Abstract
Introduction
COVID-19 has disrupted how ophthalmic practice is conducted worldwide. One patient population that may suffer from poor outcomes during the pandemic are those with age-related macular degeneration (AMD). Many practices are performing some form of tele-ophthalmology services for their patients, and guidance is needed on how to maintain continuity of care amongst patients with AMD using tele-ophthalmology.
Methods
A literature search was conducted, ending 1 August 2020, to identify AMD outcomes and telecare management strategies that could be used during the COVID-19 pandemic
Results
237 total articles were retrieved, 56 of which were included for analysis. Four American Academy of Ophthalmology and Center for Disease Control web resources were also included.
Discussion
Risk-stratification models have been developed that let providers readily screen existing patients for their future risk of neovascular AMD (nAMD). When used with at-home monitoring devices to detect nAMD, providers may be able to determine who should be contacted via tele-ophthalmology for screening. Telemedicine triage can be used for new complaints of vision loss to determine who should be referred to a retinal specialist for management of suspected nAMD. To increase access and provider flexibility, smartphone fundus photography images sent to a centralized tele-ophthalmology service can aid in the detection of nAMD. Considerations should also be made for COVID-19 transmission, and tele-ophthalmology can be used to screen patients for the presence of COVID-19 prior to in-person office visits. Tele-ophthalmology has additional utility in connecting with nursing home, rural, and socioeconomically disadvantaged patients in the post-pandemic period.
Keywords: COVID-19, age-related macular degeneration, retinal disease, telemedicine, tele-ophthalmology, retinal specialist, coronavirus, pandemic, telehealth
Introduction
Since its discovery in late 2019, coronavirus disease (COVID-19) has become a global pandemic.1 COVID-19 is highly transmissible, and infection can result in hospitalization or death, especially amongst the elderly or those with pre-existing health conditions.2,3 To help combat the spread of COVID-19, governments across the world have implemented policies which require social distancing. Such policies are aimed at reducing person–person contact, minimizing the transmission of COVID-19.4
To aid in the public health response, local governments and the Centers for Disease Control and Prevention (CDC) mandated that ambulatory medical practices delay non-urgent in-person visits and postpone elective procedures.4 Providers are encouraged to see patients via telemedicine and find alternative means to see and treat patients.4 Many ophthalmic practices have also had to delay routine appointments and are now conducting their practices via tele-ophthalmology.5 The reduction in face-to-face appointments prevents ophthalmic providers from performing tests which can detect eye pathologies. One such disease is age-related macular degeneration (AMD).
AMD is generally regarded as a disease of ageing, as 25% of all individuals older than 75 have some form of the disease.6 Neovascular AMD (nAMD) has a 10–15% prevalence amongst all subtypes of AMD and is characterized by the presence of choroidal neovascularization (CNV).6,7 Older adults are most likely to be affected by AMD, however they are also the most likely to experience death or disability as a result of COVID-19 infection.4,6,8 Prior to the COVID-19 pandemic, patients with AMD faced barriers to receiving timely care, such as delays in treatment initiation, difficulty getting specialists referrals, transportation issues or poor general health.9–13 For many patients, these barriers are at risk of being exacerbated by the current pandemic as providers and patients adapt to new means of conducting practice via tele-ophthalmology.
Although many governments are considering reducing practice restrictions, COVID-19 continues to be highly prevalent across the world. Furthermore, some COVID-19 models predict that infections will wax and wane throughout the fall, suggesting that COVID-19 risk will be heightened for the foreseeable future and ophthalmic practices may continue to use tele-ophthalmology in the months to come.14,15 Therefore, a discussion about the ways ophthalmic providers can use tele-ophthalmology to safely maintain continuity of care amongst AMD patients is urgently needed.
Methods
A literature review was performed by JM, CL, MD, and EM of Google Scholar, Medline, Web of Science and the NCBI affiliated Pubmed and LitCovid ending 1 August 2020 with the keywords: age related macular degeneration, telemedicine, tele-ophthalmology, COVID-19, SARS-COV-2, retina, anti-VEGF injections, home monitoring, neovascular, artificial intelligence (AI), fundus photography, fundus angiography, optical coherence tomography, Age Related Eye Disease Study, home monitoring, primary care eye imaging, Topcon Maestro2, and smartphone. AND was used as a Boolean operator to focus search results. The literature search’s goal was to identify the current clinical course and management of patients with AMD, and causes and outcomes related to diagnostic or treatment delay, in addition to potential diagnostic or therapeutic advancements that could be deployed during the COVID-19 pandemic. No limits were applied on the publication date. In total, 226 articles were found using these criteria, 14 of which were duplicates. For each retrieved article, JM and CL screened the title and abstract. Articles that did not match the study goals were excluded (n=135). The full text of (n=77) eligible articles was obtained for assessment by JM. Non-COVID-19 editorials, studies missing patient outcomes, conference abstracts, retracted studies, and case reports were not selected (n=21). Eleven articles were retrieved from direct query: two articles regarding COVID-19 provider safety and testing and nine articles regarding COVID-19 transmission dynamics and clinical features. Finally, 56 studies were included in the analysis, and data were supplemented with information from American Academy of Ophthalmology (AAO) and CDC web resources (n=4). A literature review flowchart is shown in Supplemental Figure 1.
