Abstract
Aims: We aimed to determine the characteristics and vascular outcomes of stroke in renal transplant (RT) recipients and compare them with those in patients on hemodialysis (HD) and those with no renal replacement therapy (RRT).
Methods: In this prospective observational study, 717 patients (mean age, 70.8 years; male, 60.5%) with acute ischemic stroke within one week of onset were consecutively enrolled and followed for one year. The patients were classified into three groups: (1) living donor RT recipients (n=27); (2) patients on maintenance HD before the index stroke (n=39); and (3) those with no history of RRT (n=651). The primary outcome was a composite of major adverse cardiovascular events (MACE).
Results: Diabetic nephropathy was the most common reason for RRT in both RT and HD patients. RT patients were more likely to have embolic stroke of undetermined source (33.3%) than others, whereas HD patients more often had cardioembolism (51.3%). No difference was observed in the MACE risk between the patients in RT and non-RRT groups (annual rate, 11.3% vs. 13.1%; log-rankP=0.82; hazard ratio [95% confidence interval], 0.92 [0.29-2.98]). In contrast, HD patients had a greater risk of MACE than those with no RRT (annual rate, 28.2% vs. 13.1%; log-rankP=0.019; hazard ratio [95% confidence interval], 2.24 [1.16-4.3]).
Conclusions: The underlying etiologies of stroke differed in RT and HD patients. The one-year risk of MACE for stroke patients who had received an RT was lower than that for patients undergoing HD and comparable with that of patients with no RRT.
Keywords: Embolic stroke of undetermined source, End stage kidney disease, Hemodialysis, Renal transplantation, Stroke
Introduction
Renal replacement therapy (RRT), including renal transplant (RT) and hemodialysis (HD), remarkably increases the life expectancy of patients with end-stage kidney disease 1 , 2) . However, RRT patients have a substantial risk of cerebro- and cardiovascular events, which should be a major consideration for life-long managements after RRT. According to data from the United States Renal Data Systems 3) , the prevalence of stroke and coronary artery disease were 26% and 9%, respectively, in patients with a functioning RT. For patients undergoing HD, the prevalence was even higher; 44% and 17% had experienced stroke and coronary events, respectively. Furthermore, the leading cause of death is vascular accidents for both RT and HD patients 3) . Hence, there is the need to develop a better approach to prevent and treat vascular diseases in patients undergoing RRT.
To date, stroke in HD patients have been well studied in terms of clinical aspects and prognosis 4 - 8) . On the other hand, limited data are available on stroke in RT recipients. Given the recent increase in the number of patients with renal failure receiving RT 3) , a better understanding of stroke after RT is of utmost importance. Living donor RT is much more common than cadaveric RT in Japan, and it accounts for approximately 85.9 % of all RT cases, whereas cadaveric RT is more common in the majority of countries 3) . In the present study, we aimed to characterize stroke in living donor RT recipients based on robust clinical, laboratory, and imaging data and determine their one-year prognosis compared with those receiving HD as well as those with no history of RRT.
Methods
Study Design and Patients
The Tokyo Women’s Medical University (TWMU) Stroke Registry is a single-center prospective observational study, in which patients with acute ischemic stroke or transient ischemic attack hospitalized at our center within one week of onset were consecutively enrolled. The study adhered to the ethical principles of the 1975 Declaration of Helsinki, as well as the Ethical Guidelines for Epidemiological Research by the Japanese government and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The study protocol was approved by the ethics committee of Tokyo Women’s Medical University Hospital (approval no. 2955-R2). Written informed consent was obtained from all the patients. The TWMU Stroke Registry is registered at UMIN000031913 (https://upload.umin.ac.jp). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Between December 2013 and September 2019, 806 patients were enrolled in the study. After excluding 7 patients who met the exclusion criteria (e.g., stroke mimics as final diagnosis or more than one week after stroke onset) and 82 patients with transient ischemic attack, the data of 717 patients with acute ischemic stroke were included for the present analysis ( Supplementary Fig.1 ) .
Supplementary Fig.1.
Study flowchart
All the cases of stroke were diagnosed by board-certified stroke neurologists based on neurological and radiological findings. Upon admission, the neurological symptoms were assessed using the National Institutes of Health Stroke Scale (NIHSS) score. The patient data collected included demographic data, clinical symptoms during the qualifying event, medical history, medications, investigations (including blood tests, brain and cerebral artery imaging, 24-hour Holter electrocardiogram, and ultrasonic echocardiography), management (medical treatment, revascularization procedure, and surgery), and the occurrence of clinical events after the qualifying event using a structured case report form.
Renal Disease
We identified patients who had a history of living donor RT or were undergoing maintenance HD before the index stroke (RT and HD groups, respectively). Information on primary kidney diseases that necessitated RRT was obtained from previous medical records. None of the patients in our cohort received cadaveric RT. The estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease formula with the Japanese coefficient; chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2.
Evaluation of Atherosclerotic Disease
The intracranial arteries were examined using time-of-flight magnetic resonance angiography (n=686) and/or computed tomography angiography (n=146). The narrowest diameter of each stenosed vessel was measured and divided by the diameter of the normal vessel proximal to the lesion or distal to the lesion if the proximal artery was diseased. Significant intracranial artery stenosis was defined as >50% stenosis or occlusion.
Extracranial carotid atherosclerosis was evaluated using ultrasonography (n=675) and/or computed tomography angiography (n=81) and/or time-of-flight magnetic resonance angiography (n=66). We defined significant extracranial artery stenosis as the presence of atherosclerotic stenosis of >50% or occlusion according to the European Carotid Surgery Trial criteria 9) .
Aortic atherosclerosis was evaluated using transesophageal echocardiography (n=226). Mobile plaques were diagnosed as mobile components that swung on their peduncles. An ulcerative plaque was diagnosed as a discrete indentation of the luminal surface of the plaque with a base width, as well as a maximum depth, of at least 2 mm. Complex aortic atheroma was defined as any plaque with a thickness of ≥ 4 mm or ulceration or mobile components 10) .
