Abstract
Aim
This study aimed to clarify the clinical effects of the indocyanine green (ICG)‐fluorescence imaging (FI) technique for determination of liver transection lines during laparoscopic partial liver resection for liver tumors.
Methods
This was a retrospective study including 112 patients who underwent laparoscopic partial liver resection for liver tumors. These enrolled patients were divided into an ICG‐FI group (n = 55) and a non‐ICG‐FI group (n = 57) according to the availability of the ICG‐FI. The clinicopathological characteristics of patients between two groups were compared before and after propensity score matching.
Results
The ICG‐FI and non‐ICG‐FI groups differed at baseline in terms of ICG retention rate at 15 min. After propensity score matching, two comparable groups of 32 patients each were obtained. The negativity rated of the pathological surgical margins were comparable between the two groups before and after propensity score matching. However, the surgical margins were significantly wider in the ICG‐FI group before and after propensity score matching (P = .039 and P = .047, respectively).
Conclusion
The ICG‐fluorescence imaging technique may offer clinical benefits in terms of a secure surgical margin in laparoscopic partial liver resection.
Keywords: indocyanine green fluorescence imaging, laparoscopic partial liver resection
Propensity score matching analysis between patients who underwent and those who did not undergo indocyanine green fluorescence imaging demonstrated that the indocyanine green fluorescence imaging technique using near‐infrared light facilitated the attainment of a wide surgical margin from liver tumors during laparoscopic partial liver resection. To the best of our knowledge, this is the first study focusing on laparoscopic partial liver resection to use propensity score matching to show the use of the indocyanine green fluorescence imaging technique is associated with a macroscopically wider surgical margin in patients with liver tumor.
1. INTRODUCTION
Laparoscopic liver resection is widely accepted for patients with liver tumors including hepatocellular carcinoma, liver metastasis, and other liver tumors because of technical innovations. 1 Intraoperative ultrasonography has proven to be a useful instrument in the evaluation of liver lesions in open liver resection. 2 However, during laparoscopic liver resection, the information obtained from intraoperative ultrasonography is extremely limited, and may result in positive surgical margins.
The indocyanine green fluorescence imaging (ICG‐FI) technique, employed with near‐infrared light, has been used to visualize the blood supply of the gastrointestinal tract in gastrointestinal surgery 3 and detect sentinel lymph nodes in breast cancer during breast surgery. 4 The ICG‐FI technique has also been applied for the intraoperative identification of liver tumors 5 and visualization of hepatic segmental boundaries, 6 the drainage area of the hepatic vein, 7 and extrahepatic bile duct anatomy 8 in hepatobiliary surgery.
The retrospective study aimed to investigate the clinical effects of using ICG‐FI during laparoscopic partial liver resection.
2. METHODS
2.1. Patients
This study included 112 patients with liver tumors who had been preoperatively diagnosed with malignancy and undergone laparoscopic partial liver resection at the Department of Surgery and Science, Kyushu University Hospital, between January 2017 and December 2020. The ICG‐FI technique came into wide use from January 2019, and 55 patients underwent laparoscopic partial liver resection with the ICG‐FI technique. These enrolled 112 patients were divided into ICG‐FI and non‐ICG‐FN groups, in accordance with the availability of ICG‐FI.
The study protocol was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the Institutional Review Board of Kyushu University Hospital (approval codes: 2020‐671).
2.2. Procedures
Patients were intravenously injected with ICG (Diagnogreen; Daiichi Sankyo, Tokyo, Japan) at a dose of .5 mg/kg body weight within 2–7 d preoperatively. The Visera Elite II (Olympus, Tokyo, Japan) or IMAGE1 S Camera Systems (KARL STORZ, El Segundo, CA, USA) was used as the near‐infrared imaging system for ICG‐FI.
The patient was placed in the supine or left semilateral position. After mobilization of the liver for partial liver resection, intraoperative ultrasonography using a flexible laparoscopic probe (Hitachi‐Aloka Medical, Ltd., Mitaka, Japan) was performed to identify the tumor location. The laparoscopic liver parenchyma was transected using an ultrasonic dissector, a cavitron ultrasonic surgical aspirator, and a soft‐coagulation system with a monopolar electrode. In the ICG‐FI group, fluorescence imaging was performed to detect hepatic tumors, determine the hepatic transection lines, and obtain the surgical margins during laparoscopic parenchymal transection. The resected surface under laparoscopic liver transection was observed by fluorescence imaging, depending on each surgeon's manner (Figure 1). The fluorescence area around the tumor of the cut surfaces of the specimens was observed and measured by using the near‐infrared image system immediately after liver resection (Figure 2).
