Abstract
Aim
Using nationwide data collected over the past 20 years, we aimed to investigate deceased donor liver transplantation (DDLT) outcomes to develop a unique risk model that can be used to establish a standard for organ acceptance in Japan.
Methods
Data were collected for 449 recipients aged ≥18 years who underwent DDLT between 1999 and 2019. Least absolute shrinkage and selection operator (LASSO) regression analysis was utilized to develop an original risk score model for 1‐year graft loss (termed the Japan Risk Index [JRI]). We developed risk indices according to recipient, donor, and surgery components (termed JRI‐R, D, and S, respectively). The JRI was validated via a 5‐fold cross‐validation. We also compared DDLT outcomes and risk indices among Era1 (−2011), Era2 (−2015), and Era3 (−2019).
Results
The 1‐year graft survival rate was 89.5% and improved significantly, reaching 84.7%, 87.6%, and 93.9% in Era1, Era2, and Era3, respectively. The JRI was calculated as JRI‐R (re‐transplantation, Model for End‐Stage Liver Disease score, medical condition in intensive care unit) × JRI‐D (age, catecholamine index, maximum sodium, maximum total bilirubin) × JRI‐S (total ischemic time) × 0.84. The risk model achieved a mean C‐statistic value of 0.81 in the validation analysis. The risk index was significantly lower in Era3 than in Era2.
Conclusion
Changes in the risk index over time indicated that avoiding risks contributed to the improved outcomes in Era3. The JRI is unique to adult DDLT in Japan and may be useful as a reference for organ acceptance in the future.
Keywords: brain death, database, Japan, liver transplantation, risk assessment
Using data from the national databases of the Japan Organ Transplant Network and Japanese Liver Transplantation Society, we investigated the outcomes of DDLT over the past 20 years in Japan and developed a new risk score model consisting of the identified risk factors for graft loss within 1 year after DDLT by LASSO regression analysis. Moreover, the 1‐year graft survival rate improved significantly over time and the risk index significantly decreased. Changes in the risk index over time suggest avoiding risks contributed to the improved outcomes.

Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BA
biliary atresia
- BAR
balance of risk
- BMI
body mass index
- DDLT
deceased donor liver transplantation
- DLI
donor liver index
- DLI1
donor liver index for 1‐year graft survival
- D‐MELD
donor age and recipient model for end‐stage liver disease
- DQI
donor quality index
- DRI
donor risk index
- ET‐DRI
eurotransplant donor risk index
- GGT
γ‐glutamyl transpeptidase
- ICU
intensive care unit
- JLTS
Japanese Liver Transplantation Society
- JOTNW
Japan Organ Transplant Network
- JRI (‐D, ‐R, ‐S)
Japan Risk Index (for donor, for recipient, for surgery)
- LT
liver transplantation
- MELD
model for end‐stage liver disease
- ROC
receiver operating characteristic
- SOFT
survival outcomes following liver transplantation
1. INTRODUCTION
For successful deceased donor liver transplantation (DDLT), complete and accurate knowledge of risk factors before transplantation is essential. Numerous countries and regions have identified risk factors for DDLT. However, the risk to patient and graft survival in DDLT varies by country and region. Thus, it is not possible to perform a universal risk factor analysis. 1 , 2 , 3 , 4 , 5 , 6 In fact, previously reported indices often included region‐specific factors, such as race or regional allocation systems, and differed across regions. 1 , 2 , 3 , 4 Therefore, we believe it is necessary for each country or region to perform its own analysis of risk factors for DDLT.
In Japan, the first DDLT from a brain‐dead donor under the Organ Transplant Law was performed in 1999. Furukawa and colleagues analyzed the first 85 adult cases who underwent DDLT from 1999 to 2011 in Japan and identified the recipient's Mayo End‐Stage Liver Disease (MELD) score, donor age, and cold ischemic time as independent risk factors for 3‐month recipient survival. 7 After revision of the law on transplantation in 2010, which allowed organ donation with family consent, the number of donations increased, and the cumulative number of DDLTs reached 500 cases in 2019. However, there have been no standards or indicators relevant to the Japanese population. To increase the success rate of liver transplantation (LT), there is an urgent need for Japanese transplant surgeons to summarize past outcomes of Japanese DDLT and develop a practical national risk model for predicting organ acceptance.
In the present study, we collected DDLT‐related recipient, donor, and surgical factors from the national databases of the Japanese Liver Transplantation Society (JLTS) and the Japan Organ Transplant Network (JOTNW). Using these data collected over the past 20 years, we aimed to develop a unique risk model that can be used to establish a nationwide standard for organ acceptance in Japan.
2. MATERIALS AND METHODS
2.1. Data collection from the nationwide JLTS and JOTNW databases
We collected data related to recipient, donor, and surgical factors from the JLTS and JOTNW databases. We combined these two national databases while ensuring anonymization to protect personal information. The transplantation year, transplantation prefecture, recipient age, sex, and blood type were used to identify the corresponding patients in the two databases. Regarding graft survival, status as of March 2020 was also collected. Regarding recipient factors, hepatic encephalopathy was evaluated according to the grade of hepatic coma proposed by the Inuyama Symposium in 1972. 8 Regarding donor factors, average alcohol intake ≥60 g/day was defined as alcohol history positive, and Brinkman index ≥100 was defined as smoking history positive. Since the JOTNW database lacks information on the length of intensive care unit (ICU) stays for donors, the duration on a respirator was used as an indicator of long‐term donor management. The catecholamine level at procurement was calculated using the catecholamine index, which was defined as dopamine + dobutamine + (adrenaline + noradrenaline) × 100 μg/kg/min. 9 For blood biochemical tests, the maximum value from admission to procurement and the last value before procurement were collected. Pathological data regarding the presence of fibrosis (degree indicated by the Inuyama classification) 10 and steatosis were collected from the biopsy report, if intraoperative biopsy was performed during procurement.
This study was approved by the project committee of the JLTS and the Institutional Review Board of the Keio University School of Medicine (#20180301, #20140289). The JOTNW data were provided for this study with the approval of the Institutional Review Board of JOTNW (#4). There was no need to acquire written informed consent from each patient given the nature of the study.
2.2. Organ allocation system in Japan
The Japanese organ allocation system has previously been reported in detail. 11 , 12 Donors are allocated on a national basis, and there is no local or regional priority. Waiting patients are prioritized based on the following criteria: blood type, disease severity, and duration on the waiting list. Disease severity is determined using the medical emergency score, a unique point system in Japan. Patients with acute liver failure or early graft failure after LT receive top priority (9 points), followed by patients with liver cirrhosis with Child–Pugh grades of C, B, and A 13 (6, 3, and 1 points), respectively. Since 2011, the most severe cases with Child‐Pugh grade C (MELD score ≥25 and Child‐Pugh score ≥13) have been upgraded to 8 points on the medical emergency score, and patients with scores of 9 points have been re‐registered with scores of 10 points.
2.3. Study population, era definition, and outcome
This study included DDLTs performed in Japan from January 1999 to March 2019 for recipients aged ≥18 years. The study period was divided by case number into three eras according to the accumulated case number. The first 100 cases reported by Furukawa and colleagues were categorized as Era1. 7 The remaining cases were equally divided into Era2 and Era3. Subsequently, patients aged <18 years and those who died intraoperatively were excluded.
The main outcome was defined as 1‐year graft loss after DDLT.
2.4. Development of a Japanese risk model for 1‐year graft loss
For construction of the risk model, univariate logistic regression analyses were performed first. Recipient, donor, and surgical factors that were considered known or predictable at the time of organ acceptance were subjected to univariate analysis. Regarding the surgical factors, split LT, simultaneous liver and kidney transplantation, and total ischemic time were included in the analysis, as split LT and simultaneous liver and kidney transplantation are known at pre‐transplant condition, and the total ischemic time is a predictable factor.
