Abstract
Background:
Recent investigations of malignant peripheral nerve sheath tumor (MPNST) survival have reported higher mortality among non-White individuals. However, previous analyses have not examined the impact of socioeconomic status on these observations. This study aims to characterize factors associated with cause-specific MPNST survival, including information related to census-tract level socioeconomic status (CT-SES).
Methods:
We identified 2432 primary MPNSTs using the SEER 18 (2000-2016) database. We used Cox proportional hazards modeling to estimate the effects of sex, race/ethnicity, CT-SES quintile, metastasis at diagnosis, tumor site, age at diagnosis, and treatment by surgery on survival. Models were fit in both the full population and, separately, stratified by race/ethnicity and age at diagnosis (<40 vs ≥40).
Results:
In adjusted models, age at diagnosis, CT-SES, and metastasis at diagnosis were associated with mortality. In race/ethnicity stratified analysis, higher CT-SES was found to improve survival only in the White population. Among those diagnosed before age 40, metastasis at diagnosis and American Indian/Alaska Native race/ethnicity were associated with mortality, and both Hispanic ethnicity and Asian/Pacific Islander race were suggestive for increased mortality. Among cases diagnoses at age 40 and above, age at diagnosis, male sex, and CT-SES were associated with mortality.
Conclusions:
This analysis provides evidence that among pediatric and young adult patients, non-White populations experience inferior survival compared to Whites, independent of CT-SES. Our findings also suggest that the effect of CT-SES on MPNST survival may differ by racial/ethnic group.
Impact:
These findings suggest that barriers to healthcare for certain racial/ethnic groups extend beyond SES.
Introduction
Malignant peripheral nerve sheath tumors (MPNSTs) are rare soft tissue sarcomas of the peripheral nervous system that are estimated to make up 5-10% of the approximately 6000 soft tissue sarcomas diagnosed in the United States annually 1. MPNSTs arise primarily from Schwann cells—the cells forming the myelin sheath in the peripheral nervous system—and can occur throughout the body.
Though MPNSTs are rare in the general population, with an incidence rate of approximately 0.001% 1, individuals with Neurofibromatosis Type 1 (NF1) are at substantially increased risk. NF1 is an autosomal dominant cancer predisposition syndrome caused by inherited or de novo mutations of the NF1 tumor suppressor gene, occurring in approximately 1:2500 to 1:3500 births 2. The incidence of MPNSTs among individuals with NF1 is 1000-2000 times higher than that of the general population 3, and approximately 50% of all MPNSTs are NF1-related. Additionally, MPNSTs that occur among NF1 patients are typically diagnosed at earlier ages than those that arise sporadically; one study of MPNSTs found that the median age at diagnosis of MPNST in NF1 patients was 26 years, compared to 62 years in the those without NF1 4. Lifetime risk of MPNSTs in individuals with NF1 is estimated to be between 8 and 13% 1,4.
MPNSTs have poor prognosis, with a 5-year survival estimated at just over 50% overall 5, and lower survival rates among individuals with NF1 6. Past investigations of MPNST survival have examined the influence of tumor location, tumor size, tumor grade, age at diagnosis, race/ethnicity, sex, and therapy on MPNST survival 5,7-16. A recent analysis reported increased mortality among Black children 7 while another reported lowered overall survival in Black individuals of all ages 16, and a third analysis reported lower survival in individuals who identified as a race other than White, Black, or Asian/Pacific Islander 5. Socioeconomic factors, such as access to preventative health care, health literacy, economic stability, insurance status, stress, and provider linguistic and cultural competency may also influence MPNST survival. Although socioeconomic factors are intertwined with race/ethnicity and may also influence MPNST outcomes, previous studies have not investigated the role of socioeconomic status (SES) in MPNST survival. This analysis aims to further elucidate factors affecting MPNST survival including census-tract level SES (CT-SES), through an analysis of data from the Surveillance, Epidemiology, and End Results program.
Materials and Methods
Study population:
Incident MPNST cases were identified in the Surveillance, Epidemiology, and End Results 18 database along with relevant treatment and demographic variables. The SEER 18 database includes data from the following states/regions: Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco/Oakland, Louisiana, Utah, Seattle-Puget Sound, Atlanta, San Jose/Monterey, Los Angeles, Rural Georgia, Alaska Natives, Greater California, Kentucky, New Jersey, and Greater Georgia. Due to limitations on the availability of SES related variables, this analysis was conducted on incident cases diagnosed between 2000 and 2016.
Case definition:
MPNST cases were identified for this analysis using the following ICD-O-3 histologic codes: 9540 (MPNST); 9560 (Neurilemoma); and 9561 (Triton tumor). Epithelioid MPNSTs (9542) and perineuriomas (9571) were not included in this analysis.
Variables of Interest:
The following variables were obtained as variables of interest: sex (male, female), race/ethnicity (Hispanic, non-Hispanic White [NHW], non-Hispanic Black [NHB], non-Hispanic Asian/Pacific Islander [API], non-Hispanic American Indian/Alaska Native [AI/AN], non-Hispanic Unknown), Census tract SES (CT-SES) as measured by Yost quintile, metastasis at diagnosis (yes, no), number of lifetime in situ/malignant tumors (one, two or greater), tumor site (Central nervous system/CNS [primary sites in the cerebrum, cerebellum, brain stem, spinal cord, or meninges], head/neck, trunk/core, limbs, other [nervous system not otherwise specified; subcutaneous, connective and other soft tissues not otherwise specified; and unknown]), tumor size (<4 cm, 4-7 cm, >7 cm), age at diagnosis, and treatment by surgery. The primary sites included in each tumor site category have been more fully described in Supplemental Table 1. Due to high levels of missingness, treatment by radiation and tumor grade were excluded from analysis. Tumor size was likewise excluded from the main analysis due to missingness.
The outcome of interest was cause-specific mortality.