FIGURE 1.
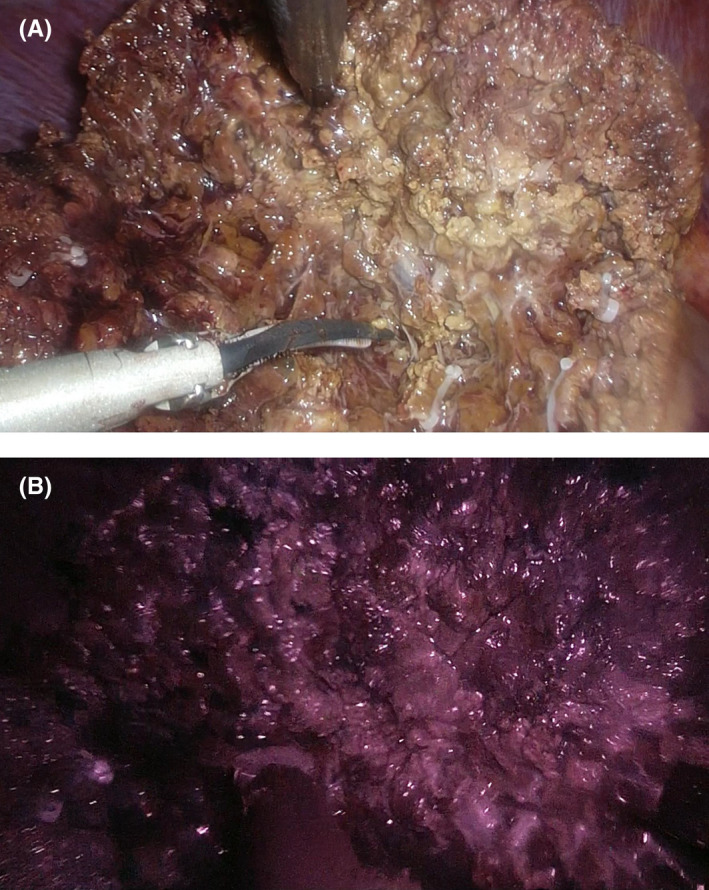
Observation of the resected surface using indocyanine green (ICG)‐fluorescence imaging during laparoscopic liver transection. (A) Normal laparoscopic image. (B) Fluorescence image
FIGURE 2.
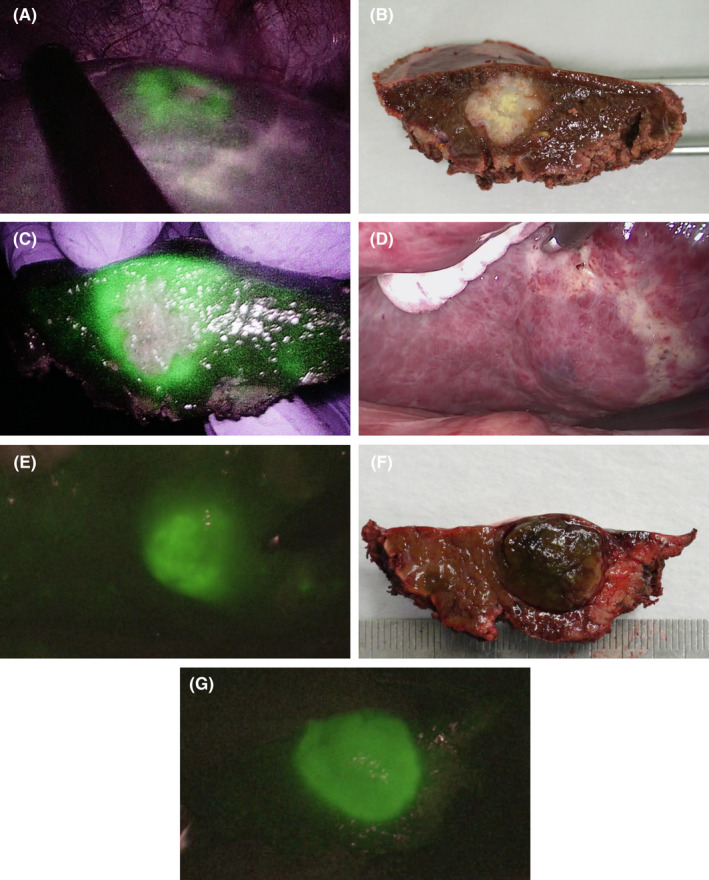
Laparoscopic partial liver resection using indocyanine green (ICG)‐fluorescence imaging. (A) Colorectal liver metastasis in segment 8. Hepatic transection line based on intraoperative ultrasonography and fluorescence imaging of the tumor. (B) Surgical specimen shown with normal laparoscopic color image. (C) Fluorescence image of the surgical specimen. (D) Hepatocellular carcinoma in segment 6. Normal laparoscopic color image. (E) Fluorescence image. (F) Normal image of the surgical specimen. (G) Fluorescence image of the surgical specimen
Postoperative complications were categorized using the Clavien–Dindo classification. 9 Postoperative complications at 30 d after hepatic resection were classified as grade ≥3a.
