Abstract
Objective:
Corticosteroids are commonly used in the treatment of pediatric septic shock without clear evidence of the potential benefits or risks. This study examined the association of early corticosteroid therapy with patient-centered clinically meaningful outcomes.
Design:
Subsequent cohort analysis of data derived from the prospective Life After Pediatric Sepsis Evaluation (LAPSE) investigation. Outcomes among patients receiving hydrocortisone or methylprednisolone on study Day 0 or1 were compared to those who did not using a propensity score-weighted analysis that controlled for age, sex, study site, and measures of first-day illness severity.
Setting:
Twelve academic PICUs in the United States
Patients:
Children with community-acquired septic shock ages 1 month to 18 years enrolled in LAPSE, 2013–2017. Exclusion criteria included a history of chronic corticosteroid administration.
Interventions:
None
Measurements and Main Results:
Among children enrolled in LAPSE, 352/392 met analysis inclusion criteria and 155/352 (44%) received early corticosteroid therapy. After weighting corticosteroid therapy administration propensity across potentially confounding baseline characteristics, differences in outcomes associated with treatment were not statistically significant [adjusted effect or odds ratio, (95% CI)]: vasoactive-inotropic support duration [−0.37 days (−1.47, 0.72), p=0.503], short-term survival without new morbidity [1.37, (0.83, 2.28), p=0.218], new morbidity among Month-1 survivors [0.70, (0.39, 1.23), p=0.218], and persistent severe deterioration of health-related quality of life or mortality at Month 1 [0.70 (0.40, 1.23), p=0.212].
Conclusions:
This study examined the association of early corticosteroid therapy with mortality and morbidity among children encountering septic shock. After adjusting for variables with the potential to confound the relationship between early corticosteroid administration and clinically meaningful endpoints, there was no improvement in outcomes associated with this therapy. Results from this propensity analysis provide additional justification for equipoise regarding corticosteroid therapy for pediatric septic shock, and ascertain the need for a well-designed clinical trial to examine benefit/risk for this intervention.
Keywords: children, corticosteroids, sepsis, septic shock, functional status, health-related quality of life, vasoactive-inotropic support, propensity score weighting, outcomes
INTRODUCTION
Sepsis is one of the leading causes of pediatric morbidity and mortality world-wide (1). Improving the outcomes and establishing an evidence-based standard of care for these patients remains an ongoing quest. While rigorous investigative and quality improvement efforts have reduced overall mortality in pediatric sepsis, significant, enduring morbidity burdens children surviving sepsis, with 24–35% of patients suffering significant, persistent deterioration of health-related quality of life (HRQL) and/or functional status (FS) (2–6). There is ongoing investigation in this area, with the goal of describing factors that contribute to FS and HRQL following sepsis in children.
It has been repeatedly documented that severity of sepsis illness is strongly associated with morbidity. Critical illness factors such as extent and duration of organ failure and shock are independently associated with persistent, serious deterioration of HRQL in children surviving a septic shock event (7). Patient factors, such as older age and immune compromise, have also been implicated (6). However, little is definitively known regarding the effect treatment factors, such as adjunctive pharmacologic therapies, have on mortality/morbidity outcomes related to pediatric septic shock.
Corticosteroids are one of the most commonly used adjunctive therapies for children with septic shock (8–12). The true effect of this therapy on sepsis mortality and morbidity is unknown as large, well-designed pediatric interventional trials are lacking, albeit in progress [NCT03401398]. Corticosteroid side effects, such as immune-suppression, myopathy, hyperglycemia, and salt/water retention have the potential to worsen the outcomes of children with septic shock (13). In fact, elegant gene expression analyses of children with sepsis have identified endotypes associated with increased or decreased risk of mortality with corticosteroid prescription (14, 15).
Although several previous investigations (mostly retrospective descriptive cohort studies) (2, 8–11, 14, 16, 17) have examined the potential benefits and risks ofcorticosteroid therapy for pediatric septic shock, we sought to examine this same question in a planned secondary analysis of the Life After Pediatric Sepsis Evalution (LAPSE) database utilizing propensity balancing methodology with a focus on mortality/morbidity outcomes assessed at one month following PICU admission.