Statistical Analysis:
We investigated and visualized survival trends over time using Kaplan-Meier curves and differences in survival by grouping was determined through log-rank testing. We used Cox proportional hazards modeling to estimate unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals for the association between survival and sex, race/ethnicity, CT-SES (Yost) quintile, metastasis at diagnosis, tumor site, age at diagnosis (continuous), and treatment by surgery. Analyses were conducted stratified by racial/ethnic group in order to understand the effect of SES within these groupings. We also fit models stratified into those diagnosed before age 40 and those diagnosed at ages 40 and older in order to assess what differences may exist between the general population and those with a higher probability of NF1. 40 was selected as an approximate cut point as previous studies have estimated the median age of diagnosis for individuals with NF1 as between 26 and 38 years 1,2 while those without NF1 tend to be diagnosed at later ages. Additional sub analyses were conducted stratified by CT-SES quintile as well as in the subset of data for which tumor size was available. The proportionality assumption was investigated and verified through visual inspection of Schoenfeld residuals. All analyses were conducted using R (version 4.0.3).
Data Availability:
The data for this analysis is available through the Surveillance, Epidemiology, and End Results program (SEER) and may be accessed through application.
Results
2432 MPNST cases diagnosed between 2000 and 2016 were identified from the SEER 18 database. By the end of follow-up, 1274 (52.4%) individuals had died of MPNST related causes. 62.9% of cases were diagnosed in individuals ages 40 and over, while 37.1% were diagnosed in those under age 40. Additional demographic information on the study population can be found in Table 1.
Table 1:
Demographic and tumor characteristics of individuals diagnosed with primary MPNSTs in the SEEER 18 database 2000-2016
| Age at Diagnosis | ||||
|---|---|---|---|---|
| Under 40 (N=903) |
40 and older (N=1529) |
Overall (N=2432) |
Median Survival Time from Diagnosis (months) |
|
| Sex | ||||
| Female | 390 (43.2%) | 700 (45.8%) | 1090 (44.8%) | 33.5 |
| Male | 513 (56.8%) | 829 (54.2%) | 1342 (55.2%) | 26 |
| Age | ||||
| <1 year | 3 (0.3%) | 0 (0%) | 3 (0.1%) | 54 |
| 1-4 years | 20 (2.2%) | 0 (0%) | 20 (0.8%) | 62.5 |
| 5-9 years | 33 (3.7%) | 0 (0%) | 33 (1.4%) | 43 |
| 10-14 years | 73 (8.1%) | 0 (0%) | 73 (3.0%) | 41 |
| 15-19 years | 112 (12.4%) | 0 (0%) | 112 (4.6%) | 29 |
| 20-24 years | 161 (17.8%) | 0 (0%) | 161 (6.6%) | 30 |
| 25-29 years | 147 (16.3%) | 0 (0%) | 147 (6.0%) | 21 |
| 30-34 years | 152 (16.8%) | 0 (0%) | 152 (6.2%) | 22 |
| 35-39 years | 202 (22.4%) | 0 (0%) | 202 (8.3%) | 26.5 |
| 40-44 years | 0 (0%) | 200 (13.1%) | 200 (8.2%) | 33 |
| 45-49 years | 0 (0%) | 200 (13.1%) | 200 (8.2%) | 53 |
| 50-54 years | 0 (0%) | 192 (12.6%) | 192 (7.9%) | 41.5 |
| 55-59 years | 0 (0%) | 212 (13.9%) | 212 (8.7%) | 34 |
| 60-64 years | 0 (0%) | 169 (11.1%) | 169 (6.9%) | 34 |
| 65-69 years | 0 (0%) | 151 (9.9%) | 151 (6.2%) | 19 |
| 70-74 years | 0 (0%) | 124 (8.1%) | 124 (5.1%) | 24 |
| 75-79 years | 0 (0%) | 133 (8.7%) | 133 (5.5%) | 17.5 |
| 80-84 years | 0 (0%) | 77 (5.0%) | 77 (3.2%) | 27 |
| ≥85 years | 0 (0%) | 71 (4.6%) | 71 (2.9%) | 17.5 |
| Race | ||||
| Non-Hispanic White | 465 (51.5%) | 1064 (69.6%) | 1529 (62.9%) | 30 |
| Hispanic (All Races) | 208 (23.0%) | 147 (9.6%) | 355 (14.6%) | 25.5 |
| Non-Hispanic American Indian/Alaska Native | 10 (1.1%) | 10 (0.7%) | 20 (0.8%) | 41 |
| Non-Hispanic Asian or Pacific Islander | 72 (8.0%) | 106 (6.9%) | 178 (7.3%) | 33.5 |
| Non-Hispanic Black | 141 (15.6%) | 194 (12.7%) | 335 (13.8%) | 30 |