2.3. Statistical analysis
Continuous variables are presented as the median and were compared using the Mann–Whitney U test. Categorical variables were reported as percentages and compared using the χ2 test or Fisher's exact test. A P‐value of <0.05 was considered as indicative of statistical significance. All statistical analyses were performed using JMP software (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Baseline characteristics
There were 55 patients in the ICG‐FI group and 57 patients in the non‐ICG‐FI group. Regarding pathological findings, 73 of 112 patients (65.2%) had hepatocellular carcinomas, 34 (30.3%) had metastatic liver tumors, which were derived from colorectal cancer in 30 cases, gastric cancer in one case, pancreatic cancer in one case, breast cancer in one case, and epithelioid hamangioendothelioma in one case. Five patients (4.5%) had intrahepatic cholangiocarcinomas. The ICG‐FI group had a significantly better ICG retention rate at 15 min (P = .006) than the non‐ICG‐FI group (Table 1). Other background characteristics did not significantly differ between the two groups.
TABLE 1.
Background characteristics of patients who underwent laparoscopic partial liver resection
| Variable | non‐ICG‐FI (n = 57) | ICG‐FI (n = 55) | P‐value |
|---|---|---|---|
| Age (y) | 68 (44–87) | 68 (43–92) | .720 |
| Sex, male/female | 36/21 | 35/20 | .958 |
| BMI (kg/m2) | 23.5 (17.2–37.8) | 23.3 (16.9–37.8) | .618 |
| Diabetes mellitus (%) | 19 (33.3%) | 13 (23.6%) | .256 |
| Hypertension (%) | 31 (54.3%) | 31 (56.3%) | .833 |
| Histology | |||
| Hepatocellular carcinoma | 36 | 35 | .366 |
| Intrahepatic cholangiocarcinoma | 1 | 4 | |
| Metastatic liver tumor | 18 | 16 | |
| Child–Pugh classification A/B | 51/6 | 54/1 | .113 |
| ICGR15 (%) | 10.8 (0.1–60.1) | 7.8 (0.1–45.9) | .007 |
| Platelet (×104/μL) | 18.2 (4.6–33.9) | 18.0 (8.5–34.3) | .322 |
| Tumor size (cm) | 2.0 (0.7–5.5) | 1.7 (0.8–5.3) | .391 |
| Solitary/Multiple | 47/10 | 44/11 | .739 |
| Tumor location | |||
| Segment1/2/3/4/5/6/7/8 | 1/6/6/5/8/10/8/13 | 2/3/3/7/6/13/3/18 | .324 |
| Fibrosis, F3 or F4 | 24 (42.1%) | 15 (27.2%) | .099 |
Data are presented as n (%) or median (range).
Abbreviations: BMI, body mass index; FI, fluorescence imaging; ICG, indocyanine green; ICGR15, indocyanine green retention rate at 15 min.