Ischemic Stroke Subtype
The etiologies of ischemic stroke were classified into atherothrombosis, cardioembolism, small-vessel disease, other determined causes, and undetermined causes, according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification 11) . Strokes of undetermined causes were further divided into embolic stroke of undetemined source (ESUS), stroke with coexisting etiologies, and incomplete investigations. ESUS was diagnosed based on the proposed criteria by the cryptogenic stroke/ESUS international working group (i.e., stroke detected by computed tomography or magnetic resonance imaging that is not lacunar; absence of extracranial or intracranial atherosclerosis causing ≥ 50% luminal stenosis in arteries supplying the area of ischemia; no major-risk cardioembolic source of embolism or no other specific cause of stroke identified) 12) .
Follow-Up and Outcomes
Patients visited our center after 3 months and thereafter every 1 year for 3 years after enrollment. This study reports one-year outcomes. At follow-up visits, findings from physical examinations, treatments, any occurrence of clinical events, and the modified Rankin Scale (mRS) scores were recorded. If the patient could not be reached for follow-up, a relative or caregiver was interviewed via telephone. The primary outcome was a composite of major adverse cardiovascular events (MACE), including nonfatal stroke (ischemic or hemorrhagic), nonfatal acute coronary syndrome, major peripheral artery disease, and vascular death. Vascular death was defined as fatal acute coronary artery disease, fatal stroke, and other cardiovascular deaths. Secondary outcomes included stroke subtype and all-cause mortality. Stroke-related functional outcomes were assessed using the mRS score at one year. A poor functional outcome was defined as an mRS score of ≥ 3.
Statistical Analysis
Quantitative variables were expressed as mean (standard deviation) for normally distributed data or median (interquartile range). The qualitative variables were presented as frequencies (percentages). The patients were classified into the RT, HD, and non-RRT groups. We further divided the non-RRT patients into those with and without CKD. The comparisons of the groups were performed using the t-test, Mann-Whitney U test, one-way analysis of variance, or Kruskal-Wallis test for quantitative variables and test for qualitative variables, as appropriate. The event rates were estimated using the Kaplan-Meier method, and the inter-group differences were assessed using the log-rank test. Cox proportional hazard regression models were used to calculate the age- and sex-adjusted hazard ratios and 95% confidence intervals for the patients with RT and HD, in comparison with those without RRT. The data for the patients with no information at one year were censored at the time of the last available follow-up. For a given outcome, the patients who died of causes other than the outcome were censored at the time of death. Events that occurred after one year of follow-up were not included in the current analysis. To identify the predictors of poor functional outcomes, we performed multiple logistic regression analysis with adjustments for age, sex, RRT modality, and admission NIHSS score. For all the analyses, statistical significance was set at P<0.05.
Results
Of the 717 patients (mean age, 70.8 years; male, 60.5%), 27 patients (3.8 %) had received living donor RT and 39 patients (5.4 %) were undergoing maintenance HD; the remaining 651 patients (90.8 %) had no history of RRT. The median duration from RT surgery to stroke onset was 33 months (interquartile range, 7-150 months) in the RT group. The median duration from HD initiation to stroke onset was 124 months (interquartile range, 63-157 months) in the HD patients. Diabetic nephropathy was the most frequent reason for RRT in both the RT and HD groups ( Table 1 ) . The usage rates of immunosuppressive agents in patients with RT are shown in Supplementary Table 1 . Immunosuppressive regimens were not changed after the index stroke.
Table 1. Primary renal diagnosis.
| Renal transplant (n = 27) | Hemodialysis (n = 39) | |
|---|---|---|
| Diabetic nephropathy | 9 | 16 |
| IgA nephropathy | 4 | 2 |
| Renal sclerosis | 2 | 6 |
| Chronic glomerulonephritis | 1 | 6 |
| Polycystic kidney | 1 | 1 |
| Undetermined | 6 | 6 |
| Others | Lupus nephritis (n = 1) | CIN (n = 1) |
| HUS (n = 1) | MPGN (n = 1) | |
| Gouty kidney (n = 1) | ||
| Nephrotic syndrome (n = 1) |
Abbreviations: CIN = contrast induced nephropathy; HUS = hemolytic uremic syndrome; MPGN = membranoproliferative glomerulonephritis.
Supplementary Table 1. Usage of immunosuppressive agents among 27 patients after renal transplant.
| No. of patients (%) | |
|---|---|
| Prednisolone | 23 (85.2) |
| Tacrolimus | 21 (77.8) |
| Mycophenolate mofetil | 21 (77.8) |
| Cyclosporine | 2 (7.4) |
| Mizoribine | 2 (7.4) |
| Everolimus | 1 (3.7) |
Table 2 shows the baseline characteristics of the patients. Patients in the RT group were younger, and those in the HD group more often had chronic heart failure and peripheral artery disease than the others. There were no differences in the prevalence of atherosclerotic diseases in the intra- and extracranial arteries and aortas among the 3 groups. The low-density lipoprotein concentrations were lower, and the homocysteine concentrations were higher, in the RT and HD groups than in the non-RRT group. The brain natriuretic peptide level was highest in the HD group. The comparisons of the baseline characteristics of the patients with the RT, HD, no RRT with CKD, and no RRT without CKD groups are shown in Supplementary Table 2 . Regarding the etiologic subtype ( Fig.1 and Supplementary Fig.2 ) , ESUS was more frequent in the RT patients than in the others. As shown in Supplementary Table 3 , there were no relationships between specific immunosuppressive agents with the development of ESUS. On the other hand, cardioembolism was more frequent in the HD patients. In the HD group, unfractionated heparin and low-molecular-weight heparin were used in 32 and 6 patients, respectively, for anticoagulation of the extracorporeal circuit. There were no significant associations of the type of anticoagulant for HD with a specific subtype of ischemic stroke.