| Non-Hispanic Unknown Race | 7 (0.8%) | 8 (0.5%) | 15 (0.6%) | 60 |
| Surgery | ||||
| No Surgery | 123 (13.6%) | 270 (17.7%) | 393 (16.2%) | 9 |
| Surgery | 772 (85.5%) | 1244 (81.4%) | 2016 (82.9%) | 34.5 |
| Site | ||||
| Central Nervous System | 45 (5.0%) | 96 (6.3%) | 141 (5.8%) | 25 |
| Limb | 309 (34.2%) | 461 (30.2%) | 770 (31.7%) | 41.5 |
| Other | 54 (6.0%) | 97 (6.3%) | 151 (6.2%) | 16 |
| Trunk/Core | 367 (40.6%) | 570 (37.3%) | 937 (38.5%) | 21 |
| Yost Quintile | ||||
| Group 1 | 187 (20.7%) | 251 (16.4%) | 438 (18.0%) | 24 |
| Group 2 | 174 (19.3%) | 258 (16.9%) | 432 (17.8%) | 25 |
| Group 3 | 186 (20.6%) | 279 (18.2%) | 465 (19.1%) | 34.5 |
| Group 4 | 158 (17.5%) | 305 (19.9%) | 463 (19.0%) | 36 |
| Group 5 | 147 (16.3%) | 325 (21.3%) | 472 (19.4%) | 33 |
| Metastasis at Diagnosis | ||||
| No Metastasis | 721 (79.8%) | 1211 (79.2%) | 1932 (79.4%) | 36 |
| Metastasis at Diagnosis | 122 (13.5%) | 183 (12.0%) | 305 (12.5%) | 7 |
| Number in situ/malignant tumors | ||||
| One | 759 (84.1%) | 996 (65.1%) | 1755 (72.2%) | 30 |
| Two or More | 144 (15.9%) | 533 (34.9%) | 677 (27.8%) | 30 |
| Tumor Size | ||||
| <4 cm | 137 (15.2%) | 322 (21.1%) | 459 (18.9%) | 50.5 |
| 4-7 cm | 172 (19.0%) | 310 (20.3%) | 482 (19.8%) | 38 |
| >7 cm | 372 (41.2%) | 424 (27.7%) | 796 (32.7%) | 20 |
| Radiation | ||||
| No Radiation | 3 (0.3%) | 9 (0.6%) | 12 (0.5%) | 24 |
| Radiation | 399 (44.2%) | 606 (39.6%) | 1005 (41.3%) | 25 |
| Grade | ||||
| Well differentiated; Grade I | 118 (13.1%) | 186 (12.2%) | 304 (12.5%) | 69 |
| Moderately differentiated; Grade II | 167 (18.5%) | 237 (15.5%) | 404 (16.6%) | 58 |
| Poorly differentiated; Grade III | 236 (26.1%) | 294 (19.2%) | 530 (21.8%) | 23 |
| Undifferentiated; anaplastic; Grade IV | 53 (5.9%) | 70 (4.6%) | 123 (5.1%) | 20 |
| Histologic Type ICD-O-3 | ||||
| 9450 (MPNST) | 765 (84.7%) | 1251 (81.8%) | 2016 (82.9%) | 28 |
| 9560 (Neurilemoma) | 86 (9.5%) | 221 (14.5%) | 307 (12.6%) | 73 |
| 9561 (Triton Tumor) | 52 (5.8%) | 57 (3.7%) | 109 (4.5%) | 16 |
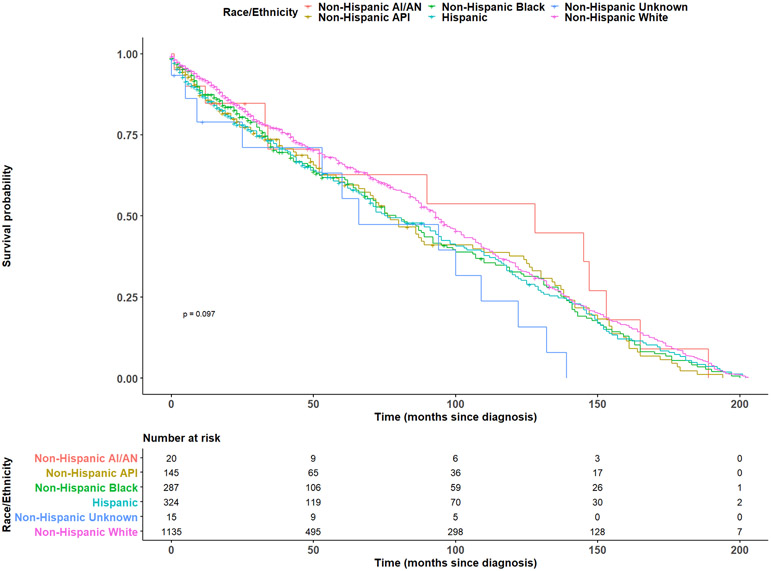
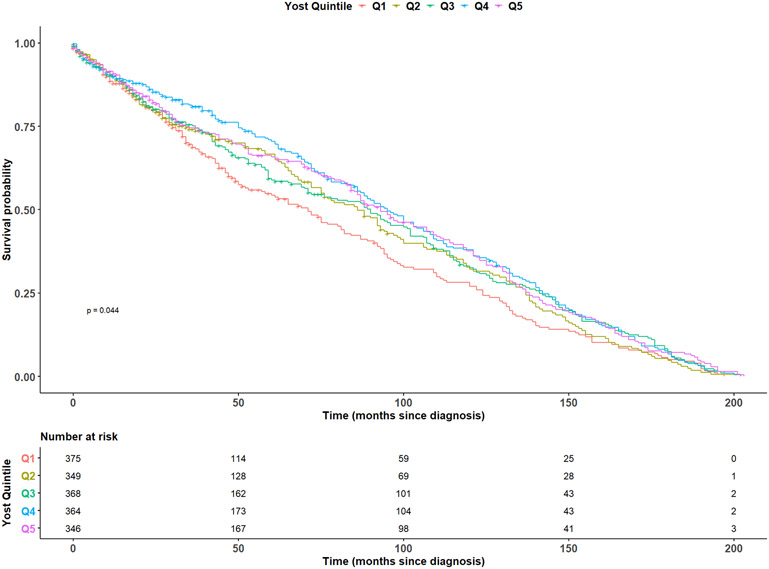
In fully adjusted models, age at diagnosis (HR: 1.01, 95% CI: 1.01-1.01), and metastasis at diagnosis (HR: 1.57, 95% CI: 1.16-2.11) were observed to be statistically significantly associated with an increased risk of MPNST mortality, while CT-SES quintile was inversely associated with mortality (Q2 HR: 0.81 95% CI: 0.66-1.00; Q3 HR: 0.82, 95% CI: 0.67-1.00; Q4 HR: 0.77, 95% CI: 0.63-0.95; Q5 HR: 0.74, 95% CI: 0.60-0.92) (Table 2). We visualized survival trends by race/ethnicity and CT-SES in the full population using Kaplan-Meier estimation in Figures 1 and 2. Race/ethnicity stratified models were fit in the non-Hispanic White, non-Hispanic Black, non-Hispanic Asian/Pacific Islander, and Hispanic racial/ethnic subgroups (Table 3). Models were not fit in the American Indian/Alaska Native population due to sample size constraints. In White individuals, age at diagnosis (HR: 1.01, 95% CI: 1.01-1.02) was associated with an increase in the risk of MPNST mortality, while increasing CT-SES (Q2 HR: 0.71, 95% CI: 0.52-0.99; Q3 HR: 0.68, 95% CI: 0.51-0.92; Q4 HR: 0.71, 95% CI: 0.53-0.96; Q5 HR: 0.65, 95% CI: 0.49-0.88) and two or more lifetime tumors (HR: 0.78, 95% CI: 0.61-1.00) were inversely associated with mortality. No variables were found to reach statistical significance at the 0.05 significance level in the Black or Hispanic populations. In the Asian/Pacific Islander population, male sex (HR: 1.64, 95% CI: 1.02-2.62) was statistically significantly associated with an increased risk of mortality and tumor location in the head/neck (HR: 0.32, 95% CI: 0.13-0.80) or locations other than CNS, head/neck, trunk, or limbs (HR: 0.21, 95% CI: 0.04-0.96) were significantly inversely associated with mortality.