Literature results were categorized into four clinical domains: screening, prevention, triage, and diagnosis. These clinical domains represent the key modalities of non-therapeutic management of patients with any underlying medical complaint, including AMD. Furthermore, articles that discuss expansion of patient access to ophthalmic imaging, a critical component of AMD diagnosis, using innovative methods were grouped separately. A summary of our findings is shown in Table 1.
Table 1.
Summary of evidence-based recommendations.
| Finding | Rationale | |
|---|---|---|
| Screening | Delaying in-person AMD screening in the general public is safe for duration of COVID-19 pandemic | US Preventative Task Force suggests that annual eye exams are of unclear benefit17 |
| Employ validated risk-stratification models amongst existing patients with AMD and use tele-ophthalmology to maintain appointments for high-risk patients, including those with previous history of nAMD | Risk of nAMD is high amongst certain subgroups with AMD19,22 Appointments may be backlogged post-pandemic which can further exacerbate delays in screening of high-risk patients |
|
| Patients with subjective complaints of metamorphopsia, dyschromatopsia without micropsia elicited via telemedicine should be referred promptly to a retinal specialist. | Clinical symptoms of metamorphopsia and dyschromatopsia without micropsia is strongly correlated to nAMD, even in the presence of other subjective symptoms36 |
|
| Prevention | Use FDA-approved in-home monitoring devices amongst existing patients with AMD to detect emergence of CNV | In-home monitoring devices are more likely to detect CNV with less visual impairment than regular in-person clinical visits29–31 In-home monitoring had higher rates of patient compliance29–31 |
| Diagnosis | Conduct initial patient visits via telemedicine or tele-ophthalmology | Tele-visits limit the number of face-to-face interactions with patients and limit the risk of COVID-19 transmission |
| Utilize smartphone FP and/or primary care imaging to facilitate the initial detection of nAMD | Smartphone FP and/or primary care retinal imaging provides a means of determining who should be seen in-office by the retinal specialist46,48–50 AAO endorsed instructions on how to perform smartphone FP are readily available 47 |
|
| Limit patients in-office visits unless clinical suspicion for nAMD is high | Minimizing the number of face-to-face visits reduces the risk of COVID-19 transmission Maximize utility of scarce testing resources amongst patients with highest need |
|
| Safety | Promote RT-PCR testing for COVID-19 for patients and providers before providing care | Asymptomatic carriers may drive COVID-19 spread52 Expanded COVID-19 screening may reduce the risk of transmission during interactions Ophthalmologists are at an increased risk of COVID-19 transmission54,55 |
AMD: Age-related macular degeneration, nAMD: neovascular age-related macular degeneration, COVID 19: Corona virus novel disease 2019, CNV: Choroidal neovascularization, FDA: food and drug administration, FP: Fundus photo, AAO: American Academy of Ophthalmology, RT-PCR: reverse transcriptase polymerase chain reaction.
Screening for nAMD
AMD is traditionally screened for during regular optometrist or primary care appointments. The AAO recommends screening for adults older than 65 every 1–2 years. Screening is designed to identify AMD before the development of clinical symptoms and CNV.16 Current evidence suggests that screening in the general population for AMD and other causes of vision loss is of unclear benefit in the United States.17 Therefore, annual in-person eye exams can be safely delayed for patients that have not been previously diagnosed with AMD until an effective therapy or vaccine is introduced.
For those previously diagnosed with early AMD, there is a 1–18% progression to late stage AMD over 5 years.18 Providers can benefit from applying a simple risk-stratification model in determining who to screen for nAMD. One study proposed a risk-stratification model on the presence of large drusen (≥125 μm) and retinal pigment epithelial changes.19 Each patient was given a score between 0 and 4 based on the presence of either feature in each eye. Patients with scores of 1–2 had a 3–9% of progression to nAMD at 5 years.19 However, patients with higher scores of 3–4 had a 5–10% risk to progress to nAMD at 1 year.19 In addition, previous diagnosis of nAMD is a strong independent risk factor for the development of contralateral nAMD, especially with predisposing risk factors.20,21 Risk for a second diagnosis of nAMD increases approximately 10%, 28%, and 42% at 1, 3, and 5 years, respectively. The risk of nAMD amongst patients with high-risk clinical features and previous diagnosis of nAMD suggests that they may strongly benefit from continued screening through tele-ophthalmology during the pandemic, although their model did not consider specific risk factors, such as smoking status.22 Those at intermediate risk of progression and other risk factors can likely be screened after local provider restrictions are lifted, although tele-ophthalmology may still be beneficial as a first-line contact with these patients as COVID-19 remains prevalent. Importantly, patients can be stratified quickly using chart review, which eases provider burden when determining which patients to contact via tele-ophthalmology.
Prevention of nAMD
Patients with non-nAMD should be maintained on existing treatment plans.16,23 Existing patients should be counselled on smoking cessation, weight loss, and antioxidant supplementation in concordance with the Age-Related Eye Disease Study 2 (AREDS2), all of which can help prevent progression to nAMD.24 Consultations with existing patients can be provided via a tele-ophthalmology discussion, eliminating the need for physical face-to-face interactions.