Using the R package “glmnet,” a logistic regression model was applied using the least absolute shrinkage and selection operator (LASSO) method for variable selection and shrinkage in order to develop a risk model. 14 Variables with P values <0.10 in the univariate analyses were included. The penalty regularization parameter (λ) was determined via the 5‐fold cross‐validation method using “cv.glmnet.” The parameter (λ) was given by the least mean square error. 14 A series of nonzero variables combined with the corresponding efficiency were identified and used to construct a prediction model for 1‐year graft loss. We also divided the nonzero variables into donor, recipient, and surgery components and developed the risk indices based on each component.
2.5. Validation of the risk score model
In order to confirm the robustness and accuracy of the risk score model, 5‐fold cross‐validation was used for internal validation. The ability to predict 1‐year graft loss was comprehensively evaluated by plotting a receiver operating characteristic (ROC) curve in each validation set and by calculating a mean area under the curve (C‐statistic) using the R package “ROCR.” 15
2.6. Ability of previously reported models to discriminate 1‐year graft loss
We then evaluated the discrimination ability of the following previously developed models in our cohort: Donor Risk Index (DRI), Eurotransplant DRI (ET‐DRI), Donor Liver Index (DLI), DLI for 1‐year graft survival (DLI1), Donor Quality Index (DQI), Survival Outcomes Following Liver Transplantation (SOFT) score, adjusted Balance of Risk (BAR) score, Donor Age and Recipient MELD (D‐MELD) score, and the risk model proposed by Molinari et al. 1 , 2 , 3 , 4 , 5 , 6 , 16 , 17 The C‐statistic was then calculated for each model. To estimate DQI, we used the estimated glomerular filtration rate for the Japanese population rather than diet modification in renal disease creatinine clearance. 18 In the estimation of SOFT scores, “life support,” “portal venous thrombosis,” and “previous abdominal surgery” were estimated as “none” because this information was not available. To estimate DRI, ET‐DRI, and SOFT scores, geographic region was fixed to “national sharing” because the Japanese allocation system does not introduce regionality.
2.7. Graft survival rates according to risk evaluations of donor, recipient, and surgical factors
To examine the survival rate according to risk evaluations in detail, we assessed 1‐year graft survival rates according to recipient, donor, and surgical indices.
2.8. Changes in risk factors and risk indices over time
The number of DDLTs for patients with donor, recipient, and surgical factors, as determined using the LASSO method and the developed risk indices, were compared among the eras.
2.9. Graft survival with and without liver steatosis and fibrosis
Since liver biopsy was performed in less than two‐thirds of all patients in this study, microscopic findings of steatosis and fibrosis were not included in the multivariate analysis for 1‐year graft loss and were subjected to another analysis of graft survival and risk score assessments with and without steatosis/fibrosis.
2.10. Statistical analyses
Continuous data are represented as mean values and standard deviations, while categorical data are represented as numbers and percentages. Student's t test was used to compare continuous data, and chi‐square test was used to compare categorical data. Graft survival was calculated using the Kaplan‐Meier method, and 1‐year graft survival was compared using the log‐rank test. P values <0.05 were considered statistically significant. R software v 4.1.1 (R Foundation for Statistical Computing) was used for LASSO regression and 5‐fold cross‐validation, while the Statistical Package for the Social Sciences version 27.0 software (IBM Corp) was used for other statistical analyses.
3. RESULTS
3.1. Characteristics and graft survival of DDLT in Japan
Within the study period, 523 DDLTs were performed in Japan. All donors were brain dead. After integrating the two databases and excluding recipients aged <18 years (n = 73) and intraoperative death (n = 1), 449 recipients were enrolled in this study. Finally, Era1 (January 1999‐January 2011, n = 85), Era2 (January 2011‐December 2015, n = 185), and Era3 (December 2015‐March 2019, n = 179) were analyzed (Figure 1).
FIGURE 1.
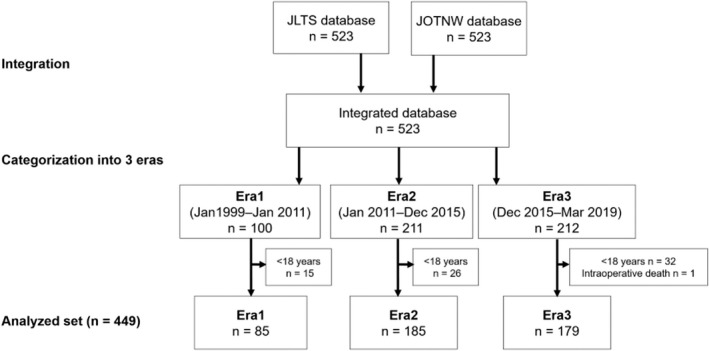
Integration of two nationwide databases and categorization of data into transplantation eras. The JLTS and JOTNW databases were integrated and then categorized into three eras. The first 100 cases were categorized as Era1 (January 1999‐January 2011). The next 423 cases were divided into Era2 (January 2011‐December 2015) and Era3 (December 2015‐March 2019). Subsequently, patients aged <18 years (n = 73) and a patient who died intraoperatively (n = 1) were excluded from all categories. Finally, 449 cases, consisting of Era1 (n = 85), Era2 (n = 185), and Era3 (n = 179), were included in this study. JLTS, Japanese Liver Transplantation Society; JOTNW, Japan Organ Transplant Network
Characteristics of DDLT are shown in Table 1. The 1‐, 3‐, and 5‐year graft survival rates were 89.5%, 86.1%, and 83.0%, respectively. (Figure 2A) The 1‐year graft survival rate was significantly higher in Era3 (93.9%) than in Era1 (84.7%, P = 0.01) and Era2 (87.6%, P = 0.03; Figure 2B).
TABLE 1.
Characteristics of adult DDLT in Japan
| Variables | n (%) or mean ± SD |
|---|---|
| Recipient factor | |
| Sex (male) | 250 (55.7) |
| Age, years | 46.3 ± 12.3 |
| Primary liver disease | |
| Acute liver failure | 112 (24.9) |
| Cirrhosis (hepatocellular, viral) | 103 (22.9) |
| Cirrhosis (hepatocellular, non‐viral) | 57 (12.7) |
| Cirrhosis (cholestatic, BA) | 30 (6.7) |
| Cirrhosis (cholestatic, non‐BA) | 92 (20.5) |
| Metabolic disease | 30 (6.7) |
| Others | 25 (5.6) |
| Re‐transplantation | 76 (16.9) |
| Ascites: Moderate | 218 (48.6) |
| Encephalopathy: ≥Ⅲ | 58 (12.9) |
| Medical condition: in ICU | 126 (28.1) |
| Dialysis | 118 (26.3) |
| Anti‐hepatitis C antibody positive | 86 (19.2) |
| BMI, kg/m2 | 23.4 ± 4.5 |
| MELD score | 23.1 ± 8.0 |
| Donor factor | |
| Sex (Male) | 263 (58.6) |
| Age, years | 44.2 ± 14.3 |
| Hypertension | 81 (18.0) |
| Diabetes mellites | 14 (3.1) |
| BMI, kg/m2 | 22.7 ± 3.9 |
| Alcohol history | 53 (12.2) |
| Smoking history | 198 (45.3) |
| Anti‐hepatitis B core antibody positive | 47 (10.5) |
| Cause of death | |
| Trauma | 84 (18.7) |
| Cerebrovascular disease | 213 (47.4) |
| Anoxia | 149 (33.2) |
| Others | 3 (0.7) |
| Duration on respirator, days | 9.9 ± 8.2 |
| Catecholamine index | 5.9 ± 7.6 |
| Sodium, maximum/ last mEq/L | 156.4 ± 10.6/ 141.0 ± 9.7 |
| Total bilirubin, maximum/ last, mg/dL | 1.9 ± 2.0/ 1.4 ± 1.7 |
| AST, maximum/ last, IU/L | 327 ± 653/ 63 ± 118 |
| ALT, maximum/ last, IU/L | 229 ± 517/ 58 ± 82 |
| GGT, maximum/ last, IU/L | 134 ± 134/ 97 ± 101 |
| Creatinine, last, mg/dL | 1.3 ± 1.6 |
| Image findings of steatosis | 45 (10.0) |
| Pathological findings | |
| Fibrosis (F0‒1/ F2/ not examined) | 250/6/193 |
| Steatosis (<30%/ ≥30%/ not examined) | 246/12/191 |
| Number of declines for donor reason ≥3 | 40 (8.9) |
| Surgical factor | |
| Split liver transplantation | 37 (8.2) |
| Simultaneous liver and kidney transplantation | 19 (4.2) |
| Total ischemic time, h | 8.9 ± 2.5 |
Data were presented as mean values ± SD and numbers (%) for continuous and categorical variables, respectively. B
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BA, biliary atresia; BMI, body mass index; DDLT, deceased‐donor liver transplantation; GGT, gamma‐glutamyl transpeptidase; ICU, intensive care unit; MELD, Model for End‐stage Liver Disease; SD, standard deviations.