Table 2:
Unadjusted hazard ratios and adjusted hazard ratios and 95% confidence intervals from Cox proportional hazards modeling
| Age at Diagnosis | ||||||
|---|---|---|---|---|---|---|
| All Ages | <40 Years at Diagnosis | ≥40 Years at Diagnosis | ||||
| Crude HR | Adjusted HRa (95% CI) |
Crude HR | Adjusted HRa (95% CI) |
Crude HR |
Adjusted HRa (95% CI) |
|
| Sex | ||||||
| Female | ref | ref | ref | ref | ref | ref |
| Male | 0.99 | 1.08 (0.95, 1.23) | 0.93 | 0.96 (0.78, 1.17) | 1.05 | 1.21 (1.02, 1.43) |
| Age at Diagnosis | 1.01 | 1.01 (1.01, 1.01) | 1.00 | 1.00 (0.99, 1.01) | 1.02 | 1.02 (1.01, 1.03) |
| Race/Ethnicity | ||||||
| White | ref | ref | ref | ref | ref | ref |
| Hispanic (All races) | 1.14 | 1.16 (0.96, 1.40) | 1.30 | 1.30 (1.00, 1.68) | 1.04 | 1.02 (0.77, 1.36) |
| American Indian/Alaska Native | 0.94 | 0.92 (0.43, 1.97) | 2.07 | 2.78 (1.13, 6.84) | 0.48 | 0.35 (0.09, 1.43) |
| Asian/Pacific Islander | 1.19 | 1.24 (0.98, 1.56) | 1.28 | 1.49 (0.98, 2.27) | 1.14 | 1.20 (0.90, 1.59) |
| Black | 1.16 | 1.07 (0.88, 1.30) | 1.24 | 1.15 (0.85, 1.55) | 1.12 | 1.04 (0.80, 1.35) |
| Non-Hispanic Unknown | 1.71 | 1.01 (0.48, 2.16) | 1.40 | 1.11 (0.35, 3.54) | 2.09 | 0.89 (0.32, 2.44) |
| Surgery | ||||||
| No Surgery | ref | ref | ref | ref | ref | ref |
| Surgery | 0.89 | 0.94 (0.75, 1.18) | 0.71 | 0.83 (0.53, 1.30) | 1.02 | 1.00 (0.76, 1.32) |
| Site | ||||||
| Central Nervous System | ref | ref | ref | ref | ref | ref |
| Head/Neck | 0.83 | 0.83 (0.62, 1.12) | 0.83 | 1.00 (0.61, 1.64) | 0.83 | 0.73 (0.50, 1.07) |
| Limb | 0.90 | 0.87 (0.66, 1.15) | 0.87 | 1.00 (0.63, 1.58) | 0.95 | 0.83 (0.58, 1.18) |
| Other | 0.97 | 0.95 (0.62, 1.44) | 0.99 | 0.89 (0.44, 1.77) | 0.97 | 1.01 (0.59, 1.74) |
| Trunk/Core | 0.94 | 0.93 (0.70, 1.22) | 0.93 | 1.12 (0.71, 1.76) | 0.97 | 0.84 (0.59, 1.19) |
| Yost Quintile | ||||||
| Yost Group 1 | ref | ref | ref | ref | ref | ref |
| Yost Group 2 | 0.89 | 0.81 (0.66, 1.00) | 0.93 | 0.87 (0.64, 1.19) | 0.85 | 0.72 (0.54, 0.97) |
| Yost Group 3 | 0.83 | 0.82 (0.67, 1.00) | 0.83 | 0.81 (0.61, 1.09) | 0.82 | 0.78 (0.59, 1.03) |
| Yost Group 4 | 0.77 | 0.77 (0.63, 0.95) | 0.74 | 0.79 (0.58, 1.09) | 0.77 | 0.74 (0.56, 0.98) |
| Yost Group 5 | 0.78 | 0.74 (0.60, 0.92) | 0.78 | 0.83 (0.59, 1.16) | 0.76 | 0.69 (0.52, 0.93) |
| Metastasis at Diagnosis | ||||||
| No Metastasis at Diagnosis | ref | ref | ref | ref | ref | ref |
| Metastasis at Diagnosis | 1.46 | 1.57 (1.16, 2.11) | 1.50 | 1.71 (1.11, 2.65) | 1.57 | 1.39 (0.92, 2.12) |
| Number in situ/malignant tumors | ||||||
| One | ref | ref | ref | ref | ref | ref |
| Two or more | 0.94 | 0.83 (0.68, 1.01) | 0.89 | 0.95 (0.62, 1.45) | 0.92 | 0.79 (0.63, 0.99) |
Hazard ratios for each variable adjusted for all other covariates in table
Abbreviations: Ref= Referent Group
Figure 1. Kaplan-Meier survival by race/ethnicity.
Kaplan-Meier survival curves for individuals diagnosed with MPNSTs between 2000 and 2016, stratified by racial/ethnic group. Number at risk by category at select time points is additionally displayed below. Abbreviations: AI/AN=American Indian/Alaska Native, API=Asian/Pacific Islander
Figure 2. Kaplan-Meier survival by Yost quintile.