Early detection of CNV is the greatest determinant of future visual acuity in the years following diagnosis of nAMD.25–27 Physicians can suggest one of the two FDA-approved in-home nAMD monitoring devices for patients with existing AMD such as the Forseehome home device (Notal Vision, Tel Aviv, Israel) and MyVisionTrack (Genentech, San Francisco USA) smartphone application, which both use hyperacuity tests to monitor for nAMD.28,29 The Forseehome device requires patients detect linear distortions delivered through an industry-sponsored platform, whereas the MyVisionTrack application utilizes radial frequency patterns to have patients perform shape discrimination tasks.28,29 One study of the Forseehome device demonstrated that patients who used the home device were more likely to have higher visual acuity at time of CNV recurrence, although the MyVisionTrack application and other methods have also shown efficacy.26,28–31 However, their effectiveness outside of monitoring at-risk patients for nAMD is unknown, as patients enrolled in one study all had existing AMD and those with existing visual defects had a 20% screen failure rate.29 To aid in tele-ophthalmology visits with at-risk patients, we suggest that providers discuss in-home monitoring for all patients diagnosed with AMD and facilitate their use in all at-risk patients.
Triage for nAMD
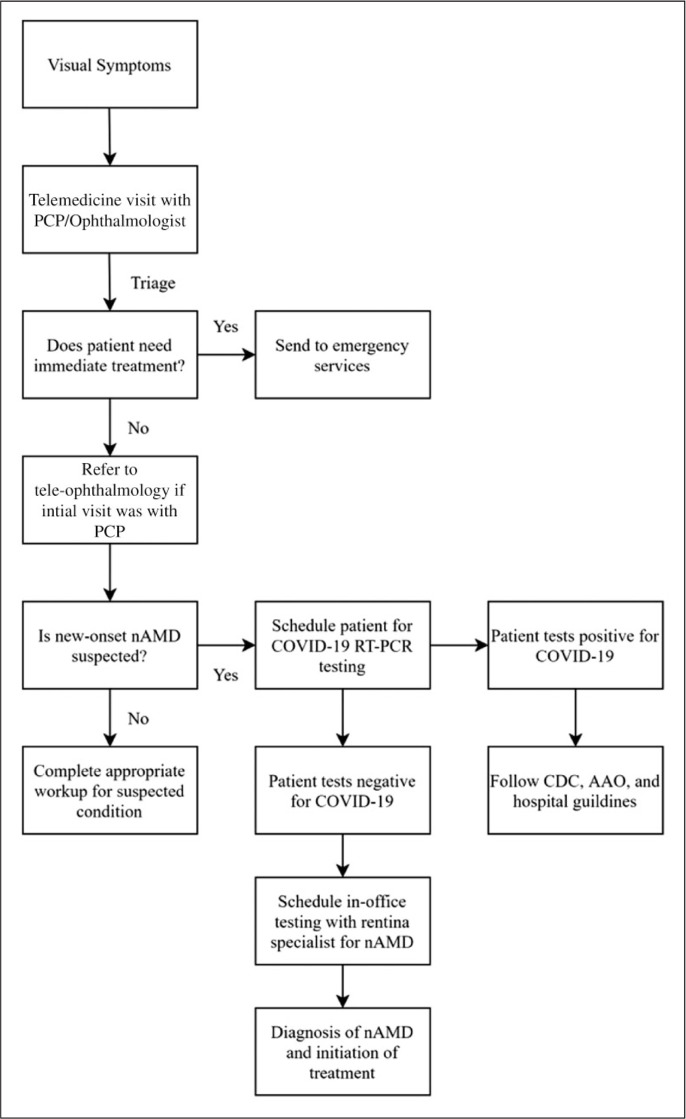
The AAO and CDC both recommend that all non-urgent services be provided via telemedicine or tele-ophthalmology.4,5 Tele-ophthalmology is an evolving means of expanding access to ophthalmic care, especially for those who may not have access to regular care, such as rural and underserved patients.32 In addition, the AAO developed well-defined guidelines for provider reimbursement and billing using tele-ophthalmology, although they are less clear on how to proceed about conducting teleophthalmic practice for providers who are unfamiliar with it.33 For those with new complaints of vision loss and no previous ophthalmic care, initial telemedicine encounters may be performed with a primary care provider (Figure 1). If the complaint is not deemed an emergent, sight-threatening condition, then a referral can be generated to tele-ophthalmology for diagnostic evaluation and confirmation of AMD.
Figure 1.
Flow algorithm to evaluate the complaint of vision loss due to nAMD during the COVID-19 pandemic. Initial telemedicine encounters with a primary care provider can determine the need for immediate treatment and generate referrals to tele-ophthalmology if no immediate threat to vision is present. For patients who have existing ophthalmic care, the initial tele-visit can take place via tele-ophthalmology directly. Tele-ophthalmology can determine the need for in-person testing and schedule COVID-19 RT-PCR testing for those in need of in-person testing. Positive titers should follow local protocols about how to proceed, before initiating in-office testing.