Table 2. Baseline characteristics.
| Renal transplant (n = 27) | Hemodialysis (n = 39) | P-value* | No RRT (n = 651) | P-value† | |
|---|---|---|---|---|---|
| Age, y | 59±12 | 67±13 | 0.018 | 71±13 | <0.001 |
| Male | 22 (81.5) | 23 (59.0) | 0.049 | 389 (59.8) | 0.058 |
| Medical history | |||||
| Hypertension | 20 (74.1) | 34 (87.2) | 0.18 | 483 (74.2) | 0.15 |
| Diabetes mellitus | 12 (44.4) | 19 (48.7) | 0.73 | 250 (38.4) | 0.38 |
| Dyslipidemia | 14 (51.9) | 18 (46.1) | 0.65 | 308 (47.3) | 0.89 |
| Current smoking | 3 (11.1) | 3 (7.7) | 0.64 | 120 (18.5) | 0.11 |
| Atrial fibrillation | 6 (22.2) | 15 (38.5) | 0.16 | 142 (21.8) | 0.075 |
| Chronic heart failure | 2 (7.4) | 12 (30.8) | 0.016 | 65 (10.0) | 0.002 |
| Stroke | 4 (14.8) | 10 (25.6) | 0.28 | 130 (20.0) | 0.55 |
| Coronary artery disease | 2 (7.4) | 8 (20.5) | 0.13 | 92 (14.1) | 0.31 |
| Peripheral artery disease | 2 (7.4) | 8 (20.5) | 0.13 | 30 (4.6) | 0.003 |
| Investigations | |||||
| Intracranial artery | |||||
| > 50% stenosis | 5 (18.5) | 13 (33.3) | 0.18 | 184 (28.4) | 0.39 |
| Extracranial artery | |||||
| > 50% stenosis | 2 (7.4) | 4 (10.3) | 0.69 | 96 (14.9) | 0.37 |
| Extracranial artery | |||||
| 30%-50% stenosis | 4 (14.8) | 6 (15.4) | 0.95 | 84 (13.0) | 0.89 |
| Complex aortic atheroma | 2 (20.0) | 4 (33.3) | 0.48 | 51 (27.7) | 0.78 |
| Laboratory data | |||||
| Creatinine mg/dL | 2.4±2.5 | 7.6±2.7 | <0.001 | 1.0±0.6 | <0.001 |
| LDL-c, mg/dL | 111±41 | 98±38 | 0.18 | 117±38 | 0.009 |
| HDL-c, mg/dL | 50±22 | 51±15 | 0.74 | 55±17 | 0.12 |
| Triglycerides, mg/dL | 139±64 | 117±58 | 0.16 | 132±96 | 0.60 |
| HbA1c, % | 6.0±0.7 | 6.2±1.7 | 0.55 | 6.8±5.4 | 0.59 |
| BNP, pg/mL | 99±168 | 728±790 | <0.001 | 136±231 | <0.001 |
| Homocysteine, nmol/mL | 15.2 (11.6-19.4) | 21.5 (14.5-26.9) | 0.064 | 9.4 (7.2-12.6) | <0.001 |
| hsCRP, mg/mL | 1.3 (0.2-2.3) | 5.2 (1.5-14.8) | 0.44 | 1.4 (0.4-5.4) | 0.80 |
| IL-6, pg/mL | 4.0 (1.5-11.3) | 9.3 (4.3-13.5) | 0.14 | 3.5 (1.9-8.2) | 0.52 |
| NIHSS | 2 (1-4) | 4 (2-6) | 0.13 | 3 (1-5) | 0.40 |
Figures are expressed as n (%), mean±standard deviation, or median (interquartile range).
*Comparisons between patients with renal transplant and hemodialysis.
†Comparisons among patients with renal transplant, hemodialysis, and no RRT.
Abbreviations: BNP = brain natriuretic peptide; HDL-c = high density lipoprotein cholesterol; hsCRP = high sensitivity C-reactive protein; IL-6 = interleukin-6; LDL-c = low density lipoprotein cholesterol; NIHSS = National Institute of Health Stroke Scale; RRT = renal replacement therapy.
Supplementary Table 2. Baseline characteristics.
| Renal transplant (n = 27) | Hemodialysis (n = 39) | No RRT (n = 651) | P-value* | ||
|---|---|---|---|---|---|
| With CKD† (n = 139) | Without CKD (n = 512) | ||||
| Age, y | 59±12 | 67±13 | 76±10 | 70±13 | <0.001 |
| Male | 22 (81.5) | 23 (59.0) | 89 (64.0) | 300 (58.6) | 0.069 |
| Medical history | |||||
| Hypertension | 20 (74.1) | 34 (87.2) | 123 (88.4) | 360 (70.3) | <0.001 |
| Diabetes mellitus | 12 (44.4) | 19 (48.7) | 70 (50.4) | 180 (35.2) | 0.006 |
| Dyslipidemia | 14 (51.9) | 18 (46.1) | 84 (60.4) | 224 (43.8) | 0.005 |
| Current smoking | 3 (11.1) | 3 (7.7) | 17 (12.2) | 103 (20.2) | 0.024 |
| Atrial fibrillation | 6 (22.2) | 15 (38.5) | 46 (33.1) | 96 (18.8) | 0.006 |
| Chronic heart failure | 2 (7.4) | 12 (30.8) | 33 (23.7) | 32 (6.3) | <0.001 |
| Stroke | 4 (14.8) | 10 (25.6) | 31 (22.3) | 99 (19.3) | 0.61 |
| Coronary artery disease | 2 (7.4) | 8 (20.5) | 37 (26.6) | 55 (10.7) | <0.001 |
| Peripheral artery disease | 2 (7.4) | 8 (20.5) | 15 (10.8) | 15 (2.9) | <0.001 |
| Investigations | |||||
| Intracranial artery | 5 (18.5) | 13 (33.3) | 44 (32.1) | 140 (27.5) | 0.39 |
| > 50% stenosis | |||||
|
Extracranial artery > 50% stenosis |
2 (7.4) | 4 (10.3) | 29 (21.5) | 67 (13.1) | 0.059 |
| Extracranial artery | 4 (14.8) | 6 (15.4) | 18 (13.3) | 66 (12.9) | 0.97 |
| 30%-50% stenosis | |||||
| Complex aortic atheroma | 2 (20.0) | 4 (33.3) | 12 (40.0) | 39 (25.3) | 0.39 |
| Laboratory data | |||||
| Creatinine mg/dL | 2.4±2.5 | 7.6±2.7 | 1.6±0.1 | 0.8±0.1 | <0.001 |