METHODS
LAPSE (R01HD073362) was a prospective, observational cohort investigation conducted at 12 major pediatric critical care centers across the US from 2013–2017 (5, 7). Children ages 1 month to 18 years with community-acquired septic shock were enrolled. Septic shock was defined as: documented or suspected infection with onset within 48 hours of hospital admission; and presence of at least two systemic inflammatory response syndrome criteria, including abnormal leukocyte count and/or abnormal body temperature (18), and need for fluid resuscitation, and vasoactive/inotropic support initiated within 72 hours of admission to the hospital and 48 hours of PICU admission. Other details of the LAPSE protocol may be found in reference 5, Supplemental Electronic Content [ http://links.lww.com/CCM/F145 ]. Patients identified by research personnel as immunocompromised due to corticosteroid use prior to hospitalization were considered at high risk for absolute adrenal insufficiency and excluded from this secondary analysis.
Initial demographic information, initial illness severity, and baseline functional status and HRQL scores (reflecting patient status in the month prior to the sepsis event) were collected around the time of PICU admission. Pediatric RISk of Mortality (PRISM) III was evaluated at the time of PICU admission using the worst physiologic values from -2 to 4 hours after PICU admission (19). Vasoactive-Inotropic Score (VIS) was calculated twice daily (0800 and 2000) and Pediatric Logistic Organ Dysfunction (PELOD-2) scores were collected daily until PICU discharge, death, or Day 28, whichever came first (20).
Under institutional review board oversight (Supplemental Digital Content [SDC], eText 1), documented, informed parental permission was obtained before any LAPSE study procedures were undertaken. Developmentally appropriate patients were requested to provide assent for their own continued study participation following PICU discharge. Management of patients, including the use of corticosteroids, was left to attending physician discretion.
The primary aim of this LAPSE ancillary investigation was to evaluate the association of early corticosteroid therapy with resolution of shock (vasoactive-inotropic support-free days), Month-1 survival, Month-1 survival without new morbidity, and survival to Month 1 with no severe reduction in HRQL compared to pre-hospital baseline. Vasoactive-inotropic support-free days was defined as 28 minus the number of days (up to 28) of vasoactive-inotropic support use for 28-day survivors. Patients who died before Day 28 were assigned zero vasoactive-inotropic support-free days. New morbidity was defined as a worsening in Functional Status Score (FSS) by 3 or more points from baseline to hospital discharge or Day 28, whichever came first (21). Health-related quality of life (HRQL) was measured using the Stein Jessop Functional Status (FSII-R) for severely developmentally delayed subjects and the Pediatric Quality of Life Inventory (PedsQL™) for others according to parent preference (5, 22–24). Data quantifying HRQL was collected on enrollment and at the Month-1 follow-up (Day 21–42). Persistent, severe deterioration (PSD) in HRQL was defined as more than a 25% decrease compared to pre-hospital baseline. Secondary outcomes were mechanical ventilation-free days, PICU-free days, hospital-free days, and sum of PELOD-2 scores during PICU stay, truncated at 28 days. These outcomes were selected a-priori as the primary and secondary outcomes that might be affected by the use of corticosteroids.
Early corticosteroid therapy was defined as initiation of hydrocortisone or methylprednisolone on study Day 0 or 1. Study Day 0 was defined as the time of PICU admission to 2359 that day. All other days described a 24 hour time period from 0000 to 2359. If steroids were prescribed, the name of the corticosteroid and the total 24 hour dose was recorded for each study day. Time of initiation was not recorded, limiting a more narrow time period for the inclusion criteria. Patients who received early corticosteroid therapy were included in the “Early Corticosteroid” group. Patients who did not receive early corticosteroid therapy were included in the “No Early Corticosteroid” group, regardless of steroid use after Day 0–1 or systemic steroid use other than hydrocortisone or methylprednisolone on Day 0–1 (such as dexamethasone). Patients receiving methylprednisolone were included in the Early Corticosteroid group because at the dose most commonly prescribed (1–2 mg/kg/day), potentially beneficial hemodynamic and anti-inflammatory actions might be expected, although the mineralocorticoid and glucocorticoid effects of methylprednisolone are considered to be 5 times as potent as hydrocortisone (25). In order to more fully understand how this approach might impact results, a sensitivity analysis was conducted in which the patients receiving methylprednisolone only (no hydrocortisone) during the treatment window were included in the “No Early Corticosteroid” group and primary outcomes were analyzed. In the pediatric ICU, dexamethasone is most commonly used for its anti-inflammatory properties to decrease airway swelling in preparation for extubation. Dexamethasone is not considered adjunctive therapy for sepsis. Thus, patients who received dexamethasone on Day 0 or 1 were included in the “No Early Corticosteroid” group.