3.2. Perioperative characteristics
There was no significant difference in repeat liver resection, duration of surgery and Pringle maneuver, estimated blood loss, or intraoperative blood transfusion between the non‐ICG‐FI and ICG‐FI groups (Table 2). The pathological positivity rates of the surgical margin did not significantly differ between the two groups. The characteristics of patients with tumor that had the pathological surgical margin positivity are shown in Table S1. Postoperative complication rates and length of postoperative hospital stay were comparable in both groups. The surgical margin in the ICG‐FI group was significantly wider than that in the non‐ICG‐FI group (P = .039). In terms of pathological findings, the surgical margin did not significantly differ among patients with hepatocellular carcinoma, metastatic liver tumor, or intrahepatic cholangiocarcinoma. Pathological positivity of the surgical margin was associated with tumor location at segments 7 or 8 of the liver (positivity, 70.0% vs negativity, 34.3%; P = .038).
TABLE 2.
Perioperative characteristics of patients who underwent laparoscopic partial liver resection
| Variable | non‐ICG‐FI (n = 57) | ICG‐FI (n = 55) | P‐value |
|---|---|---|---|
| Repeat liver resection (%) | 10 (17.5%) | 16 (29.0%) | .147 |
| Duration of surgery (min) | 202 (70–499) | 186 (106–380) | .700 |
| Duration of Pringle maneuver (min) | 44 (0–136) | 39 (0–150) | .720 |
| Estimated blood loss (g) | 100 (1–2000) | 70 (5–1060) | .571 |
| Intraoperative blood transfusion (%) | 2 (3.5%) | 1 (1.8%) | .579 |
| Macroscopic surgical margin (mm) | 2 (0–20) | 3 (0–20) | .039 |
| Pathological surgical margin positivity | 5 (8.7%) | 5 (9.0%) | .952 |
| Hepatocellular carcinoma | 2 | 2 | |
| Intrahepatic cholangiocarcinoma | 0 | 0 | |
| Metastatic liver tumor | 3 | 3 | |
| Postoperative complication grade ≥3a | 4 (7.0%) | 1 (1.8%) | .364 |
| Postoperative hospital stay (d) | 7 (4–51) | 8 (5–87) | .265 |
Data are presented as n (%) or median (range).
Abbreviations: FI, fluorescence imaging; ICG, indocyanine green.
3.3. Propensity score matching
Propensity score matching was used to overcome the confounding effects of these differences between the two groups. Overall, the surgical margin was significantly wider in the ICG‐FI group (median, 3.5 mm) than in the non‐ICG‐FI group (median, 2.5 mm; P = .047), and there were no significant differences in the background and perioperative characteristics between the two groups (Tables 3 and 4).
TABLE 3.
Background characteristics of patients who underwent laparoscopic partial liver resection after propensity score matching
| Variable | non‐ICG‐FI (n = 32) | ICG‐FI (n = 32) | P‐value |
|---|---|---|---|
| Age (y) | 69 (44–87) | 67 (44–83) | .256 |
| Sex, male/female | 25/7 | 20/12 | .171 |
| BMI (kg/m2) | 23.3 (17.2–37.8) | 23.8 (16.9–37.8) | .232 |
| Diabetes mellitus (%) | 11 (34.3%) | 7 (21.8%) | .266 |
| Hypertension (%) | 22 (68.7%) | 19 (59.3%) | .434 |
| Histology | |||
| Hepatocellular carcinoma | 20 | 16 | .427 |
| Intrahepatic cholangiocarcinoma | 1 | 4 | |
| Metastatic liver tumor | 9 | 11 | |
| Child–Pugh classification A/B | 32/0 | 31/1 | .313 |
| ICGR15 (%) | 10.1 (0.1–28.3) | 7.5 (0.1–45.9) | .190 |
| Platelet (×104/μL) | 19.1 (6.1–33.9) | 19.8 (8.5–34.3) | .412 |
| Tumor size (cm) | 2.0 (0.7–4.3) | 1.9 (1.0–5.3) | .962 |
| Solitary/Multiple | 28/4 | 25/7 | .509 |
| Tumor location | |||
| Segment1/2/3/4/5/6/7/8 | 1/3/1/2/8/7/3/7 | 1/3/2/5/4/5/2/10 | .778 |
| Fibrosis, F3 or F4 | 12 (37.5%) | 11 (34.3%) | .794 |
Data are presented as n (%) or median (range).