| LDL-c, mg/dL | 111±41 | 98±38 | 110±47 | 119±36 | 0.001 |
| HDL-c, mg/dL | 50±22 | 51±15 | 53±19 | 56±17 | 0.097 |
| Triglycerides, mg/dL | 139±64 | 117±58 | 137±92 | 130±97 | 0.64 |
| HbA1c, % | 6.0±0.7 | 6.2±1.7 | 6.6±1.3 | 6.9±6.0 | 0.71 |
| Homocysteine, nmol/mL | 15.2 (11.6-19.4) | 21.5 (14.5-26.9) | 12.7 (10.4-16.8) | 8.8 (6.9-11.7) | <0.001 |
| hsCRP, mg/mL | 1.3 (0.2-2.3) | 5.2 (1.5-14.8) | 2.6 (0.5-8.4) | 1.2 (0.4-4.8) | 0.83 |
| IL-6, pg/mL | 4.0 (1.48-11.3) | 9.3 (4.3-13.5) | 5.2 (2.5-11.0) | 3.1 (1.7-7.1) | 0.73 |
| BNP, pg/mL | 99±168 | 728±790 | 219±25 | 114±186 | <0.001 |
| NIHSS | 2 (1-4) | 4 (2-6) | 3 (2-6) | 3 (1-5) | 0.42 |
Figures are expressed as n (%), mean±standard deviation, or median (interquartile range).
*Comparisons among four groups. †CKD was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2.
Comparisons among patients with renal transplant, hemodialysis and no RRT.
Abbreviations: BNP = brain natriuretic peptide; CKD = chronic kidney disease; HDL-c = high density lipoprotein cholesterol; hsCRP = high sensitivity C-reactive protein; IL-6 = interleukin- 6; LDL-c = low density lipoprotein cholesterol; NIHSS = National Institute of Health Stroke Scale; RRT = renal replacement therapy.
Fig.1. Etiologic subtype of ischemic stroke.
Distributions of etiologic subtype were significantly different between the groups (P=0.031).
Abbreviations: ESUS=embolic stroke of undetermined source; RRT=renal replacement therapy.
Supplementary Fig.2. Etiologic subtype of ischemic stroke.
Distributions of etiologic subtype were significantly different between the groups (P=0.002).
Abbreviations: CKD=chronic kidney disease; ESUS=embolic stroke of undetermined source; RRT=renal replacement therapy.
Supplementary Table 3. Immunosuppressive agent use in ESUS and Non-ESUS patients in the renal transplant group.
| ESUS (n = 9) | No ESUS (n = 18) | P-value | |
|---|---|---|---|
| Prednisolone | 7 (77.8) | 16 (88.9) | 0.45 |
| Tacrolimus | 7 (77.8) | 14 (77.8) | 1.00 |
| Mycophenolate mofetil | 7 (77.8) | 14 (77.8) | 1.00 |
| Cyclosporine | 0 | 2 (11.1) | 0.19 |
| Mizoribine | 1 (11.1) | 1 (5.6) | 0.61 |
| Everolimus | 0 | 1 (5.6) | 0.36 |
ESUS = embolic stroke of undetermined source.
The data on medication use at discharge and surgery are presented in Supplementary Table 4 . The usage rates of antiplatelet and anticoagulant agents were 74.1% and 32.1% in the RT group and 59.0% and 46.5% in the HD group, respectively. Among the RT and HD patients with 74.1% and 87.2% prevalence of hypertension, ≥ 1 antihypertensive agents were used in 61.5% and 82.1%, respectively at discharge.
Supplementary Table 4. Medication use at discharge and surgical treatments.
| Renal transplant (n = 27) | Hemodialysis (n = 39) | P-value* | No RRT (n = 651) | P-value† | |
|---|---|---|---|---|---|
| Antiplatelet agent, | 20 (74.1) | 23 (59.0) | 0.20 | 457 (70.3) | 0.31 |
| Single | 9 (33.3) | 16 (41.0) | 0.53 | 267 (41.1) | 0.72 |
| Dual or triple | 11 (40.7) | 7 (18.0) | 0.042 | 190 (29.2) | 0.12 |
| Anticoagulant agent | 9 (32.1) | 20 (46.5) | 0.15 | 237 (32.8) | 0.72 |
| Warfarin | 6 (22.2) | 20 (51.2) | 0.016 | 87 (13.5) | <0.001 |
| Direct oral anticoagulant agent | 3 (11.1) | 0 | 0.018 | 128 (19.9) | <0.001 |
| Antihypertensive agent | 16 (61.5) | 32 (82.1) | 0.067 | 357 (54.9) | 0.002 |
| Ca channel blocker | 11 (40.7) | 22 (56.4) | 0.21 | 192 (29.5) | 0.002 |
| ACEl/ARB | 8 (29.6) | 18 (46.2) | 0.17 | 190 (29.1) | 0.10 |
| Beta blocker | 6 (22.2) | 15 (38.4) | 0.16 | 99 (15.2) | 0.002 |
| Diuretics | 2 (7.4) | 6 (15.4) | 0.32 | 78 (12.0) | 0.60 |
| Alpha blocker | 0 | 4 (10.3) | 0.036 | 18 (2.8) | 0.046 |
| RAS inhibitor | 2 (7.4) | 1 (2.6) | 0.36 | 4 (0.6) | 0.039 |
| Lipid lowering agent | 15 (55.6) | 25 (64.1) | 0.49 | 404 (62.2) | 0.76 |
| Statin | 14 (51.9) | 22 (56.4) | 0.71 | 443 (60.7) | 0.55 |
| Others | 4 (14.8) | 9 (23.1) | 0.40 | 247 (38.0) | 0.006 |
| Glucose lowering agent or insulin | 7 (25.9) | 14 (35.9) | 0.39 | 283 (28.1) | 0.57 |
| Carotid endarterectomy | 0 | 0 | - | 11 (1.7) | 0.34 |
| Carotid artery stent | 0 | 0 | - | 17 (2.6) | 0.19 |
Figures are expressed as n (%).