FIGURE 2.
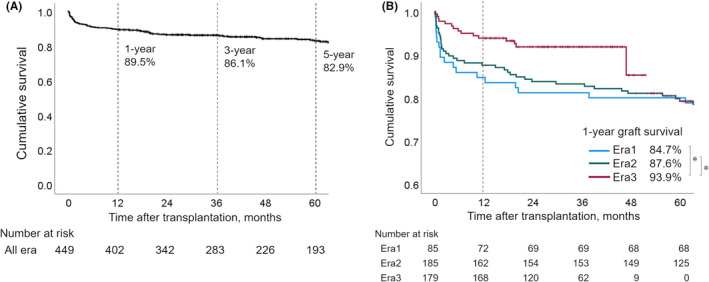
Graft survival curves for DDLT in Japan. (A) The Kaplan‐Meier curve shows graft survival for the total cohort (n = 449) in Japan. The 1‐, 3‐, and 5‐year graft survival rates were 89.5%, 86.1%, and 83.0%, respectively. (B) The 1‐year graft survival rate was significantly higher in Era3 than in Era1 (P = 0.01) and Era2 (P = 0.03). There was no significant difference in the 1‐year graft survival rate between Era1 and Era2 (P = 0.51; *P < 0.05). DDLT, deceased donor liver transplantation
3.2. Risk score model developed via LASSO regression
Variables with P values <0.10 in the univariate logistic analysis were subjected to LASSO regression. Table 2 and Figure 3A,B demonstrate the results of the LASSO procedure. The optimal tuning parameter of lambda (λ) was determined as 0.018905 via 5‐fold cross‐validation. The selected nonzero variables were re‐transplantation, recipient MELD, recipient medical condition in ICU, donor age, donor catecholamine index, donor maximum sodium level, donor maximum total bilirubin level, total ischemic time, and era. The risk model for 1‐year graft loss (i.e. the Japan Risk Index [JRI]) and the estimated 1‐year graft loss rate were defined based on the nonzero coefficient of β expressed in Table 2. Moreover, we also developed risk indices according to each component. The index and each component are expressed as follows:
TABLE 2.
Univariate analyses and LASSO regression analysis for 1‐year graft loss
| A | 1‐year graft survival, % | Univariate | ||
|---|---|---|---|---|
| Variables | P value | OR | 95%CI | |
| Recipient factor | ||||
| Sex: male (vs female) | 89.2 (vs 89.9) | 0.80 | 1.08 | 0.59‐2.00 |
| Age (vs <40 years) | (vs 88.1) | 0.51 | 1.30 | 0.60‐2.82 |
| 40‐59 years | 90.9 | 0.39 | 0.74 | 0.38‐1.46 |
| ≥60 years | 87.3 | 0.86 | 1.08 | 0.45‐2.58 |
| Primary liver disease (vs acute liver failure) | (vs 86.6) | reference | ||
| Cirrhosis (hepatocellular, viral) | 88.3 | 0.70 | 0.85 | 0.38‐1.92 |
| Cirrhosis (hepatocellular, non–viral) | 89.5 | 0.59 | 0.76 | 0.28‐2.08 |
| Cirrhosis (cholestatic, BA) | 91.3 | 0.37 | 1.62 | 0.57‐4.61 |
| Cirrhosis (cholestatic, non–BA) | 80.0 | 0.30 | 0.62 | 0.25‐1.53 |
| Metabolic disease | 100.0 | 1.00 | 0.00 | 0.0–∞ |
| Others | 100.0 | 1.00 | 0.00 | 0.0–∞ |
| Re–transplantation: yes (vs no) | 77.6 (vs 92.0) | <0.001 | 3.29 | 1.71‐6.35 |
| Ascites: moderate (vs none‐mild) | 87.2 (vs 91.8) | 0.11 | 1.64 | 0.89‐3.04 |
| Encephalopathy: ≥Ⅲ (vs ≤Ⅱ) | 82.8 (vs 90.5) | 0.08 | 1.99 | 0.93‐4.27 |
| Medical condition: in ICU (vs not in ICU) | 83.3 (vs 91.9) | 0.01 | 2.28 | 1.23‐4.22 |
| Dialysis: yes (vs no) | 89.0 (vs 90.0) | 0.76 | 1.11 | 0.56‐2.19 |
| Anti‐hepatitis C antibody positive (vs negative) | 90.7 (vs 89.3) | 0.69 | 0.85 | 0.38‐1.90 |
| BMI (vs <25.0 kg/m2) | (vs 90.4) | reference | ||
| 25.0‐34.9 kg/m2 | 86.8 | 0.29 | 1.44 | 0.74‐2.81 |
| ≥35.0 kg/m2 | 100.0 | 1.00 | 0.00 | 0.0–∞ |
| MELD score (vs <20) | (vs 91.5) | |||
| 20‐29 | 91.4 | 0.96 | 1.02 | 0.48‐2.17 |
| 30‐39 | 82.4 | 0.05 | 2.31 | 1.00‐5.36 |
| ≥40 | 72.7 | 0.06 | 4.06 | 0.95‐17.4 |
| Donor factor | ||||
| Sex: male (vs female) | 89.7 (vs 89.2) | 0.87 | 0.95 | 0.52‐1.75 |
| Age (vs <40 years) | (vs 90.9) | reference | ||
| 40‐59 years | 91.8 | 0.76 | 0.90 | 0.44‐1.83 |
| 60‐69 years | 81.7 | 0.06 | 2.25 | 0.97‐5.21 |
| ≥70 years | 40.0 | 0.004 | 15.0 | 2.3‐97.0 |
| Hypertension: yes (vs no) | 91.4 (vs 89.0) | 0.74 | 0.87 | 0.39‐1.94 |
| Diabetes mellites: yes (vs no) | 100.0 (vs 89.1) | 1.00 | 0.00 | 0–∞ |
| BMI (vs <25.0 kg/m2) | (vs 90.6) | reference | ||
| 25.0‐34.9 kg/m2 | 85.1 | 0.13 | 1.69 | 0.87‐3.30 |
| ≥35.0 kg/m2 | 100.0 | 1.00 | 0.00 | 0–∞ |
| Alcohol history: yes (vs no) | 87.8 (vs 89.8) | 0.21 | 2.07 | 0.66‐6.46 |
| Smoking history: yes (vs no) | 90.9 (vs 89.1) | 0.54 | 0.82 | 0.44‐1.54 |
| Anti‐hepatitis B core antibody positive (vs negative) | 91.5 (vs 89.3) | 0.64 | 0.78 | 0.27‐2.27 |
| Cause of death (vs Trauma) | (vs 89.3) | reference | ||
| Cerebrovascular disease | 88.3 | 0.80 | 1.11 | 0.49‐2.48 |