Kaplan-Meier survival curves for individuals diagnosed with MPNSTs between 2000 and 2016, stratified by Yost quintile. Number at risk by category at select time points is additionally displayed below. Abbreviations: Q1=Quintile 1, Q2=Quintile 2, Q3=Quintile 3, Q4=Quintile 4, Q5=Quintile 5
Table 3:
Adjusted hazard ratios and 95% confidence intervals in Cox proportional hazards models stratified by race/ethnicity
| Race/Ethnicity | ||||
|---|---|---|---|---|
| White HRa (95% CI) |
Black HRa (95% CI) |
Hispanic HRa (95% CI) |
Asian/Pacific Islander HRa (95% CI) |
|
| Sex | ||||
| Female | ref | ref | ref | ref |
| Male | 0.95 (0.81, 1.12) | 1.15 (0.80, 1.66) | 1.29 (0.93, 1.80) | 1.64 (1.02, 2.62) |
| Age at Diagnosis | 1.01 (1.01, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| Surgery | ||||
| No Surgery | ref | ref | ref | ref |
| Surgery | 0.89 (0.66, 1.20) | 1.11 (0.62, 2.01) | 0.85 (0.43, 1.68) | 0.68 (0.26, 1.78) |
| Site | ||||
| Central Nervous System | ref | ref | ref | ref |
| Head/Neck | 0.95 (0.64, 1.41) | 0.51 (0.19, 1.36) | 0.94 (0.43, 2.08) | 0.32 (0.13, 0.80) |
| Limb | 1.04 (0.72, 1.50) | 0.47 (0.20, 1.13) | 1.05 (0.50, 2.21) | 0.60 (0.24, 1.49) |
| Other | 1.33 (0.77, 2.28) | 0.68 (0.08, 5.93) | 0.67 (0.24, 1.84) | 0.21 (0.04, 0.96) |
| Trunk/Core | 0.95 (0.66, 1.36) | 0.85 (0.35, 2.06) | 1.05 (0.50, 2.18) | 0.61 (0.28, 1.33) |
| Yost Quintile | ||||
| Yost Group 1 | ref | ref | ref | ref |
| Yost Group 2 | 0.71 (0.52, 0.99) | 0.93 (0.61, 1.42) | 0.84 (0.55, 1.29) | 0.76 (0.26, 2.22) |
| Yost Group 3 | 0.68 (0.51, 0.92) | 0.76 (0.47, 1.23) | 1.02 (0.66, 1.57) | 1.08 (0.49, 2.41) |
| Yost Group 4 | 0.71 (0.53, 0.96) | 0.62 (0.35, 1.07) | 0.81 (0.49, 1.34) | 0.82 (0.38, 1.78) |
| Yost Group 5 | 0.65 (0.49, 0.88) | 1.15 (0.50, 2.66) | 1.13 (0.59, 2.19) | 0.62 (0.27, 1.43) |
| Metastasis at Diagnosis | ||||
| No Metastasis at Diagnosis | ref | ref | ref | ref |
| Metastasis at Diagnosis | 1.37 (0.92, 2.04) | 1.24 (0.58, 2.63) | 1.97 (0.93, 4.16) | 0.53 (0.18, 1.56) |
| Number in situ/malignant tumors | ||||
| One | ref | ref | ref | ref |
| Two or more | 0.78 (0.61, 1.00) | 0.86 (0.52, 1.45) | 0.87 (0.43, 1.76) | 0.61 (0.20, 1.86) |
Hazard ratios for each variable adjusted for all other covariates in table
Abbreviations: Ref= Referent Group
In age-stratified analysis, among those diagnosed before age 40, metastasis at diagnosis (HR: 1.71, 95% CI: 1.11-2.65), and American Indian/Alaska Native race/ethnicity (HR: 2.78, 95% CI: 1.13-6.84) were statistically significantly associated with an increased risk of mortality. Point estimates for Hispanic (HR: 1.30, 95% CI: 1.00-1.68) and Asian/Pacific Islander (HR: 1.49, 95% CI: 0.98-2.27) individuals were elevated but did not reach statistical significance. Among those diagnosed at ages 40 and above, age at diagnosis (HR: 1.02, 95% CI: 1.01-1.03) and male sex (HR: 1.21, 95% CI: 1.02-1.43) were associated with an increased risk of mortality, while increasing CT-SES (Q2 HR: 0.72, 95% CI: 0.54-0.97; Q3 HR: 0.78, 95% CI: 0.59-1.03; Q4 HR: 0.74, 95% CI: 0.56-0.98; Q5 HR: 0.69, 95% CI: 0.52-0.93) was significantly inversely associated with mortality (Table 2).
Discussion
In this population-based study of MPNST survival in cases diagnosed 2000-2016 in the SEER 18 database, we found that CT-SES, age at diagnosis, and metastasis at diagnosis are all important factors influencing cause-specific mortality. Factors predicting mortality were also investigated in models stratified by race/ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic Asian/Pacific Islander, and Hispanic), in order to further understand the relationship between SES and race/ethnicity in terms of mortality. In these stratified analyses, CT-SES was found to predict mortality in White individuals only. Additional analyses investigated factors affecting mortality separately amongst individuals diagnosed before age 40, and those diagnosed at ages 40 and above, in order to assess whether factors affecting mortality may differ between those with a higher probability of having NF1 and those with a lower probability. In individuals diagnosed before age 40, metastasis at diagnosis and American Indian/Alaska Native race predicted higher risk of mortality. In individuals ages 40 and above at diagnosis, age at diagnosis, male sex, and CT-SES predicted mortality.
Previous literature has reported lower MPNST survival among non-White populations 5,7,16. Two previous SEER-based analyses have reported lowered MPNST survival in Black individuals 7,16 while an analysis of data from the National Cancer Database reported lower survival in individuals who identified as a race other than White, Black, or Asian/Pacific Islander 5. Other analyses have included race/ethnicity as a covariate in their analyses without observing significant results 9,10. However, none of these previous analyses have included SES in their models. In the current analysis, we observed an association between American Indian/Alaska Native race and mortality as well as a trend towards worsened survival in Hispanic individuals in fully adjusted models among individuals diagnosed before age 40. Additionally, in race/ethnicity stratified analysis, higher CT-SES was found to improve survival only in White individuals. In previous literature, the most frequently implicated factor affecting MPNST survival is tumor size 3-12 . Tumor size was missing in almost 30% of cases included in this analysis; however, in the subset of cases for which this information was available, larger tumor size (>7 cm) was associated with mortality. Past studies have also found an association between metastasis at diagnosis 11,12, and older age 5,8,9,16 with poorer prognosis, findings that were replicated in this analysis. However, multiple past analyses have found an association between tumor location and mortality 4,9,11,13, a finding that was not replicated in this study. Additionally, multiple previous studies have found an association between tumor grade and mortality 4,7,8,10,11, but tumor grade was not included in this analysis due to missingness.