PCP: Primary care provider, nAMD: neovascular age-related macular degeneration, COVID 19: Corona virus novel disease 2019, CDC: Centers for Disease Control and Prevention, AAO: American Academy of Ophthalmology, RT-PCR: reverse transcriptase polymerase chain reaction
Triage of patients who call with complaints of vision loss should be a priority. Multiple studies have found that increasing the time interval between diagnosis and treatment of nAMD leads to a higher degree of visual acuity decline.9,34,35 Extrapolating this information, it is prudent to assume that vision loss is also occurring during the interval between onset of symptoms and time of patient presentation. Therefore, distinguishing nAMD and other forms of acute vision loss from earlier stages of AMD via telemedicine is imperative.
Worsening vision loss is a prominent feature of nAMD but is not a distinctive feature. One study of subjective symptoms in nAMD found that the presence of metamorphopsia, dyschromatopsia in absence of micropsia were strongly correlated with nAMD.36 However, the presence or absence of other features, such as a central dark spot, should not exclude the diagnosis of nAMD, but they are less specific.36 Any provider who discovers this combination of symptoms via telemedicine may benefit from generating a direct tele-ophthalmology referral to a retinal specialist for further evaluation. Prompt referral to a retinal specialist can minimize diagnostic and treatment delays, while maximizing the patient’s current visual acuity.
All patients should receive a health telemedicine consultation and if nAMD is suspected, providers should initiate an ophthalmology referral for diagnostic confirmation and treatment. Traditional tele-ophthalmology programs have focused on using telehealth to arrange and read optical coherence tomography (OCT) images to confirm the diagnosis of nAMD; however, other models that utilize centralized ophthalmologists to evaluate complaints have also been proposed.32,37 Clinical trials of tele-ophthalmology for detection of AMD and diabetic retinopathy have been shown to be equal to visiting an in-person retinal specialist, in terms of diagnostic accuracy, time to referral, and visual outcomes, while also increasing patient participation in follow-up care.37 Although providers should utilize tele-ophthalmology services whenever possible, many proposed programmes rely on intermediates, such as imaging facilities, whose availability may vary, and patients may eventually have to come into a physical ophthalmic practice to receive diagnostic confirmation or treatment of nAMD.
Diagnosis of nAMD
The diagnosis of AMD is made through a combination of clinical symptoms, visual acuity testing, and fundus imaging.7 AMD is diagnosed via colour fundus photography (FP) which shows the presence of drusen.38 While signs of CNV may be detected via FP, fluorescein angiography (FA) can confirm the presence of CNV. OCT is commonly used in conjunction with FA, which provides a means of evaluating retinal thickness and sub-occult CNV.38 OCT is often used to non-invasively measure disease progression and monitor response to treatment.38
OCT is indispensable in the current management of AMD, as it can readily recognize CNV before the presence of symptoms.23 OCT provides a similar sensitivity and specificity in the initial detection of nAMD as compared with FA at 94–100% and 80.8–98%, respectively.39,40 However, FP also provides a means to diagnose nAMD, and one study reported 100% specificity and 78% sensitivity as compared with FA.39 The high specificity suggests that FP can be used to quickly rule in nAMD, especially in patient populations with a high pretest probability.
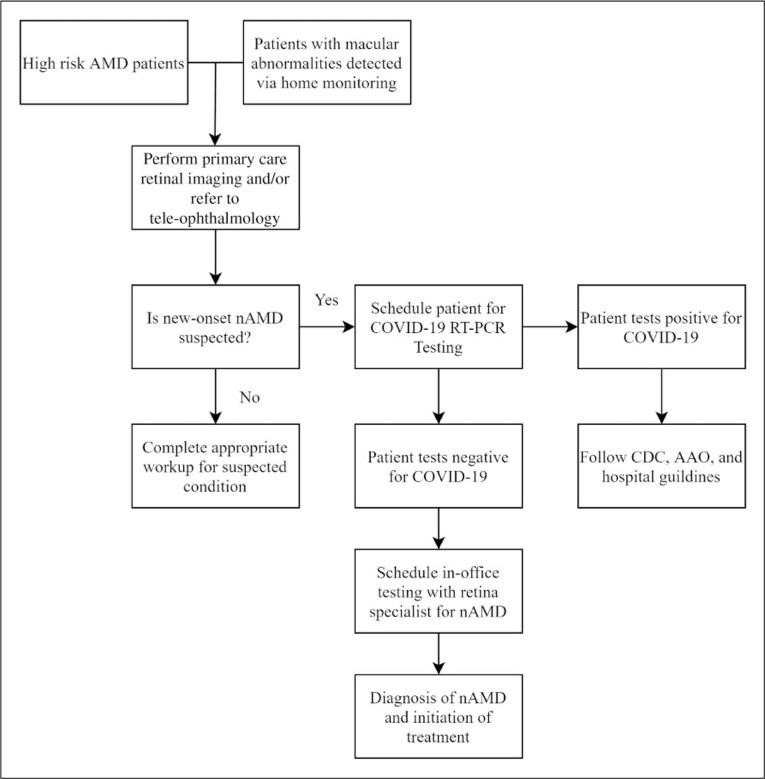
Two groups that have an increased probability for nAMD are patients who had an elevated risk via the presence of clinical features who have upcoming scheduled appointments and patients with home monitoring devices that detect nAMD. Providers can perform a tele-ophthalmology consultation with these patients to determine if they are able to come in directly for imaging (Figure 2). Some primary care providers can take retinal images directly in the office; however, results should be sent to a centralized ophthalmologist who can help interpret imaging results and guide next steps in care. If clinical suspicion is high, the primary care team or ophthalmologist can discuss the benefits and risks of an in-person visit with a retina specialist for further testing or treatment. Risk stratification has been discussed previously, and those who are at the highest risk may benefit from bypassing tele-ophthalmology or smartphone FP and proceeding directly to COVID-19 RT-PCR testing in preparation for in-office testing.