| Anoxia | 91.3 | 0.62 | 0.80 | 0.33‐1.95 |
| Others | 100.0 | 1.00 | 0.00 | 0.0–∞ |
| Duration on respirator (vs <7 days) | (vs 90.1) | reference | ||
| 7‐27 days | 88.9 | 0.69 | 1.13 | 0.61‐2.11 |
| ≥28 days | 92.3 | 0.80 | 0.76 | 0.09‐6.16 |
| Catecholamine index (vs <10.0) | (vs 91.8) | reference | ||
| 10.0‐29.9 | 84.1 | 0.04 | 2.11 | 1.04‐4.26 |
| ≥30.0 | 63.6 | 0.005 | 6.38 | 1.77‐23.1 |
| Sodium, maximum (vs <160 mEq/L) | (vs 91.2) | reference | ||
| 160‐179 mEq/L | 87.6 | 0.23 | 1.47 | 0.78‐2.77 |
| ≥180 mEq/L | 75.0 | 0.07 | 3.45 | 0.88‐13.6 |
| Sodium, last ≥160 mEq/L (vs <160 mEq/L) | 88.9 (vs 89.6) | 0.11 | 1.03 | 0.99‐1.06 |
| Total bilirubin, maximum (vs <3.0 mg/dL) | (vs 91.1) | reference | ||
| 3.0‐4.9 mg/dL | 82.4 | 0.10 | 2.20 | 0.86‐5.69 |
| ≥5.0 mg/dL | 70.0 | 0.004 | 4.41 | 1.59‐12.2 |
| Total bilirubin, last (vs <3.0 mg/dL) | (vs 91.1) | reference | ||
| 3.0‐4.9 mg/dL | 80.8 | 0.12 | 2.27 | 0.81‐6.34 |
| ≥5.0 mg/dL | 70.0 | 0.12 | 2.85 | 0.75‐10.8 |
| AST, maximum (vs <100 IU/L) | (vs 89.0) | reference | ||
| 100‐999 IU/L | 90.8 | 0.55 | 0.82 | 0.43‐1.56 |
| ≥1000 IU/L | 82.1 | 0.30 | 1.76 | 0.60‐5.15 |
| AST, last ≥100 IU/L (vs <100 IU/L) | 86.9 (vs 89.9) | 0.47 | 1.35 | 0.60‐3.05 |
| ALT, maximum (vs <100 IU/L) | (vs 89.1) | reference | ||
| 100‐999 IU/L | 90.3 | 0.68 | 0.88 | 0.47‐1.63 |
| ≥1000 IU/L | 86.7 | 0.77 | 1.25 | 0.27‐5.87 |
| ALT, last ≥100 IU/L (vs <100 IU/L) | 87.9 (vs 89.8) | 0.67 | 1.20 | 0.51‐2.83 |
| GGT, maximum ≥150 IU/L (vs <150 IU/L) | 87.3 (vs 91.0) | 0.25 | 1.46 | 0.77‐2.80 |
| GGT, last ≥150 IU/L (vs <150 IU/L) | 86.7 (vs 90.6) | 0.31 | 1.47 | 0.71‐3.05 |
| Creatinine, last ≥1.5 mg/dL (vs <1.5 mg/dL) | 89.3 (vs 89.6) | 0.94 | 1.03 | 0.48‐2.23 |
| Image findings of steatosis: present (vs absent) | 89.3 (vs 91.1) | 0.71 | 0.82 | 0.28‐2.40 |
| Number of declines for donor reason ≥3 (vs <3) | 82.5 (vs 90.2) | 0.13 | 1.96 | 0.81‐4.71 |
| Surgical factor | ||||
| Split liver transplantation: yes (vs no) | 91.9 (vs 89.3) | 0.63 | 0.74 | 0.22‐2.50 |
| Simultaneous liver and kidney transplantation: yes (vs no) | 100.0 (vs 89.1) | 1.00 | 0.00 | 0–∞ |
| Total ischemic time (vs <9.0 h) | (vs 95.2) | reference | ||
| 9.0‐10.9 h | 90.3 | 0.08 | 2.12 | 0.91‐4.96 |
| 11.0‐12.9 h | 77.0 | <0.001 | 5.91 | 2.62‐13.3 |
| ≥13.0 h | 68.2 | <0.001 | 9.25 | 3.13‐27.3 |
| Others | ||||
| Transplantation era (vs Era1) | (vs 84.7) | reference | ||
| Era2 | 87.6 | 0.52 | 0.79 | 0.37‐1.65 |
| Era3 | 93.9 | 0.02 | 0.38 | 0.17‐0.87 |
| B | Coefficient β | OR exp (β) |
|---|---|---|
| Variables | ||
| Recipient factor | ||
| Re–transplantation: yes (vs no) | 0.545 | 1.724 |
| Encephalopathy: ≥Ⅲ (vs ≤Ⅱ) | 0.000 | 1.000 |
| Medical condition: in ICU (vs not in ICU) | 0.383 | 1.466 |
| MELD score (vs <20) | reference | |
| 20‐29 | 0.000 | 1.000 |
| 30‐39 | 0.291 | 1.338 |
| ≥40 | 0.473 | 1.605 |
| Donor factor | ||
| Age (vs <40 years) | reference | |
| 40‐59 years | 0.000 | 1.000 |
| 60‐69 years | 0.390 | 1.476 |
| ≥70 years | 0.869 | 2.385 |
| Catecholamine index (vs <10.0) | reference | |
| 10.0‐29.9 | 0.262 | 1.300 |
| ≥30.0 | 0.642 | 1.901 |
| Sodium, maximum (vs <160 mEq/L) | reference | |
| 160‐179 mEq/L | 0.000 | 1.000 |
| ≥180 mEq/L | 0.518 | 1.678 |
| Total bilirubin, maximum (vs <3.0 mg/dL) | reference | |
| 3.0‐4.9 mg/dL | 0.079 | 1.082 |
| ≥5.0 mg/dL | 0.544 | 1.723 |
| Surgical factor | ||
| Total ischemic time (vs <9.0 h) | reference | |
| 9.0‐10.9 h | 0.000 | 1.000 |
| 11.0‐12.9 h | 0.923 | 2.541 |
| ≥13.0 h | 1.152 | 3.164 |
| Others | ||
| Transplantation era (vs Era1) | reference | |
| Era2 | 0.000 | 1.000 |
| Era3 | – 0.170 | 0.842 |
| Intercept | – 2.876 | 0.056 |
Bold emphasis indicates variables with P values <0.10 in the univariate analyses (A) and variables with non‐zero coefficient in the LASSO analysis (B). Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BA, biliary atresia; BMI, body mass index; CI, confidential intervals; GGT, gamma‐glutamyl transpeptidase; ICU, intensive care unit; LASSO, the least absolute shrinkage and selection operator; MELD, Model for End‐stage Liver Disease.
FIGURE 3.
