national average, none of these states has reached the Healthy People 2020 vaccination goal of 80%. A possible contributor to these results may be due to a “survivorship effect”, that is, as vaccination coverage increases, the pool of unvaccinated decreases, and as a result, the proportion of hesitant individuals among the unvaccinated increases due to the smaller number of remaining individuals (26). From the formula for PAF, as the proportion of hesitant individuals among the unvaccinated increases, PAF will increase, holding rr constant. Furthermore, in a successful vaccination program, as coverage among the non-hesitant increases, the prevalence of unvaccinated among the non-hesitant decreases, which may result in an increase in the relative risk comparing the prevalence of unvaccinated among the hesitant group with the prevalence of unvaccinated among the non-hesitant group (e.g., assuming no change in the prevalence of unvaccinated among the hesitant, or a lesser decrease compared to the prevalence of unvaccinated among the non-hesitant). States with higher overall vaccination coverage have higher coverage among the non-hesitant, and also tend to have higher hesitancy among the non-vaccinated (survivorship effect). Thus, the variation across states in PAF could be due to a combination of factors related to variations in the prevalence of hesitancy among the non-vaccinated, and the relative risk comparing the prevalence of unvaccinated among the hesitant group with the prevalence of unvaccinated among the non-hesitant group.
States like Louisiana, Utah, and Virginia had PAF less than 10% and influenza vaccination coverage ranging from 45.2% to 69.1%. States with low PAF could have a combination of low vaccination coverage, low prevalence of hesitancy among the non-vaccinated due to a larger pool of unvaccinated individuals, and lower relative risk of unvaccinated among the hesitant group compared with unvaccinated among the non-hesitant group. Other barriers to vaccination, such as access, missed opportunities or other factors, could contribute the low vaccination coverage and low PAF found in these states (27). Addressing all barriers to vaccination and targeting efforts to reduce vaccine hesitancy particularly is important for increasing uptake throughout the United States.
Recent studies have found that vaccine hesitant parents were less likely to vaccinate their children for influenza than non-hesitant parents (1, 3, 28). Approximately 1 in 5 parents were hesitant, and children of hesitant parents had 26 percentage points lower influenza vaccination coverage than children of non-hesitant parents (1). While vaccination coverage was lower for children of hesitant parents than non-hesitant parents, it is unclear from previous studies the role of hesitancy and other factors on under-vaccination and non-vaccination. This study demonstrated that hesitancy is not evenly distributed across all populations but rather is higher for some vaccines, states, and populations than others. The PAF values can be used to estimate the burden of non-vaccination due to vaccine hesitancy which could be reduced if vaccine hesitancy is eliminated. In areas where hesitancy is low, other barriers should be considered when addressing coverage gaps in the population, particularly among low-income households. Studies have found that coverage with influenza and most other vaccines was lower among children living in poverty and children with Medicaid or no health insurance (14). To increase access to vaccines, the Vaccines for Children (VFC) program can provide recommended vaccines at no cost to children aged ≤18 years who are Medicaid-eligible, uninsured, American Indian/Alaska Native, or insured by health plans that do not fully cover all routine immunizations. Increased awareness of the VFC program and assistance locating VFC providers could facilitate improved vaccination coverage among eligible children. Efforts to increase vaccination should focus on responding to vaccine hesitancy as well as access barriers to vaccination.
Several strategies within the CDC Vaccinate with Confidence framework were developed to respond to vaccine hesitancy (29). These strategies include activities designed to increase vaccination coverage by helping to protect communities, empower families, and stop vaccination-related myths. Public health partners should work together to identify under-vaccinated communities using surveillance tools and vaccine coverage monitoring systems, characterize populations at risk for under-vaccination, and develop tailored strategies to promote vaccination, while continuing to remove barriers to vaccine access. To ensure parents are confident in their decision to vaccinate their children, healthcare providers should have appropriate resources to initiate early vaccine conversations with parents of young children and with pregnant women. Healthcare providers are positioned to increase acceptance for vaccines by strongly recommending routine vaccinations for children (30). Lastly, overcoming myths and misinformation on vaccines requires educating the public and policymakers about vaccines and engaging trusted messengers to repeatedly share accurate and easily understandable information, for example using social media platforms to promote accurate information, dispel myths, and counter misinformation.