In analyses conducted by racial/ethnic grouping, higher CT-SES was found to improve survival only in the White population. This finding suggests that White individuals may experience benefits from higher SES, that other racial and ethnic groups do not. Improved access to care may be one such benefit. Minorities report greater difficulty with access to and use of healthcare compared to non-Hispanic Whites 18. Though SES and race/ethnicity are intertwined variables, there is evidence that barriers to quality health care persist in minority populations even when controlling for SES 19,20. One of these barriers may take the form of bias in the healthcare system, both implicit and systemic 19. Due to these biases, it is reported that even after controlling for socioeconomic factors such as education and income, minority populations still do not receive comparable cancer treatment when compared to White individuals and are additionally less likely to receive follow-up care post-treatment 19. Perceived bias in the healthcare system has been linked to lower rates of cancer screening 21, delays in seeking care, underutilization of healthcare, and avoidance of the healthcare system altogether 22. These barriers to care may prevent minority individuals with MPNSTs from receiving high level care regardless of CT-SES, resulting in these populations witnessing no benefits at higher SES in terms of mortality as was observed in this analysis. Sub analyses conducted by CT-SES quintile (Table 4) found that within the highest CT-SES quintile, Hispanic individuals had a hazard ratio of 1.75 (95% CI: 0.95-3.24) compared to non-Hispanic Whites, while Black individuals had a hazard ratio of 1.77 (95% CI: 0.79-3.96) when compared to non-Hispanic Whites. Though these hazard ratios did not reach statistical significance, these point estimates further suggest the existence of survival disparities in minority populations that cannot be explained by SES. Though access to and use of healthcare is one potential explanation for these observed associations, SEER does not collect data on variables related to health systems or healthcare utilization and thus we were unable to explore these hypotheses.
Table 4:
Adjusted hazard ratios and 95% confidence intervals in Cox proportional hazards models stratified by Yost quintile
| Yost Quintile (SES) | |||||
|---|---|---|---|---|---|
| Yost Quintile 1 HRa (95% CI) |
Yost Quintile 2 HRa (95% CI) |
Yost Quintile 3 HRa (95% CI) |
Yost Quintile 4 HRa (95% CI) |
Yost Quintile 5 HRa (95% CI) |
|
| Sex | |||||
| Female | ref | ref | ref | ref | ref |
| Male | 1.17 (0.87, 1.57) | 0.70 (0.51, 0.96) | 1.14 (0.87, 1.49) | 1.07 (0.80, 1.44) | 1.19 (0.87, 1.62) |
| Age at Diagnosis | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.01, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) |
| Race/Ethnicity | |||||
| White | ref | ref | ref | ref | ref |
| Hispanic (All races) | 1.00 (0.69, 1.44) | 1.00 (0.66, 1.52) | 1.50 (1.01, 2.22) | 1.01 (0.64, 1.61) | 1.75 (0.95, 3.24) |
| American Indian/Alaska Native | 4.62 (0.55, 38.86) | NA | 0.89 (0.36, 2.24) | 3.46 (0.47, 25.50) | NA |
| Asian/Pacific Islander | 1.19 (0.62, 2.27) | 1.59 (0.71, 3.55) | 1.55 (0.94, 2.53) | 1.21 (0.76, 1.92) | 1.21 (0.75, 1.95) |
| Black | 1.07 (0.74, 1.55) | 1.34 (0.90, 1.97) | 1.16 (0.75, 1.79) | 0.84 (0.50, 1.40) | 1.77 (0.79, 3.96) |
| Non-Hispanic Unknown | 0.82 (0.25, 2.69) | 0.56 (0.08, 4.12) | NA | NA | 1.29 (0.40, 4.10) |
| Surgery | |||||
| No Surgery | ref | ref | ref | ref | ref |
| Surgery | 1.00 (0.59, 1.68) | 1.08 (0.57, 2.02) | 1.31 (0.78, 2.20) | 0.79 (0.46, 1.35) | 0.72 (0.44, 1.20) |
| Site | |||||
| Central Nervous System | ref | ref | ref | ref | ref |
| Head/Neck | 0.42 (0.19, 0.94) | 1.50 (0.64, 3.50) | 0.79 (0.41, 1.51) | 0.68 (0.36, 1.29) | 1.05 (0.58, 1.88) |
| Limb | 0.46 (0.21, 1.01) | 1.58 (0.73, 3.43) | 0.77 (0.43, 1.37) | 0.69 (0.37, 1.27) | 1.20 (0.69, 2.08) |
| Other | 0.87 (0.29, 2.60) | 2.78 (0.89, 8.63) | 1.11 (0.47, 2.61) | 0.77 (0.25, 2.39) | 0.49 (0.20, 1.22) |
| Trunk/Core | 0.64 (0.30, 1.39) | 1.30 (0.60, 2.82) | 0.90 (0.50, 1.62) | 0.81 (0.44, 1.49) | 0.97 (0.56, 1.66) |
| Metastasis at Diagnosis | |||||
| No Metastasis at Diagnosis | ref | ref | ref | ref | ref |
| Metastasis at Diagnosis | 1.15 (0.61, 2.16) | 1.28 (0.55, 2.99) | 2.72 (1.36, 5.44) | 1.71 (0.88, 3.34) | 1.14 (0.56, 2.31) |
| Number in situ/malignant tumors | |||||
| One | ref | ref | ref | ref | ref |
| Two or more | 0.75 (0.46, 1.25) | 0.56 (0.33, 0.94) | 1.12 (0.75, 1.69) | 1.29 (0.84, 1.98) | 0.58 (0.37, 0.88) |
Hazard ratios for each variable adjusted for all other covariates in table
Abbreviations: Ref= Referent Group
NF1 associated MPNSTs are known to have worse survival outcomes when compared to sporadic MPNSTs6. Additionally, familial NF1 is associated with lower SES when compared to individuals with sporadic NF123 and it is also likely that individuals with NF1 may be of lower SES when compared to the general population due to the cognitive and physical complications associated with the disorder23-25, which can result in higher than average medical costs as well as limited employment opportunities. Thus, it is possible that some of the observed differences in survival by CT-SES quintile could be due to NF1 status, a variable which is not available in the SEER database. However, as NF1 prevalence is not known to differ by race/ethnicity2, this does not explain the differential effect of SES by racial/ethnic grouping.