*Comparisons between patients with renal transplant and hemodialysis.
†Comparisons among patients with renal transplant, hemodialysis, and no RRT.
Abbreviations: ACEl = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor blocker; RAS = renin angiotensin aldosterone system; RRT = renal replacement therapy.
One-Year Event Risk
Among the 717 patients, 96 had at least one vascular event within one year, giving an event rate of 13.4% (95% confidence interval, 11.1 %–16.1%). As shown in Fig.2 and Table 3 , patients in the HD group had a significantly higher risk of MACE than those in the non-RRT group. No differences were observed between the MACE risks of the patients in the RT and non-RRT groups. These results were consistent when patients with no RRT history were further divided according to the presence or absence of CKD ( Supplementary Fig.3 ) . There was no difference in the risk of all-cause mortality across the three groups.
Fig.2. Kaplan-Meier curves for major cardiovascular events.

Abbreviations: HD=hemodialysis; RRT=renal replacement therapy; RT= renal transplantation.
Table 3. Event risk at one year.
| Event rate, n (%/year) | Log rank P-value | Adjusted HR (95% CI) | P-value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Major cardiovascular events | ||||
| No RRT | 82 (13.1) | - | 1.00 (ref ) | - |
| Renal transplant | 3 (11.3) | 0.82 | 0.92 (0.29-2.98) | 0.89 |
| Hemodialysis | 10 (28.2) | 0.019 | 2.14 (1.11-4.14) | 0.020 |
| Secondary outcome | ||||
| Stroke | ||||
| No RRT | 71 (11.3) | - | 1.00 (ref ) | - |
| Renal transplant | 3 (11.3) | 0.97 | 1.09 (0.33-3.54) | 0.89 |
| Hemodialysis | 6 (15.8) | 0.39 | 1.49 (0.65-3.45) | 0.35 |
| Ischemic stroke | ||||
| No RRT | 64 (10.1) | - | 1.00 (ref ) | - |
| Renal transplant | 3 (11.3) | 0.83 | 1.13 (0.35-3.70) | 0.84 |
| Hemodialysis | 5 (13.2) | 0.55 | 1.39 (0.56-3.47) | 0.48 |
| All-cause death | ||||
| No RRT | 46 (7.3) | - | 1.00 (ref ) | - |
| Renal transplant | 1 (3.7) | 0.49 | 0.57 (0.08-4.22) | 0.58 |
| Hemodialysis | 4 (10.4) | 0.48 | 1.57 (0.58-4.39) | 0.39 |
Abbreviations: CI = confidence interval; HR = hazard ratio; RRT = renal replacement therapy.
Supplementary Fig.3. Kaplan-Meier curves for major cardiovascular events.

Abbreviations: CKD=chronic kidney disease; HD=hemodialysis; RRT=renal replacement therapy; RT=renal transplantation.
There were 3 and 5 patients who experienced recurrent stroke in the RT and HD groups, respectively. Subtypes of the index and recurrent strokes were coincident, except for 1 patient in the HD group who had atherothrombosis at first and then developed cardioembolism during follow-up ( Supplementary Table 5 ) . Supplementary Table 6 shows comparisons of the 1-year MACE risk between each immunosuppressive agent users and non-users among the RT patients. Cyclosporine and mizoribine tended to raise the risk of MACE with no statistical differences, whereas tacrolimus and mycophenolate mofetil were associated with a lower risk. In the HD group, 32 (84.2%) and 6 (15.8%) patients used unfractionated heparin and low-molecular-weight heparin during the HD procedure, respectively. As shown in Supplementary Table 7 , the event rates of MACE and ischemic stroke were not significantly different between the anticoagulant drugs.
Supplementary Table 5. Etiologic subtype of initial and recurrent ischemic stroke in patients receiving renal replacement therapy.
| Index stroke subtype | Recurrent stroke subtype | |
|---|---|---|
| Renal transplant group | ||
| Patient 1 | Atherothrombosis | Atherothrombosis |
| Patient 2 | Other determined cause | Other determined cause |
| Patient 3 | Coexisting cause | Coexisting cause |
| Hemodialysis group | ||
| Patient 1 | Cardioembolism | Cardioembolism |
| Patient 2 | Cardioembolism | Cardioembolism |
| Patient 3 | Atherothrombosis | Cardioembolism |
| Patient 4 | ESUS | ESUS |
| Patient 5 | Small vessel disease | Small vessel disease |
Abbreviations: ESUS = embolic stroke of undetermined source.
Supplementary Table 6. Risk of major adverse cardiovascular events at one year by the use of each immunosuppressive agent.
| Event rate, n (%/year) | Log rank P-value | |
|---|---|---|
| Prednisolone | ||
| No (n = 4) | 0 | |
| Yes (n = 23) | 3 (13.0) | 0.48 |
| Tacrolimus | ||
| No (n = 6) | 2 (33.3) | |
| Yes (n = 21) | 1 (5.0) | 0.038 |
| Mycophenolate mofetil | ||
| No (n = 6) | 2 (33.3) | |
| Yes (n = 21) | 1 (5.0) | 0.038 |
| Cyclosporine | ||
| No (n = 25) | 2 (8.2) | |
| Yes (n = 2) | 1 (50.0) | 0.057 |
| Mizoribine | ||
| No (n = 25) | 2 (8.2) | |
| Yes (n = 2) | 1 (50.0) | 0.057 |
| Everolimus | ||
| No (n = 26) | 3 (11.7) | |
| Yes (n = 1) | 0 | 0.73 |
Supplementary Table 7. Event risk at one year by the type of anticoagulant used for hemodialysis.
