Abstract
Aim: short-term blood pressure variability (BPV) as a risk factor of atherosclerosis and cardiovascular events has been investigated. However, its association with atherosclerotic plaque vulnerability remains unknown. The objective of this study was to determine the association between short-term BPV and intracranial atherosclerotic plaque vulnerability.
Methods: this is a cross-sectional analysis of 267 ischemic stroke patients with symptomatic intracranial atherosclerosis (mean age, 65±12 years old; 60.3% male), which were prospectively recruited in a comprehensive stroke center. Systolic and diastolic BP SD, CV, and BP variability ratio (BPVR) from 24 hours, daytime, and nighttime were calculated from 24-h ambulatory blood pressure monitoring, intracranial atherosclerotic plaque burden and vulnerability were evaluated by high-resolution magnetic resonance vessel wall imaging. Logistic regression analysis was used to locate the correlation between short-term BPV and plaque vulnerability.
Results: a total of 36.3% subjects presented with intraplaque hemorrhage (IPH) in this study. Multivariate logistic regression suggested that nighttime diastolic BP CV and 24-h BPVR were associated with intracranial IPH independently after adjusted for cardiovascular risk factors, odds ratio (OR) and 95% confidence interval (CI) for per SD BPV changes were 1.418 (1.051, 1.914) and 0.731 (0.548, 0.976), respectively, and this association also independent of atherosclerosis burden and 24-h mean systolic BP level. Further subgroup analysis by age and hypertension history demonstrated that the statistical correlation could only establish in the elder, and subjects with hypertension.
Conclusion: nighttime diastolic BP CV and 24-h BPVR were associated with intracranial IPH independently, especially in the elderly and subjects with hypertension.
Keywords: Ischemic stroke, Intracranial atherosclerosis, Intraplaque hemorrhage, BP variability
Introduction
Blood pressure variability (BPV) has been demonstrated to be a potential trigger for cardiovascular events. A meta-analysis including 18 studies (1359 subjects) analyzed the effects of short-term BPV on stroke outcome, and concluded that higher systolic BP SD is associated with stroke prognosis 1) . BP and BPV could possibly induced cardiovascular events by atherosclerosis and vulnerable plaque. The association between vulnerable plaque and first or recurrent cerebrovascular events has already been established in a growing number of studies 2 - 4) .
The association between BPV and carotid artery atherosclerosis had been investigated in several studies. A Japanese study (Ohasama) investigated the correlation between short-term BPV and carotid atherosclerosis in 775 subjects. BPV parameters were obtained from ambulatory blood pressure monitoring (ABPM), and carotid atherosclerosis were measured as intima-media thickness (IMT) and plaque on ultrasound. The results demonstrated that nighttime systolic BP variability was positively associated with carotid atherosclerotic plaque 5) . Another study in Chinese population also reported that the contributing roles of systolic BPV in the progression of carotid IMT 6) . Moreover, BPV could also cause considerable impact on carotid plaque vulnerability 7 , 8) . Whereas, few studies concerning BP parameters and carotid artery plaque vulnerability revealed inconsistent results 9 , 10) . And the association between short-term BPV and intracranial plaque vulnerability has rarely been investigated.
High-resolution magnetic resonance (MR) vessel wall imaging (VWI) has been extensively used in assessing atherosclerosis, especially in intracranial arteries. T1-hyperintensity within intracranial plaque indicates intraplaque hemorrhage (IPH), which is one of the typical features of vulnerable plaques. The purpose of this study was to determine the relationship between short-term BPV and intracranial plaque vulnerability evaluated by high-resolution MR vessel wall imaging.
Methods
Study Design
Data were derived from a high-resolution magnetic resonance vessel wall imaging cohort, which enrolled ischemic stroke patients with symptomatic intracranial atherosclerosis prospectively in a comprehensive stroke center. The primary objectives of this study were to determine the etiology of intracranial artery stenosis and explore the associated risk factors of atherosclerotic vulnerable plaque 11) . For this cross-sectional analysis, baseline 24-hour ABPM and intracranial atherosclerotic plaque characteristics were obtained.