Figure 2.
Flow algorithm of nAMD workup to maintain continuity of case for AMD patients during the COVID-19 pandemic. High-risk AMD patients and those with macular abnormalities detected via home monitoring should have initial encounters performed via tele-ophthalmology. Smartphone FP can also be scheduled to rule in the potential diagnosis of new onset nAMD. If nAMD is suspected, patients can be referred for COVID-19 RT-PCR testing in preparation for in-office testing and treatment.
AMD: age-related macular degeneration, nAMD: neovascular age-related macular degeneration, COVID 19: Corona virus novel disease 2019, CDC: Centers for Disease Control and Prevention, AAO: American Academy of Ophthalmology, RT-PCR: reverse transcriptase polymerase chain reaction
Diagnosis of nAMD may be complicated by delays in patient presentation. Often, patients with nAMD are asymptomatic or do not recognize the onset of vision loss. Therefore, many patients present acutely with nAMD.41 One study found that amongst patients diagnosed with nAMD, approximately half waited 1–4 weeks from the onset of symptoms to visit a provider and over 25% waited longer than 1 month.13 These delays may be further complicated by COVID-19. Evidence from the United Kingdom suggests that emergency department visits for non-COVID-19 issues have decreased.42 Although the relationship between COVID-19 prevalence and patient presentation for complaints of acute vision loss is unknown, it may compound on other factors that cause patients to delay seeking medical care, even when care is performed via telemedicine.
Increasing access to ophthalmic imaging during the COVID-19 pandemic
FP, FA, and OCT are traditionally performed in an in-office setting. However, recent developments suggest all three techniques can be performed remotely, using handheld devices or smartphones.43–46 Smartphone-based FP has been proposed as a mobile alternative to traditional in-office FP. The AAO endorses an online wiki-how for providers to conduct smartphone FP, using free software and a 20-diopter lens.47 Smartphone FP showed similar rates of detection of diabetic retinopathy when compared with traditional FP and represents a promising potential option for diagnosing nAMD.48 However, smartphone FP has not been validated in the diagnosis of AMD. Smartphone FA has been developed, although mainly as a proof of concept.44 Smartphone camera quality has improved since the development of smartphone-based retinal imaging; however, advanced training may be necessary to take high-quality macular images, making smartphone FP potentially unsuitable for community-dwelling patients.49 OCT can also be performed remotely, using a handheld device.43 However, unlike smartphones, OCT scanners may not be cost-effective or readily accessible for all providers. The major upside of these innovations is their portability and ability to easily integrate with tele-ophthalmology, which may expand access to care for underserved patients or those in regions particularly afflicted by COVID-19.43 Nevertheless, at-home retinal imaging largely remains an unmet clinical need.
Primary care OCT imaging can limit the number of provider visits and reduce diagnostic delay, but still requires patients to come in for a face-to-face visit, which may be unpalatable during the COVID-19 pandemic, especially for patients with underlying comorbidities associated with poor COVID-19 outcome, such as cardiovascular disease or diabetes mellitus.3,50 Therefore, it may be useful to employ alternative models where ophthalmic imaging can be delivered directly to the patient. Handheld devices or smartphones can be employed for this purpose; however, image quality remains a limiting factor. Like a mobile health clinic, OCT can also be retrofitted for mobile use in a vehicle or other public place. During the COVID-19 pandemic, mobile retinal imaging may be particularly useful for nursing home patients, where many at-risk patients can be screened in series, increasing patient throughput while minimizing the total number of potential COVID-19 exposures. Importantly, differences in clinical outcomes between currently commercially available OCTs are unclear, and technological adoption of primary care or mobile OCTs is not free.51 Furthermore, a current limitation of telecare platforms is their inability to delivery sight-saving procedural treatments alongside imaging in the same visit, which may also shift costs of care. Before widespread implementation of alternative imaging modalities, cost-benefit analyses should be conducted to estimate true cost savings and determine economic feasibility. Such studies are further complicated by technological change within ophthalmology, which can simultaneously impact patient outcomes, care delivery and healthcare costs.