Abbreviations: BMI, body mass index; FI, fluorescence imaging; ICG, indocyanine green; ICGR15, indocyanine green retention rate at 15 min.
TABLE 4.
Perioperative characteristics of patients who underwent laparoscopic partial liver resection after propensity score matching
| Variable | non‐ICG‐FI (n = 32) | ICG‐FI (n = 32) | P‐value |
|---|---|---|---|
| Repeat liver resection (%) | 7 (21.8%) | 7 (21.8%) | 1.000 |
| Duration of Surgery (min) | 169 (70–332) | 177 (106–318) | .298 |
| Duration of Pringle maneuver (min) | 38 (0–93) | 44 (0–95) | .294 |
| Estimated blood loss (g) | 85 (1–1100) | 54 (5–1060) | .793 |
| Intraoperative blood transfusion (%) | 1 (3.1%) | 1 (3.1%) | 1.000 |
| Macroscopic surgical margin (mm) | 2.5 (0–20) | 3.5 (0–20) | .047 |
| Pathological surgical margin positivity | 3 (9.3%) | 2 (6.2%) | .641 |
| Hepatocellular carcinoma | 1 | 0 | |
| Intrahepatic cholangiocarcinoma | 0 | 0 | |
| Metastatic liver tumor | 2 | 2 | |
| Postoperative complication grade ≥3a | 2 (6.2%) | 0 (0.0%) | .492 |
| Postoperative hospital stay (d) | 7 (4–51) | 8 (5–21) | .349 |
Data are presented as n (%) or median (range).
Abbreviations: FI, fluorescence imaging; ICG, indocyanine green.
4. DISCUSSION
Propensity score matching analysis between patients who underwent and those who did not undergo ICG‐FI demonstrated that the ICG‐FI technique using near‐infrared light facilitated the attainment of a wide surgical margin from liver tumors during laparoscopic partial liver resection. To the best of our knowledge, this is the first study focusing on laparoscopic partial liver resection to use propensity score matching to show that the use of the ICG‐FI technique is associated with macroscopically wider surgical margin in patients with liver tumor.
Several studies have investigated the clinical usefulness of laparoscopic liver resection using the near‐infrared ICG navigation system. Lu et al 10 showed that using ICG‐FI navigation in 57 patients who underwent laparoscopic liver resection, including anatomical liver resection, achieved a significantly wider surgical margin compared with that in 63 patients who did not use ICG‐FI navigation. Of these, 47 of 120 patients (39.2%) underwent laparoscopic anatomical liver resection, and 58 (48.3%) were diagnosed with hepatocellular carcinoma. In another recent study, no significant differences were found in the short‐ and long‐term effects of a surgical margin between anatomical and nonanatomical liver resections in patients with hepatocellular carcinoma. 11 Given this lack of conclusive results, we undertook the current study, which included only patients who underwent laparoscopic partial hepatic resection.
Ensuring pathological negativity of the surgical margin is important for obtaining better surgical outcomes for malignant liver tumors. 11 , 12 , 13 Aoki et al reported that adequate and secure surgical margins were attained in 25 patients who underwent laparoscopic limited liver resection with ICG‐FI navigation technology. The same study found no significant difference in the length of surgical margin and pathological surgical margin positivity between 25 and 72 patients who underwent laparoscopic limited liver resection with and without ICG‐FI navigation technology, respectively. 14 A wider surgical margin was associated with significantly improved surgical outcome for various cancers. 13 , 15 , 16 In the current study, a wide surgical margin was obtained in the ICG‐FI group at a significantly higher rare compared with the non‐ICG‐FI group before and after propensity score matching. Nevertheless, the rate of pathologically negative surgical margins was comparable between the two groups. These results may indicate better surgical outcomes in terms of local recurrence and long‐term survival in patients following laparoscopic partial liver resection with ICG‐FI. Further long‐term follow‐up is needed.