| Event rate, n (%/year) |
Log rank P-value |
||
|---|---|---|---|
| UFH (n = 32) | LWMH (n = 6) | ||
| Major cardiovascular events | 9 (30.0) | 1 (20.0) | 0.49 |
| lschemic stroke | 5 (16.0) | 0 | 0.32 |
Abbreviations: LWMH = low-molecular-weight heparin; UFH = unfractionated heparin
One-Year Functional Prognosis
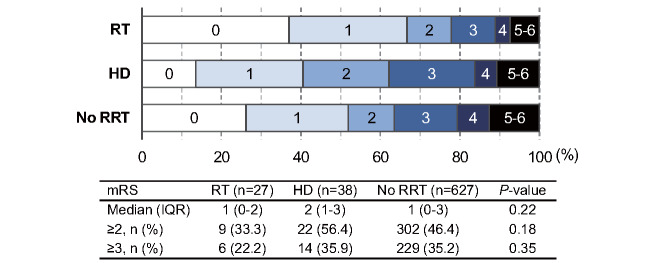
Fig.3 shows the distributions of the mRS scores at one-year follow-up. The percentages of patients with poor functional outcomes tended to be lower in the RT group, although the difference was not statistically significant. The inter-group difference was significant when the patients with no RRT were subdivided into those with CKD and those without CKD ( Supplementary Fig.4 ) . The multivariable analysis showed that RT or HD was not independently associated with poor functional prognosis at one year; age and NIHSS were independent determinants of disability ( Table 4 ) .
Fig.3. Modified Rankin Scale score at one year.
Abbreviations: HD=hemodialysis; IQR=interquartile range; mRS=modified Rankin Scale; RRT=renal replacement therapy; RT= renal transplantation.
Supplementary Fig.4. Modified Rankin Scale score at one year.
Abbreviations: CKD=chronic kidney disease; HD=hemodialysis; IQR=interquartile range; mRS=modified Rankin Scale; RRT=renal replacement therapy; RT=renal transplantation.
Table 4. Multivariable analysis for disability at one year.
| OR (95% CI) | P-value | |
|---|---|---|
| Age, per 1 year increase | 1.05 (1.04-1.07) | <0.001 |
| Male | 0.98 (0.68-1.39) | 0.91 |
| RRT | ||
| No RRT | 1.00 (ref ) | - |
| Renal transplant | 1.11 (0.41-3.04) | 0.83 |
| Hemodialysis | 1.33 (0.64-2.76) | 0.44 |
| NIHSS, per 1 point increase | 1.16 (1.11-1.20) | <0.001 |
Disability was defined as an modified Rankin Scale score of ≥ 3 at 1 year after index stroke.
Abbreviations: CI = confidence interval; OR = odds ratio; RRT = renal replacement therapy; NIHSS = National
Institute of Health Stroke Scale.
Discussion
Our main findings were as follows: (1) ESUS and cardioembolism were the most frequent stroke subtypes in RT and HD patients, respectively; (2) the one-year risk of MACE in stroke patients with RT was significantly lower than that in the HD patients and as low as that in the non-RRT patients; and (3) the one-year functional outcomes tended to be better in RT patients than in HD patients. While both risk factor management and appropriate antithrombotic therapies are essential at all stages of CKD 13) , the benefits of targeted therapies may differ in the RT and HD patients, given the substantial disparities in characteristics and outcomes of stroke between them.
Ischemic Stroke Subtype
Our results are consistent with a prior study reporting that 66% of ischemic strokes after RT were cryptogenic and 31% were likely to be embolic strokes 14) . The underlying mechanisms of ESUS can be classified as cardioembolic and non-cardioembolic 15) . Although we performed a standardized cardiac workup, latent paroxysmal atrial fibrillation might be a cause of ESUS in RT patients. Another possibility is embolism from subclinical non-stenotic atherosclerotic lesions. The atherosclerotic process is generally accelerated after RT, resulting in thromboembolic events 16) . Several studies have reported an increase in the prevalence of atherosclerotic wall changes in RT patients 17 , 18) . In our study, ipsilateral 30–50% stenosis of the extracranial carotid artery was found in 15% of RT patients, which was comparable to that in HD patients. Additionally, the serum homocysteine levels were significantly higher in the RT patients than in those without a history of RRT, whereas the prevalence of common vascular risk factors (i.e., hypertension, diabetes, or dyslipidemia) were similar. Previous studies demonstrated that an elevated homocysteine concentration was a predictor of increased cardiovascular morbidity or mortality among patients with end-stage kidney disease 19 , 20) . High concentrations of homocysteine are known to have deleterious effects on the vascular endothelium through oxidant stress and promote atherosclerosis and thrombus formation 21 , 22) . Moreover, homocysteine showed a predilection towards the promotion of platelet adhesion to endothelial cells and was also associated with higher levels of prothrombotic factors 23) . Recent studies indicated that ESUS cases result from non-stenotic atherosclerosis, atrial cardiopathy, patent foramen ovale, etc., which are generally regarded as low- to moderate-risk embolic sources 12) . We speculated that thrombotic tendency may be enhanced by the presence of hyperhomocysteinemia even in such non-high-risk diseases. The treatments for lowering homocysteine concentrations may have cardiovascular benefits in RT patients, although further studies are needed to verify this.
Cardioembolism was the most common subtype in patients undergoing HD, presumably because of their high prevalence of atrial fibrillation. This is consistent with the report of a previous study 5) , whereas some studies found that small vessel occlusion was the most frequent subtype for those receiving HD 6 , 24) . As renal function declines, patients are more likely to develop atrial fibrillation 25) . The prevalence of atrial fibrillation was reported to be particularly high in HD patients, at 7–27% 26 - 28) . Most HD patients experience chronic volume overload and its rapid fluctuation, which can increase the incidence of atrial fibrillation. In addition, the calcification of the cardiac valve by the dysregulation of calcium and phosphate metabolism can cause valvular heart disease, leading to the development of atrial fibrillation 29) . In our study, the 38.5% prevalence of atrial fibrillation in HD patients was the highest among the groups.
Vascular Event Risk
RT patients have a higher cardiovascular risk than the general population, since they usually have, in addition to traditional risk factors such as hypertension, diabetes, hyperlipidemia, or smoking, nontraditional cardiovascular risk factors, including adverse metabolic effects of immunosuppression, chronic anemia, hyperhomocysteinemia, chronic inflammation, proteinuria, and chronic allograft nephropathy 30) . The United States Renal Data Systems reported that the three-year incidence of de novo cerebrovascular events after RT was 6.8% 31) . Meanwhile, few data are available on the vascular risks in secondary prevention settings. In our study, the one-year MACE risk after stroke was 11.3% in RT patients, which was comparable with that in non-RRT patients (13.1%) and lower than that in HD patients (28.2%). Our patients in the RT group seemed to undergo rigorous preventive treatment during follow-up. Specifically, the usage rates of antiplatelet, anticoagulant, antihypertensive, and lipid-lowering agents were 74%, 32%, 62%, and 56%, respectively. These appropriate interventions may have lowered the MACE risk in RT patients to the level of the patients with no RRT history.
Functional Prognosis
Although RRT modalities were not independent determinants of functional prognosis, RT patients seemed to show better outcomes than HD patients; 22% and 36% were handicapped or dead one year after stroke in RT and HD patients, respectively. To date, few studies have assessed disability after stroke among RT recipients. Toyoda et al. reported that HD was an independent indicator for an mRS of ≥ 3 at 4 weeks 4) . The case fatality of stroke in RT patients has been reported to be very high, with a mortality rate of approximately 40% at one year 32 , 33) . The good prognosis in our study may be partly attributed to the different patient backgrounds as well as the improved secondary prevention measures. In addition, the Japanese RT patients showed excellent long-term outcomes with longer survival periods than the non-Japanese RT patients 34) , which could be consistent with our results.
Limitations
First, the sample was relatively small, especially for the RRT groups; thus, our analysis is susceptible to type II error. However, this is inevitable given that stroke after RT is a rare condition. We could not perform extensive multivariable adjustments because of the small number of outcome events. Second, we had no data available on the exact change of blood pressure levels during follow-up, which could affect the vascular outcomes. Third, because of the single-center setting, generalizability is limited. The annual MACE rate of 11.3% in the non-RRT group was higher than that reported in previous clinical trials on general stroke populations 35 , 36) . This may be in part due to our consecutive enrollment of patients regardless of their age, general conditions, or comorbidities, and clinical trials usually select patients whose systemic condition is fair. In addition, patients were included within one week of onset, ensuring that early recurrent events were captured. It is plausible that our patients represent the “real world” stroke cohort rather than those who would be included in clinical trials. Our results should be interpreted with caution and reproduced in larger multicenter studies.
Conclusions
RT patients were more likely to have ESUS and were at a lower risk of recurrent vascular events than HD patients. Our study provides useful information for developing optimal secondary prevention strategies for patients undergoing RT as well as HD.
Acknowledgements
None.
Grant Support
None.
Conflict of Interest
Dr. Kitagawa reports personal fees from Kyowa Kirin, grants and personal fees from Daiichi Sankyo, grants from Bayer, and grants from Dainihon Sumitomo outside the submitted work. Other authors have nothing to disclose.
References
- 1).Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol, 2020; 16: 573-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Reese PP, Boudville N, Garg AX. Living kidney donation: outcomes, ethics, and uncertainty. Lancet, 2015; 385: 2003-2013 [DOI] [PubMed] [Google Scholar]
- 3).United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2020 [Google Scholar]
- 4).Toyoda K, Fujii K, Fujimi S, Kumai Y, Tsuchimochi H, Ibayashi S, Iida M. Stroke in patients on maintenance hemodialysis: a 22-year single-center study. Am J Kidney Dis, 2005; 45: 1058-1066 [DOI] [PubMed] [Google Scholar]
- 5).Sozio SM, Armstrong PA, Coresh J, Jaar BG, Fink NE, Plantinga LC, Powe NR, Parekh RS. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis, 2009; 54: 468-477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Jung S, Kwon SB, Hwang SH, Noh JW, Lee YK. Ischemic stroke among the patients with end-stage renal disease who were undergoing maintenance dialysis. Yonsei Med J, 2012; 53: 894-900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Herrington W, Haynes R, Staplin N, Emberson J, Baigent C, Landray M. Evidence for the prevention and treatment of stroke in dialysis patients. Semin Dial, 2015; 28: 35-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis, 1998; 31: 991-996 [DOI] [PubMed] [Google Scholar]
- 9).Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet, 1998; 351: 1379-1387 [PubMed] [Google Scholar]
- 10).Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation, 2006; 114: 63-75 [DOI] [PubMed] [Google Scholar]
- 11).Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 1993; 24: 35-41 [DOI] [PubMed] [Google Scholar]
- 12).Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, Sacco RL, Connolly SJ, Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol, 2014; 13: 429-438 [DOI] [PubMed] [Google Scholar]
- 13).Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet, 2013; 382: 339-352 [DOI] [PubMed] [Google Scholar]