Provider and patient safety
Patient and provider safety should be made the highest priority. Patients should be questioned for symptoms of COVID-19 via tele-ophthalmology prior to any in-person visit. For those with symptoms of COVID-19, testing or treatment should only be initiated if an immediate threat to vision is present. Asymptomatic carriers are hypothesized to be drivers of COVID-19 spread and RT-PCR testing of patients prior to all in-person patient visits is recommended, which can be arranged via tele-ophthalmology.52 Providers may also benefit from receiving COVID-19 RT-PCR and antibody testing before returning to see patients. Although local protocols may vary for how to proceed after a positive test, it may be beneficial to have at least one negative test 48 hours prior to visits to confirm infection eradication before initiating in-person testing or therapy, consistent with the Stanford University Protocol.53,54 Ophthalmologists spend a considerable time performing procedures around the nares, placing them at high risk of COVID-19 infection.54 Furthermore, COVID-19 can cause ocular symptoms and may be transmissible through the mucus membranes of the eye, so extreme caution is warranted if a patient is determined to need in-person testing through telemedicine or tele-ophthalmology.55 All providers should wear complete protective gear during patient visits, including a gown, gloves, eye protection and a N95 mask whenever possible. Given current supply shortages, providers should adhere to previous AAO and CDC guidance concerning use of PPE.4,5
Future directions
Innovation in ophthalmology is driven by financial and technological investment from industry. Technologies integrating artificial intelligence (AI) with ophthalmic imaging may increase patient throughput and cost efficiency of retinal imaging. For example, one study of an industry-created deep learning algorithm of retinal imaging databases revealed a 93.2% sensitivity and 88.7% specificity, respectively, for detecting nAMD.56 In fact, a machine-learning algorithm received FDA clearance for detection of diabetic oedema, the first machine-learning algorithm approved for any diagnostic use.57 Machine learning increases clinician autonomy and provides instantaneous image interpretation, arguably expanding retinal imaging for use in primary care and resource-poor settings.58 Importantly, AI has yet to be externally validated for diagnosing AMD.58 Algorithms often reject low-quality images and struggle to identify rare conditions, such as ocular tumours, so AI may not always be superior to visiting a trained retinal specialist.58 Other potential applications of AI include risk stratification, predicting who is at high risk of progression to nAMD.59,60 AI can likely modify costs of care delivery, although more studies are needed to examine how the costs of AI systems gets passed on to the patient and their effect on provider reimbursement.
During the COVID-19 pandemic, strong partnerships between industry and clinicians may benefit patients by speeding up the pace and implementation of new innovations. Although business partnerships can shift how ophthalmic care is delivered during the pandemic and beyond, ethical, legal, and socioeconomic concerns should be addressed prior to engaging with industry partners. The exact modality of how to do this may be subject to legal or regional variation, and further research is needed to examine the most efficacious means by which to improve future delivery of quality eye care beyond the COVID-19 pandemic.
Conclusion
Here, we have reviewed existing evidence and made practical suggestions to help facilitate continuity of care of patients with AMD during the COVID-19 pandemic using telemedicine and tele-ophthalmology. Ophthalmic providers can benefit from using existing technology and telehealth models to expedite diagnosis and treatment of nAMD. Ophthalmological practices can refer to AAO or local guidelines for provider reimbursement and billing using tele-ophthalmology.
Tele-ophthalmology may be implemented post-COVID-19 pandemic for patients who are traditionally underserved, such as patients in nursing homes or rural and low socioeconomic status patients who lack access to ophthalmic care. Ophthalmology practices should view the COVID-19 pandemic as an opportunity to employ tele-ophthalmology strategies that may broaden access to ophthalmic care for future patients, while reducing adverse visual outcomes.
Supplemental Material
Supplemental material, sj-pdf-1-jtt-10.1177_1357633X20960636 for Teleophthalmology for age-related macular degeneration during the COVID-19 pandemic and beyond by Joel Mintz, Chase Labiste, Michael V DiCaro, Evan McElroy, Reza Alizadeh and Kunyong Xu in Journal of Telemedicine and Telecare
Authors' contributions
All authors contributed to the study conception and design. Literature searches were performed by Joel Mintz, Chase Labiste, Michael V Dicaro, and Evan McElroy. Figure creation was done by Evan McElroy. The manuscript first draft was created by Joel Mintz and all authors contributed towards previous versions of the manuscript. Revisions were done with consultation from all authors. The final manuscript was read and approved by all authors.
Availability of data and material
All referenced material is published literature or organizational information.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs
Joel Mintz https://orcid.org/0000-0002-8121-1736
Chase Labiste https://orcid.org/0000-0002-4117-6286
Evan McElroy https://orcid.org/0000-0001-8899-8685
Supplemental material
Supplemental material for this article is available online.
References
- 1.Khachfe HH, Chahrour M, Sammouri J, et al. An epidemiological study on COVID-19: A rapidly spreading disease. Cureus 2020; 12: e7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med Epub ahead of print 2020. DOI: 10.1056/nejmoa2005412. [DOI] [PMC free article] [PubMed]
- 3.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. Coronavirus Disease 2019 (COVID-19) in the U.S. Centers for Disease Control and Prevention, https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html (2020, accessed 23 April 2020).
- 5.AAO. Important coronavirus updates for ophthalmologists. American Academy of Ophthalmology, https://www.aao.org/headline/alert-important-coronavirus-context (2020, accessed 24 April 2020).