This analysis found differences in the factors affecting mortality between those diagnosed at earlier ages (under age 40 at diagnosis) who have a higher likelihood of NF1 and those diagnosed at later ages (40 and older) with a lower probability of NF1. In those under 40 at diagnosis, only American Indian/Alaska Native race and metastasis at diagnosis were associated with higher mortality, although results were also suggestive for Hispanic ethnicity and Asian/Pacific Islander populations. None of these factors was statistically significant in the older population. NF1 rates are not known to differ between racial/ethnic groups 2, thus, though NF1 status is unavailable in this dataset, it is unlikely that differential NF1 rates are driving these results. Proper access to medical care is important to the management of NF1 26, which is more likely in those diagnosed under age 40, and earlier detection of MPNSTs is critical to survival, as earlier detection means smaller tumor size as well as a higher likelihood of successful complete tumor removal via surgical resection 27. American Indian and Alaska Native populations are less likely to have access to adequate healthcare and more likely to face barriers to care 28,29. The observed association between American Indian/Alaska Native race and poorer survival seen in the younger population may indicate that American Indian/Alaska Native individuals in this age group have more difficulties accessing adequate medical care than other populations. Though some of the difficulties in access to quality health care should be captured by the inclusion of CT-SES in these models, there are likely additional factors contributing to the observed differential survival such as previously mentioned medical bias and poor experiences with the healthcare system.
In the older population, age at diagnosis, male sex, and CT-SES were associated with survival. Older age may be associated with mortality due to higher rates of comorbidities. Additionally, men are less likely to utilize healthcare than women 30, which could have implications in terms of early cancer detection and treatment seeking, especially in individuals without NF1 who would not necessarily be aware of symptoms regarding MPNSTs.
Higher CT-SES was associated with better survival outcomes in the older population as well. Individuals with higher SES likely have improved access to regular healthcare, as well as improved access to treatment. CT-SES was not found to significantly affect mortality in those diagnosed before age 40. As noted previously, these individuals have a higher likelihood of NF1 when compared to those diagnosed after age 40; thus, this finding may suggest that the benefits of higher CT-SES in terms of medical care may not be realized in individuals with NF1.
This analysis benefits from the population-based nature of the SEER 18 database, which captures incident cases in approximately 28% of the U.S population 31. Additionally, the SEER 18 database collects information on SES at the census tract level, allowing a relatively granular investigation of the effect of SES on MPNST survival. However, there are limitations to this dataset and thus this analysis as well. SEER has only collected information on CT-SES starting in 2000, meaning this study could only analyze recent trends in MPNST mortality. Additionally, the SEER database does not have information on NF1 status; thus, we used age at diagnosis as a proxy for probable NF1-related cases. Other variables that likely affect MPNST outcomes, such as whether or not a MPNST was encapsulated at time of surgery are also not available in the database, and thus could not be investigated in this analysis. The SEER database also does not have any information related to health behaviors such as seeking recommended preventative care and cancer screenings, which may differ between age groups32 and likely also impacts stage at diagnosis. Thus, there are potential mediators to the observed associations that cannot be accounted for in this analysis. Additionally, as information in the SEER database does not undergo central pathologic review, it is possible that some cancers within this database have been misclassified. Lastly, tumor size, tumor grade, and radiation treatment, which have been shown to affect MPNST mortality in previous literature 3-12, had high levels of missingness in this dataset and thus were excluded from analyses.
In conclusion, this analysis provides additional evidence of the importance of metastasis at diagnosis—and tumor size in the subset of data for which this variable is available—in MPNST survival. The findings of this analysis further suggest that certain populations—racial/ethnic minorities and individuals diagnosed before age 40—do not receive the same benefits of higher SES as the non-Hispanic White general population, as CT-SES was only significantly inversely associated with mortality in those diagnosed at ages 40 and above and White individuals. This indicates that there may be barriers to healthcare in minority and younger populations that extend beyond SES. Additionally, even if such barriers were overcome, both race/ethnicity 20 and NF1 23, a strong risk factor for MPNST, are associated with lower SES. Thus, it is possible that minority and genetically susceptible populations may continue to experience survival disparities even if these barriers are addressed. Further analysis into the pathways underlying decreased survival among certain subpopulations of MPNST patients is warranted.
Supplementary Material
Acknowledgements
EL Marcotte was supported by the Children's Cancer Research Fund (CCRF). AM Domingues was supported by the National Cancer Institute (T32CA099936).
Footnotes
Disclosures: Authors declare no potential conflicts of interests.