Statistical Analysis
Baseline factors have the potential to confound the relationship between early corticosteroid therapy and outcomes. Accordingly, a propensity score method was used to minimize the effects of confounding by constructing a weighted cohort of subjects balanced with respect to baseline characteristics, but differing with respect to corticosteroid therapy (26). Baseline characteristics identified a priori as likely confounders included age, sex, study site, PRISM III, and highest First-Day Vasoactive-Inotropic Score (VIS). Effect estimates for these characteristics were not reported or investigated as they were solely used in a logistic regression model to predict the probability (propensity) of corticosteroid therapy for each subject in the cohort. A balanced cohort was constructed in which subjects were weighted using stabilized inverse probability of treatment weighting (IPTW). This approach more heavily weights subjects receiving therapy different from what was predicted by the logistic regression model considering baseline characteristics. Minimum, maximum, mean, and standard deviation of the weights were assessed. Absolute standardized differences between treatment and control groups were reported for baseline characteristics in the original cohort. Absolute standardized differences measure the difference of means for continuous variables and the difference of proportions for categorical variables between those receiving corticosteroid therapy versus those who did not. Differences in each baseline characteristic were standardized in terms of the standard deviations in both the treated and the non-treated groups. Absolute standardized differences of ≤0.10 were considered to indicate a well-balanced cohort in which the effects of confounding have been removed. If the absolute standardized difference exceeded 0.10, the covariate was additionally controlled for in the outcome model.
After achieving satisfactory balance and finalizing the propensity model, logistic regression models for the different outcomes were created using the weighted cohort, with early corticosteroid therapy included as the primary predictor. These weighted regression models also controlled for PRISM III, as it is known to be a strong predictor of outcomes in question (8). By addressing confounding in both ways (weighted cohort and covariates) bias is less dependent on model specification. Additionally, by controlling for variables explicitly that are considered strong predictors of outcomes, we potentially reduce the variability of our estimated effect, improving power and narrowing confidence intervals (27). The estimated effect of therapy on outcomes was summarized using odds ratios for binary outcomes and effect-sizes for continuous variables such as vasoactive-inotropic support-free days.
Substantial loss to follow-up was observed at Month 1 in the original LAPSE cohort. To investigate the potential bias missing Month-1 HRQL measures may have introduced, sensitivity analyses were performed using multiply-imputed data. The multiple imputation techniques used in this investigation closely mirrored those of previously published LAPSE articles (5, 7), with the exception that a more robust number of imputed data sets were generated, n=50.
Reported p-values were based on a two-sided alternative hypothesis and considered significant if less than 0.05. No adjustment was made for multiple comparisons. Analyses were performed using SAS 9.4 (SAS Institute; Cary, NC).
RESULTS
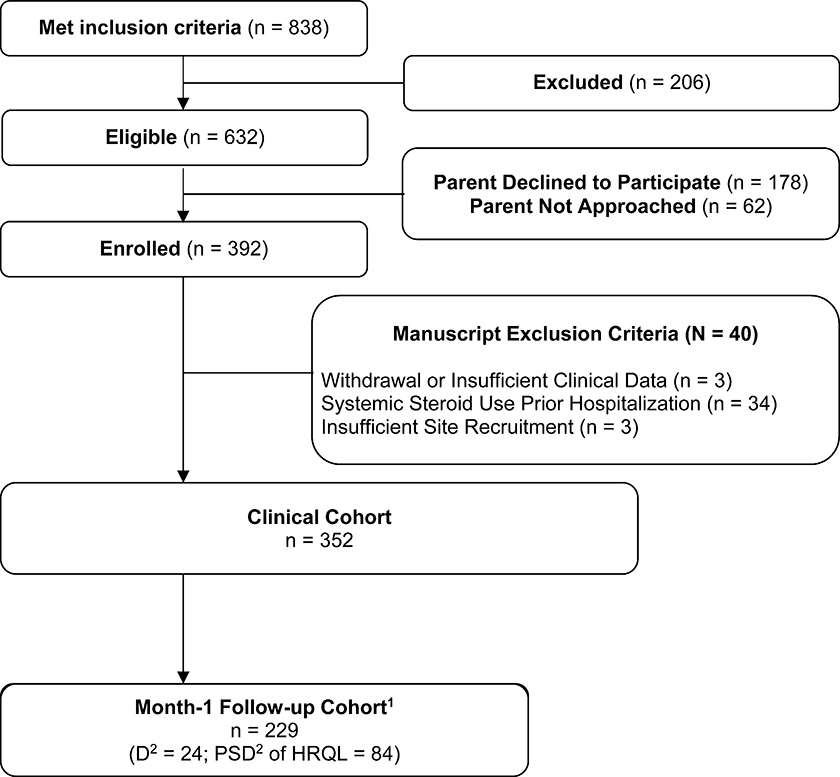
Out of 392 patients enrolled in the LAPSE study, 352 patients met analysis eligibility criteria (Figure 1). Thirty-four patients were excluded due to pre-hospitalization chronic corticosteroid administration. Six patients withdrew or had insufficient baseline clinical data. Month-1 follow-up HRQL data was available for 205 patients. Twenty-four patients died during or before the Month-1 follow-up period.