- 6.Al-Zamil W, Yassin S.Recent developments in age-related macular degeneration: A review. Clin Intervent Aging 2017; 12: 1313–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jager RD, Mieler WF, Miller JW.Age-related macular degeneration. N Engl J Med 2008; 358: 2606–2617. [DOI] [PubMed] [Google Scholar]
- 8.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. Epub ahead of print 3 March 2020. DOI: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed]
- 9.Muether PS, Hoerster R, Hermann MM, et al. Long-term effects of ranibizumab treatment delay in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2013; 251: 453–458. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Ohkubo Y, Sato A, et al. Relationship between visual prognosis and delay of intravitreal injection of ranibizumab when treating age-related macular degeneration. Retina 2015; 35: 1331–1338. [DOI] [PubMed] [Google Scholar]
- 11.Wintergerst MWM, Bouws J, Loss J, et al. [ Reasons for delayed and discontinued therapy in age-related macular degeneration]. Ophthalmologe 2018; 115: 1035–1041. [DOI] [PubMed] [Google Scholar]
- 12.Sim PY, Gajree S, Dhillon B, et al. Investigation of time to first presentation and extrahospital factors in the treatment of neovascular age-related macular degeneration: A retrospective cross-sectional study. BMJ Open 2017; 7: e017771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varano M, Eter N, Winyard S, et al. Current barriers to treatment for wet age-related macular degeneration (wAMD): Findings from the wAMD patient and caregiver survey. Clin Ophthalmol 2015; 9: 2243–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. Epub ahead of print 14 April 2020. DOI: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed]
- 15.Parke DW., II. Ophthalmology after Coronavirus Disease 2019 (COVID-19): Transition back to patient care. JAMA Ophthalmol. Epub ahead of print 4 May 2020. DOI: 10.1001/jamaophthalmol.2020.2004. [DOI] [PubMed]
- 16.Flaxel CJ, Adelman RA, Bailey ST, et al. Age-related macular degeneration preferred practice pattern®. Ophthalmology 2020; 127: P1–P65. [DOI] [PubMed] [Google Scholar]
- 17.US Preventive Services Task Force (USPSTF), Siu AL, Bibbins-Domingo K, et al. Screening for impaired visual acuity in older adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315: 908–914. [DOI] [PubMed] [Google Scholar]
- 18.Cook HL, Patel PJ, Tufail A.Age-related macular degeneration: Diagnosis and management. Br Med Bull 2008; 85: 127–149. [DOI] [PubMed] [Google Scholar]
- 19.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol 2005; 123: 1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Macular Photocoagulation Study Group. Arch Ophthalmol 1997; 115: 741–747. [DOI] [PubMed] [Google Scholar]
- 21.Yanagi Y, Mohla A, Lee SY, et al. Incidence of fellow eye involvement in patients with unilateral exudative age-related macular degeneration. JAMA Ophthalmol 2018; 136: 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Risk factors associated with age-related macular degeneration. Ophthalmology 2000; 107: 2224–2232. [DOI] [PMC free article] [PubMed]
- 23.Schmidt-Erfurth U, Chong V, Loewenstein A, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol 2014; 98: 1144–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh N, Srinivasan S, Muralidharan V, et al. Prevention of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 2017; 6: 520–526. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen A, Brandi S, Fuchs J, et al. Visual outcomes in relation to time to treatment in neovascular age-related macular degeneration. Acta Ophthalmol 2015; 93: 616–620. [DOI] [PubMed] [Google Scholar]
- 26.Keane PA, de Salvo G, Sim DA, et al. Strategies for improving early detection and diagnosis of neovascular age-related macular degeneration. Clin Ophthalmol 2015; 9: 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta H, Tufail A, Daien V, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res 2018; 65: 127–146. [DOI] [PubMed] [Google Scholar]
- 28.Mathew R, Sivaprasad S.Environmental Amsler test as a monitoring tool for retreatment with ranibizumab for neovascular age-related macular degeneration. Eye 2012; 26: 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AREDS2-HOME Study Research Group, Chew EY, Clemons TE, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology 2014; 121: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y-Z, He Y-G, Mitzel G, et al. Handheld shape discrimination hyperacuity test on a mobile device for remote monitoring of visual function in maculopathy. Invest Ophthalmol Vis Sci 2013; 54: 5497–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trevino R.Recent progress in macular function self-assessment. Ophthalmic Physiol Opt 2008; 28: 183–192. [DOI] [PubMed] [Google Scholar]
- 32.Rathi S, Tsui E, Mehta N, et al. The current state of teleophthalmology in the United States. Ophthalmology 2017; 124: 1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coding for Phone Calls, Internet and Telehealth Consultations. American Academy of Ophthalmology, https://www.aao.org/practice-management/news-detail/coding-phone-calls-internet-telehealth-consult (2020, accessed 24 April 2020).