Several studies showed attainment of pathologically negative surgical margins in patients who underwent laparoscopic liver resection with ICG‐FI navigation. 10 , 14 In our study, the pathological positivity rate of surgical margins in laparoscopic partial liver resection using the ICG‐FI technique was 9.0% (5 of 55 patients). The frequency of switching to fluorescence imaging for observation of the resected surface during laparoscopic liver transection might have been low in these patients. The real‐time ICG‐FI system, which allows a green color to be overlayed on a white‐light picture during laparoscopic surgery, would be required to minimize pathological surgical margin positivity. We would need to conduct a clinical trial to identify the usefulness of the real‐time ICG‐FI system during laparoscopic partial liver resection. Moreover, a significant association between positive surgical margin and location in segments 7 or 8 of the liver was found. Generally, laparoscopic partial liver resection is more difficult for tumors located in segments 7 or 8 of the liver than those of the anterolateral segments or the left sector of the liver. Laparoscopic anatomical liver resection, such as segmentectomy of the liver for metastatic liver tumors located in segments 7 or 8, might be an option to obtain a secure surgical margin if the liver function is to be preserved.
In contrast, Alfano et al 17 showed that 1 of 27 patients (3.7%) with colorectal cancer liver metastasis had a pathologically positive surgical margin following intraoperative ICG‐FI, although that study included only patients who underwent open liver resection. Recently, Achterberg et al 18 reported that in patients with colorectal cancer liver metastasis, one of eight nodules (12.5%) had a pathologically positive surgical margin following laparoscopic or robotic‐assisted liver resection; nevertheless, these eight nodules were determined to have macroscopically negative surgical margins by near‐infrared imaging with ICG‐FI. In our study, the pathological positivity rate of surgical margins in laparoscopic partial liver resection was 8.9% (10 of all 112 patients). Using the ICG‐FI technique, 2 of 35 patients with hepatocellular carcinomas (5.7%), 3 of 16 (18.7%) with metastatic liver tumors, and 0 (0%) with intrahepatic cholangiocarcinoma were determined to have pathologically positive surgical margins.
The present study had several limitations. First, this was a single‐institution, retrospective study. Second, we included a relatively small number of patients. Further studies with a greater number of patients are required to confirm the results of the present study. Third, we had 16 surgeons responsible for liver transection during the study period. Surgeon‐associated factors or learning curve bias cannot be eliminated. Nevertheless, no significant differences for perioperative factors between the ICG‐FI and non‐ICG‐FI groups and less experienced surgeons with wide surgical margin under more application of ICG‐FI technique might be the clues to implicate the positive impacts of ICG‐FI technique.
In conclusion, this retrospective propensity score matching study demonstrated that laparoscopic partial liver resection using the ICG‐FI technique was clinically useful in obtaining a secure surgical margin, which might result in a favorable surgical outcome with regard to local recurrence.
DISCLOSURES
Conflict of Interest: The authors declare no conflicts of interest for this article.
Author Contributions: SI participated in the study conception, design, acquisition of data, analysis, interpretation of data, and drafting of the article. TT, AM, TK, YN, TT, KM, and NH participated in the acquisition of data. MM, and TY participated in critical revision of the article.
Funding: This study was supported by JSPS KAKENHI Grant Number JP‐19K09198. The funding source had no role in the collection, analysis, or interpretation of the data or in the decision to submit the article for publication.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of the Ethics Committee, National Research Committee, as well as with the 1964 Helsinki Declaration and its later amendments. The study protocol was approved by the Institutional Review Board (No. 2020‐671).
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Supporting information
Table S1
ACKNOWLEDGEMENT
We thank Edanz Group (https://en‐author‐services.edanzgroup.com/ac) for editing a draft of this article.