Figure 1. Flow Diagram.
1Cohort includes patients with a month-1 health-related quality of life (HRQL) assessment (collected day 21-day 42) or patients who died during or before the month-1 follow-up period. 2D represents cumulative deaths among the entire LAPSE clinical cohort at month 1. PSD = persistent, severe deterioration of HRQL below baseline, specifically, HRQL scores (Pediatric Quality of Life Inventory [PedsQL] or Functional Status version II-R [FSII-R]) persisting greater than 25% below the baseline HRQL.
Patient Characteristics
Baseline characteristics of the 352 patients included in the propensity analysis can be found in Table 1. These characteristics (sex, clinical institution, age, PRISM III, and highest First-Day VIS) were determined a priori to be potentially confounding factors associated with both early adjunctive corticosteroid therapy and outcome. After propensity weighting, the absolute standardized difference between the treatment and no treatment group was ≤0.10 for all identified potentially confounding variables other than Clinical Site I, which achieved 0.11 absolute standardized difference. Thus, Site I was included as a covariate in the outcome model along with PRISM III.
Table 1.
Baseline Characteristics Determined to be Potentially Confounding Factors Associated with Both Early Corticosteroid Therapy and Outcome
| Cohort Before ITP Weighting | Cohort After ITP Weighting1 | |||||
|---|---|---|---|---|---|---|
| Early Corticosteroid (N = 155) | No Early Corticosteroid (N = 197) | Absolute Standardized Difference | Early Corticosteroid | No Early Corticosteroid | Absolute Standardized Difference | |
| Female | 76 (49%) | 87 (44%) | 0.10 | 68 (44%) | 88 (45%) | 0.02 |
| Clinical Institution | ||||||
| Site A | 41 (26%) | 39 (20%) | 0.16 | 37 (24%) | 51 (26%) | 0.05 |
| Site B | 4 (3%) | 10 (5%) | 0.13 | 5 (3%) | 7 (4%) | 0.02 |
| Site C | 7 (5%) | 11 (6%) | 0.05 | 8 (5%) | 10 (5%) | <0.01 |
| Site D | 19 (12%) | 6 (3%) | 0.35 | 11 (7%) | 14 (7%) | <0.01 |
| Site E | 9 (6%) | 31 (16%) | 0.32 | 19 (12%) | 23 (12%) | 0.02 |
| Site F | 14 (9%) | 8 (4%) | 0.20 | 9 (6%) | 10 (5%) | 0.04 |
| Site G | 18 (12%) | 19 (10%) | 0.06 | 15 (10%) | 18 (9%) | 0.01 |
| Site H | 11 (7%) | 16 (8%) | 0.04 | 10 (6%) | 13 (7%) | 0.02 |
| Site I | 9 (6%) | 23 (12%) | 0.21 | 19 (12%) | 18 (9%) | 0.11 |
| Site J | 5 (3%) | 15 (8%) | 0.19 | 7 (5%) | 11 (6%) | 0.05 |
| Site K | 12 (8%) | 8 (4%) | 0.16 | 9 (6%) | 11 (6%) | <0.01 |
| Site L | 6 (4%) | 11 (6%) | 0.08 | 7 (5%) | 10 (5%) | 0.02 |
| Age | ||||||
| 0–24 months | 37 (24%) | 65 (33%) | 0.20 | 49 (32%) | 61 (31%) | 0.02 |
| 2–17 years | 118 (76%) | 132 (67%) | 0.20 | 106 (68%) | 136 (69%) | 0.02 |
| PRISM III | 13.6 ± 8.62 | 10.2 ± 7.14 | 0.43 | 11.3 ± 8.20 | 11.0 ± 7.20 | 0.04 |
| Highest VIS, First Day 2 | 17.5 ± 19.12 | 8.5 ± 8.52 | 0.60 | 11.9 ± 14.96 | 11.0 ± 11.46 | 0.06 |
Counts may not sum to expected totals and percentages may not total 100 due to rounding.
First day was defined as day of admission if admission time was before 12:00 pm or following day if admission was after 12:00 pm.
Continuous variables are represented as mean ± standard deviation, while dichotomous variables are represented as n (percentage).
Corticosteroid Use
Overall, 65% (229/352) of patients received any systemic corticosteroid during their PICU stay. Among those who received adjunctive corticosteroids, hydrocortisone was most commonly reported (143/229, 62%), followed by dexamethasone (77/229, 33.6%), and methylprednisolone (65/229, 28%). Of the patients who received hydrocortisone or methylprednisolone, therapy was initiated on Day 0–1 in 155/208 (7575%) of the cases. Thus, 155 patients (44%) were placed in the Early Corticosteroid Group. Twelve of these patients received both hydrocortisone and methylprednisolone on Day 0–1. As defined by study criteria, 197 patients did not receive early corticosteroid therapy. Patients treated with hydrocortisone received a median dose of 1.9 mg/kg/day for a median of 5 calendar days on study. The median dose of methylprednisolone was also 1.9 mg/kg/day administered for a median duration of 6 calendar days. Additional information, including systemic steroid administration during the treatment window, average mg/kg/day dosing and duration of therapy for each systemic corticosteroid can be found in Table 2.
Table 2.
Corticosteroid Characterization
| Frequency of Use Days 0–1 n/N (%) | Frequency of Use on Study n/N (%) | Amount1 (mg/kg/day) | Duration2 (Days) | |
|---|---|---|---|---|
| Any Steroid | 160/352 (45%) | 229/352 (65%) | -- | -- |
| Hydrocortisone | 120/160 (75%) | 143/229 (63%) | 1.9 [0.9, 3.0] | 5 [3, 8] |
| Methylprednisolone | 47/160 (29%) | 65/229 (28%) | 1.9 [1.0, 2.9] | 6 [3, 8] |
| Dexamethasone | 5/160 (3%) | 77/229 (34%) | 0.5 [0.3, 1.0] | 2 [1, 3] |
| Prednisone or Prednisolone | 0/160 (0%) | 18/229 (8%) | 1.1 [0.9, 2.0] | 2 [1, 6] |
There were 25 of 1664 daily corticosteroid administration totals where the value entered was unable to be converted to mg/kg/day.
Corticosteroid administrations were recorded on study Days 0–28 inclusive. Duration is defined as the sum of calendar days the patient was administered steroids. Each steroid duration summary only includes patients receiving the medication.
Median [Q1, Q2] are reported for continuous summaries.
Effect of Adjunctive Corticosteroid Use on Outcomes
The estimated effect of adjunctive corticosteroid use on all outcomes is summarized in Tables 3 and 4. There was no significant difference in vasoactive-inotropic support-free days, duration of vasoactive-inotropic support, survival to Month 1, or survival to Month 1 without new morbidity associated with early adjunctive corticosteroid therapy. In those with complete Month-1 follow-up data (n=205), there was no significant difference in HRQL outcomes between the two groups. Additionally, there was no significant difference in hospital-free days, PICU-free days, sum of PELOD-2 during PICU admission, or ventilator-free days between the groups. Outcome data prior to propensity analysis is available for reference in eTable 4. Results from the sensitivity analysis, in which patients receiving only methylprednisolone were placed in the “No Early Corticosteroid” group, were similar (eTables 5 and 6), again with no significant estimated effect of early hydrocortisone therapy demonstrated for any of the primary outcomes.
Table 3.
Estimated Effect of Early Corticosteroid Therapy on Primary Outcomes
| Adjusted odds ratio (95% CI) | Adjusted effect (95% CI) | P-value | |
|---|---|---|---|
| Outcomes (N=352) | |||
| Vasoactive-inotropic support-free days | 0.72 (−0.85, 2.29) | 0.370 | |
| Duration of vasoactive-inotropic support* | −0.37 (−1.47, 0.72) | 0.503 | |
| Survival to Month 1 without new morbidity1,2 | 1.37 (0.83, 2.28) | 0.218 | |
| Survival to Month 1 | 1.46 (0.62, 3.60) | 0.387 | |
| New morbidity* | 0.70 (0.39, 1.23) | 0.218 | |
| Outcomes (HRQL cohort, N=229) | |||
| PSD3 of HRQL or mortality at Month 1 | 0.70 (0.40, 1.23) | 0.212 | |
| Mortality at Month 1 | 0.63 (0.25, 1.50) | 0.298 | |
| PSD of HRQL among Month-1 survivors | 0.74 (0.38, 1.41) | 0.360 |
Models were weighted using stabilized inverse probability of treatment weights. Additionally, all models control for PRISM III and Site I.
Among Month-1 survivors.
Month 1 refers to 21–42 calendar days following the hospital admission.
There were four subjects missing FSS at Day 28 or hospital discharge. New morbidity was defined as a worsening in Functional Status Score (FSS) by 3 or more points from baseline to hospital discharge or Day 28, whichever came first.
PSD, persistent, severe deterioration of HRQL below baseline, specifically, HRQL scores (PedsQL™ or FSII-R) persisting > 25% below the baseline HRQL.
Table 4.
Estimated Effect of Early Corticosteroid Therapy on Secondary Outcomes
| Adjusted effect (95% CI) | P-value | |
|---|---|---|
| Outcomes (N=352) | ||
| PICU-free days | −0.21 (−1.87, 1.44) | 0.799 |
| Hospital-free days | −1.48 (−3.34, 0.37) | 0.117 |
| Sum of PELOD-2 | −8.68 (−22.01, 4.64) | 0.201 |
| Ventilator-free days | 1.14 (−0.70, 2.97) | 0.223 |
Models were weighted using stabilized inverse probability of treatment weights. Additionally, all models control for PRISM III and Site I.
Because loss to follow-up at Month-1 was substantial, we utilized the imputation methods described above to analyze an additional 80 patients for the composite outcome of mortality or PSD (n=328) and an additional 72 patients for PSD (n=308) at Month 1. Odds ratio estimates and 95% CIs for early corticosteroid therapy using imputed data (eTables 1 and 2) compared to the complete case analysis (Table 3) showed no evidence of substantial bias due to missing HRQL data at Month 1. Cohort characteristics of those with complete HRQL data were compared to those who were lost to follow up (eTable 3). The patients who were lost to follow up differed only by study site, while markers of illness severity were not statistically different.
DISCUSSION
This study examined the association of early corticosteroid therapy with clinically meaningful outcomes such as functional status and HRQL among children encountering septic shock using a propensity-weighted analysis. The results of this study did not reveal any significant associated improvement or worsening of survival, HRQL, or functional status among patients who received early corticosteroid therapy. Additionally, there was no associated improvement in the duration of vasoactive-inotropic support, ventilator-free days, duration of organ dysfunction, or resource utilization.
The most common reason pediatric critical care practitioners prescribe adjunctive corticosteroids in septic shock is to achieve hemodynamic stability in the setting of escalating vasoactive-inotropic support. Corticosteroids may improve the hemodynamics of an individual in shock in multiple waysincluding improving myocardial contractility and vasomotor tone by increasing calcium availability (28), increasing β-adrenergic receptor sensitivity and expression (29), decreasing the re-uptake of norepinephrine (28), and decreasing production of nitric oxide and prostacyclin (30). This drug class stimulates intracellular adhesion factors which may play a role in capillary leak (31). Clinically, early hydrocortisone supplementation has been shown to increase blood pressure and hasten the resolution of shock in adults and children (32–38). El-Nawawy et al. corroborated these findings in children with septic shock, but failed to prove that corticosteroids improved patient-centered outcomes such as mortality (39). Our study failed to document a decrease in vasoactive-inotropic support. This may be due to the limited (twice-daily) reporting of the VIS with more subtle VIS differences potentially missed. Similarly, reporting the duration of vasoactive-inotropic support in units of nearest days increases the likelihood of missing a significant difference, despite previous studies being able to do so (39). It is plausible that more granular vasoactive-inotropic support data may have allowed us to demonstrate a difference with early corticosteroid therapy, but the LAPSE investigation was not designed to do so. It is also plausible that there truly is no effect to find. The only large, high quality studies that have reported an improvement in mortality attributable to corticosteroids are adult-based, but these results are not reliably reproducible (34–36, 40). Our results are similar to other observational cohort studies that have either demonstrated harm or failed to show a benefit of adjunctive corticosteroid therapy in children with septic shock (2, 8–11, 14). Several of these studies were not able to account for illness severity at PICU admission to the extent we were able to do with our propensity score-weighting approach.
The findings of this study are significant for a number of reasons. First, they add to the literature regarding the use of early adjunctive corticosteroids in septic shock. Despite a wealth of randomized control trials, observational cohort studies, and meta-analyses, there remains significant controversy regarding corticosteroid use for sepsis within the medical community. This debate persists for a number of reasons. Studies that examine the effect of corticosteroids in patients with septic shock are notoriously heterogeneous in terms of patient population (degree of shock, etiology of sepsis, underlying medical conditions, etc.) and treatment regimen, making it difficult to draw a unified conclusion regarding the effectiveness of adjunctive corticosteroid therapy in these patients. Additionally, given the low rate of mortality of pediatric septic shock in resource-rich nations, an interventional trial that is adequately powered to evaluate the effect of early corticosteroid therapy on mortality would require significant resources, time, equipoise, and large-scale collaboration because of the need to enroll thousands of research subjects.
Second, we demonstrate that adjunctive corticosteroids continue to be commonly administered to pediatric patients with septic shock. In this cohort, 155/352 (44%) of patients received either hydrocortisone or methylprednisolone on study Day 0 or 1. In the entire cohort (n=352), 229 (65%) patients received at least one systemic steroid (methylprednisolone, hydrocortisone, dexamethasone, prednisone/prednisolone) during their PICU stay. This frequency of corticosteroid usage, despite inadequate evidence of benefit, is similar to previous studies (2, 8–11) and reinforces the need for high quality data from a randomized controlled trial in children.
Third, our analysis focuses on clinically meaningful outcomes, such as survival without new morbidity and HRQL as primary outcomes. The results from the LAPSE trial revealed that while 9% of the cohort suffered in-hospital mortality, 35% of survivors suffered a significant deterioration in their HRQL that persisted at least one year following their hospitalization (5). While we were unable to demonstrate a difference in HRQL outcomes among those treated with early corticosteroid therapy, the profound, persistent burden of morbidity in these patients warrants further investigation to reveal how these outcomes are affected by any current or future therapies.
Fourth, our statistical approach likely provided more precise estimates of the effect of early corticosteroid therapy on patient outcomes than previous studies. By using a propensity score modelling approach, we were able to account for a larger number of clinical institutions and illness severity measures at PICU admission without compromising model integrity. Our results derive from a sizable cohort of 352 patients admitted to 12 different pediatric hospitals across the United States.
This study has important limitations. First, the LAPSE investigation was not primarily designed to detect or evaluate the risks or benefits of early corticosteroid therapy. The potential adverse effects of corticosteroids were not systematically recorded. Time of first corticosteroid administration was not recorded, which limited the ability to narrow the treatment window. Limiting the treatment window to Day 0 may have wrongly categorized patients admitted close to midnight, even if corticosteroids were prescribed within the first few hours of admission. This is a limitation of our study. Second, our study included methylprednisolone in the treatment definition. We recognize that hydrocortisone is the only steroid with any evidence to support its use in septic shock. However, given the prevalence, dose and pharmacologic properties of methylprednisolone administration during the treatment window in this cohort, these patients were included in the treatment group. Excluding patients who received methylprednisolone in the treatment window would ignore a sizeable subcohort subjected to most of the same corticosteroid effects as the subcohort who received hydrocortisone. Additionally, we could not ascertain the indication for methylprednisolone use in this cohort. This is a significant limitation of our study. Thus, a sensitivity analysis was performed in which the patients receiving only methylprednisolone on Day 0 or 1 were placed in the “No Early Corticosteroid” group. There remained no significant difference in any of the outcomes associated with early corticosteroid (hydrocortisone) therapy. The duration of corticosteroid use has the potential to affect the outcomes of interest. However, we were unable to stratify results based on duration of steroid use with our approach. Third, it is unclear how corticosteroids administered outside of the study definition of early corticosteroid therapy, such as dexamethasone or systemic steroids initiated after study Day 1, affected outcomes. Fourth, while our imputation calculation did not suggest significant bias due to missing HRQL data at Month 1, potential bias due to loss of follow up remains. And fifth, it is possible that the confounding variables identified for the propensity analysis were incomplete.