- 14).Adams HP, Dawson G, Coffman TJ, Corry RJ. Stroke in renal transplant recipients. Arch Neurol, 1986; 43: 113-115 [DOI] [PubMed] [Google Scholar]
- 15).Kamel H, Merkler AE, Iadecola C, Gupta A, Navi BB. Tailoring the Approach to Embolic Stroke of Undetermined Source: A Review. JAMA Neurol, 2019; 76: 855-861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Bruno A, Adams HP. Neurologic problems in renal transplant recipients. Neurol Clin, 1988; 6: 305-325 [PubMed] [Google Scholar]
- 17).Jogestrand T, Fehrman-Ekholm I, Angelin B, Berglund L, Gäbel H. Increased prevalence of atherosclerotic wall changes in patients with hyperlipidaemia after renal transplantation. J Intern Med, 1996; 239: 177-180 [PubMed] [Google Scholar]
- 18).Barbagallo CM, Pinto A, Gallo S, Parrinello G, Caputo F, Sparacino V, Cefalù AB, Novo S, Licata G, Notarbartolo A, Averna MR. Carotid atherosclerosis in renal transplant recipients: relationships with cardiovascular risk factors and plasma lipoproteins. Transplantation, 1999; 67: 366-371 [DOI] [PubMed] [Google Scholar]
- 19).Moustapha A, Naso A, Nahlawi M, Gupta A, Arheart KL, Jacobsen DW, Robinson K, Dennis VW. Prospective study of hyperhomocysteinemia as an adverse cardiovascular risk factor in end-stage renal disease. Circulation, 1998; 97: 138-141 [DOI] [PubMed] [Google Scholar]
- 20).Mallamaci F, Zoccali C, Tripepi G, Fermo I, Benedetto FA, Cataliotti A, Bellanuova I, Malatino LS, Soldarini A, CREED Investigators. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int, 2002; 61: 609-614 [DOI] [PubMed] [Google Scholar]
- 21).Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation, 1999; 100: 1161-1168 [DOI] [PubMed] [Google Scholar]
- 22).Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest, 1996; 98: 5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Zhang S, Bai YY, Luo LM, Xiao WK, Wu HM, Ye P. Association between serum homocysteine and arterial stiffness in elderly: a community-based study. J Geriatr Cardiol, 2014; 11: 32-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Yeh SJ, Tang SC, Tsai LK, Chen CH, Hsu SP, Sun Y, Lien LM, Wei CY, Lai TC, Chen PL, Chen CC, Huang PH, Lin CH, Liu CH, Lin HJ, Hu CJ, Lin CL, Jeng JS, Hsu CY, and Taiwan Stroke Registry Investigators. Renal Function-Dependent Associations of Statins with Outcomes of Ischemic Stroke. J Atheroscler Thromb, 2021; 28: 146-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Liao JN, Chao TF, Liu CJ, Wang KL, Chen SJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chung FP, Chen TJ, Chen SA. Incidence and risk factors for new-onset atrial fibrillation among patients with end-stage renal disease undergoing renal replacement therapy. Kidney Int, 2015; 87: 1209-1215 [DOI] [PubMed] [Google Scholar]
- 26).Wetmore JB, Mahnken JD, Rigler SK, Ellerbeck EF, Mukhopadhyay P, Spertus JA, Hou Q, Shireman TI. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int, 2012; 81: 469-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, Acquistapace I, Stella A, Bonforte G, DeVecchi A, DeCristofaro V, Buccianti G, Vincenti A. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis, 2005; 46: 897-902 [DOI] [PubMed] [Google Scholar]
- 28).Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis M, Rigler SK, Mukhopadhyay P, Spertus JA, Zhou X, Hou Q, Shireman TI. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol, 2013; 23: 112-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Shen CH, Zheng CM, Kiu KT, Chen HA, Wu CC, Lu KC, Hsu YH, Lin YF, Wang YH. Increased risk of atrial fibrillation in end-stage renal disease patients on dialysis: A nationwide, population-based study in Taiwan. Medicine (Baltimore), 2016; 95: e3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Rangaswami J, Mathew RO, Parasuraman R, Tantisattamo E, Lubetzky M, Rao S, Yaqub MS, Birdwell KA, Bennett W, Dalal P, Kapoor R, Lerma EV, Lerman M, McCormick N, Bangalore S, McCullough PA, Dadhania DM. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant, 2019; 34: 760-773 [DOI] [PubMed] [Google Scholar]
- 31).Lentine KL, Rocca Rey LA, Kolli S, Bacchi G, Schnitzler MA, Abbott KC, Xiao H, Brennan DC. Variations in the risk for cerebrovascular events after kidney transplant compared with experience on the waiting list and after graft failure. Clin J Am Soc Nephrol, 2008; 3: 1090-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Oliveras A, Roquer J, Puig JM, Rodríguez A, Mir M, Orfila MA, Masramon J, Lloveras J. Stroke in renal transplant recipients: epidemiology, predictive risk factors and outcome. Clin Transplant, 2003; 17: 1-8 [DOI] [PubMed] [Google Scholar]
- 33).Findlay MD, Thomson PC, MacIsaac R, Jardine AG, Patel RK, Stevens KK, Rutherford E, Clancy M, Geddes CC, Dawson J, Mark PB. Risk factors and outcome of stroke in renal transplant recipients. Clin Transplant, 2016; 30: 918-924 [DOI] [PubMed] [Google Scholar]
- 34).Takahashi K, Saito K, Takahara S, Okuyama A, Tanabe K, Toma H, Uchida K, Hasegawa A, Yoshimura N, Kamiryo Y, Japanese ABO-Incompatible Kidney Transplantation Committee. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant, 2004; 4: 1089-1096 [DOI] [PubMed] [Google Scholar]
- 35).Hoshino T, Sissani L, Labreuche J, Bousser MG, Chamorro A, Fisher M, Ford I, Fox KM, Hennerici MG, Mattle HP, Rothwell PM, Gabriel Steg P, Vicaut E, Amarenco P. Non-cardioembolic stroke/transient ischaemic attack in Asians and non-Asians: A post-hoc analysis of the PERFORM study. Eur Stroke J, 2019; 4: 65-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Hoshino T, Uchiyama S, Wong LKS, Kitagawa K, Charles H, Labreuche J, Lavallée PC, Albers GW, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, Molina C, Rothwell P M, Steg PG, Touboul PJ, Vicaut É, Amarenco P, TIA registry.org Investigators. Five-Year Prognosis After TIA or Minor Ischemic Stroke in Asian and Non-Asian Populations. Neurology, 2021; 96: e54-e66 [DOI] [PubMed] [Google Scholar]