- 34.Oliver-Fernandez A, Bakal J, Segal S, et al. Progression of visual loss and time between initial assessment and treatment of wet age-related macular degeneration. Can J Ophthalmol 2005; 40: 313–319. [DOI] [PubMed] [Google Scholar]
- 35.Lim JH, Wickremasinghe SS, Xie J, et al. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am J Ophthalmol 2012; 153: 678–86, 686.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessellund A, Larsen DA, Bek T.The predictive value of subjective symptoms and clinical signs for the presence of treatment-requiring exudative age-related macular degeneration. Acta Ophthalmol 2012; 90: 471–475. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Powell A-M, Hooper PL, et al. Prospective evaluation of teleophthalmology in screening and recurrence monitoring of neovascular age-related macular degeneration: A randomized clinical trial. JAMA Ophthalmol 2015; 133: 276–282. [DOI] [PubMed] [Google Scholar]
- 38.Gong J, Yu S, Gong Y, et al. The diagnostic accuracy of optical coherence tomography angiography for neovascular age-related macular degeneration: A comparison with fundus fluorescein angiography. J Ophthalmol 2016; 2016: 7521478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mokwa NF, Ristau T, Keane PA, et al. Grading of age-related macular degeneration: Comparison between color fundus photography, fluorescein angiography, and spectral domain optical coherence tomography. J Ophthalmol 2013; 2013: 385915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilde C, Patel M, Lakshmanan A, et al. The diagnostic accuracy of spectral-domain optical coherence tomography for neovascular age-related macular degeneration: A comparison with fundus fluorescein angiography. Eye 2015; 29: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zawinka C, Ergun E, Stur M.Prevalence of patients presenting with neovascular age-related macular degeneration in an urban population. Retina 2005; 25: 324–331. [DOI] [PubMed] [Google Scholar]
- 42.Thornton J.Covid-19: A&E visits in England fall by 25% in week after lockdown. BMJ 2020; m1401. [DOI] [PubMed] [Google Scholar]
- 43.Keane PA, Sadda SR.Retinal imaging in the twenty-first century: State of the art and future directions. Ophthalmology 2014; 121: 2489–2500. [DOI] [PubMed] [Google Scholar]
- 44.Suto S, Hiraoka T, Oshika T.Fluorescein fundus angiography with smartphone. Retina 2014; 34: 203–205. [DOI] [PubMed] [Google Scholar]
- 45.Lord RK, Shah VA, San Filippo AN, et al. Novel uses of smartphones in ophthalmology. Ophthalmology 2010; 117: 1274–1274.e3. [DOI] [PubMed] [Google Scholar]
- 46.Toy BC, Myung DJ, He L, et al. Smartphone-based dilated fundus photography and near visual acuity testing as inexpensive screening tools to detect referral warranted diabetic eye disease. Retina 2016; 36: 1000–1008. [DOI] [PubMed] [Google Scholar]
- 47.Smartphone Funduscopy-How to use smartphone to take fundus photographs - EyeWiki, https://eyewiki.aao.org/Smartphone_Funduscopy-How_to_use_smartphone_to_take_fundus_photographs (2018, accessed 24 April 2020).
- 48.Rajalakshmi R, Arulmalar S, Usha M, et al. Validation of smartphone based retinal photography for diabetic retinopathy screening. PLoS One 2015; 10: e0138285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bifolck E, Fink A, Pedersen D, et al. Smartphone imaging for the ophthalmic examination in primary care. JAAPA 2018; 31: 34–38. [DOI] [PubMed] [Google Scholar]
- 50.Wilson C, Horton M, Cavallerano J, et al. Addition of primary care-based retinal imaging technology to an existing eye care professional referral program increased the rate of surveillance and treatment of diabetic retinopathy. Diabetes Care 2005; 28: 318–322. [DOI] [PubMed] [Google Scholar]
- 51.Huang J, Liu X, Wu Z, et al. Macular and retinal nerve fiber layer thickness measurements in normal eyes with the Stratus OCT, the Cirrus HD-OCT, and the Topcon 3D OCT-1000. J Glaucoma 2011; 20: 118–125. [DOI] [PubMed] [Google Scholar]
- 52.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med. Epub ahead of print 24 April 2020. DOI: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed]
- 53.Forrester JD, Nassar AK, Maggio PM, et al. Precautions for operating room team members during the COVID-19 pandemic. J Am Coll Surg 2020; 230: 1098–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel ZM, Fernandez-Miranda J, Hwang PH, et al. Letter: precautions for endoscopic transnasal skull base surgery during the COVID-19 pandemic. Neurosurgery 2020; 87: E66–E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu K, Patel J, Patel BC. Ophthalmic Manifestations Of Coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2020. [PubMed]
- 56.Abràmoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med 2018; 1: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ting DSW, Cheung CY-L, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017; 318: 2211–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ting DSW, Pasquale LR, Peng L, et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol 2019; 103: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt-Erfurth U, Waldstein SM, Klimscha S, et al. Prediction of individual disease conversion in early AMD using artificial intelligence. Invest Ophthalmol Vis Sci 2018; 59: 3199–3208. [DOI] [PubMed] [Google Scholar]
- 60.Bhuiyan A, Wong TY, Ting DSW, et al. Artificial intelligence to stratify severity of age-related macular degeneration (AMD) and predict risk of progression to late AMD. Transl Vis Sci Technol 2020; 9: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jtt-10.1177_1357633X20960636 for Teleophthalmology for age-related macular degeneration during the COVID-19 pandemic and beyond by Joel Mintz, Chase Labiste, Michael V DiCaro, Evan McElroy, Reza Alizadeh and Kunyong Xu in Journal of Telemedicine and Telecare
Data Availability Statement
All referenced material is published literature or organizational information.