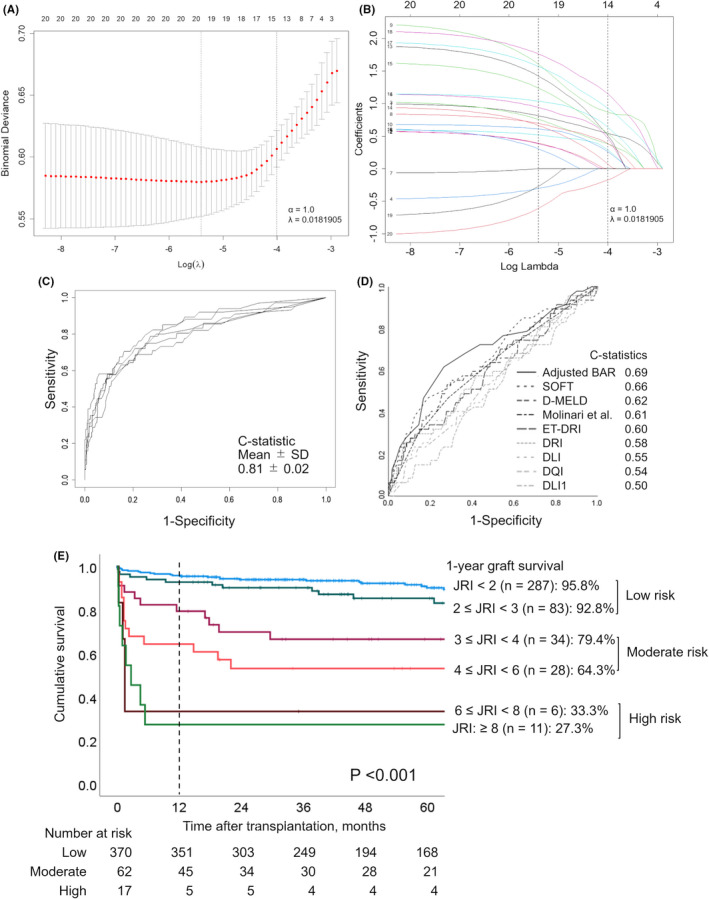
Development and validation of a new risk model for 1‐year graft loss in Japan. (A) Variable selection using the least absolute shrinkage and selection operator (LASSO) logistic regression model. Fourteen variables were selected by LASSO logistic regression analysis. Two dotted vertical lines mark the optimal values of lambda (λ) by minimum criteria and 1‐standard error criteria. (B) LASSO coefficient profiles of the 20 variables. The numbers assigned for each curve show each variable as follows: re‐transplantation (1), encephalopathy: ≥Ⅲ (2), medical condition in ICU (3), MELD score (20‐29 [4], 30‐39 [5], ≥40 [6]), donor age (40‐59 years [7], 60‐69 years [8], ≥70 years [9]), catecholamine index (10.0‐29.9 [10], ≥30.0 [11]), maximum sodium level (160‐179 mEq/L [12], ≥180 mEq/L [13]), maximum total bilirubin level (3.0‐4.9 mg/dL [14], ≥5.0 mg/dL [15]), total ischemic time (9.0‐10.9 h [16], 11.0‐12.9 h [17], ≥13.0 h [18]), and transplantation era (Era2 [19], Era3 [20]). A vertical line indicates the optimal value of lambda based on by the least mean square error, which gives 14 nonzero coefficients. (C) The Japan Risk Index (JRI) was assessed through 5‐fold cross‐validation using the full sample of 449 participants for internal validation. Across the folds, the model had a mean C‐statistic of 0.81 and a standard deviation of 0.02. (D) Ability of the previous risk models to discriminate 1‐year graft loss. The C‐statistic was calculated for each model. The C‐statistics for all previously reported models were below 0.70. (E) Graft survival following DDLT according to risk scores. The 1‐year graft survival rate worsened significantly as the risk score increased (P < 0.001). The following three groups were obtained based on survival rates: low‐risk (JRI < 3), moderate‐risk (3 ≤ JRI < 6), and high‐risk (JRI ≥6). BAR, Balance of Risk; DDLT, deceased donor liver transplantation; DLI, Donor Liver Index; DLI1, Donor Liver Index for 1‐year graft survival; D‐MELD, Donor Age and Recipient Model for End‐Stage Liver Disease; DQI, Donor Quality Index; DRI, Donor Risk Index; ET‐DRI, Eurotransplant Donor Risk Index; ICU, intensive care unit; JRI, Japan Risk Index; LASSO, least absolute shrinkage and selection operator; MELD, Model for End–stage Liver Disease; SOFT, Survival Outcomes Following Liver Transplantation
JRI = (JRI for recipient [JRI‐R]) × (JRI for donor [JRI‐D]) × (JRI for surgery [JRI‐S]) × (0.84 [if Era3])
Estimated 1‐year graft loss rate (%) = JRI × Intercept (0.056) × 100
JRI‐R = exp ([0.545, if re‐transplantation] + [0.291, if MELD 30‒39] + [0.473, if MELD ≥40] + [0.383, if recipient ICU])
JRI‐D = exp ([0.390, if donor age 60‒69 years] + [0.869, if donor age ≥70 years] + [0.262, if catecholamine index 10.0‒29.9] + [0.642, if catecholamine index ≥30.0] + [0.518, if donor maximum sodium ≥180 mEq/L] + [0.079, if donor maximum total bilirubin 3.0‒4.9 mg/dL] + [0.544, if donor maximum total bilirubin ≥5.0 mg/dL])
JRI‐S = exp ([0.923, if total ischemic time 11.0‒12.9 h] + [1.152, if total ischemic time ≥13.0 h])
Then, an internal validation of the JRI was performed through 5‐fold cross‐validation using the full sample of 449 participants. Across the folds, the model achieved a mean C‐statistic of 0.81 and a standard deviation of 0.02 (Figure 3C). On the other hand, the C‐statistic values for all previous models were <0.70 (Figure 3D).
The Kaplan‐Meier curves for the JRI are shown in Figure 3E. Three groups were obtained according to the following values: low risk (JRI <3), moderate risk (3 ≤ JRI < 6), and high risk (JRI ≥6). JRI scores clearly corresponded to excellent, acceptable, and poor 1‐year graft survival outcomes, respectively.
3.3. Graft survival rates and number of DDLTs according to donor, recipient, and surgical risk
JRI‐D, JRI‐R, and JRI‐S were categorized into 3, 4, and 2 subclasses [(JRI‐D = 1, 1 < JRI‐D < 1.5, and JRI‐D ≥ 1.5), (JRI‐R = 1, 1 < JRI‐R < 1.5, 1.5 < JRI‐R < 2, and JRI‐R ≥ 2), and (JRI‐S = 1 and >1)], respectively. The number of DDLTs and 1‐year graft survival rate for each risk class are presented in Table 3. Poor 1‐year graft survival was consistent with overlap of the three risk assessments, and excessive risk duplication was limited. In particular, the significance of JRI‐S was notable when it overlapped with other poor prognostic factors.
TABLE 3.