Itoh S, Tomiyama T, Morinaga A, Kurihara T, Nagao Y, Toshima T, et al. Clinical effects of the use of the indocyanine green fluorescence imaging technique in laparoscopic partial liver resection. Ann Gastroenterol Surg. 2022;6:688–694. doi: 10.1002/ags3.12563
REFERENCES
- 1. Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative short‐term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg. 2016;263(4):761–77. [DOI] [PubMed] [Google Scholar]
- 2. Kokudo N, Takemura N, Ito K, Mihara F. The history of liver surgery: achievements over the past 50 years. Ann Gastroenterol Surg. 2020;4(2):109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nakashima Y, Saeki H, Yukaya T, Tsutsumi S, Nakanishi R, Sugiyama M, et al. Blood flow assessment with indocyanine green fluorescence angiography for pedicled omental flap on cervical esophagogastric anastomosis after esophagectomy. J Am Coll Surg. 2016;222(5):e67–9. [DOI] [PubMed] [Google Scholar]
- 4. Wishart GC, Loh SW, Jones L, Benson JR. A feasibility study (ICG‐10) of indocyanine green (ICG) fluorescence mapping for sentinel lymph node detection in early breast cancer. Eur J Surg Oncol. 2012;38(8):651–6. [DOI] [PubMed] [Google Scholar]
- 5. Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green‐fluorescence imaging during hepatectomy. HepatoBiliary Surg Nutr. 2016;5(4):322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terasawa M, Ishizawa T, Mise Y, Inoue Y, Ito H, Takahashi Y, et al. Applications of fusion‐fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg Endosc. 2017;31(12):5111–8. [DOI] [PubMed] [Google Scholar]
- 7. Kurihara T, Yamashita Y, Yoshida Y, Takeishi K, Itoh S, Harimoto N, et al. Indocyanine green fluorescent imaging for hepatic resection of the right hepatic vein drainage area. J Am Coll Surg. 2015;221(3):e49–53. [DOI] [PubMed] [Google Scholar]
- 8. Yoshiya S, Minagawa R, Kamo K, Kasai M, Taketani K, Yukaya T, et al. Usability of intraoperative fluorescence imaging with indocyanine green during laparoscopic cholecystectomy after percutaneous transhepatic gallbladder drainage. World J Surg. 2019;43(1):127–33. [DOI] [PubMed] [Google Scholar]
- 9. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Ann Surg. 2009;250(2):187–96. [DOI] [PubMed] [Google Scholar]
- 10. Lu H, Gu J, Qian XF, Dai XZ. Indocyanine green fluorescence navigation in laparoscopic hepatectomy: a retrospective single‐center study of 120 cases. Surg Today. 2021;51(5):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aoki T, Kubota K, Hasegawa K, Kubo S, Izumi N, Kokudo N, et al. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br J Surg. 2020;107(1):113–20. [DOI] [PubMed] [Google Scholar]
- 12. Wu L, Tsilimigras DI, Paredes AZ, Mehta R, Hyer JM, Merath K, et al. Trends in the incidence, treatment and outcomes of patients with intrahepatic cholangiocarcinoma in the USA: facility type is associated with margin status, use of lymphadenectomy and overall survival. World J Surg. 2019;43(7):1777–87. [DOI] [PubMed] [Google Scholar]
- 13. Sadot E, Groot Koerkamp B, Leal JN, Shia J, Gonen M, Allen PJ, et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: surgical technique or biologic surrogate? Ann Surg. 2015;262(3):476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aoki T, Murakami M, Koizumi T, Matsuda K, Fujimori A, Kusano T, et al. Determination of the surgical margin in laparoscopic liver resections using infrared indocyanine green fluorescence. Langenbecks Arch Surg. 2018;403(5):671–80. [DOI] [PubMed] [Google Scholar]
- 15. Tsilimigras DI, Sahara K, Moris D, Hyer JM, Paredes AZ, Bagante F, et al. Effect of surgical margin width on patterns of recurrence among patients undergoing R0 hepatectomy for T1 hepatocellular carcinoma: an international multi‐institutional analysis. J Gastrointest Surg. 2020;24(7):1552–60. [DOI] [PubMed] [Google Scholar]
- 16. Watanabe Y, Matsuyama Y, Izumi N, Kubo S, Kokudo N, Sakamoto M, et al. Effect of surgical margin width after R0 resection for intrahepatic cholangiocarcinoma: a nationwide survey of the liver cancer study group of Japan. Surgery. 2020;167(5):793–802. [DOI] [PubMed] [Google Scholar]
- 17. Alfano MS, Molfino S, Benedicenti S, Molteni B, Porsio P, Arici E, et al. Intraoperative ICG‐based imaging of liver neoplasms: a simple yet powerful tool. Preliminary results. Surg Endosc. 2019;33(1):126–34. [DOI] [PubMed] [Google Scholar]
- 18. Achterberg FB, Sibinga Mulder BG, Meijer RPJ, Bonsing BA, Hartgrink HH, Mieog JSD, et al. Real‐time surgical margin assessment using ICG‐fluorescence during laparoscopic and robot‐assisted resections of colorectal liver metastases. Ann Transl Med. 2020;8(21):1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


