Abstract
Purpose:
To assess safety of gene therapy in G11778A Leber hereditary optic neuropathy (LHON).
Design:
Phase 1 clinical trial.
Methods:
Setting:
single institution.
Participants:
Patients with G11778A LHON and chronic bilateral visual loss >12 months (Group 1,n=11), acute bilateral visual loss <12 months (Group 2,n=9), or unilateral visual loss (Group 3,n=8).
Intervention:
unilateral intravitreal AAV2(Y444,500,730F)-P1ND4v2 injection with low, medium, high and higher doses to worse eye for Groups 1 and 2 and better eye for Group 3.
Outcome Measures:
Best-corrected visual acuity (BCVA), adverse events, and vector antibody responses. Mean follow-up was 24 months (range 12–36), BCVAs were compared with a published prospective natural history cohort with designated surrogate study and fellow eyes.
Results:
Incident uveitis (8/28,29%), the only vector-related adverse event, resulted in no attributable vision sequelae and was related to vector dose; 5/7(71%) higher-dose eyes versus 3/21(14%) low-, medium-, or high-dose eyes (p<0.001). Incident uveitis requiring treatment was associated with increased serum AAV2 neutralizing antibody titers (p=0.007) but not serum PCR. Improvements of ≥15 letter BCVA occurred in some treated and fellow eyes of Groups 1 and 2 and some surrogate study and fellow eyes of natural history subjects. All study eyes (BCVA ≥20/40) in Group 3 lost ≥15 letters within the first year despite treatment.
Conclusions:
G11778A LHON gene therapy has a favorable safety profile. Our results suggest if there is an efficacy effect, it is likely small and not dose-related. Demonstration of efficacy requires randomization of patients to a group not receiving vector in either eye.
Keywords: Leber hereditary optic neuropathy G11778A, Gene therapy, AAV2
Precis
In this phase 1 G11778A LHON gene therapy clinical trial, a favorable safety profile was found. Incident uveitis was the only vector-related adverse event, resulted in no attributable vision sequelae and was related to vector dose. Our results suggest if there is an efficacy effect, it is likely small and not dose-related. Demonstration of efficacy requires randomization of patients to a group not receiving vector in either eye.
INTRODUCTION
Leber hereditary optic neuropathy (LHON) is a maternally inherited mitochondrial DNA (mDNA) disease associated with severe visual loss.1,2 Approximately 95% of LHON is caused by one of three pathogenic point mDNA mutations coding for the respiratory chain subunits of the nicotinamide adenine dinucleotide ubiquinone-oxidoreductase (complex I) genes: 3460G>A ND1, 11778G>A ND4, and 14484T>C ND6.3 Carriers of LHON genotypes have incomplete penetrance and only a portion of LHON genotype carriers, more likely males than females, will manifest the disease with a low annual conversion rate. Affected individuals usually are young adults and have severe bilateral visual loss typically starting in one eye followed by the fellow eye within days to months. A pre-symptomatic stage before visual loss, consisting of optic disc edema and retinal ganglion cell (RGC) layer thinning often occurs. A prospective natural history study found spontaneous partial recovery of visual acuity of 3 lines or more in approximately 18% of patients occurring months to years after the onset of visual loss.4 No established effective treatment of LHON is yet available.
We report the safety and efficacy results of our phase 1 G11778A LHON gene therapy that included three study groups in which each participant was treated with a unilateral intravitreal injection of low, medium, high, or higher dose of gene therapy vector AAV2(Y444,500,730F)-P1ND4v2.5 The treatment uses allotopic expression by delivering a normal nuclear-encoded ND4 gene (OMIM 516003.0001) into the nuclei of RGCs via an adeno-associated virus vector.6–8 The nuclear version of the mitochondrial gene ND4 subunit is recoded for expression and is imported into mitochondria to normalize the defective adenosine triphosphatase synthesis of RGCs and improve their survival.
METHODS
Study design and intervention
This Phase 1, open-label gene therapy clinical trial for G11778A LHON (NCT02161380) was approved by the University of Miami Institutional Review Board and the United States Food and Drug Administration and was conducted at the Bascom Palmer Eye Institute (Miami, FL). The study was designed as a 3+3 dose escalation of unilateral intravitreal gene therapy injection of AAV2(Y444,500,730F)-P1ND4v2 for each of the three groups of G11778A LHON patients with visual loss.
Patient Groups
Three groups of patients with G11778A mitochondrial DNA and visual loss were enrolled. The chronic bilateral group (Group 1) had onset of visual loss in 1 eye of ≥ 12 months and ≥ 6 months in the more recently affected eye with best-corrected visual acuity (BCVA) reduced to ≤ 35 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (Snellen equivalent, 20/200) in each eye. The acute bilateral group (Group 2) had onset of visual loss in both eyes of ≤12 months to a BCVA of ≤ 35 ETDRS letters. The unilateral acute group (Group 3) had visual loss in one eye to ≤ 35 ETDRS letters, with mildly impaired but good acuity of 70 letters or more (Snellen equivalent, 20/40) in the contralateral eye. Mean follow-up was 24 months (median 36 months, range 12–36 months). To avoid possible confounding effect of idebenone, a washout period of at least 1 week was utilized before gene therapy injection if the patient was taking idebenone at baseline 1 examination (n=6).
Intervention
The treatment consisted of a single unilateral intravitreal 100 μl injection of AAV2(Y444,500,730F)-P1ND4v2 to the worse eye for Group 1 and 2, and to the better eye for Group 3. Each group independently had dose escalations from low, to medium, to high, and to higher dose.9 Table 1 summarizes the number of patients enrolled and the AAV2(Y444,500,730F)-P1ND4v2 doses received. The study was initially designed with treatment of low (5.0×108 viral genomes(vg)/eye), medium (2.5×109 vg/eye), and high (2.4×1010 vg/eye) doses of AAV2(Y444,500,730F)-P1ND4v2 manufactured by the University of Florida (UF). Manufacturing of the high dose was changed to Children’s Hospital of Pennsylvania (CHOP) because UF was unable to provide the vector at the high dose. The CHOP high-dose vector was 2.4×109 vg/eye; CHOP determination of the UF low dose was 1.2×108 vg/eye and of the UF medium dose was 5.8×108 vg/eye. Given that the CHOP high dose was similar to the UF medium dose as initially determined by UF, the Food and Drug Administration (FDA) recommended labeling the CHOP 2.4×109 vg/eye dose as the high dose and a CHOP 1.0×1010 vg/eye vector as a higher dose. Due to discrepancies between UF and CHOP dose assessment, the FDA Center for Biologics Evaluation and Research (CBER) required injection of three chronic bilateral patients at the high dose before proceeding to the higher dose in three acute bilateral patients. No safety concerns emerged in these high-dose chronic bilateral patients and administration of higher dose investigational product (IP) began in the acute bilateral group. Subsequently, the data safety monitoring committee (DSMC) and the FDA approved higher-dose injection of three chronic bilateral patients in addition to the planned three unilateral acute patients. However, the higher-dose injections were suspended after two chronic bilateral, three acute bilateral, and two acute unilateral patients were injected due to DSMC concerns related to incident uveitis requiring treatment in eyes injected with the higher dose vector.
Table 1.
Summary of Patients Enrolled and Doses of LHON Gene Therapy Received
| Study Group | Expected doses (vg/eye) by manufacturing vector core Certificate of activity and protocol dilution procedures | |||
|---|---|---|---|---|
| UF Low 5.0 x 1008†vg/eye |
UF Medium 2.5 x 1009‡ vg/eye |
CHOP High 2.4 x 1009 vg/eye |
CHOP Higher 1.0 x 1010 vg/eye |
|
| 1: Chronic bilateral | N=3 | N=3 | N=3 | N=2 |
| 2: Acute bilateral | N=3 | N=3 | None | N=3 |
| 3: Acute unilateral | N=3 | N=3 | None | N=2 |
1.2×108vg/eye CHOP titers
5.8×108vg/eye CHOP titers
UF: University of Florida, CHOP: Children Hospital of Pennsylvania
Clinical Assessments
The primary safety outcome measure for Groups 1 and 2 was a 15 ETDRS letter loss of visual acuity in the injected eye from the value recorded on the day before vector injection (the 2nd baseline visit). For group 3, the primary outcome measure was a 3-line drop in acuity in the injected eye, relative to the worst vision of the fellow eye recorded during the study. The first baseline visit occurred at study entry after determination of a qualifying visual acuity and Group assignment. Change in BCVA during follow-up was determined from the second baseline measurement to guard against regression to the mean. In addition to the first and second baseline visits, serial ETDRS BCVA was performed by a masked certified ophthalmic technician on post-injection days 1, 7, 30, 60, 90, 180, 270, 365, 548, 730, 1096. Additional testing included periodic Humphrey 30–2 white-on-white standard visual field, steady-state pattern electroretinography (SS-PERG), and spectral-domain optical coherence tomography (SD-OCT) assessment of peripapillary retinal nerve fiber layer (RNFL) and retinal ganglion cell layer (GCL) thickness. The RNFL and GCL were measured as described previously9 and were collected only at baseline, day 7, day 60, month 12, and annually thereafter.
Immunologic Assessment
The neutralizing antibody (NAb) titer was reported as the highest serum or anterior chamber sample dilution that inhibited self-complementary AAV2(Y444,500,730F)-smCBA-mCherry transduction by 50% or more compared with no serum control.9 Quantitative SYBR green polymerase chain reaction analysis was performed using the bGH poly A primers (bovine growth hormone polyadenylation signal): F1–5’-AGCCTCGACTGTGCCTTCTAGTT-3’ and R1–5’0-GGGTTCCTGCTATTGTCTTCCCAA-3’ with SsoAdvanced universal SYBR Green Supermix (cat. no. 172–5271; SsoAdvanced, BioRad, Hercules, CA) according to a standard protocol as described previously.9
Statistical Analysis
Longitudinal analysis of changes was performed with generalized estimating equation (GEE) analysis, with logistic link function for dichotomous variables and linear link function for continuous variables. To avoid reporting partial results in a dose-group cohort for which follow-up is still being accrued, we used the maximum common follow-up for each dose-group. This phase 1 study was not randomized and did not include an uninjected control group. Therefore, we compared changes from baseline with those observed in our previously published natural history cohort.4 For these analyses, we used the 26 of 44 natural history patients who would have been eligible for this gene therapy trial at baseline. We assigned the worse eye of natural history patients as the study eye to act as a surrogate for the treated eye in the current study, and we assigned the better eye as the fellow eye. When both eyes of eligible natural history patients had identical baseline acuities, we randomly selected the study eye. Of note, visits of our natural history patients occurred every 6 months, limiting the follow-up times that could be compared directly with the gene therapy patients.
Changes from baseline in the study eyes versus the fellow eyes were compared between the gene therapy subjects and the natural history cohort stratified by onset group with 2-factor analysis of variance. Missing and out-of-window visits became a substantial issue during 2020, due to the Covid-19 pandemic. Linear interpolation was approved by the DSMC as a method for estimation of 14 missed visits and to adjust 14 out-of-window visits to the window midpoint. This procedure was applied only to follow-up visits between month 6 and month 36. No measurements were estimated for missed visits unless bracketed by two existing measurements. The dataset used in these analyses was closed on 4/20/2021.
RESULTS
Patient Demographics and Follow-Up
28 patients (5 females, 23 white, 4 white Hispanic, 1 Native American) age 16 to 56 years were treated (details in Supplemental Table 1). Twenty-seven (96%) patients completed the final month 36 visit or are being actively followed beyond the month-18 visit (details in Supplemental Table 2). One patient was lost to follow-up after the 180-day visit.
Safety Evaluations
Participants experienced transient ocular irritation due to the injection procedure and one patient required treatment for elevated post-injection IOP that resolved within 24 hours. The only IP-related adverse event was incident uveitis, which was not related to patient group but was significantly related to vector dose: 1/9 (11%), low dose; 1/9 (11%) medium dose; 1/3 (33%), high dose; and 5/7 (71%), higher dose (p=0.006, exact test of trend in proportions) (Table 2). Four of the total eight uveitis cases resolved spontaneously without treatment. All four cases of incident uveitis that required treatment occurred in eyes injected with the higher-dose vector.