One‐year graft survival rate (number of DDLTs) according to the risk indices by donor, recipient, and surgical factors
| JRI‐D a = 1.0 | 1.0 < JRI‐D < 1.5 | JRI‐D ≥ 1.5 | Total | ||||
|---|---|---|---|---|---|---|---|
| JRI‐S c = 1 | JRI‐S > 1 | JRI‐S = 1 | JRI‐S > 1 | JRI‐S = 1 | JRI‐S > 1 | ||
| JRI‐R b = 1.0 | 97.7% (n = 131) | 90.9% (n = 33) | 95.2% (n = 42) | 75.0% (n = 12) | 88.9% (n = 18) | 60.0% (n = 5) | 93.8% (n = 226) |
| 1.0 < JRI‐R < 1.5 | 94.3% (n = 53) | 92.3% (n = 13) | 100.0% (n = 18) | 0.0% (n = 3) | 80.0% (n = 10) | 100.0% (n = 1) | 90.8% (n = 98) |
| 1.5 < JRI‐R < 2.0 | 91.2% (n = 34) | 76.9% (n = 13) | 92.9% (n = 14) | 66.7% (n = 3) | 100% (n = 1) | 50.0% (n = 2) | 86.6% (n = 67) |
| JRI‐R ≥ 2 | 85.7% (n = 21) | 33.3% (n = 3) | 66.7% (n = 9) | 50.0% (n = 4) | 50.0% (n = 2) | 25.0% (n = 4) | 67.4% (n = 43) |
| Total | 95.0% (n = 239) | 85.5% (n = 62) | 92.8% (n = 83) | 59.1% (n = 22) | 83.9% (n = 31) | 50.0% (n = 12) | 89.1% (n = 449) |
Dark and light gray color shade indicate that 1‐year graft survival rate ≤50% and 50% < 1‐year graft survival rate <80%, respectively. Abbreviations: DDLT, deceased donor liver transplantation; JRI, Japan Risk index; JRI‐D, JRI for donor; JRI‐S, JRI for surgery; JRI‐R, JRI for recipient; MELD, Model for End‐Stage Liver Disease.
JRI‐D = exp ([0.390 if donor age 60‒69] + [0.869 if donor age ≥70] + [0.262 if catecholamine index 10.0‒29.9] + [0.642 if catecholamine index ≥30.0] + [0.518 if donor maximum sodium ≥180 mEq/L] + [0.079 if donor maximum total bilirubin 3.0‒4.9 mg/dL] + [0.544 if donor maximum total bilirubin ≥5.0 mg/dL]).
JRI‐R = exp ([0.545 if re‐transplantation] + [0.291 if MELD30‒39] + [0.473 if MELD ≥40 ]+ [0.383 if recipient ICU]).
JRI‐S = exp ([0.923 if total ischemic time 11.0‒12.9 h] + [1.152 if total ischemic time ≥13.0 h]).
3.4. Changes in risk factors and risk indices over time
The number of DDLTs with the determined risk factors and the developed risk indices were compared among the eras in Table 4. We observed a significant increase in the number of patients with MELD scores ≥30 and significant decreases in numbers of patients with catecholamine index ≥10 and total ischemic time ≥11 h from Era1 to Era3. The mean JRI (without era) and the number of DDLTs with a JRI (without era) ≥6 was significantly lower in Era3 than in Era2 (P = 0.02). The mean JRI‐S value was significantly lower in Era3 than in Era1 (P = 0.007).
TABLE 4.
Changes in risk factors and risk indices over the eras
| All era n = 449 | Era1 n = 85 | Era2 n = 185 | Era3 n = 179 |
Era3 vs Era1 P value |
Era3 vs Era2 P value | |
|---|---|---|---|---|---|---|
| Recipient factor | ||||||
| Re‐transplantation | 76 (16.9) | 13 (15.3) | 37 (20.0) | 26 (14.5) | 0.87 | 0.17 |
| Medical condition: in ICU | 126 (28.1) | 15 (17.6) | 61 (33.2) | 50 (27.9) | 0.07 | 0.28 |
| MELD score ≥30 | 85 (19.0) | 10 (11.9) | 32 (17.3) | 43 (24.0) | 0.02 | 0.11 |
| Donor factor | ||||||
| Age ≥60 years | 65 (14.5) | 7 (8.2) | 34 (18.4) | 24 (13.4) | 0.22 | 0.20 |
| Catecholamine index ≥10 | 93 (20.9) | 29 (35.4) | 35 (18.9) | 29 (16.2) | 0.001 | 0.50 |
| Sodium, maximum ≥180 mEq/L | 12 (2.7) | 0 (0.0) | 6 (3.2) | 6 (3.4) | 0.19 | 0.73 |
| Total bilirubin, maximum ≥3 mg/dL | 54 (12.0) | 7 (8.2) | 28 (15.1) | 19 (10.6) | 0.54 | 0.20 |
| Surgical factor | ||||||
| Total ischemic time ≥11 h | 96 (21.4) | 26 (30.6) | 42 (22.7) | 28 (15.6) | 0.01 | 0.09 |
| The proposed risk indices | ||||||
| JRI (without era) a | 2.48 ± 3.84 | 2.36 ± 1.90 | 3.01 ± 5.70 | 1.98 ± 1.18 | 0.09 | 0.02 |
| JRI (without era) ≥6 | 20 (3.3) | 3 (3.5) | 13 (7.0) | 4 (2.2) | 0.54 | 0.03 |
| JRI‐R b | 1.37 ± 0.54 | 1.27 ± 0.48 | 1.43 ± 0.56 | 1.37 ± 0.68 | 0.11 | 0.35 |
| JRI‐D c | 1.17 ± 0.48 | 1.18 ± 0.29 | 1.21 ± 0.68 | 1.11 ± 0.22 | 0.07 | 0.06 |
| JRI‐S d | 1.28 ± 0.68 | 1.52 ± 0.80 | 1.38 ± 0.72 | 1.25 ± 0.59 | 0.007 | 0.06 |
Data were presented as mean values ± standard deviations and numbers (%) for continuous and categorical variables, respectively. Bold emphasis indicates statistical significance (P <0.05).
Abbreviations: ICU, intensive care unit; JRI, Japan Risk index; JRI‐D, JRI for donor; JRI‐R, JRI for recipient; JRI‐S, JRI for surgery; MELD, Model for End‐stage Liver Disease.
JRI (without era) = (JRI for recipient [JRI‐R]) × (JRI for donor [JRI‐D]) × (JRI for surgery [JRI‐S]).
JRI‐R = exp ([0.545 if re‐transplantation] + [0.291 if MELD30‒39] + [0.473 if MELD ≥40] + [0.383 if recipient ICU]).
JRI‐D = exp ([0.390 if donor age 60‒69] + [0.869 if donor age ≥70] + [0.262 if catecholamine index 10.0‒29.9] + [0.642 if catecholamine index ≥30.0] + [0.518 if donor maximum sodium ≥180 mEq/L] + [0.079 if donor maximum total bilirubin 3.0‒4.9 mg/dL] + [0.544 if donor maximum total bilirubin ≥5.0 mg/dL]).
JRI‐S = exp ([0.923 if total ischemic time 11.0‒12.9 h] + [1.152 if total ischemic time ≥13.0 h]).
3.5. Graft survival in patients with and without liver steatosis and fibrosis
Graft survival curves for cases with and without liver steatosis and fibrosis are shown in Figure 4. There was no graft loss in DDLTs with ≥30% steatosis (n = 12) and F2 fibrosis (n = 6). The proportion of DDLTs with selected risk factors and corresponding JRI values are shown in Table S1. There was no case with risk factors of maximum total bilirubin ≥3 mg/dL or total ischemic time ≥11 h among DDLTs involving livers with ≥30% steatosis. There was also no case with risk factors of donor age ≥60 years, maximum total bilirubin ≥3 mg/dL, or total ischemic time ≥11 h among DDLTs involving livers with F2 fibrosis. Moreover, there was also no case with a JRI ≥3 among DDLTs with ≥30% steatosis, and there was only one patient with a JRI ≥3 among DDLTs involving livers with F2 fibrosis.
FIGURE 4.