Subjects
Patients were included if they fulfilled the following criteria: (1) age ≥ 18 years old; (2) ischemic stroke with more than one cardiovascular risk factor; (3) stroke was caused by intracranial atherosclerosis detected by computed tomography angiography or MR angiography; (4) MRI was conducted within 4 weeks of symptoms onset; and (5) patients who performed 24-h ABPM during hospitalization, and the interval between ABPM and VWI was within 4 weeks. Patients were excluded if: (1) stroke was caused by non-atherosclerotic intracranial artery diseases, such as dissection, vasculitis, or moyamoya disease; (2) MR vessel wall imaging with inadequate image quality (image quality score <2). The scales for image quality 12) were: 1=poor, 2=adequate, 3=good, and 4=excellent; (3) invalid BP readings (<70% of daytime or nighttime readings); (4) patients who had underwent endovascular procedures before MR vessel wall imaging were also excluded.
This study was conducted within the framework of the 1964 ethical guidelines of the Helsinki Declaration, and the protocol was also approved by the local ethics committee. Written informed consent were obtained in this cohort.
Demographic Characteristics Collection and Cardiovascular Risk Factors Definition
Demographic characteristics of age and sex, atherosclerotic risk factors (hypertension, diabetes, hyperlipidemia, current smoking, and previous medications) were obtained from medical records. In this study, hypertension was counted if patients presented with systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg, or patients who were on anti-hypertensive medication. Diabetes was considered if patients had fasting glucose ≥ 7 mmol/L or nonfasting glucose ≥ 11.1 mmol/L or were on anti-diabetic medication. Hyperlipidemia was determined if subjects presented with total cholesterol ≥ 240 mg/dL, low-density lipoprotein cholesterol ≥ 160 mg/dL, or high density lipoprotein cholesterol <40 mg/dL, patients who have been on statins were also defined as hyperlipidemia. In addition, previous history of anti-platelet medication and current smoking were also collected.
BP Measurements and BPV Calculation
A 24-hour ABPM was performed using unified device (Welch-Allyn 7100, Chicago, USA), which was set to record BP readings automatically at 30-minute intervals. Daytime was determined as from 8 am to 9 pm, and nighttime as 10 pm to 7 am in this analysis.
The following BP parameters and their short-term variability were calculated: (1) SDs of all systolic and diastolic BP readings during 24 hours, daytime, and nighttime, 24-h BP SDs were calculated as weighted SD=(day SD*daytime+night SD*nighttime)/(daytime+nighttime); (2) CVs, which were defined as the ratio between the SD and Mean at the same periods; (3) Blood pressure variability ratio (BPVR) for 24-hour, daytime, and nighttime equals to the ratio between systolic and diastolic variability (SD) 13) ; (4) Average real variability (ARV) was calculated as the average of the differences (in absolute value) between consecutive BP measurements obtained throughout the 24-hour period 14) .
MR Imaging Protocol and Image Analysis
All intracranial artery vessel wall imaging was performed on a 3.0 T MR scanner (Discovery 750, GE Healthcare, Milwaukee, USA) with 8-channel head coil. The imaging protocol includes 3D time-of-flight (TOF)-MRA and CUBE T1-weighted imaging. The MR vessel wall imaging parameters for this study were as follows: 3D TOF-MRA obtained in axial plane, fast spoiled grass (SPGR), repetition time (TR)/echo time (TE) 22/2.5 ms, flip angle 20°, field of view (FOV) 22×18 cm2, and spatial resolution 0.6×1.0×1.2 mm3; 3D CUBE-T1W was scanned in coronal plane: fast spin echo, TR/ TE 800/16 ms, optimized flip angle, FOV 23×18.4 cm2, and spatial resolution 0.7×0.6×0.6 mm3.
MR vessel wall images of intracranial arteries were interpreted by two neuroradiologists independently after post-processing using the GE-Extend Workstation. The two observers had 5 years’ experience in neurovascular imaging and were blinded to clinical information. A third senior neuroradiologist who had 10 years’ experience in neurovascular imaging would perform peer review when there was disagreement between two observers. The two observers had excellent agreement in identifying IPH, the degree of stenosis, and measuring maximum wall thickness (max WT) 11) .