Under- and non-vaccination can result in outbreaks of vaccine-preventable diseases, hospitalizations, and deaths (31). Multiple outbreaks have occurred in under-vaccinated communities across the United States in recent years (4). For example, in 2019, there were 1,282 cases of measles reported in the United States, the highest number seen since 1992, and the United States almost lost its measles elimination status (32). Furthermore, COVID-19 cases are rising in some parts of the U.S. (33). Increasing confidence in vaccines and reducing barriers to vaccination is important for preventing outbreaks, hospitalizations, and deaths from vaccine-preventable diseases. Current efforts to increase influenza vaccination coverage are especially important, particularly during the COVID-19 pandemic. The SARS-CoV-2 virus in conjunction with the influenza virus and other potential viruses could cause significant morbidity and mortality, and could impose a significant strain on public health and medical systems (34–35).
The findings in this report are subject to several limitations. First, the low response rate and exclusion of phoneless and landline-only households may introduce the possibility of bias if study participants are not representative of U.S. children (18,19). While data weighting was designed to produce nationally representative results, bias in estimates might remain even after weighting for household and provider non-response and non-coverage. Second, influenza vaccination status and vaccine hesitancy were self-reported and may be subject to recall or social desirability bias. Third, the vaccine hesitancy questions were only asked for three months out of the year, which limited the analyses that we could do for all of the vaccines. Fourth, the survey asked about hesitancy toward vaccines in general and not about a particular vaccine. It is possible that the results might have been different had the survey asked about vaccine-specific hesitancy. Finally, data are from a cross-sectional survey with potentially unmeasured confounding and imprecise measures; thus, the PAF estimates are only an approximation to the unknown association between hesitancy and vaccination (36).
As the pandemic progresses, it is more important than ever to address all potential barriers to vaccination, including hesitancy, access and other factors to reduce the spread of influenza, COVID-19, or other vaccine preventable diseases among children. Further efforts are needed to understand the contribution of these factors on under- or non-vaccination, and to address these barriers appropriately. Tailoring messages to address barriers to vaccination is needed, particularly among populations with the lowest vaccination coverage. Health professionals can address hesitancy by communicating with families about how vaccinations can be provided safely during the pandemic, address any concerns or questions, remind parents of vaccinations that are due or overdue for their children, and administer all recommended vaccinations to children during clinic visits. Increased communication and partnerships between healthcare providers and families can protect the health of children and families and protect the entire nation from outbreaks of vaccine-preventable diseases.
Figure. Population attributable fraction (PAF)* of influenza non-vaccination among children 6 months – 17 years attributed to parental vaccine hesitancy by state, National Immunization Survey-Flu, April-June interviews, 2018–2019.
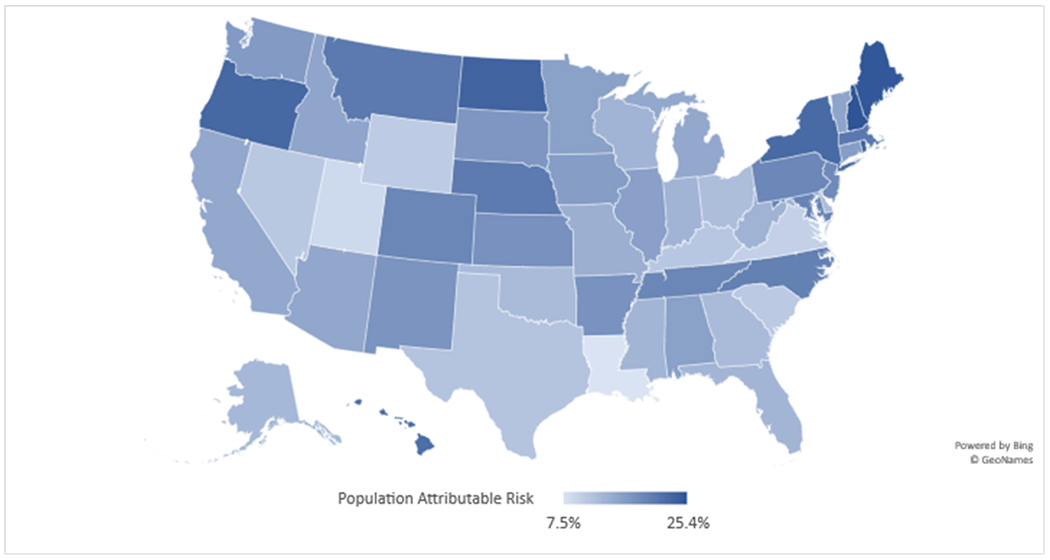
* PAF = p (rr-1)/rr, where p is the proportion of hesitant individuals among the not vaccinated group of individuals and rr denotes the relative risk comparing the proportion of those who are not vaccinated among the hesitant group with the proportion of those who are not vaccinated among the non-hesitant group.
Funding/Support:
No funding was secured for this study.
Footnotes
See e.g., 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Conflict of Interest Disclosures (includes financial disclosures): The authors have no conflicts of interest relevant to this article to disclose. None of the authors have financial relationships relevant to this article to disclose.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Santibanez TA, Nguyen KH, Greby SM, Fisher A, Scanlon P, Bhatt A, Srivastav A, Singleton JA. Parental vaccine hesitancy and childhood influenza vaccination. Pediatrics. 2020. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen KH, Santibanez TA, Stokley S, Lindley MC, Fisher A, Kim D, Greby S, Srivastav A and Singleton J, 2021. Parental vaccine hesitancy and its association with adolescent HPV vaccination. Vaccine, pp.S0264–410X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith PJ, Humiston SG, Marcuse EK, Zhao Z, Dorell CG, Howes C, Hibbs B. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public health reports. 2011. Jul;126(2_suppl):135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbaeyi S, Cohn A, Messonnier N. A call to action: strengthening vaccine confidence in the United States. Pediatrics. 2020;145(6):e20200390. doi: 10.1542/peds.2020-0390 [DOI] [PubMed] [Google Scholar]
- 5.Bedford H, Attwell K, Danchin M, Marshall H, Corben P, Leask J. Vaccine hesitancy, refusal and access barriers: the need for clarity in terminology. Vaccine. 2018;36(44):6556–6558. doi: 10.1016/j.vaccine.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 6.Atwell JE, Salmon DA. Pertussis resurgence and vaccine uptake: implications for reducing vaccine hesitancy. Pediatrics. 2014;134(3):602–604. doi: 10.1542/peds.2014-1883 [DOI] [PubMed] [Google Scholar]
- 7.Gowda C, Dempsey AF. (2013). The rise (and fall?) of parental vaccine hesitancy. Hum Vaccin Immunother;9(8):1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy A, Lavail K, Nowak G, Basket M, Landry S. (2011). Confidence about vaccines in the United States: understanding parents’ perceptions. Health Aff (Millwood);30(6): 1151–1159. [DOI] [PubMed] [Google Scholar]
- 9.Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine. 2015;33 Suppl 4:D66–D71. [DOI] [PubMed] [Google Scholar]
- 10.Bedford H, Attwell K, Danchin M, Marshall H, Corben P, Leask J. Vaccine hesitancy, refusal and access barriers: the need for clarity in terminology. Vaccine. 2018;36(44):6556–6558. doi: 10.1016/j.vaccine.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 11.Nadeau JA, Bednarczyk RA, Masawi MR, Meldrum MD, Santilli L, Zansky SM, Blog DS, Birkhead GS, McNutt LA. (2015). Vaccinating my way—Use of alternative vaccination schedules in New York state. The Journal of pediatrics;166(1):151–6. [DOI] [PubMed] [Google Scholar]
- 12.Wallace AS, Mantel C, Mayers G, Mansoor O, Gindler JS, Hyde TB. (2014). Experiences with provider and parental attitudes and practices regarding the administration of multiple injections during infant vaccination visits: lessons for vaccine introduction. Vaccine;;32(41):5301–5310. [DOI] [PubMed] [Google Scholar]
- 13.Robinson CL, Bernstein H, Poehling K, Romero JR, Szilagyi P. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Pingali SC, Santibanez TA. Vaccination Coverage by Age 24 Months Among Children Born in 2016 and 2017—National Immunization Survey-Child, United States, 2017–2019. Morbidity and Mortality Weekly Report. 2020. Oct 23;69(42):1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healthy People. 2020. Available at https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives
- 16.Elam-Evans LD, Yankey D, Singleton JA, Sterrett N, Markowitz LE, Williams CL, Fredua B, McNamara L, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. Morbidity and Mortality Weekly Report. 2020. Aug 21;69(33):1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Improving vaccination demand and addressing hesitancy. World Health Organization. Available at: https://www.who.int/immunization/programmes_systems/vaccine_hesitancy/en/ [Google Scholar]
- 18.A user’s guide for the 2018 public-use data file. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/vaccines/imz-managers/nis/downloads/NIS-TEEN-PUF18-DUG.pdf [Google Scholar]
- 19.A user’s guide for the 2019 public-use data file. Centers for Disease Control and Prevention. Available at https://www.cdc.gov/vaccines/imz-managers/nis/downloads/NIS-TEEN-PUF19-DUG.pdf [Google Scholar]
- 20.Scanlon P, Jamoom E. 2020. The cognitive evaluation of survey items related to vaccine hesitance and confidence for inclusion on a series of short question sets. Accessed April 11, 2020. https://wwwn.cdc.gov/QBank/report/Scanlon_NCHS_2019_VAX.pdf
- 21.Metropolitan and micropolitan. U.S. Census Bureau. Available at https://www.census.gov/programs-surveys/metro-micro.html [Google Scholar]
- 22.Regional offices. Health and Human Services. Available at https://www.hhs.gov/about/agencies/iea/regional-offices/index.html [Google Scholar]
- 23.Poverty thresholds. U.S. Census Bureau. Available at https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-poverty-thresholds.html [Google Scholar]
- 24.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic research: principles and quantitative methods. John Wiley & Sons; 1982. May 15. [Google Scholar]
- 25.Vaish AK, & Khavjou O (2017). Confidence intervals for population attributable fractions using complex survey data. Proceedings of the 2017 Joint Statistical Meetings, 1351–1357. [Google Scholar]
- 26.Nguyen KH, Santibanez TA, Stokley S, Lindley MC, Fisher A, Kim D, Greby S, Srivastav A and Singleton J, 2021. Parental vaccine hesitancy and its association with adolescent HPV vaccination. Vaccine, pp.S0264–410X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen KH., Srivastav A, Lindley M, Fisher A, Kim D, Greby S, Lee J, Singleton J. Parental Vaccine Hesitancy and Association With Childhood Diphtheria, Tetanus Toxoid, and Acellular Pertussis; Measles, Mumps and Rubella; Rotavirus; and Combined 7-Series Vaccination. American Journal of Preventative Medicine. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewer NT, Gilkey MB, Thompson P. RE: Progress in HPV Vaccine Hesitancy. Pediatrics. 2021. Jun 1;147(6). [DOI] [PubMed] [Google Scholar]
- 29.Esposito S, Principi N, Cornaglia G; ESCMID Vaccine Study Group (EVASG). Barriers to the vaccination of children and adolescents and possible solutions. Clin Microbiol Infect. 2014;20 Suppl 5:25–31. doi: 10.1111/1469-0691.12447 [DOI] [PubMed] [Google Scholar]
- 30.Strelitz B, Gritton J, Klein EJ, Bradford MC, Follmer K, Zerr DM, Englund JA, Opel DJ. Parental vaccine hesitancy and acceptance of seasonal influenza vaccine in the pediatric emergency department. Vaccine. 2015. Apr 8;33(15):1802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaccine with Confidence. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/vaccines/partners/vaccinate-with-confidence.html [Google Scholar]
- 32.Making a Strong Flu Vaccine Recommendation (SHARE). Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/flu/professionals/vaccination/flu-vaccine-recommendation.htm [Google Scholar]
- 33.Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020–21 influenza season. MMWR Recomm Rep 2020;69(No. RR-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel M, Lee AD, Clemmons NS, Redd SB, Poser S, Blog D, Zucker JR, Leung J, Link-Gelles R, Pham H and Arciuolo RJ (2019). National update on measles cases and outbreaks—United States, January 1–October 1, 2019. Morbidity and Mortality Weekly Report, 68(40), p.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Covid cases are rising in the states with low vaccination rates. NBC News. Available at: https://www.nbcnews.com/news/us-news/map-covid-cases-are-rising-states-low-vaccination-rates-n1275322 [Google Scholar]
- 36.Singer BD. COVID-19 and the next influenza season. Sci Adv 2020;6:eabd0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon DA, Sherman AC, Kanjilal S. Influenza in the COVID-19 Era. JAMA 2020;324:1342. [DOI] [PubMed] [Google Scholar]
- 38.Greenland S, 2015. Concepts and pitfalls in measuring and interpreting attributable fractions, prevented fractions, and causation probabilities. Annals of Epidemiology, 25(3), pp.155–161. [DOI] [PubMed] [Google Scholar]