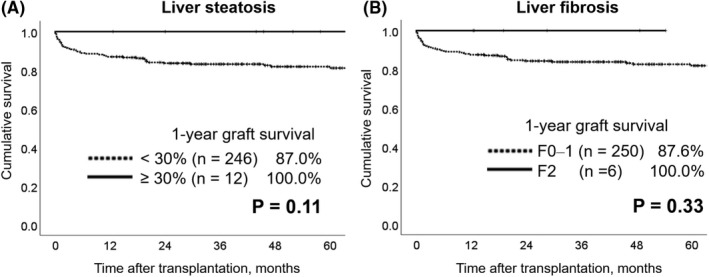
Graft survival with and without liver steatosis or fibrosis. Kaplan‐Meier curves of graft survival with (≥30%) and without (<30%) liver steatosis are shown in (A), and those with (F2) and without (F0‒1) liver fibrosis are shown in (B). There was no graft loss for livers with steatosis and those with fibrosis
4. DISCUSSION
This nationwide study investigated outcomes of Japanese DDLTs performed over the past 20 years. To help future researchers in developing a strategy for successful Japanese DDLT, we examined the DDLT graft survival rate in Japan to develop a new risk model. The risk model proposed in this study provides insight into the reasons underlying improved graft survival over time in Japan. Prospective use of this risk model may help to facilitate graft survival and enable efficient utilization of marginal donors.
The 1‐year graft survival throughout all eras was 89.5%, and era‐specific survival analysis revealed that the 1‐year graft survival rate reached 93.9% in the most recent era. Thus, although the number of transplants remains small, Japan's DDLT success rate has reached a level comparable with those of other countries and regions. 3 , 19 , 20 In establishing the risk model, we also identified risk factors and non‐risk factors for 1‐year graft survival unique to DDLT in Japan. Although nine risk factors were included in the model for 1‐year graft survival, variables not included among these nine factors should also be noted. As shown in Table 2, duration on a respirator (an indicator of long‐term donor management as well as ICU stay) was not significant in our univariate analysis for 1‐year graft survival, which is in disagreement with some previous studies. 21 , 22 This result suggests that the Japanese donor evaluation and management system, which employs professional transplant management doctors (medical consultants) to assess donor organ eligibility and advise the physicians of the donor hospital regarding donor management, 23 is now well‐functioning. Another point of interest is split LT, which has also been reported to be a poor prognostic factor. 1 , 4 , 24 In Japan, Sakamoto and colleagues already reported that 36 adult and pediatric recipients who underwent split LT from 1999 to 2014 achieved an acceptable 1‐year graft survival rate (91.0%). 25 Our study focused on adults and expanded the patient population to recent cases. Our current results suggest that there is little effect of split LT on 1‐year graft survival in adult patients, although these patients typically receive a partial liver graft that remains after a pediatric patient has first received a desired graft from a whole donor liver.
The main objective of this study was to establish a nationwide standard for organ acceptance in Japan. According to the C‐statistics, none of the previous models had an adequately high discriminative ability to predict the 1‐year graft survival of the Japanese DDLT cohort. In this study, we developed a risk index based on the national data collected from Era1 to Era3 and proposed using the index (JRI = JRI‐R × JRI‐D × JRI‐S × 0.84) as a new prognostic risk model for 1‐year graft survival. As shown in Figure 3E, the JRI clearly stratified the survival rates. Considering the 1‐year graft survival rate of DDLT in other countries (88.2% from 2010 to 2014 in the United States and 83.0% from 2009 to 2013 in France), 3 , 19 we suggest organ acceptance based on the following criteria, at least until achieving a sufficient increase in the number of donor organs:
JRI < 3: recommended
3 ≤ JRI < 6: controversial but acceptable when the patient's general condition does not allow further waiting
JRI ≥ 6: discouraged
The proposed risk model, JRI, and Table 3 may be useful references for recipients with extremely high risk and marginal donors in the future.
Interestingly, the total ischemic time is not a definite known factor at the time of organ acceptance decision, but has the highest odds ratio among the selected variables in the LASSO regression analysis for 1‐year graft loss (Table 2B). As shown in Table 3, 1‐year graft survival was extremely poor when recipient, donor, and especially surgical factors overlapped. We attempted to develop a risk index using variables without including the total ischemic time, but the developed index did not maintain sufficient predictive power compared to that with total ischemic time (mean C‐statistic ± SD = 0.73 ± 0.03 vs 0.81 ± 0.02, respectively; detailed data are not presented). It is very important for transplant surgeons to accurately estimate the total ischemic time collecting traffic information and understand the difficulty of liver removal. The most important issue may be that the total ischemic time is shortened by introducing a regional allocation system so that the impact of ischemic time becomes negligible.
We also measured the changes in risk factors and risk index according to era. As shown in Table 4, the DDLT environment has changed significantly over time. Recipients were more severely ill in Era3 than in Era1. In 2011, the Japanese DDLT allocation system modified the medical emergency score to prioritize not only the Child‐Pugh score but also the MELD score, which may have contributed to the increase in the number of DDLTs with MELD scores ≥30 after Era2. In contrast, our results indicate that transplant surgeons succeeded in performing DDLT under favorable conditions in terms of ischemic time, and intensive care physicians were also successful in reducing catecholamine usage during donor management in Era3. The analysis of the JRI revealed that several indices were significantly decreased in Era3, suggesting that transplant surgeons had become more cautious about challenging preoperative conditions and intended to avoid overlapping potential risk factors in Era3. Such alterations in the attitudes of transplant surgeons may have contributed to the excellent 1‐year graft survival rate in Era3, along with advances in transplant surgery and postoperative management.
The pathological findings of a graft (steatosis or fibrosis) are medically definite factors affecting graft function and are among the main reasons for declining a graft. 26 , 27 , 28 , 29 The analysis focusing on these pathological findings revealed that the 1‐year graft survival rate was 100% in cases involving livers with moderate steatosis and fibrosis, implying that steatosis and fibrosis have little effect on 1‐year graft survival in Japan. However, the comparison of risk factors between patients with and without each finding indicated that DDLTs using steatotic and fibrotic livers were performed under favorable conditions. Thus, acceptance of steatotic and fibrotic livers is possible but should be approached carefully. Future studies should investigate the detailed criteria for the acceptance of steatotic and fibrotic livers in DDLT.
This study had some limitations, the most substantial of which was the low number of included DDLTs. This study covered all past DDLTs in Japan and collected as much information as possible from the national databases, but the sample size was relatively smaller than those for studies conducted in other countries and regions, limiting the statistical power of our analysis. In particular, the number of donors at high risk (JRI ≥6) was limited. Therefore, we discourage organ acceptance in the high‐risk group in this study; however, this may need to be reconsidered with the increase in the number of DDLTs. Since it is unclear whether Japan's DDLT performance is mature enough to prospectively use the model developed based on previous data, our suggestion to use the model with a coefficient of 0.84 presupposes that the conditions of the coming era are equivalent to those of Era3. Therefore, continued data collection is essential for validating and updating the risk index proposed in this study.
In conclusion, through a detailed analysis of DDLT cases in Japan over 20 years, we developed a risk model for 1‐year graft loss unique to Japan. Retrospective analysis of risk index transitions was useful in understanding the reasons for improved graft survival over time. Based on past outcomes according to risk index, a JRI of <3 should be preferred for organ acceptance in Japan. Prospective use of this risk score may further improve graft survival and allow for effective utilization of marginal donors.
CONFLICT OF INTEREST
Author YK is Editor in Chief of Annals of Gastroenterological Surgery. Author HO and SE are current editors of Annals of Gastroenterological Surgery.
ETHICAL APPROVAL AND CONSENT
This study was approved by the project committee of the JLTS and the Institutional Review Board of the Keio University School of Medicine (#20180301, #20140289). The JOTNW data were provided for this study with the approval of the Institutional Review Board of the JOTNW (#4). There was no need to acquire written informed consent from each patient given the nature of the study.
Supporting information
Table S1
ACKNOWLEDGEMENTS
We would like to thank the volunteers and JOTNW staff for their cooperation in constructing the JLTS and JOTNW databases.
Takemura Y, Shinoda M, Takemura R, Hasegawa Y, Yamada Y, Obara H, et al. Development of a risk score model for 1‐year graft loss after adult deceased donor liver transplantation in Japan based on a 20‐year nationwide cohort. Ann Gastroenterol Surg. 2022;6:712–725. 10.1002/ags3.12573
Funding information
This work was partially supported by Grants‐in‐Aid from the Ministry of Education, Science, and Culture of Japan (#20K08967) and a donation from family members of patients who were on the waiting list
DATA AVAILABILITY STATEMENT
The datasets used during this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Feng S, Goodrich NP, Bragg‐Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–90. [DOI] [PubMed] [Google Scholar]
- 2. Braat AE, Blok JJ, Putter H, Adam R, Burroughs AK, Rahmel AO, et al. The Eurotransplant donor risk index in liver transplantation: ET‐DRI. Am J Transplant. 2012;12(10):2789–96. [DOI] [PubMed] [Google Scholar]
- 3. Winter A, Feray C, Audureau E, Azoulay D, Antoine C, Daurès J‐P, et al. A donor quality index for liver transplantation: development, internal and external validation. Sci Rep. 2018;8(1):9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collett D, Friend PJ, Watson CJE. Factors associated with short‐ and long‐term liver graft survival in the United Kingdom: development of a UK donor liver index. Transplantation. 2017;101(4):786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, et al. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8(12):2537–46. [DOI] [PubMed] [Google Scholar]
- 6. Dutkowski P, Schlegel A, Slankamenac K, Oberkofler CE, Adam R, Burroughs AK, et al. The use of fatty liver grafts in modern allocation systems: risk assessment by the balance of risk (BAR) score. Ann Surg. 2012;256(5):861–9. [DOI] [PubMed] [Google Scholar]
- 7. Furukawa H, Taniguchi M, Fujiyoshi M, Oota M. Experience using extended criteria donors in first 100 cases of deceased donor liver transplantation in Japan. Transplant Proc. 2012;44(2):373–5. [DOI] [PubMed] [Google Scholar]
- 8. Mochida S, Takikawa Y, Nakayama N, Oketani M, Naiki T, Yamagishi Y, et al. Diagnostic criteria of acute liver failure: a report by the intractable hepato‐biliary diseases study group of Japan. Hepatol Res. 2011;41(9):805–12. [DOI] [PubMed] [Google Scholar]
- 9. Yamashita C, Hara Y, Kuriyama N, Nakamura T, Nishida O. Clinical effects of a longer duration of polymyxin B‐immobilized fiber column direct hemoperfusion therapy for severe sepsis and septic shock. Ther Apher Dial. 2015;19(4):316–23. [DOI] [PubMed] [Google Scholar]
- 10. Ichida F, Tsuji T, Omata M, Ichida T, Inoue K, Kamimura T, et al. New inuyama classification; new criteria for histological assessment of chronic hepatitis. Int Hepatol Commun. 1996;6(2):112–9. [Google Scholar]
- 11. Kasahara M, Katono M, Schlegel A, Kubota T, Nakazato Y, Uchida H, et al. Waiting list mortality for pediatric deceased donor liver transplantation in a Japanese living‐donor‐dominant program. Pediatr Transplant. 2019;23(8):e13578. [DOI] [PubMed] [Google Scholar]
- 12. Sakamoto S, Uchida H, Shimizu S, Yanagi Y, Takeda M, Kubota T, et al. Current status of pediatric deceased donor liver transplantation: lessons learned from a high‐volume center in Japan where living donation remains predominant. J Hepatobiliary Pancreat Sci. 2020;28(11):1–9. [DOI] [PubMed] [Google Scholar]
- 13. Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–9. [DOI] [PubMed] [Google Scholar]
- 14. Chen Z, Li J, Sun Y, Wang C, Yang W, Ma M, et al. A novel predictive model for poor in‐hospital outcomes in patients with acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2021;S0022–5223(21):778–9. [DOI] [PubMed] [Google Scholar]
- 15. Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. 2005;21(20):3940–1. [DOI] [PubMed] [Google Scholar]
- 16. Halldorson JB, Bakthavatsalam R, Fix O, Reyes JD, Perkins JD. D‐MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9(2):318–26. [DOI] [PubMed] [Google Scholar]
- 17. Molinari M, Ayloo S, Tsung A, Jorgensen D, Tevar A, Rahman SH, et al. Prediction of perioperative mortality of cadaveric liver transplant recipients during their evaluations. Transplantation. 2019;103(10):e297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92. [DOI] [PubMed] [Google Scholar]
- 19. Gao Q, Mulvihill MS, Scheuermann U, Davis RP, Yerxa J, Yerokun BA, et al. Improvement in liver transplant outcomes from older donors: a US national analysis. Ann Surg. 2019;270(2):333–9. [DOI] [PubMed] [Google Scholar]
- 20. Annual report on liver transplantation. Report for 2018/2019 [internet]. National Health Service Blood and Transplant, 2019;36–8. [updated August 2019; https://nhsbtdbe.blob.core.windows.net/ Accessed November 14, 2021.
- 21. Cuende N, Miranda B, Canon JF, Garrido G, Matesanz R. Donor characteristics associated with liver graft survival. Transplantation. 2005;79(10):1445–52. [DOI] [PubMed] [Google Scholar]
- 22. Cameron AM, Ghobrial RM, Yersiz H, Farmer DG, Lipshutz GS, Gordon SA, et al. Optimal utilization of donor grafts with extended criteria: a single‐center experience in over 1000 liver transplants. Ann Surg. 2006;243(6):748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukushima N, Ono M, Saiki Y, Minami M, Konaka S, Ashikari J. Donor evaluation and management system (medical consultant system) in Japan: experience from 200 consecutive brain‐dead organ donation. Transplant Proc. 2013;45(4):1327–30. [DOI] [PubMed] [Google Scholar]
- 24. Lee KW, Cameron AM, Maley WR, Segev DL, Montgomery RA. Factors affecting graft survival after adult/child split‐liver transplantation: analysis of the UNOS/OPTN data base. Am J Transplant. 2008;8(6):1186–96. [DOI] [PubMed] [Google Scholar]
- 25. Sakamoto S, Kasahara M, Ogura Y, Inomata Y, Uemoto S. Current status of deceased donor split liver transplantation in Japan. J Hepatobiliary Pancreat Sci. 2015;22(12):837–45. [DOI] [PubMed] [Google Scholar]
- 26. Linares I, Hamar M, Selzner N, Selzner M. Steatosis in liver transplantation: current limitations and future strategies. Transplantation. 2019;103(1):78–90. [DOI] [PubMed] [Google Scholar]
- 27. Wadhera V, Harimoto N, Lubezky N, Gomatos I, Facciuto M, Gonzalez D, et al. The impact of donor liver allograft fibrosis on patients undergoing liver transplantation. Clin Transplant. 2018;32(3):e13187. [DOI] [PubMed] [Google Scholar]
- 28. de Graaf EL, Kench J, Dilworth P, Shackel NA, Strasser SI, Joseph D, et al. Grade of deceased donor liver macrovesicular steatosis impacts graft and recipient outcomes more than the donor risk index. J Gastroenterol Hepatol. 2012;27(3):540–6. [DOI] [PubMed] [Google Scholar]
- 29. Nafidi O, Marleau D, Roy A, Bilodeau M. Identification of new donor variables associated with graft survival in a single‐center liver transplant cohort. Liver Transplant. 2010;16(12):1393–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The datasets used during this study are available from the corresponding author on reasonable request.