The characteristics of atherosclerotic plaque were assessed in all intracranial vascular beds, including the bilateral A1 segments of the anterior cerebral artery, M1-2 segments of the middle cerebral artery, terminal segments of the internal carotid artery, P1 segments of the posterior cerebral artery, V4 segments of the vertebral artery, and basilar artery. Multiplanar reconstruction was conducted perpendicular to the arterial center line. Atherosclerotic plaque was considered when there is eccentric wall thicknesses with or without significant luminal stenosis. The max WT and stenosis degree were measured in the culprit plaque, which was located in the vessels supplying the blood flow for the ischemic lesions. If multiple plaques were present in this vessel, then the most severe one is counted. Luminal stenosis was measured according to the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) criteria 15) and divided into 4 grades in this analysis (0 for <30%; 1 for 30-49%; 2 for 50-69%; and 3 for ≥ 70%). Multiple plaques were defined as more than three plaques in all detected vascular segments of each patient. IPH was defined as T1-hyperintensity with signal intensity 1.5 times higher than that of the surrounding brain parenchyma 16) .
Statistical Analysis
Continuous variables with normal distribution were presented as mean±SD or median (interquartile range, IQR) if skewed distribution. Categorical variables were described as count and percentage. Baseline characteristics were compared between subjects with and without intracranial IPH using independent t test, Mann-Whitney U test, or Chi-square test. Univariate and multivariate logistic regression analysis were conducted to assess the correlation between BPV parameters and intracranial IPH. Variables with p<0.1 in univariate analysis were included in multivariate regression model. Odds ratio (OR) and 95% confidence interval (CI) were calculated to determine the association between BPV and intracranial IPH for per SD BPV changes. All p values <0.05 were considered statistically significant, and all statistical analyses were performed by SPSS 22.0 (IBM, New York, USA).
Results
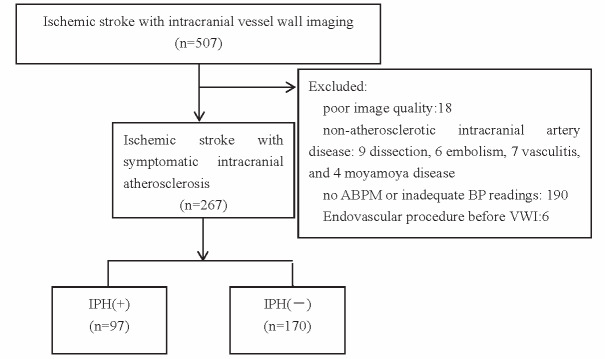
From 2017 to 2020, 507 subjects were enrolled in this database, of which, 267 subjects were included in this study (mean age 65±12 years old, 161 males). A total of 240 subjects were excluded due to the following reasons: non-atherosclerotic intracranial artery disease, inadequate image quality, and no 24-h ABPM data available. The flow chart of subjects screening was shown in Fig.1 .
Fig.1.
Flow chart of subjects screening
Clinical and Imaging Characteristics of Subjects Included
The comparison results of clinical and imaging characteristics between subjects with and without intracranial IPH are shown in Table 1 . Patients with intracranial IPH were more likely to be male and have higher percentage of anti-hypertensive medication than those without intracranial IPH (all p values <0.05). For the mean BP at daytime, nighttime and 24-h, no significant differences were detected between two groups (all p values >0.05). Patients with intracranial IPH had significantly greater maxWT (2.71 vs. 2.35 mm, p<0.001), higher prevalence of severe stenosis (42.3% vs. 28.2%, p=0.019) and multiple atherosclerotic plaques (92.8% vs. 61.8%, p<0.001) in intracranial arteries compared with subjects without intracranial IPH.
Table 1. Clinical and imaging characteristics of subjects included.
| Variables, M±SD or n (%) | IPH (+) (n = 97) | IPH (-) (n = 170) | P Value |
|---|---|---|---|
| Age (years) | 66.3±11.3 | 64.8±12.4 | 0.347 |
| Male | 67 (69.1) | 94 (55.3) | 0.027 |
| Hypertension | 82 (84.5) | 129 (75.9) | 0.095 |
| Duration of hypertension (years) | 10 (6, 20) | 10 (5, 20) | 0.241 |
| Anti-hypertensive medication | 71 (73.2) | 95 (55.9) | 0.005 |
| Diabetes mellitus | 46 (47.4) | 63 (37.1) | 0.098 |
| Hyperlipidemia | 40 (41.2) | 52 (30.6) | 0.078 |
| Smoking | 40 (41.2) | 50 (29.4) | 0.049 |
| Medication | |||
| Anti-platelet | 28 (28.9) | 53 (31.2) | 0.693 |
| Statin | 24 (24.7) | 46 (27.1) | 0.679 |
| Mean BP (mmHg) | |||
| 24-h systolic | 140.2±17.5 | 140.6±16.4 | 0.844 |
| 24-h diastolic | 78.6±11.7 | 79.4±11.6 | 0.582 |
| 24-h pulse pressure | 61.6±12.6 | 61.2±12.6 | 0.805 |
| Daytime systolic | 140.4±17.1 | 141.2±16.3 | 0.698 |
| Daytime diastolic | 79.3±12.0 | 80.3±11.7 | 0.534 |
| Daytime pulse pressure | 61.1±12.5 | 60.9±12.6 | 0.941 |
| Nighttime systolic | 140.0±19.8 | 140.0±19.3 | 0.997 |
| Nighttime diastolic | 77.6±12.5 | 78.2±12.6 | 0.679 |
| Nighttime pulse pressure | 62.4±13.7 | 61.7±14.0 | 0.704 |
| Heart rate (bps/min) | 75±12 | 76±13 | 0.661 |
| Plaque characteristics | |||
| Max WT(mm) | 2.71±0.56 | 2.35±0.62 | <0.001 |
| ≥ 50% stenosis | 41 (42.3) | 48 (28.2) | 0.019 |
| Multiple plaques | 90 (92.8) | 105 (61.8) | <0.001 |
MaxWT: maximum wall thickness.
BPV Comparison between Subjects with and without Intracranial IPH
Table 2 presented the short-term BPV comparison between subjects with and without intracranial IPH. No significant differences in 24-h, daytime, and nighttime BPV were found between the two groups, except nighttime diastolic BP CV (p=0.072) and 24-h BPVR (p=0.059) were in marginally statistical significance.
Table 2. Short-term BPV between subjects with and without intracranial IPH.
| Variables | IPH (+) (n = 97) | IPH (-) (n = 170) | P Value |
|---|---|---|---|
| Systolic | |||
| 24-h SD | 13.6±3.9 | 14.2±4.6 | 0.284 |
| 24-h CV (%) | 9.8±2.9 | 10.1±3.1 | 0.420 |
| 24-h ARV | 13.10±3.3 | 13.3±3.9 | 0.631 |
| Daytime SD | 12.3±3.8 | 12.8±4.4 | 0.349 |
| Daytime CV (%) | 8.9±3.1 | 9.1±3.1 | 0.585 |
| Nighttime SD | 13.5±5.1 | 13.5±5.9 | 0.923 |
| Nighttime CV (%) | 9.7±3.8 | 9.7±3.9 | 0.895 |
| Diastolic | |||
| 24-h SD | 10.1±3.5 | 9.8±3.2 | 0.420 |
| 24-h CV (%) | 13.0±4.4 | 12.5±4.0 | 0.280 |
| 24-h ARV | 9.9±3.3 | 9.5±3.0 | 0.246 |
| Daytime SD | 9.2±4.0 | 9.0±3.5 | 0.349 |
| Daytime CV (%) | 11.7±4.8 | 11.4±4.4 | 0.529 |
| Nighttime SD | 10.3±4.2 | 9.5±3.9 | 0.106 |
| Nighttime CV (%) | 13.5±5.4 | 12.3±4.9 | 0.072 |
| 24-h BPVR | 1.40±0.35 | 1.49±0.33 | 0.059 |
| Daytime BPVR | 1.44±0.45 | 1.51±0.43 | 0.220 |
| Nighttime BPVR | 1.44±0.66 | 1.51±0.56 | 0.315 |
Association between Short-Term BPV and Intracranial IPH
Associations between short-term BPV and intracranial IPH are shown in Table 3 . After adjusted for cardiovascular risk factors, nighttime diastolic BP CV and 24-h BPVR still have significant statistical relationship with intracranial IPH, with OR and corresponding 95% CI were 1.418 (1.051, 1.914) and 0.731 (0.548, 0.976) for per SD increment of the two variables. After further correction of plaque burden (adjusted for maxWT in model 3) and 24-h mean systolic BP level (model 4), the significant statistical association can still establish.
Table 3. Association between BPV and intracranial IPH by logistic regression analysis.
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Nighttime diastolic BP CV | 1.381 (1.029, 1.853) | 1.418 (1.051, 1.914) | 1.483 (1.087, 2.023) | 1.477 (1.081, 2.017) |
| 24-h BPVR | 0.749 (0.565, 0.992) | 0.731 (0.548, 0.976) | 0.712 (0.528, 0.960) | 0.719 (0.532, 0.972) |
Model 1: adjusted for age and sex; Model 2: Model 1+hypertension, diabetes, hyperlipidemia, smoking; Model 3: Model 2+maxWT; Model 4: Model 3+mean systolic BP
Association between BPV and Intracranial IPH by Age and Hypertension Subgroup
In addition, we also analyzed the association between nighttime diastolic BP CV, 24-h BPVR and intracranial IPH by age and hypertension subgroups, as well as by smoking status, individually. As a result, after correction of multiple confounding factors, the association between short-term BPV and intracranial IPH could only be seen in the elder patients (age ≥ 60), and hypertension subgroup ( Fig.2 and Fig.3 ) . OR and 95% CI were 1.64 (1.12, 2.42) and 1.46 (1.04, 2.05) for nighttime diastolic BP CV, 0.64 (0.45, 0.92) and 0.68 (0.49, 0.95) for 24-h BPVR, individually. The p for interaction are 0.665 for nighttime diastolic BP CV, and 0.761 for 24-h BPVR in age subgroup. In hypertension subgroup, the p for interaction are 0.472 for nighttime diastolic BP CV and 0.614 for 24-h BPVR. All p values >0.1. We didn’t find any difference of BPV and IPH in different smoking subgroups (shown in Supplementary Table 1 ).
Fig.2.
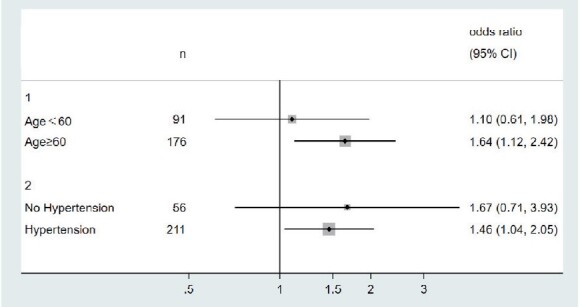
Association between nighttime diastolic BP CV and intracranial IPH by age and hypertension subgroups (adjusted for sex, hypertension, diabetes, hyperlipidemia, smoking and max WT in age subgroup, and age, sex, diabetes, hyperlipidemia, smoking and max WT in hypertension subgroup)
Fig.3.
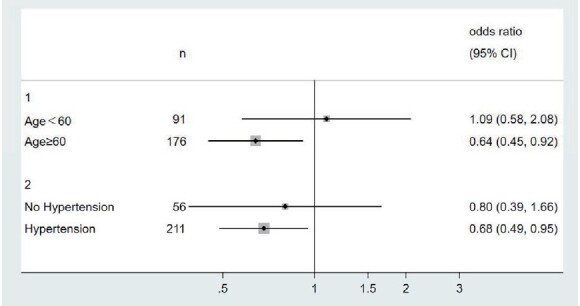
Association between 24-h BPVR and intracranial IPH by age and hypertension subgroups (adjusted for sex, hypertension, diabetes, hyperlipidemia, smoking and max WT in age subgroup, and age, sex, diabetes, hyperlipidemia, smoking and max WT in hypertension subgroup)
Supplementary Table 1. Association of short-term BPV and intracranial IPH by smoking subgroup.
| Smoking group | No-smoking group | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Night diastolic BP CV | 1.660 | 0.963, 2.864 | 1.379 | 0.940, 2.025 |
| 24-h BPVR | 0.944 | 0.544, 1.638 | 0.569 | 0.390, 0.831 |
Discussion
This study investigated the association between short-term BPV and intracranial IPH in ischemic stroke with intracranial atherosclerosis. The results in our study revealed the following key findings: (1) short-term BPV was associated with intracranial IPH; (2) of the short-term BPV parameters included, nighttime diastolic BP CV and 24-h BPVR were associated with intracranial IPH independently; (3) the significant associations between short-term BPV and IPH can only be established in subjects with older age and hypertension subgroups. The results in our study could provide new insights into the clinical significance of short-term BPV in predicting intracranial vulnerable plaque, suggesting the importance of BP management in ischemic stroke patients with intracranial atherosclerosis.
Of the short-term BPV parameters included in our study, nighttime diastolic BP CV and 24-h BPVR were associated with intracranial IPH independently. Both nighttime diastolic BP CV and 24-h BPVR are a reflection of artery stiffness 17) . Rotterdam study revealed that arterial stiffness is associated with carotid artery IPH in the general population 18) . There is also study told that diastolic BP level is associated with coronary plaque volume 19) . The JAMP study (Japan Ambulatory Blood Pressure Monitoring Prospective), enrolled 6369 subjects and followed up 4.5 years, was set to determine the association between nighttime BP dipping patterns and the occurrence of cardiovascular events. The results indicated that nighttime systolic BP was independently correlated with cardiovascular events 20) . Compared with daytime BP, the nighttime BP is less affected by daily activities, and it’s better to reflect the heart rhythm. Data from the international ABPM study also favor that nighttime BPV could add predictive value in cardiovascular events and mortality 21) . As for 24-h BPVR, although no significant discrepancies were found in systolic and diastolic BP SD between subjects with and without intracranial IPH, we can also see the statistical significance of 24-h BPVR in identify IPH, which suggest the disproportionate changes of systolic and diastolic BP SD on atherosclerosis plaque vulnerability. Another possibility is the different proportion of hypertension subtypes in this population, such as isolated systolic, isolated diastolic, and systolic-diastolic hypertension. In contrast, we didn’t find the positive association between systolic BPV and intracranial IPH. The possible explanations could be that the population included and the timing of 24-h ABPM completed, different from previous studies in the general population, we enrolled subjects with ischemic stroke, and obtained the 24-h ABPM when in hospital, the blood pressure of those patients were well in management, especially systolic BP.
In the subgroups, we can find the positive relationship between BPV and IPH in the elder subjects but not in those with younger age. Previous study suggested that the prognostic value of short-term BPV on target organ damage is correlated with age, and will increase along with age 22 - 24) . As shown by the results of Olesen’ study, the relationship between BPV and adverse outcome can be modified by age 23) . In that study, ARV of both systolic and diastolic BPs were much higher in the older than the middle-aged population (p<0.001). Other study about the association between hypertension or blood pressure variability and cardiovascular disease also suggested the distinct effects of blood pressure and its variability on atherosclerosis in different population 25 , 26) . In young patients without end organ damage, blood pressure management is more important, however, in those hypertensive patients who have already presented multi-organ damage, BPV is much more important in predicting short-term stroke risk. In addition, we can also explain this phenomenon from the perspective of different BPV mechanism in age subgroups. In the elderly, increased BPV may be a result of diffuse atherosclerotic process, which leading to increased stiffness of the large elastic arteries, the impairment of cardiovascular control mechanisms may increase BPV in the old age as well. In comparison, BPV in younger population may merely reflect the effect of random BP fluctuations in response to the activities of everyday life.
In our study, the correlation between short-term BPV and intracranial IPH can only be seen in subjects with hypertension, which is consistent with previous study about BPV and cardiovascular disease 25 , 27) . Few studies investigated the BP fluctuations in people with normotensives. Study including both hypertensive and normotensives demonstrated higher short-term BPV predict long-term cardiovascular outcome in untreated hypertension but not in normotensives 28) . While in our study, the mean BPs are comparable between groups, but the split phenomenon between subjects with and without hypertension can still be seen in subgroup analysis. The possible explanation might be that even hypertensive subjects get their BP within control after medication, the BPV caused by target organ damage and medication induced fluctuation can still not be neglected.
Our study had several limitations. First, the sample size is not large enough, which might underestimate some potential correlations. Second, this is a cross-sectional analysis which lacks follow-up data to validate the cause-effect relationship between short-term BPV and IPH. Third, no BP pattern or rhythm was included in this data analysis.
In conclusion, short-term BPV is independently associated with intracranial vulnerable plaque, especially in the elderly and patients with hypertension. Our results suggest the significance of BP management in subjects with intracranial atherosclerosis.
Acknowledgement
We thank all the subjects for their participation in this study.
Funding
This study is supported by the Beijing Municipal Science & Technology Commission (Z171100001017019), Capital Health Development Scientific Research Project (2020-1-2241), Tsinghua University Initiative Scientific Research Program (20219990033).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1).Manning LS, Rothwell PM, Potter JF, Robinson TG. Prognostic Significance of Short-Term Blood Pressure Variability in Acute Stroke: Systematic Review. Stroke, 2015; 46: 2482-2490 [DOI] [PubMed] [Google Scholar]
- 2).Xu WH, Li ML, Gao S, Ni J, Yao M, Zhou LX, Peng B, Feng F, Jin ZY, Cui LY. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol, 2012; 71(2): 195-198 [DOI] [PubMed] [Google Scholar]
- 3).Wang Y, Liu X, Wu X, Degnan AJ, Malhotra A, Zhu C. Culprit intracranial plaque without substantial stenosis in acute ischemic stroke on vessel wall MRI: A systematic review. Atherosclerosis, 2019; 287: 112-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Turan TN, Bonilha L, Morgan PS, Adams RJ, Chimowitz MI. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimaging, 2011; 21(2): e159-e161 [DOI] [PubMed] [Google Scholar]
- 5).Shintani Y, Kikuya M, Hara A, Ohkubo T, Metoki H, Asayama K, Inoue R, Obara T, Aono Y, Hashimoto T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Ambulatory blood pressure, blood pressure variability and the prevalence of carotid artery alteration: the Ohasama study. J Hypertens, 2007; 25: 1704-1710 [DOI] [PubMed] [Google Scholar]
- 6).Chen Y, Xiong H, Wu D, Pirbhulal S, Tian X, Zhang R, Lu M, Wu W, Huang W. Relationship of short-term blood pressure variability with carotid intima-media thickness in hypertensive patients. Biomed Eng Online, 2015; 14: 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Xiong H, Liu X, Tian X, Pu L, Zhang H, Lu M, Huang W, Zhang YT. A numerical study of the effect of varied blood pressure on the stability of carotid atherosclerotic plaque. Biomed Eng Online, 2014; 13: 152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Song X, Zhao X, Liebeskind DS, Xu W, Zhang J, Wei C, Xu Y, Wang L, Zheng Z, Wu J. Associations between systemic blood pressure parameters and intraplaque hemorrhage in symptomatic intracranial atherosclerosis: a high-resolution MRI-based study. Hypertens Res, 2020; 43: 688-695 [DOI] [PubMed] [Google Scholar]
- 9).Selwaness M, van den Bouwhuijsen QJ, Verwoert GC, Dehghan A, Mattace-Raso FU, Vernooij M, Franco OH, Hofman A, van der Lugt A, Wentzel JJ, Witteman JC. Blood pressure parameters and carotid intraplaque hemorrhage as measured by magnetic resonance imaging: The Rotterdam Study. Hypertension, 2013; 61: 76-81 [DOI] [PubMed] [Google Scholar]
- 10).Sun J, Canton G, Balu N, Hippe DS, Xu D, Liu J, Hatsukami TS, Yuan C. Blood Pressure Is a Major Modifiable Risk Factor Implicated in Pathogenesis of Intraplaque Hemorrhage: An In Vivo Magnetic Resonance Imaging Study. Arterioscler Thromb Vasc Biol, 2016; 36: 743-749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Song X, Zhao X, Liebeskind DS, Wang L, Xu W, Xu Y, Hou D, Zheng Z, Wu J. Incremental value of plaque enhancement in predicting stroke recurrence in symptomatic intracranial atherosclerosis. Neuroradiology, 2020; 62: 1123-1131 [DOI] [PubMed] [Google Scholar]
- 12).Li D, Zhao H, Chen X, Chen S, Qiao H, He L, Li R, Xu J, Yuan C, Zhao X. Identification of intraplaque haemorrhage in carotid artery by simultaneous non-contrast angiography and intraPlaque haemorrhage (SNAP) imaging: a magnetic resonance vessel wall imaging study. Eur Radiol, 2018; 28: 1681-1686 [DOI] [PubMed] [Google Scholar]
- 13).Gavish B, Ben-Dov IZ, Kark JD, Mekler J, Bursztyn M. The association of a simple blood pressure-independent parameter derived from ambulatory blood pressure variability with short-term mortality. Hypertens Res, 2009; 32: 488-495 [DOI] [PubMed] [Google Scholar]
- 14).Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens, 2005; 23: 505-511 [DOI] [PubMed] [Google Scholar]
- 15).Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med, 2005; 352: 1305-1316 [DOI] [PubMed] [Google Scholar]
- 16).Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, Johnson MH, Daemen MJ, Vossough A, Edjlali M, Saloner D, Ansari SA, Wasserman BA, Mikulis DJ. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol, 2017; 38: 218-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Cremer A, Doublet J, Boulestreau R, Gaudissard J, Tzourio C, Gosse P. Short-term blood pressure variability, arterial stiffness, and cardiovascular events: results from the Bordeaux cohort. J Hypertens, 2021; Publish Ahead of Print [DOI] [PubMed] [Google Scholar]
- 18).Selwaness M, van den Bouwhuijsen Q, Mattace-Raso FU, Verwoert GC, Hofman A, Franco OH, Witteman JC, van der Lugt A, Vernooij MW, Wentzel JJ. Arterial stiffness is associated with carotid intraplaque hemorrhage in the general population: the Rotterdam study. Arterioscler Thromb Vasc Biol, 2014; 34: 927-932 [DOI] [PubMed] [Google Scholar]
- 19).Saleh M, Alfaddagh A, Elajami TK, Ashfaque H, Haj-Ibrahim H, Welty FK. Diastolic blood pressure predicts coronary plaque volume in patients with coronary artery disease. Atherosclerosis, 2018; 277: 34-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Kario K, Hoshide S, Mizuno H, Kabutoya T, Nishizawa M, Yoshida T, Abe H, Katsuya T, Fujita Y, Okazaki O, Yano Y, Tomitani N, Kanegae H. Nighttime Blood Pressure Phenotype and Cardiovascular Prognosis: Practitioner-Based Nationwide JAMP Study. Circulation, 2020; 142: 1810-1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Palatini P, Reboldi G, Beilin LJ, Casiglia E, Eguchi K, Imai Y, Kario K, Ohkubo T, Pierdomenico SD, Schwartz JE, Wing L, Verdecchia P. Added predictive value of night-time blood pressure variability for cardiovascular events and mortality: the Ambulatory Blood Pressure-International Study. Hypertension, 2014; 64: 487-493 [DOI] [PubMed] [Google Scholar]
- 22).Palatini P. Short-term blood pressure variability: does its prognostic value increase with ageing? J Hypertens, 2018; 36: 1795-1797 [DOI] [PubMed] [Google Scholar]
- 23).Olesen TB, Pareek M, Stidsen JV, Blicher MK, Rasmussen S, Vishram-Nielsen J, Kjaer-Hansen K, Olsen MH. Impact of age on the association between 24-h ambulatory blood pressure measurements and target organ damage. J Hypertens, 2018; 36: 1895-1901 [DOI] [PubMed] [Google Scholar]
- 24).Satoh M, Metoki H, Asayama K, Murakami T, Inoue R, Tsubota-Utsugi M, Matsuda A, Hirose T, Hara A, Obara T, Kikuya M, Nomura K, Hozawa A, Imai Y, Ohkubo T. Age-Related Trends in Home Blood Pressure, Home Pulse Rate, and Day-to-Day Blood Pressure and Pulse Rate Variability Based on Lngitudinal Cohort Data: The Ohasama Study. J Am Heart Assoc, 2019; 81: e12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Mehlum MH, Liestol K, Kjeldsen SE, Julius S, Hua TA, Rothwell PM, Mancia G, Parati G, Weber MA, Berge E. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J, 2018; 39: 2243-2251 [DOI] [PubMed] [Google Scholar]
- 26).Chi X, Li M, Zhan X, Man H, Xu S, Zheng D, Bi J, Wang Y, Liu C. Relationship between carotid artery sclerosis and blood pressure variability in essential hypertension patients. Comput Biol Med, 2018; 92: 73-77 [DOI] [PubMed] [Google Scholar]
- 27).Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ, 2016; 354: i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Hsu PF, Cheng HM, Wu CH, Sung SH, Chuang SY, Lakatta EG, Yin FC, Chou P, Chen CH. High Short-Term Blood Pressure Variability Predicts Long-Term Cardiovascular Mortality in Untreated Hypertensives But Not in Normotensives. Am J Hypertens, 2016; 29: 806-813 [DOI] [PMC free article] [PubMed] [Google Scholar]