While this study adds to the body of literature surrounding the use of corticosteroids in pediatric sepsis, a high-quality randomized control trial is needed to fully address this issue. Currently enrolling, the Stress Hydrocortisone in Pediatric Septic Shock (SHIPSS - NCT03401398) trial is a large (enrollment target n = 1034), double-blinded, randomized controlled trial that will examine the potential benefits and risks of adjunctive hydrocortisone therapy in children with fluid and vasoactive-inotropic refractory septic shock. Until the results of this study are available, providers should continue to use corticosteroids with caution given the lack of clear benefit.
CONCLUSION
This study examined the association of early adjunctive corticosteroid therapy with mortality and health-related quality of life outcomes among children with septic shock. After adjusting for variables with the potential to confound the relationship between early corticosteroid therapy and clinically meaningful endpoints, there was no association of improved outcomes with this therapy. Results from this propensity analysis provide additional justification for equipoise regarding corticosteroid therapy for pediatric septic shock, and ascertain the need for a well-designed clinical trial that will rigorously examine benefit/risk for this intervention.
Supplementary Material
Research In Context.
Corticosteroids remain a common adjunctive treatment for pediatric septic shock, despite lack of high-quality evidence of efficacy and safety.
In the LAPSE descriptive cohort investigation examining community acquired septic shock, corticosteroids were commonly prescribed, affording the opportunity to examine associations of corticosteroid administration and outcomes.
Although previous investigations have suggested a potential benefit of corticosteroids in shortening the duration of septic shock, no studies have examined the potential association of corticosteroid therapy with morbidities among children surviving an encounter with septic shock.
At the Bedside.
In this secondary analysis of the data from the LAPSE investigation, early corticosteroid therapy was administered to 44% of patients and was not associated with an improvement in outcomes.
The propensity score methodology used in this study reduces the effect of confounding variables on the results.
Results generated in the current investigation support equipoise regarding the benefit/risk of adjunctive corticosteroids for pediatric septic shock and ascertain the need for a well-designed interventional trial.
ACKNOWLEDGMENTS
The LAPSE Investigators thank all subjects and families for participating in the LAPSE investigation.
Following is a summary of LAPSE Performance Sites, Principal Investigators (PI), Co-investigators (CI), Research Coordinators (RC), and Allied Research Personnel (AP).
Children’s Hospital of Michigan, Detroit, MI: Kathleen L. Meert, PI; Sabrina Heidemann, CI; Ann Pawluszka, RC; Melanie Lulic, RC.
Children’s Hospital of Philadelphia, Philadelphia, PA: Robert A Berg, PI; Athena Zuppa, CI; Carolann Twelves, RC; Mary Ann DiLiberto, RC.
Children’s National Medical Center, Washington, DC: Murray Pollack, PI; David Wessel, PI; John Berger, CI; Elyse Tomanio, RC; Diane Hession, RC; Ashley Wolfe, RC.
Children’s Hospital of Colorado, Denver, CO: Peter Mourani, PI; Todd Carpenter, CI; Diane Ladell, RC; Yamila Sierra, RC; Alle Rutebemberwa, RC.
Nationwide Children’s Hospital, Columbus, OH: Mark Hall, PI; Andy Yates, CI; Lisa Steele, RC; Maggie Flowers, RC; Josey Hensley, RC.
Mattel Children’s Hospital, University of California Los Angeles, Los Angeles, CA: Anil Sapru, PI; Rick Harrison, CI, Neda Ashtari, RC; Anna Ratiu, RC.
Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, PA: Joe Carcillo, PI; Michael Bell, CI; Leighann Koch, RC; Alan Abraham, RC.
Benioff Children’s Hospital, University of California, San Francisco, San Francisco, CA: Patrick McQuillen, PI; Anne McKenzie, RC; Yensy Zetino, RC.
Children’s Hospital Los Angeles, Los Angeles, CA: Christopher Newth, PI; Jeni Kwok, RC; Amy Yamakawa, RC.
CS Mott Children’s Hospital, University of Michigan, Ann Arbor, MI: Michael Quasney, PI; Thomas Shanley, CI; CJ Jayachandran, RC.
Cincinnati Children’s Hospital, Cincinnati, OH: Ranjit Chima PI; Hector Wong, CI; Kelli Krallman, RC; Erin Stoneman, RC; Laura Benken, RC; Toni Yunger, RC.
Seattle Children’s Hospital, Seattle Children’s Research Institute (LAPSE Follow-up Center), University of Washington, Seattle, WA: Jerry J Zimmerman, PI; