Abstract
Objective:
To compare the risk of COVID-19 outcomes by antiretroviral therapy (ART) regimens among men with HIV.
Design:
We included men with HIV on ART in the Veterans Aging Cohort Study who, between February 2020 and October 2021, were 18 years or older and had adequate virological control, CD4 count, and HIV viral load measured in the previous 12 months, and no previous COVID-19 diagnosis or vaccination.
Methods:
We compared the adjusted risks of documented SARS-CoV-2 infection, COVID-19-related hospitalization, and intensive care unit (ICU) admission by baseline ART regimen: tenofovir alafenamide (TAF)/emtricitabine (FTC), TDF/FTC, abacavir (ABC)/lamivudine (3TC), and other. We fit pooled logistic regressions to estimate the 18-month risks standardized by demographic and clinical factors.
Results:
Among 20,494 eligible individuals, the baseline characteristics were similar across regimens, except that TDF/FTC and TAF/FTC had lower prevalences of chronic kidney disease and eGFR <60mL/min. Compared with TAF/FTC, the estimated 18-month risk ratio (95% CI) of documented SARS-CoV-2 infection was 0.65 (0.43, 0.89) for TDF/FTC, 1.00 (0.85, 1.18) for ABC/3TC, and 0.87 (0.70, 1.04) for others. The corresponding risk ratios for COVID-19 hospitalization were 0.43 (0.07, 0.87), 1.09 (0.79, 1.48), and 1.21 (0.88, 1.62). The risk of COVID-19 ICU admission was lowest for TDF/FTC, but the estimates were imprecise.
Conclusion:
Our study suggests that, in men living with HIV, TDF/FTC may protect against COVID-19-related events. Randomized trials are needed to investigate the effectiveness of TDF as prophylaxis for, and early treatment of, COVID-19 in the general population.
Keywords: COVID-19, tenofovir, antiretroviral therapy, HIV, SARS-CoV-2
Introduction
Tenofovir is an antiviral used against HIV infection and chronic hepatitis B infection. Its two forms, tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF), are commonly used in combination with emtricitabine (FTC) as the nucleoside reverse transcriptase inhibitor (NRTI) backbone in many antiretroviral therapy (ART) regimens for the treatment of HIV infection and as pre-exposure prophylaxis for HIV infection. TDF/FTC is recommended by WHO guidelines as the preferred NRTI backbone for first-line ART regimens in adults[1].
Recent evidence suggests that TDF may have antiviral potential against severe COVID-19. Observational studies in Spain[2, 3] and South Africa[4] among persons with HIV have found that those receiving TDF/FTC had a lower risk of severe COVID-19 compared with those receiving other antiretrovirals, including TAF/FTC. Similarly, a study among individuals with hepatitis B in Spain found that those on TDF had a lower risk of severe COVID-19 than those on entecavir[5].
Both TDF and TAF may interfere with the SARS-CoV-2 RNA-dependent RNA-polymerase, but TDF results in higher plasma concentrations of tenofovir than TAF[6]. Thus, TDF may have greater effectiveness against COVID-19 because of its higher bioavailability in tissues affected by SARS-CoV-2. In addition, TDF is an inexpensive generic drug shown to be very safe, even in pregnancy[7]. After long periods of use, TDF may infrequently cause loss of bone density or renal toxicity which are fully reversible upon discontinuation of the drug[8].
Here, we compared the risk of COVID-19 outcomes by NRTI combination among men with HIV in the Veterans Health Administration, the largest integrated healthcare system in the United States (U.S.).
Methods
Population and data source
The Veterans Aging Cohort Study (VACS)[9, 10], includes a national cohort of persons with HIV and matched individuals without HIV who have received care within the Veterans Health Administration between 1997–2021. Ethical approval was received from the institutional review boards of Yale University, New Haven, CT, the Veterans Affairs (VA) Connecticut Healthcare System, West Haven, CT, and the Harvard T.H. Chan School of Public Health. For this study, we restricted our analysis to men, who comprise 96% of the cohort of persons with HIV in the VA.
Information on ART pharmacy dispensing, laboratory values, hospitalization admission and discharge dates, intensive care unit (ICU) admission dates, death dates, comorbidities (see Appendix Table 1 for International Classification of Disease codes), and demographics were ascertained using electronic health records in the Corporate Data Warehouse, a national repository that incorporates data from clinical and administrative systems in a data warehouse structure[11].
ART dispensing data included type of antiretroviral drug, start dates and end dates. To determine the ART regimen used by each individual during each month, we sorted start dates chronologically, removed duplicate drugs that appeared more than once within the same prescription period, and ignored prescription gaps shorter than 14 days. Then we classified ART regimens according to their NRTI backbone combination into TAF/FTC, TDF/FTC, ABC/3TC, other NRTI combination, and no NRTI. We combined “other NRTI combination” and “no NRTI” into an “Other” category.
Laboratory data included test dates and results for CD4 cell count, CD4 cell percentage, HIV-1 RNA viral load, and SARS-CoV-2 diagnostic tests, of which approximately 85% were polymerase chain reaction (PCR) tests. Clinical measurements included measurements of systolic and diastolic blood pressure, height, weight, and body mass index (BMI).
Study design
Our study included men with HIV on ART in VACS who between February, 2020 (when nontravel-related COVID-19 cases were first identified in the U.S.[12]) and October 2021 were aged 18 years or older, virologically suppressed (HIV viral load <200 copies/mL), and who had CD4 cell count and HIV viral load measured in the previous 12 months, no history of CD4 cell count <50 cells/μL, and no previously documented COVID-19 diagnosis or vaccination against COVID-19. We excluded a small number of individuals with inconclusive data on NRTI combination and with missing values of smoking, BMI, or blood pressure.
Eligible individuals were classified according to their NRTI combination (TAF/FTC, TDF/FTC, ABC/3TC, Other). Baseline was defined as the first month on or after February 1, 2020 when an individual met all eligibility criteria. Each eligible individual was followed from baseline until the month of the outcome of interest, vaccination against COVID-19, or October 31, 2021, whichever happened first. The outcomes of interest were any documented SARS-CoV-2 infection (confirmed by diagnostic test), COVID-19-related hospitalization, COVID-19-related ICU admission, and COVID-19-related death. COVID-19-related events were defined as events that occurred within 30 days following a positive SARS-CoV-2 test.
Statistical analysis
We estimated the 18-month risks of each outcome by NRTI combination and compared them using risk ratios and risk differences. All risks were standardized to the following baseline covariates: age (linear and quadratic terms), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic, other/unknown), smoking status (never, current, former), CD4 cell count, HIV viral load (<50, ≥50 copies/mL), maximum HIV viral load over the past 12 months (<50, ≥50 copies/mL), BMI, systolic and diastolic blood pressure, average estimated glomerular filtration rate (eGFR) over the past 12 months (<60, 60–90, >90 mL/min, missing), number of months between February 2020 and baseline, hospitalization within one month prior to baseline (yes, no), and indicators for the following comorbidities (defined as one inpatient or two outpatient diagnoses, based on outpatient visits and inpatient stays between January 2019 and baseline): cardiovascular disease, hypertension, liver disease, chronic pulmonary disease, cancer, chronic kidney disease, alcohol/substance use, diabetes, and dementia.
For each outcome, we estimated the standardized risks via a pooled logistic regression that included indicators for NRTI combination, the baseline covariates, month of follow-up (linear and quadratic terms), and product terms between NRTI combination and the terms for month of follow-up. We used nonparametric bootstrapping with 1000 samples to calculate percentile-based 95% confidence intervals (95% CI). We also conducted subgroup analyses in Whites and Blacks separately, by age (<50, ≥50 years), and by renal function (eGFR <60, ≥60 mL/min).
As sensitivity analyses, we (i) estimated conditional hazard ratios (HRs) with and without covariate adjustment by fitting separate pooled logistic regression models without the product terms described above, (ii) repeated the analysis with death as a censoring event, (iii) repeated the analysis without vaccination against COVID-19 as a censoring event, (iv) conducted the analysis among women and men combined, and (v) compared the risk of all-cause mortality by NRTI combination to explore the possibility of residual confounding.
We used SAS version 9.4 (SAS Institute, Cary, NC, USA) for data management and R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria) for data analyses.
Results
Among 20,494 eligible individuals (Figure 1) with mean age of 58.7 years, TAF/FTC was the most commonly used NRTI combination (62%), followed by ABC/3TC (19%), and TDF/FTC (5%). Table 1 shows the baseline characteristics of eligible individuals by NRTI combination. The proportion of individuals with eGFR <60mL/min was 9.2% in the TDF/FTC group and 18.0% in the TAF/FTC group. The prevalence of chronic kidney disease was 4.5% in the TDF/FTC group and 8.2% in the TAF/FTC group. The prevalence of other comorbidities was similar across all groups.
Figure 1.
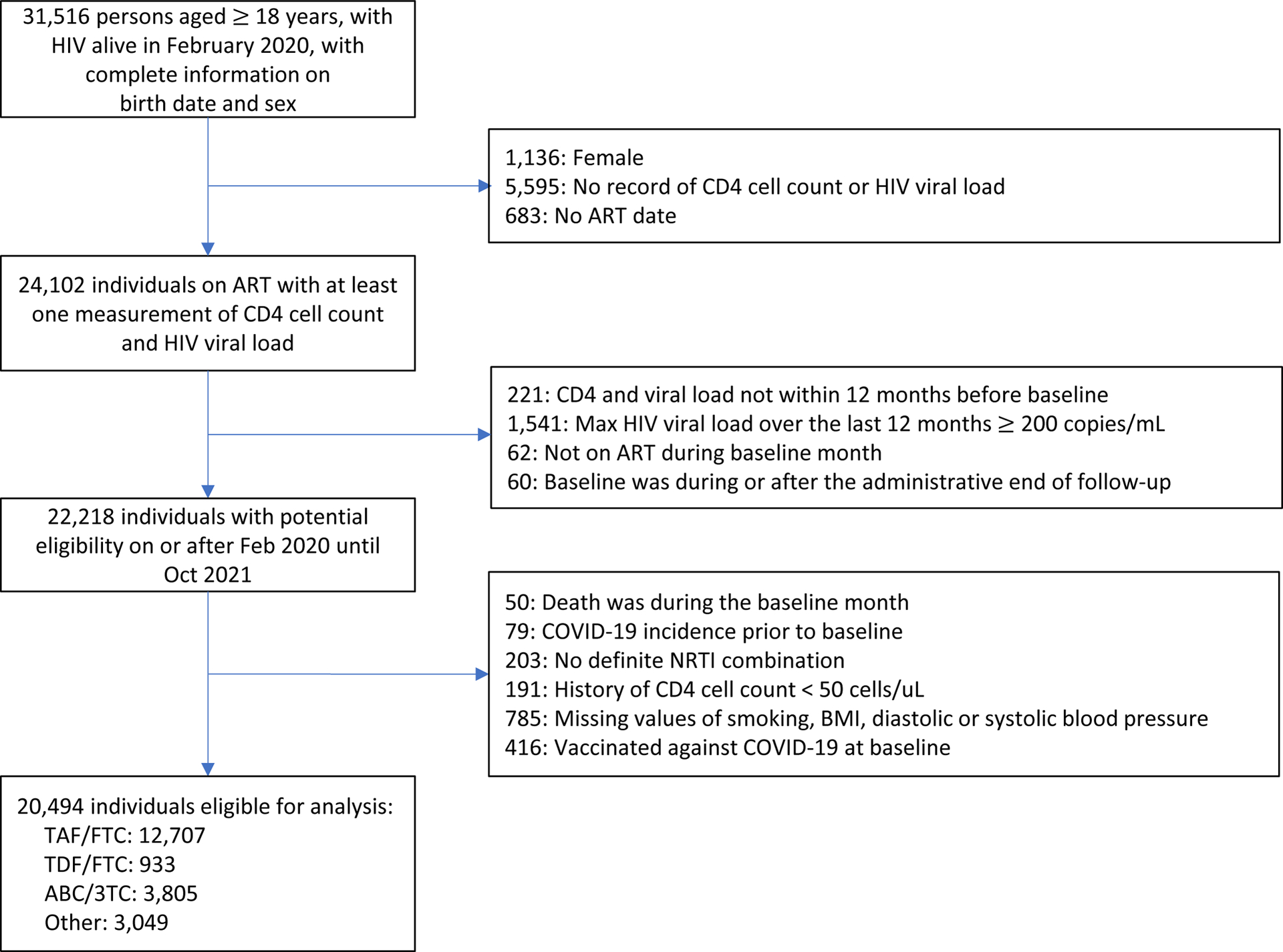
Flowchart of eligible persons with HIV, Veterans Aging Cohort Study, February 2020 – October 2021
Table 1.
Baseline characteristics of eligible men with HIV by NRTI combination group, Veterans Aging Cohort Study, February 2020 – October 2021
| TAF/FTC (N=12,707) |
TDF/FTC (N=933) |
ABC/3TC (N=3,805) |
Other (N=3,049) |
|
|---|---|---|---|---|
| Mean age (SD), years | 58.0 (12.1) | 58.7 (11.8) | 58.9 (12.5) | 61.2 (11.7) |
| Age, years | ||||
| 18–39 | 1393 (11.0%) | 84 (9.0%) | 408 (10.7%) | 224 (7.3%) |
| 40–49 | 1446 (11.4%) | 115 (12.3%) | 405 (10.6%) | 233 (7.6%) |
| 50–59 | 3695 (29.1%) | 277 (29.7%) | 992 (26.1%) | 774 (25.4%) |
| 60–69 | 4131 (32.5%) | 302 (32.4%) | 1261 (33.1%) | 1112 (36.5%) |
| 70+ | 2042 (16.1%) | 155 (16.6%) | 739 (19.4%) | 706 (23.2%) |
| Race/Ethnicity | ||||
| Non-Hispanic White | 4696 (37.0%) | 361 (38.7%) | 1292 (34.0%) | 1059 (34.7%) |
| Non-Hispanic Black | 5265 (41.4%) | 438 (46.9%) | 1873 (49.2%) | 1492 (48.9%) |
| Hispanic | 1158 (9.1%) | 75 (8.0%) | 271 (7.1%) | 227 (7.4%) |
| Other/unknown | 1588 (12.5%) | 59 (6.3%) | 369 (9.7%) | 271 (8.9%) |
| Smoking status | ||||
| Never | 4449 (35.0%) | 285 (30.5%) | 1297 (34.1%) | 985 (32.3%) |
| Current | 5711 (44.9%) | 454 (48.7%) | 1732 (45.5%) | 1454 (47.7%) |
| Former | 2547 (20.0%) | 194 (20.8%) | 776 (20.4%) | 610 (20.0%) |
| Mean CD4 cell count (SD), cells/μL | 676 (306) | 699 (311) | 699 (311) | 623 (309) |
| Detectable HIV viral load (≥50 copies/mL) | 806 (6.3%) | 52 (5.6%) | 215 (5.7%) | 198 (6.5%) |
| Detectable max viral load over the past 12 months (≥50 copies/mL) | 1834 (14.4%) | 105 (11.3%) | 451 (11.9%) | 458 (15.0%) |
| Mean BMI (SD), kg/m2 | 28.2 (5.41) | 27.5 (5.54) | 27.7 (5.34) | 27.1 (5.41) |
| Mean systolic blood pressure (SD), mmHg | 129 (11.6) | 129 (12.4) | 130 (12.2) | 130 (12.7) |
| Mean diastolic blood pressure (SD), mmHg | 77.9 (7.28) | 78.4 (7.44) | 78.2 (7.22) | 77.0 (7.80) |
| Mean eGFR within one year before baseline | ||||
| eGFR > 90 mL/min | 1821 (14.3%) | 202 (21.7%) | 368 (9.7%) | 396 (13.0%) |
| eGFR = 60–90 mL/min | 6381 (50.2%) | 504 (54.0%) | 1664 (43.7%) | 1200 (39.4%) |
| eGFR < 60 mL/min | 2292 (18.0%) | 86 (9.2%) | 1081 (28.4%) | 876 (28.7%) |
| Missing | 2213 (17.4%) | 141 (15.1%) | 692 (18.2%) | 577 (18.9%) |
| Baseline month | ||||
| Feb 2020 | 10732 (84.5%) | 811 (86.9%) | 3263 (85.8%) | 2515 (82.5%) |
| After Feb 2020 | 1975 (15.5%) | 122 (13.1%) | 542 (14.2%) | 534 (17.5%) |
| Hospitalization in previous month | 31 (0.2%) | 1 (0.1%) | 13 (0.3%) | 13 (0.4%) |
| Comorbidities | ||||
| Osteoporosis | 321 (2.5%) | 10 (1.1%) | 113 (3.0%) | 96 (3.1%) |
| Myocardial infarction | 38 (0.3%) | 2 (0.2%) | 12 (0.3%) | 18 (0.6%) |
| Heart Failure | 274 (2.2%) | 11 (1.2%) | 78 (2.1%) | 126 (4.1%) |
| Hypertension | 3962 (31.2%) | 277 (29.7%) | 1300 (34.2%) | 1061 (34.8%) |
| Ischemic stroke | 214 (1.7%) | 10 (1.1%) | 83 (2.2%) | 80 (2.6%) |
| Unstable angina | 30 (0.2%) | 0 (0%) | 6 (0.2%) | 21 (0.7%) |
| Chronic kidney disease | 1037 (8.2%) | 42 (4.5%) | 546 (14.3%) | 468 (15.3%) |
| Cancer | 648 (5.1%) | 55 (5.9%) | 215 (5.7%) | 192 (6.3%) |
| Metastatic cancer | 72 (0.6%) | 5 (0.5%) | 19 (0.5%) | 18 (0.6%) |
| Hepatitis B | 180 (1.4%) | 8 (0.9%) | 19 (0.5%) | 43 (1.4%) |
| Hepatitis C | 243 (1.9%) | 14 (1.5%) | 69 (1.8%) | 87 (2.9%) |
| Mild liver disease | 1265 (10.0%) | 87 (9.3%) | 386 (10.1%) | 343 (11.2%) |
| Severe liver disease | 55 (0.4%) | 4 (0.4%) | 20 (0.5%) | 22 (0.7%) |
| Asthma | 347 (2.7%) | 25 (2.7%) | 102 (2.7%) | 90 (3.0%) |
| Chronic obstructive pulmonary disease | 804 (6.3%) | 67 (7.2%) | 258 (6.8%) | 271 (8.9%) |
| Pneumonia | 253 (2.0%) | 13 (1.4%) | 89 (2.3%) | 112 (3.7%) |
| Bronchiectasis/ Pulmonary fibrosis/ Pulmonary hypertension | 145 (1.1%) | 5 (0.5%) | 37 (1.0%) | 42 (1.4%) |
| Alcohol use disorder | 834 (6.6%) | 43 (4.6%) | 253 (6.6%) | 202 (6.6%) |
| Opioid use disorder | 173 (1.4%) | 12 (1.3%) | 74 (1.9%) | 69 (2.3%) |
| Diabetes | 1444 (11.4%) | 75 (8.0%) | 429 (11.3%) | 368 (12.1%) |
| Dementia | 112 (0.9%) | 11 (1.2%) | 38 (1.0%) | 56 (1.8%) |
| Rheumatoid arthritis | 32 (0.3%) | 5 (0.5%) | 13 (0.3%) | 13 (0.4%) |
Abbreviations: 3TC – lamivudine; ABC – abacavir; FTC – emtricitabine; NRTI – nucleoside reverse transcriptase inhibitor; TDF – tenofovir disoproxil fumarate; TAF – tenofovir alafenamide; SD – standard deviation; BMI – body mass index; eGFR – estimated glomerular filtration rate
During 18 months of follow-up, there were 984 persons who tested positive for SARS-CoV-2 infection, 284 COVID-19-related hospitalizations, 87 COVID-19-related ICU admissions, and 31 COVID-19-related deaths. The monthly COVID-19 incidence in this study was similar to the incidence in the U.S. over the study period [13] (Appendix Figure 1). Figure 2 shows the estimated 18-month risk curves for documented SARS-CoV-2 infection, COVID-19 hospitalization, and COVID-19 ICU admission by NRTI combination. The number of SARS-CoV-2 tests proportional to persons under follow-up in each month by treatment groups is shown in Appendix Figure 2.
Figure 2.
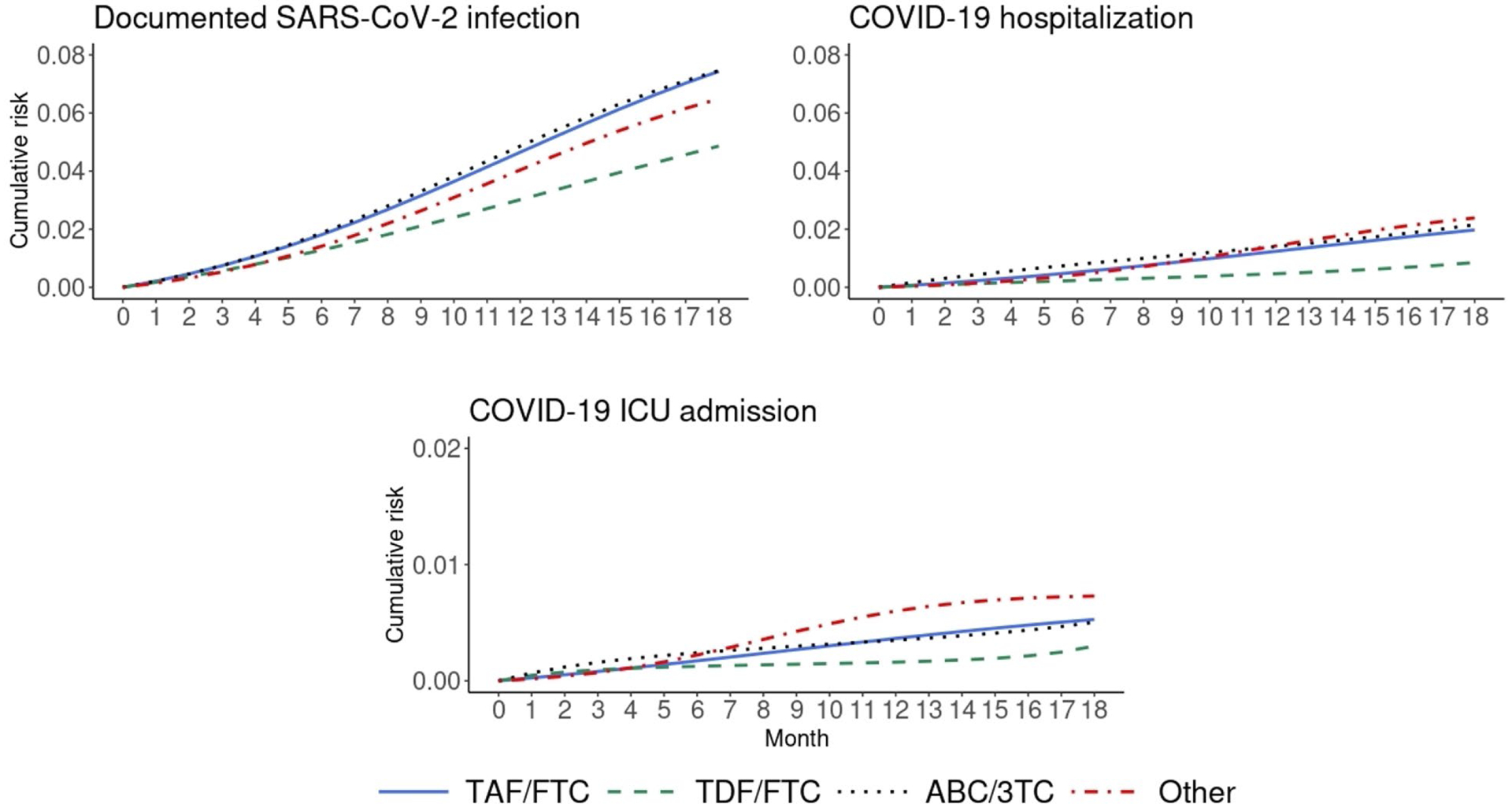
Standardized 18-month cumulative risk curves for COVID-19 outcomes by NRTI combination in men with HIV, Veterans Aging Cohort Study, February 2020 – October 2021
Compared with TAF/FTC, the estimated 18-month risk ratio (95% CI) of documented SARS-CoV-2 infection was 0.65 (0.43, 0.89) for TDF/FTC, 1.00 (0.85, 1.18) for ABC/3TC, and 0.87 (0.70, 1.04) for other regimens (Table 2). The corresponding risk ratios for COVID-19 hospitalization were 0.43 (0.07, 0.87), 1.09 (0.79, 1.48), and 1.21 (0.88, 1.62). The risk of COVID-19 ICU admission was lowest for TDF/FTC, but the estimates were imprecise. There was only one COVID-19 death in TDF/FTC users (12 in TAF/FTC users, 12 in ABC/3TC users, and six in users of other regimens), which prevented the calculation of adjusted estimates.
Table 2.
Risk estimates for COVID-19-related outcomes by NRTI combination in men with HIV, Veterans Aging Cohort Study, February 2020 – October 2021
| No. events | 18-month risk*, % (95% CI) | Risk difference, % (95% CI) | Risk ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Documented SARS-CoV-2 infection | TAF/FTC | 621 | 7.4 | (6.8, 8.0) | 0 (ref.) | .. | 1 (ref.) | .. |
| TDF/FTC | 28 | 4.9 | (3.2, 6.6) | −2.6 | (−4.3, −0.9) | 0.65 | (0.43, 0.89) | |
| ABC/3TC | 201 | 7.5 | (6.4, 8.5) | 0.0 | (−1.2, 1.2) | 1.00 | (0.85, 1.18) | |
| Other | 134 | 6.5 | (5.3, 7.6) | −0.9 | (−2.3, 0.3) | 0.87 | (0.70, 1.04) | |
| COVID-19 hospitalization | TAF/FTC | 156 | 2.0 | (1.6, 2.3) | 0 (ref.) | .. | 1 (ref.) | .. |
| TDF/FTC | 5 | 0.9 | (0.1, 1.7) | −1.1 | (−1.9, −0.2) | 0.43 | (0.07, 0.87) | |
| ABC/3TC | 63 | 2.2 | (1.6, 2.8) | 0.2 | (−0.4, 0.9) | 1.09 | (0.79, 1.48) | |
| Other | 60 | 2.4 | (1.8, 3.1) | 0.4 | (−0.3, 1.1) | 1.21 | (0.88, 1.62) | |
| COVID-19 ICU admission | TAF/FTC | 43 | 0.5 | (0.4, 0.7) | 0 (ref.) | .. | 1 (ref.) | .. |
| TDF/FTC | 2 | 0.3 | (0.0, 1.4) | −0.2 | (−0.6, 0.8) | 0.57 | (0.00, 2.63) | |
| ABC/3TC | 18 | 0.5 | (0.3, 0.8) | 0.0 | (−0.3, 0.3) | 0.96 | (0.51, 1.81) | |
| Other | 24 | 0.7 | (0.4, 1.1) | 0.2 | (−0.1, 0.6) | 1.38 | (0.75, 2.42) | |
Standardized by age, race/ethnicity, smoking status, CD4 cell count, HIV viral load, maximum HIV viral load over the past 12 months, BMI, systolic blood pressure, diastolic blood pressure, average eGFR over the past 12 months, months since February 2020, hospitalization in previous month, comorbidities including: cardiovascular disease, hypertension, liver disease, chronic pulmonary disease, cancer, chronic kidney disease, alcohol/substance use, diabetes, and dementia.
In sensitivity analyses, the hazard ratios for TDF/FTC were similar to the risk ratios above and adjustment for covariates had little impact on the estimates (Appendix Table 2). Also, risk ratio estimates were similar when censoring at death (Appendix Table 3), when not censoring at vaccination against COVID-19 (Appendix Table 4), and when both men and women were included in the analysis (Appendix Table 5). The risk of all-cause mortality was not lower for TDF/FTC compared with TAF/FTC (Appendix Table 6).
In subgroup analyses, the 18-month risk of COVID-19 hospitalization was lower for Whites than for Blacks, for individuals aged <50 than for those aged ≥50, and for individuals with eGFR ≥60 mL/min than for those with eGFR <60 mL/min. The estimated 18-month risk ratios for COVID-19 hospitalization for TDF/FTC compared with TAF/FTC were imprecise, but under 0.5 for both races and for the largest subgroups (individuals aged ≥50 and individuals with eGFR ≥60 mL/min) (Appendix Table 7).
Discussion
In this study of male veterans with HIV and with adequate virological suppression, we estimated that the risk of documented SARS-CoV-2 infection, COVID-19 hospitalization, and ICU admission was lower among those on TDF/FTC than among those using TAF/FTC, ABC/3TC, and other NRTI/no NRTI combinations.
Our findings are consistent with previous observational studies of ART treatment in persons with HIV. An early study in Spain[2, 14] found an approximately 50% lower risk of COVID-19 hospitalization among those on TDF/FTC compared with those on TAF/FTC and other NRTI combinations, but the analysis did not adjust for comorbidities. A study in South Africa[4] found an approximately 60% lower risk of COVID-19 mortality for TDF/FTC compared with ABC/zidovudine after adjustment for comorbidities, including chronic kidney disease. Similarly, a recent study in Spain (only available as a preprint) found the lowest risk of COVID-19 hospitalization among TDF/FTC users after adjustment for comorbidities[3]. A study among persons with hepatitis B in Spain also found a lower risk of severe COVID-19 in TDF users compared with entecavir users[5]. Finally, a small randomized trial among outpatients with confirmed SARS-CoV-2 infection found a reduced SARS-CoV-2 viral load and a greater proportion free of symptoms (6/30 vs. 3/30) in the TDF/FTC group than in the standard of care group[15].
Two small observational studies found a slightly higher seroprevalence of SARS-CoV-2 among users of pre- exposure prophylaxis (PrEP), including TDF/FTC users, compared with nonusers [16, 17]. However, the use of PrEP could be a marker of higher probability of exposure to SARS-CoV-2 because PrEP use is associated with a greater number of sexual partners[18–22]. Interestingly, one of these studies[16] reported that both the risk of symptomatic COVID-19 and the duration of symptoms were lower in TDF/FTC users than in TAF/FTC users, a finding that is compatible with a role of TDF/FTC in preventing disease rather than infection.
Our study has several limitations. First, like any observational study, the results may be affected by unmeasured confounding. However, the rich information in the VA electronic health records allowed us to adjust for comorbidities that are less frequent among TDF/FTC users and that may be related to COVID-19 diagnosis. Adjustment had little impact on the estimates. Additionally, the risk of all-cause mortality for TDF/FTC was comparable to that for all other NRTI combinations, indicating that after adjusting for our measured confounders, the TDF/FTC group did not appear to be healthier than other groups.
Second, there might be underreporting of SARS-CoV-2 infections, hospitalizations, ICU admissions, and deaths if individuals received care outside of the VA. However, this potential misclassification is not expected to vary by NRTI combination and thus it would likely bias our effect estimates towards the null. Moreover, because we studied regular VA users with HIV-related laboratory results and ART dispensing records over the past year, bias due to misclassification is unlikely to be high.
Third, our observational analysis emulates a target trial in which follow-up starts when participants start to be potentially exposed to SARS-CoV-2, which is generally a period after the initiation of antiretroviral treatment, i.e., the analysis excludes individuals who are lost to follow-up, die, or stop meeting the eligibility criteria between the initiation of their current NRTI combination and February 1, 2020. However, the resulting selection would only create a noncausal association (due to selection bias) if the probability of reaching February 1, 2020 depended on both the NRTI combination and on risk factors for COVID-19 diagnosis or severity. Given the comparable efficacy of TAF/FTC, TDF/FTC and ABC/3TC[23], it is reasonable to assume the probability of reaching February 1, 2020 is unlikely to differ across NRTI combinations.
Fourth, because most of the individuals in the study may have been stably on their ART regimens before the start of follow-up, we cannot determine the minimum period necessary for prophylaxis before exposure to the virus. If that period were short and our effect estimates were unbiased, our results may imply that TDF could conceivably be used for treatment of early infection rather than only pre-exposure prophylaxis, but further investigation is needed.
Last, our primary analysis study population consists of male veterans only. However, our findings were similar in sensitivity analyses that also included female veterans and compatible with those from other populations of persons with HIV who included both men and women[2–4].
In conclusion, the findings of this observational study suggest that TDF may provide protection against severe COVID-19 outcomes in persons with HIV. Our study, taken together with the available evidence, supports the conduct of randomized trials to investigate the potential role of TDF/FTC as prophylaxis and early treatment of COVID-19 in HIV-negative populations.
Acknowledgements
Funding:
This research was supported by National Institutes of Health grants R37 AI102634, U24 AA020794, U01 AA020790, U24 AA022001, U10 AA013566, K99 CA248335, and the US Department of Veterans Affairs Office of Research and Development through CSP 2032 and the use of data from the VA COVID-19 Shared Data Resource.
Additionally, we thank Marc Lipsitch for helpful comments to an earlier version of the manuscript.
Conflicts of Interest:
M.A.H. and B.A.D. report grants from the National Institutes of Health and the U.S. Department of Veterans Affairs. M.A.H. reports support from the U.S. Department of Veterans Affairs and (unrelated to this work) consulting fees from Cytel and ProPublica. Other authors declare no conflict of interests.
Appendix – Tenofovir disoproxil fumarate and COVID-19 outcomes in men with HIV
Appendix Table 1.
Definition of comorbidities and associated ICD-10 diagnosis codes
| Comorbidity | ICD10 |
|---|---|
| Acute myocardial infarction | I21.01, I21.02, I21.09, I21.11, I21.19, I21.21, I21.29, I21.3, I21.4, I21.9, I22.0, I24.0 |
| Heart failure | I11.0, I13.0, I13.2, I50.1, I50.20, I50.21, I50.22, I50.23, I50.30, I50.31, I50.32, I50.33 |
| Hypertension | I10, I11, I12, I13, I15, I16 |
| Ischemic stroke | I6302, I63.12, I63.22, I65.21, I65.22, I65.23, I65.29, I63.031, I63.032, I63.033, I63.039, I63.131, I63.132, I63.133, I63.139, I63.231, I63.232, I63.233, I63.239, I63.011, I63.012, I63.013, I63.019, I63.111, I63.112, I63.113, I63.119, I63.211, I63.212, I63.213, I63.219, I63.59, I63.09, I63.19, I63.59, I63.00, I63.10, I63.20, I63.29, I66.01, I66.02, I66.03, I66.09, I66.11, I66.12, I66.13, I66.19, I66.21, I66.22, I66.23, I66.29, I66.3, I63.30, I63.311, I63.312, I63.313, I63.319, I63.321, I63.322, I63.323, I63.329, I63.331, I63.332, I63.333, I63.339, I63.341, I63.342, I63.343, I63.349, I63.39, I63.6, I63.40, I63.411, I63.412, I63.413, I63.419, I63.421, I63.422, I63.423, I63.429, I63.431, I63.432, I63.433, I63.439, I63.441, I63.442, I63.443, I63.449, I63.49, I63.50, I63.511, I63.512, I63.513, I63.519, I63.521, I63.522, I63.523, I63.529, I63.531, I63.532, I63.533, I63.539, I63.541, I63.542, I63.543, I63.549, I63.59, I63.8, I63.9, I6789 |
| Unstable Angina | I24.1, I20.0, I25.110, I25.700, I25.710, I25.720, I25.730, I25.750, I25.760, I25.790, I24.0, I24.8, I24.9 |
| Chronic kidney disease | N19, N18, I12.0, I13.1, N03.2, N03.3, N03.4, N03.5, N03.6, N03.7, N05.2, N05.3, N05.4, N05.5, N05.6, N05.7, N25.0, Z49.0, Z49.1, Z49.2, Z94.0, Z99.2 |
| Cancers | C00-C43, C45-C76, C80-C96, C7A |
| Metastatic cancer | C77, C78, C79, C80 |
| Hepatitis B | B16, B18.0, B18.1, B19.1, Z22.51 |
| Hepatitis C | B17.10, B17.11, B18.2, B19.20, B19.21, Z22.52 |
| Mild liver disease | B18, K73, K70.3, K71.7, K74.3, K74.4, K74.5, K74.6, K70.0, K70.1, K70.2, K70.9, K71.3, K71.4, K71.5, K74.0, K74.1, K74.2, K76.0, K76.2, K76.3, K76.4, K76.8, K76.9, Z94.4 |
| Severe liver disease | K72.1, K72.9, K76.6, K76.7, I85.0, I85.9, I86.4, I98.2, K70.4, K71.1, K76.5 |
| Asthma | J45 |
| Chronic obstructive pulmonary disease | J41.0, J41.1, J41.8, J42, J43.0, J43.1, J43.2, J43.8, J43.9, J44.0, J44.1, J44.9 |
| Pneumonia, bacterial | J13, J14, J16.0, J15, J16.8, J17, J18, J09.X1, J09.X2, J10.1, J11.1, J10.0, J11.0 J12 |
| Pneumonia, NOS | |
| Pneumonia, viral | |
| Bronchiectasis | J47, J84, NOT = J84.8, J84.89, I27 |
| Pulmonary fibrosis | |
| Pulmonary hypertension | |
| Alcohol use disorder | F10.120, F10.121, F10.129, F10.150, F10.151, F10.159, F10.180, F10.181, F10.182, F10.188, F10.220, F10.221, F10.229, F10.230, F10.231, F10.232, F10.239, F10.250, F10.251, F10.259, F10.280, F10.281, F10.282, F10.288, F10.10, F10.11, F10.14, F10.19, F10.20, F10.21, F10.24, F10.25, F10.26, F10.27, F10.29 |
| Opioid use disorder | F11.120, F11.121, F11.122, F11.129, F11.150, F11.151, F11.159, F11.181, F11.182, F11.188, F11.220, F11.221, F11.222, F11.229, F11.250, F11.251, F11.259, F11.281, F11.282, F11.288, F11.920, F11.921, F11.922, F11.929, F11.950, F11.951, F11.959, F11.981, F11.982, F11.988 F11.10, F11.11, F11.14, F11.19, F11.20, F11.21, F11.23, F11.24, F11.29, F11.90, F11.93, F11.94, F11.99 |
| Diabetes | E08, E10, E11, E13 |
| Dementia | F00, F01, F02, F03, G30, F05.1, G31.1 |
| Rheumatoid arthritis | M05, M32, M34, M06.0, M06.1, M06.2, M06.3, M06.4, M06.8, M06.9, M31.5, M33.2, M35.3, M33.0, M33.1, M33.9, M35.1, M36.0 |
| Osteoporosis | M80, M81 |
Appendix Table 2.
Hazard ratio (HR) estimates for COVID-19-related outcomes by NRTI combination in men with HIV, Veterans Aging Cohort Study, February 2020 – October 2021
| No. events (up to 20 months) | Crude HR (95% CI) | Adjusted* HR (95% CI) | ||||
|---|---|---|---|---|---|---|
| Documented SARS-CoV-2 infection | TAF/FTC | 638 | 1 (ref.) | 1 (ref.) | ||
| TDF/FTC | 30 | 0.59 | (0.41, 0.85) | 0.64 | (0.44, 0.93) | |
| ABC/3TC | 205 | 1.07 | (0.91, 1.25) | 1.03 | (0.88, 1.20) | |
| Other | 138 | 0.89 | (0.74, 1.07) | 0.85 | (0.71, 1.03) | |
| COVID-19 hospitalization | TAF/FTC | 160 | 1 (ref.) | 1 (ref.) | ||
| TDF/FTC | 5 | 0.39 | (0.16, 0.96) | 0.41 | (0.17, 1.02) | |
| ABC/3TC | 64 | 1.33 | (0.99, 1.77) | 1.15 | (0.86, 1.54) | |
| Other | 62 | 1.60 | (1.20, 2.15) | 1.15 | (0.85, 1.57) | |
| COVID-19 ICU admission | TAF/FTC | 45 | 1 (ref.) | 1 (ref.) | ||
| TDF/FTC | 2 | 0.57 | (0.14, 2.35) | 0.61 | (0.15, 2.57) | |
| ABC/3TC | 18 | 1.33 | (0.77, 2.29) | 1.00 | (0.56, 1.76) | |
| Other | 24 | 2.22 | (1.35, 3.64) | 1.51 | (0.89, 2.55) | |
Adjusted for age, race/ethnicity, smoking status, CD4 cell count, HIV viral load, maximum HIV viral load over the past 12 months, BMI, systolic blood pressure, diastolic blood pressure, average eGFR over the past 12 months, months since February 2020, hospitalization in previous month, comorbidities including: cardiovascular disease, hypertension, liver disease, chronic pulmonary disease, cancer, chronic kidney disease, alcohol/substance use, diabetes, and dementia.
Abbreviations: 3TC – lamivudine; ABC – abacavir; FTC – emtricitabine; NRTI – nucleoside reverse transcriptase inhibitor; TDF – tenofovir disoproxil fumarate; TAF – tenofovir alafenamide; BMI – body mass index; eGFR – estimated glomerular filtration rate
Appendix Table 3.
Risks estimates for COVID-19-related outcomes by NRTI combination in men with HIV, with censoring at the time of death, Veterans Aging Cohort Study, February 2020 – October 2021
| No. events | 18-month risk*, % (95% CI) | Risk difference, % (95% CI) | Risk ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Documented SARS-CoV-2 infection | TAF/FTC | 621 | 7.8 | (7.1, 8.4) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 28 | 5.1 | (3.3, 7.0) | −2.6 | (−4.5, −0.8) | 0.66 | (0.43, 0.89) | |
| ABC/3TC | 201 | 7.8 | (6.6, 8.9) | 0.0 | (−1.3, 1.3) | 1.00 | (0.84, 1.17) | |
| Other | 134 | 7.0 | (5.7, 8.2) | −0.8 | (−2.3, 0.5) | 0.90 | (0.71, 1.07) | |
| COVID-19 hospitalization | TAF/FTC | 156 | 2.2 | (1.8, 2.5) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 5 | 0.9 | (0.2, 2.0) | −1.2 | (−2.1, −0.2) | 0.44 | (0.07, 0.89) | |
| ABC/3TC | 63 | 2.3 | (1.7, 3.0) | 0.2 | (−0.5, 0.9) | 1.08 | (0.79, 1.48) | |
| Other | 60 | 2.8 | (2.0, 3.7) | 0.7 | (−0.1, 1.5) | 1.31 | (0.94, 1.76) | |
| COVID-19 ICU admission | TAF/FTC | 43 | 0.6 | (0.4, 0.8) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 2 | 0.3 | (0.0, 1.6) | −0.2 | (−0.7, 1.1) | 0.57 | (0.00, 2.84) | |
| ABC/3TC | 18 | 0.5 | (0.3, 0.9) | 0.0 | (−0.3, 0.4) | 0.93 | (0.49, 1.82) | |
| Other | 24 | 0.8 | (0.5, 1.1) | 0.2 | (−0.2, 0.6) | 1.38 | (0.74, 2.44) | |
Standardized by age, race/ethnicity, smoking status, CD4 cell count, HIV viral load, maximum HIV viral load over the past 12 months, BMI, systolic blood pressure, diastolic blood pressure, average eGFR over the past 12 months, months since February 2020, hospitalization in previous month, comorbidities including: cardiovascular disease, hypertension, liver disease, chronic pulmonary disease, cancer, chronic kidney disease, alcohol/substance use, diabetes, and dementia.
Appendix Table 4.
Risks estimates for COVID-19-related outcomes by NRTI combination in men with HIV, without censoring at the time of vaccination against COVID-19, Veterans Aging Cohort Study, February 2020 – October 2021
| No. events | 18-month risk*, % (95% CI) | Risk difference, % (95% CI) | Risk ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Documented SARS-CoV-2 infection | TAF/FTC | 706 | 5.9 | (5.4, 6.3) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 31 | 3.9 | (2.7, 5.2) | −1.9 | (−3.3, −0.7) | 0.67 | (0.46, 0.88) | |
| ABC/3TC | 233 | 6.2 | (5.5, 7.0) | 0.4 | (−0.5, 1.2) | 1.06 | (0.92, 1.22) | |
| Other | 162 | 5.6 | (4.7, 6.4) | −0.3 | (−1.2, 0.6) | 0.95 | (0.80, 1.10) | |
| COVID-19 hospitalization | TAF/FTC | 175 | 1.6 | (1.3, 1.8) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 6 | 0.7 | (0.2, 1.3) | −0.9 | (−1.4, −0.2) | 0.45 | (0.14, 0.84) | |
| ABC/3TC | 69 | 1.7 | (1.3, 2.1) | 0.1 | (−0.3, 0.6) | 1.07 | (0.80, 1.39) | |
| Other | 68 | 1.9 | (1.5, 2.3) | 0.3 | (−0.2, 0.8) | 1.20 | (0.91, 1.55) | |
| COVID-19 ICU admission | TAF/FTC | 47 | 0.4 | (0.3, 0.6) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 2 | 0.2 | (0.0, 0.7) | −0.2 | (−0.5, 0.2) | 0.48 | (0.00, 1.51) | |
| ABC/3TC | 21 | 0.5 | (0.3, 0.7) | 0.0 | (−0.2, 0.3) | 1.03 | (0.60, 1.69) | |
| Other | 28 | 0.7 | (0.4, 1.0) | 0.3 | (0.0, 0.6) | 1.62 | (0.93, 2.62) | |
Standardized by age, race/ethnicity, smoking status, CD4 cell count, HIV viral load, maximum HIV viral load over the past 12 months, BMI, systolic blood pressure, diastolic blood pressure, average eGFR over the past 12 months, months since February 2020, hospitalization in previous month, comorbidities including: cardiovascular disease, hypertension, liver disease, chronic pulmonary disease, cancer, chronic kidney disease, alcohol/substance use, diabetes, and dementia.
Appendix Table 5.
Risks estimates for COVID-19-related outcomes by NRTI combination in men and women with HIV, Veterans Aging Cohort Study, February 2020 – October 2021
| No. events | 18-month risk*, % (95% CI) | Risk difference, % (95% CI) | Risk ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Documented SARS-CoV-2 infection | TAF/FTC | 638 | 7.4 | (6.8, 8.0) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 29 | 4.9 | (3.2, 6.6) | −2.5 | (−4.3, −0.7) | 0.66 | (0.41, 0.90) | |
| ABC/3TC | 208 | 7.5 | (6.5, 8.6) | 0.1 | (−1.1, 1.3) | 1.02 | (0.86, 1.18) | |
| Other | 136 | 6.4 | (5.3, 7.5) | −1.0 | (−2.2, 0.1) | 0.86 | (0.71, 1.02) | |
| COVID-19 hospitalization | TAF/FTC | 160 | 2.0 | (1.6, 2.4) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 5 | 0.8 | (0.2, 1.8) | −1.1 | (−1.9, −0.1) | 0.42 | (0.11, 0.94) | |
| ABC/3TC | 65 | 2.2 | (1.6, 2.8) | 0.2 | (−0.4, 0.8) | 1.10 | (0.80, 1.46) | |
| Other | 60 | 2.3 | (1.7, 3.0) | 0.3 | (−0.4, 1.1) | 1.17 | (0.84, 1.59) | |
| COVID-19 ICU admission | TAF/FTC | 44 | 0.5 | (0.4, 0.7) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 2 | 0.3 | (0.0, 1.2) | −0.2 | (−0.6, 0.7) | 0.54 | (0.00, 2.30) | |
| ABC/3TC | 18 | 0.5 | (0.3, 0.8) | 0.0 | (−0.3, 0.3) | 0.91 | (0.49, 1.62) | |
| Other | 24 | 0.7 | (0.4, 1.0) | 0.2 | (−0.1, 0.5) | 1.30 | (0.77, 2.21) | |
Standardized by age, race/ethnicity, smoking status, CD4 cell count, HIV viral load, maximum HIV viral load over the past 12 months, BMI, systolic blood pressure, diastolic blood pressure, average eGFR over the past 12 months, months since February 2020, hospitalization in previous month, comorbidities including: cardiovascular disease, hypertension, liver disease, chronic pulmonary disease, cancer, chronic kidney disease, alcohol/substance use, diabetes, and dementia.
Appendix Table 6.
Risks estimates for all-cause mortality by NRTI combination in men with HIV, Veterans Aging Cohort Study, February 2020 – October 2021
| No. events | 18-month risk*, % (95% CI) | Risk difference, % (95% CI) | Risk ratio (95% CI) | Crude HR (95% CI) | Adjusted** HR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TAF/FTC | 332 | 4.6 | (4.1, 5.2) | 0 (ref.) | 1 (ref.) | 1 (ref.) | 1 (ref.) | ||||
| TDF/FTC | 34 | 5.4 | (3.5, 7.3) | 0.8 | (−1.2, 2.9) | 1.18 | (0.75, 1.66) | 1.30 | (0.91, 1.85) | 1.36 | (0.95, 1.94) |
| ABC/3TC | 120 | 4.8 | (3.9, 5.9) | 0.2 | (−0.9, 1.4) | 1.04 | (0.81, 1.32) | 1.20 | (0.98, 1.48) | 1.08 | (0.87, 1.34) |
| Other | 189 | 7.2 | (6.1, 8.4) | 2.6 | (1.4, 3.8) | 1.56 | (1.29, 1.88) | 2.42 | (2.02, 2.89) | 1.62 | (1.35, 1.95) |
Standardized by age, race/ethnicity, smoking status, CD4 cell count, HIV viral load, maximum HIV viral load over the past 12 months, BMI, systolic blood pressure, diastolic blood pressure, average eGFR over the past 12 months, months since February 2020, hospitalization in previous month, comorbidities including: cardiovascular disease, hypertension, liver disease, chronic pulmonary disease, cancer, chronic kidney disease, alcohol/substance use, diabetes, and dementia.
Adjusted for all variables listed in *
Appendix Table 7.
Subgroup analyses for COVID-19-related outcomes by NRTI combination in men with HIV, Veterans Aging Cohort Study, February 2020 – October 2021
| No. events | 18-month risk*, % (95% CI) | Risk difference, % (95% CI) | Risk ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Documented SARS-CoV-2 infection |
Non-Hispanic White | TAF/FTC | 195 | 6.2 | (5.3, 7.1) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 6 | 2.1 | (0.4, 3.9) | −4.1 | (−5.8, −2.0) | 0.34 | (0.07, 0.65) | ||
| ABC/3TC | 42 | 5.3 | (3.6, 7.2) | −1.0 | (−2.8, 1.1) | 0.85 | (0.57, 1.18) | ||
| Other | 32 | 4.8 | (3.1, 6.5) | −1.4 | (−3.2, 0.7) | 0.78 | (0.50, 1.12) | ||
| Non-Hispanic Black | TAF/FTC | 286 | 8.1 | (7.0, 9.0) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 15 | 6.0 | (3.2, 9.0) | −2.1 | (−5.0, 1.2) | 0.74 | (0.39, 1.15) | ||
| ABC/3TC | 127 | 9.1 | (7.7, 10.9) | 1.1 | (−0.6, 3.1) | 1.14 | (0.93, 1.40) | ||
| Other | 73 | 7.0 | (5.4, 8.6) | −1.0 | (−3.0, 0.8) | 0.87 | (0.66, 1.10) | ||
| Age ≥ 50 years | TAF/FTC | 450 | 7.1 | (6.4, 7.7) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 19 | 4.5 | (2.6, 6.6) | −2.6 | (−4.5, −0.4) | 0.64 | (0.38, 0.95) | ||
| ABC/3TC | 150 | 7.2 | (6.1, 8.3) | 0.1 | (−1.1, 1.4) | 1.02 | (0.85, 1.21) | ||
| Other | 107 | 6.0 | (4.9, 7.2) | −1.0 | (−2.3, 0.4) | 0.86 | (0.68, 1.05) | ||
| Age < 50 years | TAF/FTC | 171 | 8.8 | (7.5, 10.2) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 9 | 5.7 | (2.3, 10.0) | −3.0 | (−6.7, 1.1) | 0.65 | (0.26, 1.13) | ||
| ABC/3TC | 51 | 8.2 | (6.0, 10.7) | −0.6 | (−3.1, 2.0) | 0.94 | (0.68, 1.24) | ||
| Other | 27 | 8.5 | (5.5, 11.4) | −0.3 | (−3.8, 2.7) | 0.97 | (0.61, 1.36) | ||
| eGFR ≥ 60 mL/min | TAF/FTC | 393 | 7.2 | (6.4, 7.9) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 18 | 3.6 | (2.1, 5.5) | −3.5 | (−5.2, −1.7) | 0.51 | (0.29, 0.76) | ||
| ABC/3TC | 99 | 7.3 | (5.8, 8.8) | 0.2 | (−1.5, 1.7) | 1.02 | (0.80, 1.24) | ||
| Other | 71 | 6.6 | (5.2, 8.2) | −0.5 | (−2.2, 1.1) | 0.93 | (0.70, 1.16) | ||
| eGFR < 60 mL/min | TAF/FTC | 122 | 8.4 | (6.9, 10.1) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 4 | 8.0 | (0.0, 18.8) | −0.5 | (−7.6, 10.2) | 0.94 | (0.00, 2.32) | ||
| ABC/3TC | 66 | 8.3 | (6.3, 10.4) | −0.2 | (−2.9, 2.3) | 0.98 | (0.70, 1.33) | ||
| Other | 41 | 6.5 | (4.6, 8.6) | −1.9 | (−4.4, 0.7) | 0.77 | (0.52, 1.10) | ||
| COVID-19 hospitalization | Non-Hispanic White | TAF/FTC | 33 | 1.0 | (0.7, 1.5) | 0 (ref.) | 1 (ref.) | ||
| TDF/FTC | 0 | 0.0 | - | −1.0 | - | 0.00 | - | ||
| ABC/3TC | 10 | 1.4 | (0.6, 2.5) | 0.4 | (−0.5, 1.5) | 1.40 | (0.56, 2.79) | ||
| Other | 14 | 2.1 | (1.0, 3.3) | 1.0 | (−0.1, 2.3) | 2.00 | (0.90, 3.77) | ||
| Non-Hispanic Black | TAF/FTC | 88 | 2.6 | (2.0, 3.2) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 3 | 1.1 | (0.0, 2.6) | −1.5 | (−2.7, 0.0) | 0.42 | (0.00, 1.00) | ||
| ABC/3TC | 47 | 3.1 | (2.2, 4.1) | 0.6 | (−0.5, 1.6) | 1.21 | (0.83, 1.73) | ||
| Other | 36 | 3.0 | (2.0, 4.0) | 0.4 | (−0.7, 1.6) | 1.15 | (0.75, 1.69) | ||
| Age ≥ 50 years | TAF/FTC | 136 | 2.2 | (1.8, 2.6) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 4 | 0.9 | (0.2, 2.3) | −1.3 | (−2.1, 0.0) | 0.42 | (0.07, 1.01) | ||
| ABC/3TC | 60 | 2.6 | (2.0, 3.4) | 0.4 | (−0.3, 1.3) | 1.19 | (0.86, 1.64) | ||
| Other | 54 | 2.5 | (1.8, 3.3) | 0.3 | (−0.5, 1.2) | 1.14 | (0.80, 1.61) | ||
| Age < 50 years | TAF/FTC | 20 | 1.1 | (0.6, 1.7) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 1 | 0.7 | (0.0, 2.6) | −0.4 | (−1.5, 1.5) | 0.66 | (0.00, 2.49) | ||
| ABC/3TC | 3 | 0.4 | (0.0, 1.4) | −0.7 | (−1.3, 0.2) | 0.35 | (0.00, 1.25) | ||
| Other | 6 | 2.3 | (0.7, 4.3) | 1.2 | (−0.5, 3.3) | 2.04 | (0.54, 5.33) | ||
| eGFR ≥ 60 mL/min | TAF/FTC | 81 | 1.7 | (1.3, 2.1) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 3 | 0.5 | (0.0, 1.1) | −1.2 | (−1.8, −0.5) | 0.28 | (0.00, 0.67) | ||
| ABC/3TC | 20 | 1.7 | (0.9, 2.5) | 0.0 | (−0.8, 0.9) | 1.00 | (0.55, 1.60) | ||
| Other | 23 | 2.1 | (1.3, 3.1) | 0.5 | (−0.4, 1.5) | 1.27 | (0.76, 2.03) | ||
| eGFR < 60 mL/min | TAF/FTC | 46 | 3.0 | (2.2, 4.1) | 0 (ref.) | 1 (ref.) | |||
| TDF/FTC | 1 | 5.5 | (0.0, 18.6) | 2.5 | (−3.7, 15.6) | 1.82 | (0.00, 6.32) | ||
| ABC/3TC | 29 | 3.4 | (2.1, 4.6) | 0.4 | (−1.2, 1.8) | 1.12 | (0.66, 1.74) | ||
| Other | 27 | 3.7 | (2.4, 5.5) | 0.7 | (−1.0, 2.5) | 1.22 | (0.72, 1.98) | ||
Standardized by age, race/ethnicity, smoking status, CD4 cell count, HIV viral load, maximum HIV viral load over the past 12 months, BMI, systolic blood pressure, diastolic blood pressure, average eGFR over the past 12 months, months since February 2020, hospitalization in previous month, comorbidities including: cardsiovascular disease, hypertension, liver disease, chronic pulmonary disease, cancer, chronic kidney disease, alcohol/substance use, diabetes, and dementia.
Appendix Figure 1.
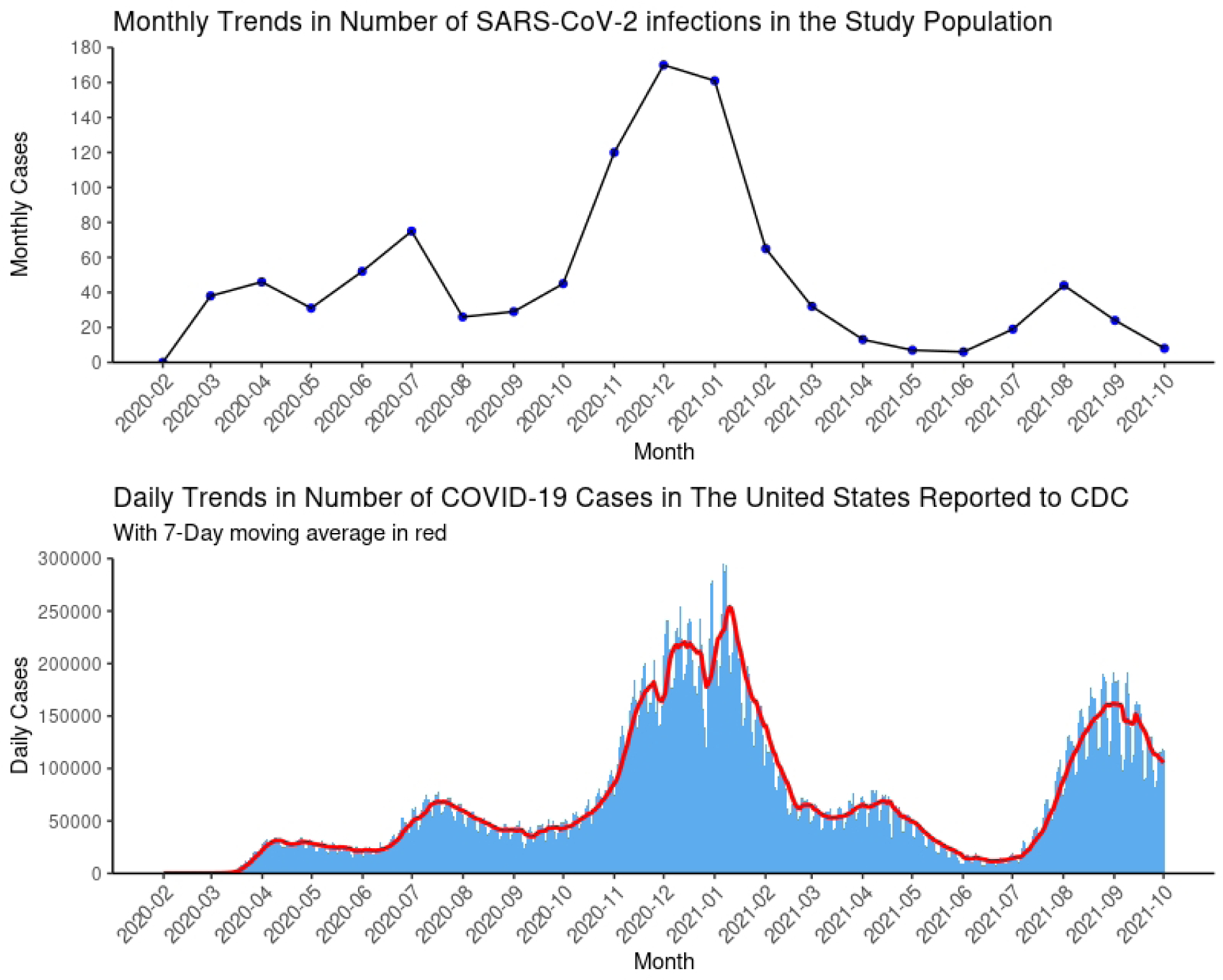
Comparison of trends of COVID-19 cases in the study population and in the United States
Appendix Figure 2.
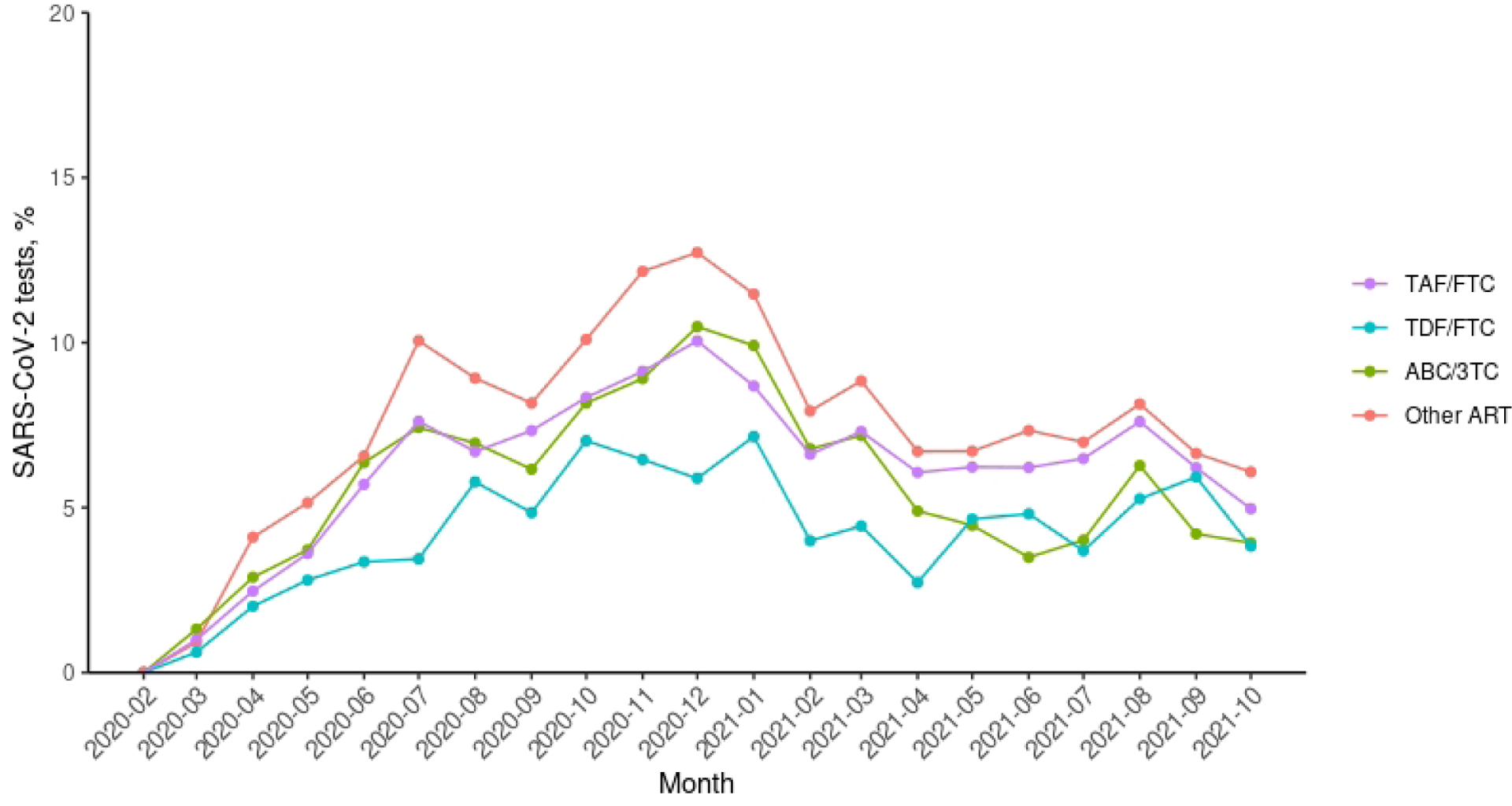
The number of SARS-CoV-2 tests proportional to persons under follow-up in each month by treatment groups
References
- 1.World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. [PubMed] [Google Scholar]
- 2.Del Amo J, Polo R, Moreno S, Díaz A, Martínez E, Arribas JR, et al. Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy : A Cohort Study. Ann Intern Med 2020; 173(7):536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Amo J, Polo R, Moreno S, Martínez E, Cabello A, Iribarren J, et al. Tenofovir Disoproxil Fumarate and severity of COVID-19 in people with HIV infection [preprint] medRxiv 2021:2021.2011.2011.. [Google Scholar]
- 4.Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA. Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2021; 73(7):e2005–e2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz B, Buti M, Vazquez I, Conde M, Monterde V, Diaz F, et al. Tenofovir reduces the severity of COVID-19 infection in chronic hepatitis B patients [abstract]. Journal of Hepatology 2021:S746–S747. [Google Scholar]
- 6.Podany AT, Bares SH, Havens J, Dyavar SR, O’Neill J, Lee S, et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018; 32(6):761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Diaz S, Bateman BT, Straub L, Zhu Y, Mogun H, Fischer M, et al. Safety of Tenofovir Disoproxil Fumarate (TDF) for Pregnant Women facing the COVID-19 Pandemic. Am J Epidemiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agbaji OO, Abah IO, Ebonyi AO, Gimba ZM, Abene EE, Gomerep SS, et al. Long Term Exposure to Tenofovir Disoproxil Fumarate-Containing Antiretroviral Therapy Is Associated with Renal Impairment in an African Cohort of HIV-Infected Adults. Journal of the International Association of Providers of AIDS Care (JIAPAC) 2019; 18:2325958218821963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care 2006; 44(8 Suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 10.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): Overview and description. Medical care 2006; 44(8 Suppl 2):S13–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Department of Veterans Affairs. 172VA10P2: VHA Corporate Data Warehouse–VA. 79 FR 4377. In: Office of the Federal Register, National Archives and Records Administration; 2020. [Google Scholar]
- 12.CDC COVID-19 Response Team, Team R, Jorden MA, Rudman SL, Villarino E, Hoferka S, et al. Evidence for limited early spread of COVID-19 within the UnitedStates, January–February 2020. Morbidity and Mortality Weekly Report 2020; 69(22):680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Trends in Number of COVID-19 Cases and Deaths in the US Reported to CDC, by State/Territory. In; 2021. [Google Scholar]
- 14.del Amo J, Polo R, Moreno S, Díaz A, Martínez E, Arribas JR, et al. Antiretrovirals and Risk of COVID-19 Diagnosis and Hospitalization in HIV-Positive Persons. Epidemiology 2020; 31(6):e49–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parienti J-J, Prazuck T, Peyro-Saint-Paul L, Fournier A, Valentin C, Brucato S, et al. Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial. EClinicalMedicine 2021; 38:100993–100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayerdi O, Puerta T, Clavo P, Vera M, Ballesteros J, Fuentes ME, et al. Preventive Efficacy of Tenofovir/Emtricitabine Against Severe Acute Respiratory Syndrome Coronavirus 2 Among Pre-Exposure Prophylaxis Users. Open Forum Infectious Diseases 2020; 7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaugerre C, Assoumou L, Maylin S, Minier M, Gabassi A, Genin M, et al. SARS CoV-2 Seroprevalence Among HIV-Negative Participants Using Tenofovir/Emtricitabine Based PrEP in 2020 – A Sub-study of ANRS PREVENIR and INSERM SAPRIS-Sero Cohorts. Open Forum Infectious Diseases 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dijk M, de Wit JBF, Guadamuz TE, Martinez JE, Jonas KJ. Slow Uptake of PrEP: Behavioral Predictors and the Influence of Price on PrEP Uptake Among MSM with a High Interest in PrEP. AIDS and behavior 2021; 25(8):2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okafor CN, Gorbach PM, Ragsdale A, Quinn B, Shoptaw S. Correlates of Preexposure Prophylaxis (PrEP) Use among Men Who Have Sex with Men (MSM) in Los Angeles, California. J Urban Health 2017; 94(5):710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eaton LA, Matthews DD, Bukowski LA, Friedman MR, Chandler CJ, Whitfield DL, et al. Elevated HIV Prevalence and Correlates of PrEP Use Among a Community Sample of Black Men Who Have Sex With Men. J Acquir Immune Defic Syndr 2018; 79(3):339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan E, Moran K, Ryan DT, Mustanski B, Newcomb ME. Threefold Increase in PrEP Uptake Over Time with High Adherence Among Young Men Who Have Sex With Men in Chicago. AIDS and behavior 2018; 22(11):3637–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traeger MW, Schroeder SE, Wright EJ, Hellard ME, Cornelisse VJ, Doyle JS, et al. Effects of Pre-exposure Prophylaxis for the Prevention of Human Immunodeficiency Virus Infection on Sexual Risk Behavior in Men Who Have Sex With Men: A Systematic Review and Meta-analysis. Clin Infect Dis 2018; 67(5):676–686. [DOI] [PubMed] [Google Scholar]
- 23.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. In: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/guidelines-adult-adolescent-arv.pdf Accessed June 2, 2022.