References
- 1.Gupta G, Maniker A. Malignant peripheral nerve sheath tumors. Neurosurg Focus. 2007;22(6). doi: 10.3171/foc.2007.22.6.13 [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Gutmann DH. Neurofibromatosis type 1. In: Handbook of Clinical Neurology. Vol 132. Elsevier B.V.; 2015:75–86. doi: 10.1016/B978-0-444-62702-5.00004-4 [DOI] [PubMed] [Google Scholar]
- 3.Uusitalo E, Rantanen M, Kallionpää RA, et al. Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol. 2016;34(17):1978–1986. doi: 10.1200/JCO.2015.65.3576 [DOI] [PubMed] [Google Scholar]
- 4.Evans DGR, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis. J Med Genet. 2002;39(5):311–314. doi: 10.1136/jmg.39.5.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowery A, Clayburgh D. Malignant peripheral nerve sheath tumors: Analysis of the national cancer database. Oral Oncol. 2019;98:13–19. doi: 10.1016/j.oraloncology.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 6.Cai Z, Tang X, Liang H, Yang R, Yan T, Guo W. Prognosis and risk factors for malignant peripheral nerve sheath tumor: A systematic review and meta-analysis. World J Surg Oncol. 2020;18(1). doi: 10.1186/s12957-020-02036-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amirian ES, Goodman JC, New P, Scheurer ME. Pediatric and adult malignant peripheral nerve sheath tumors: An analysis of data from the surveillance, epidemiology, and end results program. J Neurooncol. 2014;116(3):609–616. doi: 10.1007/s11060-013-1345-6 [DOI] [PubMed] [Google Scholar]
- 8.Martin E, Coert JH, Flucke UE, et al. A nationwide cohort study on treatment and survival in patients with malignant peripheral nerve sheath tumours. Eur J Cancer. 2020;124:77–87. doi: 10.1016/j.ejca.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 9.Arshi A, Tajudeen BA, St. John M. Malignant peripheral nerve sheath tumors of the head and neck: Demographics, clinicopathologic features, management, and treatment outcomes. Oral Oncol. 2015;51(12):1088–1094. doi: 10.1016/j.oraloncology.2015.08.012 [DOI] [PubMed] [Google Scholar]
- 10.Wanebo JE, Malik JM, Vandenberg SR, Wanebo HJ, Driesen N, Persing JA. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer. 1993;71(4):1247–1253. doi: [DOI] [PubMed] [Google Scholar]
- 11.Zou C, Smith KD, Liu J, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249(6):1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a [DOI] [PubMed] [Google Scholar]
- 12.Okada K, Hasegawa T, Tajino T, et al. Clinical relevance of pathological grades of malignant peripheral nerve sheath tumor: A multi-institution TMTS study of 56 cases in northern Japan. Ann Surg Oncol. 2007;14(2):597–604. doi: 10.1245/s10434-006-9053-5 [DOI] [PubMed] [Google Scholar]
- 13.Angelov L, Davis A, O’Sullivan B, Bell R, Guha A. Neurogenic sarcomas: Experience at the University of Toronto. Neurosurgery. 1998;43(1):56–65. doi: 10.1097/00006123-199807000-00035 [DOI] [PubMed] [Google Scholar]
- 14.Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: Prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107(5):1065–1074. doi: 10.1002/cncr.22098 [DOI] [PubMed] [Google Scholar]
- 15.Stucky CCH, Johnson KN, Gray RJ, et al. Malignant Peripheral Nerve Sheath Tumors (MPNST): The Mayo Clinic experience. Ann Surg Oncol. 2012;19(3):878–885. doi: 10.1245/s10434-011-1978-7 [DOI] [PubMed] [Google Scholar]
- 16.Martin E, Muskens IS, Coert JH, Smith TR, Broekman MLD. Treatment and survival differences across tumor sites in malignant peripheral nerve sheath tumors: A SEER database analysis and review of the literature. Neuro-Oncology Pract. 2019;6(2):134–143. doi: 10.1093/nop/npy025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCaughan JA, Holloway SM, Davidson R, Lam WWK. Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J Med Genet. 2007;44(7):463. doi: 10.1136/JMG.2006.048140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozlov N, Benzon HT. Role of Gender and Race in Patient-Reported Outcomes and Satisfaction KEYWORDS Outcome domains Measurement tools Race Socioeconomic status Gender Outcomes Patient satisfaction KEY POINTS. doi: 10.1016/j.anclin.2020.01.012 [DOI] [Google Scholar]
- 19.Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (with CD). National Academies Press; 2003. doi: 10.17226/12875 [DOI] [PubMed] [Google Scholar]
- 20.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Heal Psychol. 2016;35(4):407–411. doi: 10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouton CP, Carter-Nolan PL, Makambi KH, et al. Impact of perceived racial discrimination on health screening in Black Women. J Health Care Poor Underserved. 2010;21(1):287–300. doi: 10.1353/hpu.0.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shavers VL, Fagan P, Jones D, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health. 2012;102(5):953–966. doi: 10.2105/AJPH.2012.300773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biotteau M, Déjean S, Lelong S, et al. Sporadic and Familial Variants in NF1: An Explanation of the Wide Variability in Neurocognitive Phenotype? Front Neurol. 2020;11. doi: 10.3389/fneur.2020.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65(7):1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce [DOI] [PubMed] [Google Scholar]
- 25.Theos A, Korf BR. Pathophysiology of Neurofibromatosis Type 1.; 2006. www.annals.org. Accessed June 3, 2021. [DOI] [PubMed] [Google Scholar]
- 26.Stewart DR, Korf BR, Nathanson KL, Stevenson DA, Yohay K. Care of adults with neurofibromatosis type 1: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2018;20(7):671–682. doi: 10.1038/gim.2018.28 [DOI] [PubMed] [Google Scholar]
- 27.Park SJ, Sawitzki B, Kluwe L, Mautner VF, Holtkamp N, Kurtz A. Serum biomarkers for neurofibromatosis type 1 and early detection of malignant peripheral nerve-sheath tumors. BMC Med. 2013;11(1):1–9. doi: 10.1186/1741-7015-11-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cromer KJ, Wofford L, Wyant DK. Barriers to Healthcare Access Facing American Indian and Alaska Natives in Rural America. J Community Health Nurs. 2019;36(4):165–187. doi: 10.1080/07370016.2019.1665320 [DOI] [PubMed] [Google Scholar]
- 29.Martino SC, Elliott MN, Hambarsoomian K, et al. Disparities in care experienced by american Indian and alaska native medicare beneficiaries. In: Medical Care. Vol 58. Lippincott Williams and Wilkins; 2020:981–987. doi: 10.1097/MLR.0000000000001392 [DOI] [PubMed] [Google Scholar]
- 30.Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA. Gender Differences in the Utilization of Health Care Services. J Fam Pract. 2000;49(2):147–152. [PubMed] [Google Scholar]
- 31.Number of Persons by Race and Hispanic Ethnicity for SEER Participants - SEER Registries. https://seer.cancer.gov/registries/data.html. Accessed June 3, 2021. [Google Scholar]
- 32.Callahan ST, Cooper WO. Changes in Ambulatory Health Care Use During the Transition to Young Adulthood. J Adolesc Heal [Internet] 2010. [cited 2022 Feb 8];46:407–13. Available from: http://www.jahonline.org/article/S1054139X09003796/fulltext [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this analysis is available through the Surveillance, Epidemiology, and End Results program (SEER) and may be accessed through application.