Table 2.
Incident Uveitis and Treatments in 8 Study Patients
| Participant | Group | Dose | Inj. MM/YYYY | Onset MM/YYYY | Resolution MM/YYYY | Vision Sequelae | Treatment |
|---|---|---|---|---|---|---|---|
| 1–2–07 | 1: Chronic | Medium | 06/2015 | 08/2015 | 09/2015 | None | None |
| 2–1–11 | 2: Acute | Low | 09/2015 | 11/2015 | 03/2016 | None | None |
| 1–3–22 | 1: Chronic | High | 06/2018 | 07/2018 | 12/2018 | None | None |
| 2–4–26 | 2: Acute | Higher | 04/2019 | 05/2019 | 01/2020 | None | None |
| 2–4–27 | 2: Acute | Higher | 6/2019 | 6/2019 | 12/2019 | None | Topical prednisolone, brimonidine/timolol, cyclopentolate; oral prednisone |
| 3–4–28 | 3: Unilateral | Higher | 10/2019 | 12/2019 | 05/2020 | None | Topical prednisolone |
| 1–4–29 | 1: Chronic | Higher | 12/2019 | 1/2020 | Ongoing* | None | Topical prednisolone, timolol |
| 1–4–31 | 1: Chronic | Higher | 03/2020 | 03/2020 | 12/2020 | None | Topical prednisolone, brimonidine/timolol, bimatoprost; oral acetazolamide |
Medications discontinued in 6/2021; 1+ anterior chamber cells continuing at 24-month visit in 12–2021 with stable or decreased laser flare meter indicating no clinically significant inflammation causing breakdown of blood aqueous barrier and low risk of future complications.
For purposes of assessing IP-related loss of vision in unilateral participants injected in their better-seeing eyes, a visual acuity severe adverse event (SAE) was defined as loss of acuity in the injected eye equivalent to 15 ETDRS letters worse than the worst fellow-eye acuity measured at any study visit. This occurred in two patients: one at 9 months post-injection, which resolved at month 18, and the other at 6 months post-injection, which was still ongoing at month 36. Neither of these two patients’ eyes lost peripheral visual field (assessed by confrontation), and the DSMC and the FDA concurred with the study team that these changes were likely due to the natural history of visual loss in acute onset LHON. No unexpected concerning findings appeared on dilated exam for any patients at any follow-up visit. Blood and urine samples collected according to protocol were scrutinized and showed no indication of IP-related toxicity. A single patient reported an in-patient hospitalization during follow-up that was required to treat a pre-existing parathyroid gland condition.
Immunologic Response
Neutralizing Antibodies (Nabs):
The complete Nabs data appear in Supplemental Table 3 and are summarized in Table 3 as the means and standard deviations of logarithmic viral vector titers. Change in Nabs was derived by subtracting the follow-up visit measurements from the pre-injection baseline. There were small increases in Nabs among patients without uveitis on the order of 0.24 to 0.6 log units compared with an average increase of 0.3 to 3 log units in Nabs among cases with uveitis requiring treatment. Fitting a GEE model to these data (AR1 correlation structure) suggested a larger increase (p=0.026, 2-degree of freedom comparison of 3 category uveitis factor) in Nabs of patients with uveitis requiring treatment than in patients with no uveitis (p=0.007); however, patient numbers were very small and this was a post-hoc analysis.
Table 3.
Log Neutralizing Antibody Titers by Vector dose and Incident Uveitis at Baseline, Day 7, Day 90 and Year 1
| Dose | Low | Medium | High | Higher | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| No Uveitis | Uveitis with No Symptom | No Uveitis | Uveitis with No Symptom | No Uveitis | Uveitis with No Symptom | No Uveitis | Uveitis with No Symptom | Uveitis with No Symptomatic | ||
|
| ||||||||||
| Visit Baseline | mean (SD) |
2.28 (1.79) |
4.31 (N/A) |
1.23 (1.04) |
1.90 (N/A) |
0.70 (0.00) |
4.31 (N/A) |
0.70 (0.00) |
0.70 (N/A) |
1.45 (0.90) |
| N | 8 | 1 | 8 | 1 | 2 | 1 | 2 | 1 | 4 | |
|
| ||||||||||
| mean | 2.35 | 4.31 | 1.23 | 4.31 | 1.30 | 4.31 | 0.70 | 0.70 | 1.75 (1.24) | |
| Day 7 | (SD) | (1.73) | (N/A) | (1.04) | (N/A) | (0.85) | (N/A) | (0.00) | (N/A) | |
| N | 8 | 1 | 8 | 1 | 2 | 1 | 2 | 1 | 4 | |
|
| ||||||||||
| mean | 2.51 | 4.31 | 1.23 | 4.31 | 1.30 | 4.31 | 0.70 | 3.11 | 3.71 (0.00) | |
| Day 90 | (SD) | (1.81) | (N/A) | (0.88) | (N/A) | (0.85) | (N/A) | (0.00) | (N/A) | |
| N | 7 | 1 | 8 | 1 | 2 | 1 | 1 | 1 | 1* | |
|
| ||||||||||
| mean | 2.59 | 1.47 | 4.31 | 1.00 | 4.31 | 1.30 | 0.70 | 3.11 (1.20) | ||
| Year 1 | (SD) | (1.72) | (0.97) | (N/A) | (0/3) | (N/A) | (0.85) | (N/A) | ||
| N | 7 | 7 | 1 | 2 | 1 | 2 | 1 | 3† | ||
Two patients missed 90-day visits due to the Covid19 pandemic
One patient had year 1 follow-up with an outside physician, due to the Covid 19 pandemic, and the test wasn’t done
Blood PCR:
Given the sensitivity of the assay, any copy number <500 was considered negative. When the duplicate assays for each person-visit are averaged, all qPCR results were negative by this definition (Supplemental Table 4). Supplemental Table 5 presents summary copy numbers of AAV2P1ND4v2 in 0.5 μg blood DNA by IP dose, uveitis incidence, and visit. There were no statistically significant relationships between post-injection changes in qPCR and dose, uveitis, or visit (all p>0.1, GEE with AR1 correlation structure).
Visual Acuity Endpoints
Table 4 presents annual follow-up changes from baseline classified by the pre-specified criterion of 15 ETDRS letters for each group and dose. In both the chronic and acute bilateral groups, improvements were observed in both study and fellow eyes of the natural history and gene therapy dose cohorts at all annual follow-ups. In the unilateral acute group, injection of the IP did not prevent any study eyes with initial acuity ≥20/40 from losing 15 letters of visual acuity within the first year, whereas one of the surrogate study eyes from the previous natural history study did maintain good acuity through 3 years follow-up. Some fellow eyes in the unilateral acute group (all ≤20/200 at baseline) did exhibit improvement during follow-up.
Table 4.
15 Letter ETDRS Visual Acuity Changes in 3 Study Groups and the Natural History Cohort
|
Group 1: Chronic bilateral group
| |||||||
| Months | Study Eye | Fellow Eye | |||||
|
|
|||||||
| Followed | Dose | Worsened | Stable | Improved | Worsened | Stable | Improved |
|
| |||||||
| Nat Hx | 0 (0%) | 19 (79%) | 5 (21%) | 1 (4%) | 21 (88%) | 2 (8%) | |
| Low | 0 (0%) | 3 (100%) | 0 (0%) | 0 (0%) | 2 (67%) | 1 (33%) | |
| 12 | Medium | 0 (0%) | 2 (67%) | 1 (33%) | 0 (0%) | 2 (67%) | 1 (33%) |
| High | 0 (0%) | 1 (33%) | 2 (67%) | 0 (0%) | 2 (67%) | 1 (33%) | |
| Higher | 0 (0%) | 2 (100%) | 0 (0%) | 0 (0%) | 2 (100%) | 0 (0%) | |
|
| |||||||
| Nat Hx | 1 (5%) | 13 (68%) | 5 (26%) | 0 (0%) | 16 (84%) | 3 (16%) | |
| 24 | Low | 0 (0%) | 2 (67%) | 1 (33%) | 0 (0%) | 2 (67%) | 1 (33%) |
| Medium | 0 (0%) | 2 (67%) | 1 (33%) | 0 (0%) | 2 (67%) | 1 (33%) | |
| High | 0 (0%) | 1 (33%) | 2 (67%) | 0 (0%) | 2 (67%) | 1 (33%) | |
|
| |||||||
| Nat Hx | 0 (0%) | 9 (82%) | 2 (18%) | 0 (0%) | 10 (91%) | 1 (9%) | |
| 36 | Low | 0 (0%) | 2 (67%) | 1 (33%) | 0 (0%) | 2 (67%) | 1 (33%) |
| Medium | 0 (0%) | 2 (67%) | 1 (33%) | 0 (0%) | 2 (67%) | 1 (33%) | |
|
Group 2: Acute Bilateral group | |||||||
| Months | Study Eye | Fellow Eye | |||||
|
|
|||||||
| Followed | Dose | Worsened | Stable | Improved | Worsened | Stable | Improved |
|
| |||||||
| Nat Hx | 2 (22%) | 3 (33%) | 4 (44%) | 3 (33%) | 3 (33%) | 3 (33%) | |
| 12 | Low | 0 (0%) | 1 (33%) | 2 (67%) | 1 (33%) | 2 (67%) | 0 (0%) |
| Medium | 0 (0%) | 2 (100%) | 0 (0%) | 0 (0%) | 2 (100%) | 0 (0%) | |
| Higher | 1 (33%) | 2 (67%) | 0 (0%) | 2 (67%) | 1 (33%) | 0 (0%) | |
|
| |||||||
| Nat Hx | 1 (13%) | 4 (50%) | 3 (38%) | 3 (38%) | 3 (38%) | 2 (25%) | |
| 24 | Low | 0 (0%) | 0 (0%) | 3 (100%) | 0 (0%) | 1 (33%) | 2 (67%) |
| Medium | 0 (0%) | 2 (100%) | 0 (0%) | 0 (0%) | 2 (100%) | 0 (0%) | |
| Higher | 1 (33%) | 1 (33%) | 1 (33%) | 2 (67%) | 0 (0%) | 1 (33%) | |
|
| |||||||
| Nat Hx | 1 (14%) | 4 (57%) | 2 (29%) | 2 (29%) | 4 (57%) | 1 (14%) | |
| 36 | Low | 0 (0%) | 0 (0%) | 3 (100%) | 0 (0%) | 1 (33%) | 2 (67%) |
| Medium | 0 (0%) | 2 (100%) | 0 (0%) | 0 (0%) | 2 (100%) | 0 (0%) | |
|
Group 3: Unilateral Acute Group | |||||||
| Months | Study Eye | Fellow Eye | |||||
|
|
|||||||
| Followed | Dose | Worsened | Stable | Improved | Worsened | Stable | Improved |
|
| |||||||
| Nat Hx | 1 (50%) | 1 (50%) | 0 (0%) | 0 (0%) | 2 (100%) | 0 (0%) | |
| 12 | Low | 3 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (67%) | 1 (33%) |
| Medium | 3 (100%) | 0 (0%) | 0 (0%) | 2 (67%) | 1 (33%) | 0 (0%) | |
| Higher | 2 (100%) | 0 (0%) | 1 (0%) | 1 (50%) | 1 (50%) | 0 (0%) | |
|
| |||||||
| Nat Hx | 1 (50%) | 1 (50%) | 0 (0%) | 0 (0%) | 1 (50%) | 1 (50%) | |
| 24 | Low | 3 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (67%) | 1 (33%) |
| Medium | 3 (100%) | 0 (0%) | 0 (0%) | 2 (67%) | 1 (33%) | 0 (0%) | |
|
| |||||||
| Nat Hx | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | |
| 36 | Low | 3 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (67%) | 1 (33%) |
| Medium | 3 (100%) | 0 (0%) | 0 (0%) | 2 (67%) | 1 (33%) | 0 (0%) | |
Nat Hx = Natural History
Supplemental Table 6 presents mean logMAR ETDRS visual acuities for all groups and doses by visit, as well as corresponding visual acuities for the previously conducted prospective LHON natural history study. Supplemental Table 7 displays average improvements from baseline ETDRS acuity by group, dose, and follow-up, as well as corresponding acuities in the natural history cohort. Similarities and differences in the evolution of visual acuity over follow-up are depicted in Figure 1 (month 12) and Figure 2 (month 24). Improvements occurred in both injected and fellow eyes in both the gene therapy subjects and the natural history cohort patients. More improved eyes were noted at the 24-month follow-up.
Figure 1.

Month 12 acuity versus Baseline acuity by group, all doses included but not identified separately in these plots. A=Gene therapy treated eyes; B=Gene therapy fellow eyes; C=Natural history surrogate treated eyes; D=Natural history fellow eyes. Points within the gray region were stable at 12 months, while points above the gray region represent worsened acuity and points below represent improved vision. Of note, all unilateral acute eyes (Group 3, green dots) injected with IP worsened (plot A: green points), while two chronic injected eyes (Group 1, blue dots) improved more than 15 letters as did one acute eye. One unilateral acute, Group 3, fellow eye improved from <20/200 to nearly 20/20 (plot B, bold arrow), while this patient’s injected eye which received low dose IP worsened from 20/30 to <20/200. Two acute bilateral, (Group 2, red dots) natural history surrogate injected eyes improved from <20/200 to nearly (plot C, bold arrow), while three natural history surrogate fellow eyes also improved (plot D, bold arrow).
Figure 2.
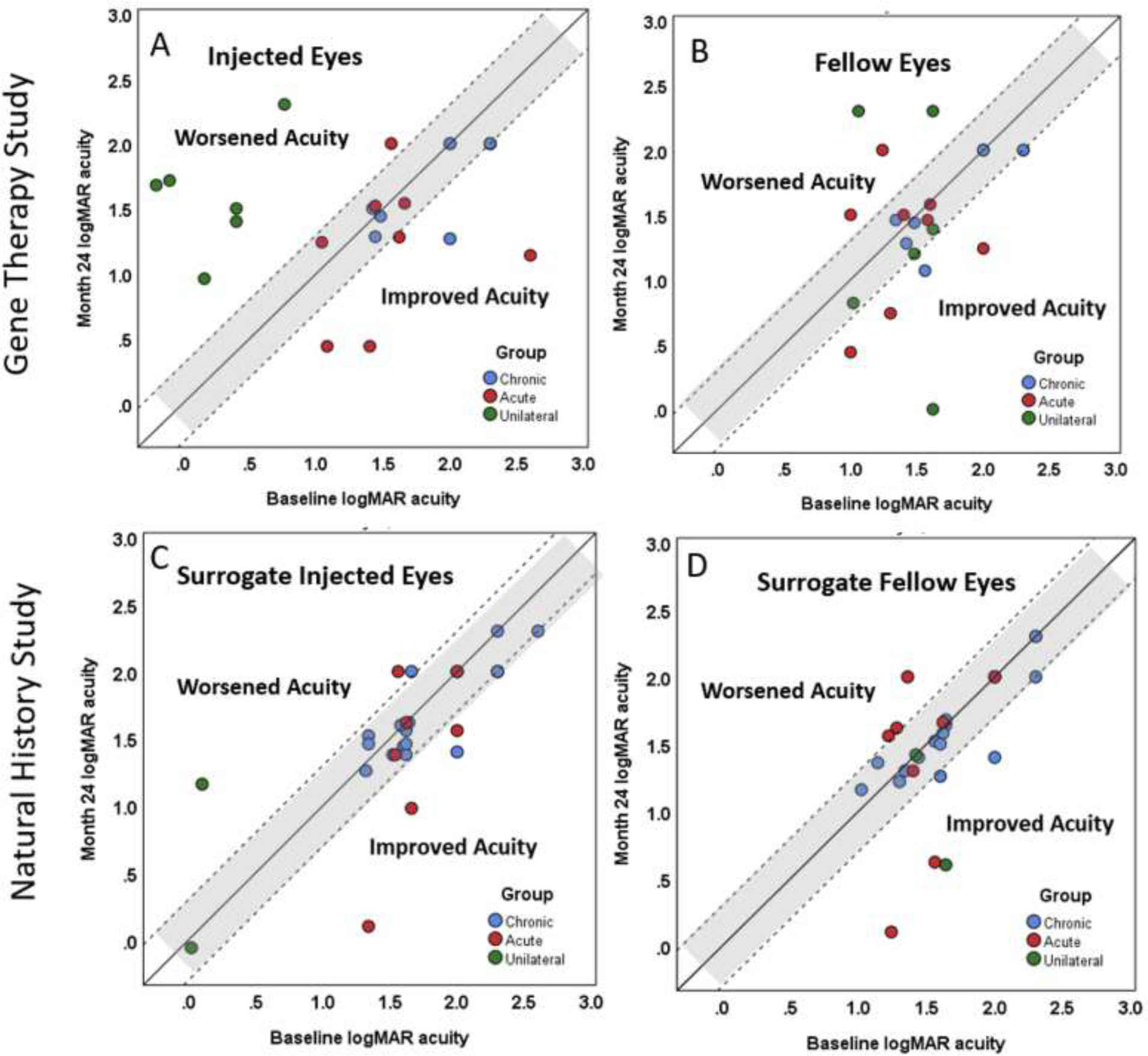
Month 24 acuity versus Baseline acuity by group, all doses included but not identified separately in these plots. A=Gene therapy treated eyes; B=Gene therapy fellow eyes; C=Natural history surrogate treated eyes; D=Natural history fellow eyes. Points within the gray region were stable at 24 months, while points above the gray region represent worsened acuity and points below represent improved vision. Further improvements in visual acuity can be appreciated in both the gene therapy and natural history patients in both injected (or surrogate injected) eyes and fellow eyes.
Vector Dose and Acuity improvement
We sought to determine if a relationship existed between IP dose and visual acuity improvement in the study eyes of chronic and acute bilateral groups. Due to small sample sizes and the low incidence of worsening among chronic and acute bilateral groups, we combined worse and stable acuities into a single category to facilitate examination for a dose-response relationship with acuity improvement. Further, due to small numbers and because examination of Supplemental Table 6 does not suggest particular efficacy of the higher dose, we combined high and higher doses. Even with these simplifications, we were unable to fit a single multivariable model to account for dose and months followed. Therefore, we performed separate analyses of percent 15 ETDRS letter acuity improvements by group for 12-month and 24-month follow-up visits as displayed in Figure 3. No consistent appearance of a relationship between acuity improvement and dose emerged (all p>0.05). Among chronic bilateral patients, the largest percent improvements occurred with the CHOP high and higher doses; however, in the acute bilateral patients, the largest percent improvements occurred with the UF-manufactured low dose.
Figure 3.
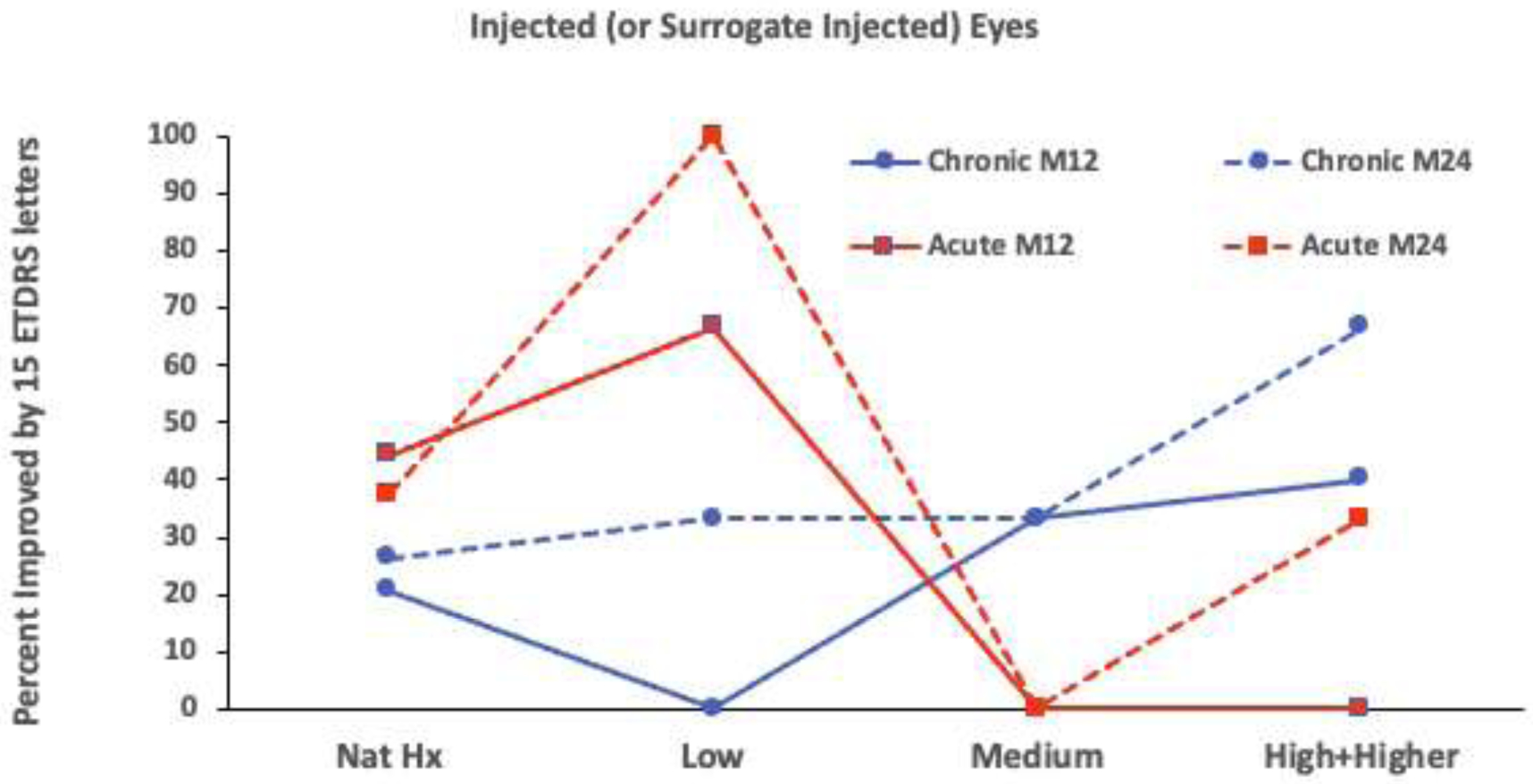
Percent with 15 letter visual improvements by dose and follow up for chronic and acute bilateral groups. No statitically significant relationships to IP dose were found for the chronic group at month 12 (M12) (p=0.66 and p=0.22, exact chi-squared test and exact test of trend in proportions, respectively) or M24 (p=0.70 and p=0.13). Neither were there statistically significant relationships in the acute bilateral group at M12 (p=0.36 and p=0.088) or at M24 (p=0.19 and p=0.41). Solid lines indicate M12 results; dashed lines indicate M24 results.
Change in acuity over time
Figure 4 displays visual acuity changes over time from baseline through month 36. No noteworthy changes in visual acuity over time were observed in either injected (or surrogate “injected”) eyes of the chronic bilateral groups of either the gene therapy or natural history patients. Despite very small sample sizes, we assessed the apparent improvement in acute bilateral eyes for statistical significance, which was seen in the injected eyes of gene therapy patients (p=0.033, GEE). No significant interaction was found between the course of acuity improvement in the gene therapy and natural history eyes (p=0.13, GEE), and when both gene therapy-injected eyes and natural history surrogate injected eyes were combined in a single analysis, acuity improvement over follow up was not quite statistically significant (p=0.070, GEE). Unilateral acute IP-injected eyes worsened substantially over follow-up (p<0.001, GEE), whereas their fellow eyes, which may be thought of as similar to the eyes of acute bilateral patients, showed no change over follow-up (p=0.65, GEE). There were insufficient unilateral acute natural history patients to attempt statistical analysis. A discussion of the analysis of visual field, SS-PERG, and GCL RNFL OCT is beyond the scope of this paper and will be reported in the future.
Figure 4.

LogMAR ETDRS visual acuity change over follow up by group and eye. All gene therapy doses combined. See supplemental table 5 for sample sizes. The apparent increase in unilateral surrogate (natural history) injected eye and fellow eye acuity is due to attrition of the two patients in this group who suffered poor visual outcomes. (GT=gene therapy, NHx=natural history)
Individual subjects demonstrated the wide range of clinical courses that can occur in LHON. For example, case 2–1-06 was a 49-year-old woman who improved the most of all subjects. Her baseline 2 BCVA was 20/500 right eye and 20/400 left eye. She was enrolled into the acute bilateral low-dose group and treated in the right eye. At the 12-month visit, BCVA was 20/250 right eye and 20/260 left eye. At the 24-month visit, BCVA had improved to 20/55 right eye and 20/100 left eye, with subsequent further improvement to 20/40 right and 20/70 left. On the other hand, Case 3–1-16 was a 52-year-old man who showed worsening of treated eye but remarkable improvement of the untreated eye. His baseline 2 BCVA was 20/800 right eye and 20/30 left eye. He was enrolled into the acute unilateral low-dose group and treated in the left eye. At the 12-month visit, BCVA of the untreated right eye had improved to 20/25 but the treated eye had worsened to 20/250. BCVA subsequently remained the same at the 24-month and 36-month visits with 20/25 right eye and 20/200 left eye. Case 2–4-26 was a 34-year-old man who showed delayed improvements in both the treated and untreated eyes. His baseline 2 BCVA was 20/200 right eye and 20/250 left eye. He was enrolled into the acute unilateral higher-dose group and treated in the left eye. At the 18-month visit, BCVA was 20/150 right eye and 20/250 left eye. At the 24-month visit, BCVA was 20/55 right eye and 20/55 left eye.
DISCUSSION
The results of this phase 1 G11778A LHON gene therapy trial showed a favorable safety and tolerability profile in eyes treated with intravitreal AAV2(Y444,500,730F)-P1ND4v2 up to the high dose. Incident uveitis (8/28 (29%) treated eyes) was the only probable IP-related adverse event and resulted in no attributable vision sequelae (Table 2). Incident uveitis was significantly related to vector dose. Three of 21 eyes (14%) treated with low-, medium-, or high-dose IP developed mild anterior uveitis that resolved spontaneously without treatment. In contrast, 5/7 (71%) of eyes treated with higher-dose (1.0 × 1010 vg/eye) IP developed anterior and intermediate uveitis (p=0.006, exact test of trend in proportions). Of these 5 eyes, 3 required topical prednisolone eye drops, and one eye required topical prednisolone and oral prednisone. All incident uveitis cases occurred within the first 2 months after intravitreal AAV2(Y444,500,730F)-P1ND4v2 and some persisted for months. One subject (1–4-29 Group 1 Chronic, higher dose) had persistent grade 1 anterior chamber cells at the last visit at 24-month with topical prednisolone discontinued at 18-month; flare meter at 24-month was stable or decreased indicating no clinically significant inflammation causing breakdown of blood aqueous barrier and low risk of future complications. Prophylactic steroid treatment was not given in any groups in the current study, and the greater occurrence of uveitis with the higher-dose vector suggests that prophylactic corticosteroid treatment and close monitoring may be beneficial when higher-dose vector treatment is utilized.
Injection-day anterior chamber AAV2 Nab titers were identical and low for all but three patients (Supplemental Table 3). These three included two patients without uveitis and one with asymptomatic uveitis not requiring treatment. Baseline and follow-up serum AAV2 Nab titers demonstrated an association between uveitis requiring treatment with injection of the higher dose IP (p=0.007); however, this was a post-hoc analysis with small numbers. No association was found between serum AAV2 PCR in subjects who developed uveitis in their treated eyes versus subjects who did not develop uveitis. Thus, increased high vector dose is the primary predictor of incident uveitis in eyes treated with intravitreal AAV2(Y444,500,730F)-P1ND4v2. Whereas an increase in the incidence of uveitis with increased doses of vector has been observed with other LHON gene therapy trials using a similar intravitreal vector, correlation with Nab and PCR were not reported.10–12 Of interest, in this study, the anterior chamber Nab titers and serum AAV2P1ND4v2 quantitative PCR values were negligible.
This phase 1 trial was designed to assess safety by measuring visual acuity and assessing adverse events. The low number of subjects provided limited power to assess efficacy of the gene therapy IP in stabilizing or improving vision. Because there was no randomized, untreated comparator arm in the trial, we compared visual acuity of untreated subjects from our previous prospective G11778A LHON natural history study4 who would have met the eligibility criteria for this trial and assigned injected eye and fellow-eye surrogates for comparison with our phase 1 trial patients. We defined visual acuity change as ≥15 ETDRS letters. Improvement of this magnitude was observed in both the study eyes and fellow eyes of some patients in the chronic bilateral and acute bilateral gene therapy groups as well as in both the surrogate study eyes and fellow eyes of some of the natural history subjects (Table 4, Figure 1, 12-month, Figure 2, 24-month). These 15-letter improvements were noted at both 12-month and 24-month follow-ups. In the unilateral acute group, gene therapy injection did not prevent any study eyes with an initial acuity ≥20/40 from losing ≥15 BCVA letters within the first year. Only two surrogate study eyes were available from the previous natural history study for comparison with the unilateral acute group, with one surrogate eye losing ≥15 letters and the other surrogate eye maintaining good acuity through 3 years of follow up, indicating that although severe visual loss may occur in acute LHON eyes with a BCVA of ≥20/40, BCVA may not worsen in all cases. Of interest, BCVA change analysis based on “nadir”, defined as the worst BCVA during the study period, has been used in other LHON gene therapy clinical trials.11–13 Because an analysis of BCVA compared with nadir BCVA will always result in improvement or no change in BCVA and a worsening of BCVA can never occur, we chose not to analyze our data based on nadir.
Baseline BCVA in the treated eye was a crude predictor of the final BCVA. Treated eyes that improved remarkably had baseline BCVA measurable on the ETDRS chart (≥20/800). Treated eyes that improved but had baseline BCVAs off the ETDRS chart (<20/800) never improved to >20/200. The amount and timing of improvement, if it occurred, in the treated and untreated eyes were highly variable and likely related to the variable clinical courses in the natural history of LHON.
In summary, G11778A LHON gene therapy with intravitreal AAV2(Y444,500,730F)-P1ND4v2 has a favorable safety and tolerability profile with dose-related incident uveitis as the only IP-related adverse event. Our results do not rule out efficacy in improving visual acuity; however, of note, the BCVA of all treated better seeing eyes in Group 3 unilateral acute patients worsened after treatment. Our results suggest if there is an efficacy effect, it is likely small and not related to IP dose. Instances of substantial improvement of ≥15 ETDRS letters were not uncommon in our natural history patients (Table 4), suggesting that a demonstration of gene therapy efficacy in LHON requires randomization of some patients to a group not receiving active IP in either eye.
Supplementary Material
ACKNOWLEDGEMENT
a. Funding/Support: Supported by the National Eye Institute, National Institutes of Health, Bethesda, Maryland (1U10EY023558-01A1 & UG1EY023558-01A1 (B.L.L. J.L.D., V.P.) 1U10EY024247-01 [W.J.F], P30EY014801 [V.P.]. We gratefully acknowledge the support of the National Heart, Lung and Blood Institute’s Gene Therapy Resource Program AAV facility at CHOP in manufacturing the higher dose investigational product used in this trial.
b. Financial Disclosures: B.L.L.: AGTC, Biogen, Foundation for Fighting Blindness, Editas, Jassen, ProQR, Spark (study grants); Biogen, Editas, ProQR, Jassen (consultant). J.L.D.: Consultant: Kodiak Sciences, 4D Molecular Therapeutics; Research Support: NIH-JHU ADVISE trial, GyroscopeTx.
c. Other Acknowledgements: The authors acknowledge the defining contributions of John Guy, M.D. (deceased), who invented the LHON gene therapy investigational product, directed pre-clinical studies, obtained the IND, and designed and implemented this phase I trial. His tireless efforts made this study possible.
We thank our NEI program officer, Maryann Redford, DDS, MPH, and the NEI appointed LHON DSMC (M Tariq Bhatti, MD, Chair; Theodore Friedmann, MD; Anne Lindblad, PhD; Neil R Miller, MD; and Maureen Neitz, PhD) for their support, guidance, many helpful contributions, and thoughtful constructive criticisms.
Footnotes
Supplemental Material available at AJO.com
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Riordan-Eva P, Sanders MD, Govan GG, Sweeney MG, Costa JD, Harding AE. The clinical features of Leber’s hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118(2):319–337. [DOI] [PubMed] [Google Scholar]
- 2.Sadun AA, La Morgia C, Carelli V. Leber’s hereditary optic neuropathy. Curr Treatment Options Neurol. 2011;13(1):109–117. [DOI] [PubMed] [Google Scholar]
- 3.Harding AE, Sweeney MG, Govan GG, Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am J Hum Genet. 1995;57(1):77–86. [PMC free article] [PubMed] [Google Scholar]
- 4.Lam BL, Feuer WJ, Schiffman JC, Porciatti V, Vandenbroucke R, Rosa PR, Gregori G, Guy J. Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy: preparation for gene therapy clinical trial. JAMA Ophthalmol. 2014;132(4):428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koilkonda RD, Yu H, Chou T-H, Feuer WJ, Ruggeri M, Porciatti V, Tse D, Hauswirth WW, Chiodo V, Boye SL, Lewin AS, Neuringer M, Renner L, Guy J. Safety and effects of the vector for the leber hereditary optic neuropathy gene therapy clinical trial. JAMA Ophthal. 2014;132(4):409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glick B, Wachter C, Schatz G. Protein import into mitochondria: two systems acting in tandem? Trends Cell Biol. 1991;1(4):99–103. [DOI] [PubMed] [Google Scholar]
- 7.Manfredi G, Fu J, Ojaimi J, Sadlock JE, Kwong JQ, Guy J, Schon EA. Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus. Nat Genet. 2002;30(4):394–399. [DOI] [PubMed] [Google Scholar]
- 8.Neupert W Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. [DOI] [PubMed] [Google Scholar]
- 9.Feuer WJ, Schiffman JC, Davis JL, Porciatti V, Gonzalez P, Koilkonda RD, Yuan H, Lalwani A, Lam BL, Guy J. Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123(3):558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vignal C, Uretsky S, Fitoussi S, Galy A, Blouin L, Girmens JF, Bidot S, Thomasson N, Bouquet C, Valero S, Meunier S, Combal JP, Gilly B, Katz B, Sahel JA. Safety of rAAV2/2-ND4 gene therapy for Leber hereditary optic neuropathy. Ophthalmology. 2018;125(6):945–947. [DOI] [PubMed] [Google Scholar]
- 11.Newman NJ, Yu-Wai-Man P, Carelli V, Moster ML, Biousse V, Vignal-Clermont C, Sergott RC, Klopstock T, Sadun AA, Barboni P, DeBusk AA, Girmens JF, Rudolph G, Karanjia R, Taiel M, Blouin L, Smits G, Katz B, Sahel JA; LHON Study Group. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within 6 months of disease onset. Ophthalmology. 2021;128(5):649–660. [DOI] [PubMed] [Google Scholar]
- 12.Yu-Wai-Man P, Newman NJ, Carelli V, Moster ML, Biousse V, Sadun AA, Klopstock T, Vignal-Clermont C, Sergott RC, Rudolph G, La Morgia C, Karanjia R, Taiel M, Blouin L, Burguière P, Smits G, Chevalier C, Masonson H, Salermo Y, Katz B, Picaud S, Calkins DJ, Sahel JA . Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. 2020;12(573). [DOI] [PubMed] [Google Scholar]
- 13.Newman NJ, Yu-Wai-Man P, Carelli V, Biousse V, Moster ML, Vignal-Clermont C, Sergott RC, Klopstock T, Sadun AA, Girmens JF, La Morgia C, DeBusk AA, Jurkute N, Priglinger C, Karanjia R, Josse C, Salzmann J, Montestruc F, Roux M, Taiel M, Sahel JA.. Intravitreal gene therapy vs. natural history in patients with Leber hereditary optic neuropathy carrying the m.11778G>A ND4 mutation: systematic review and indirect comparison. Front Neurol. 2021;12:662838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


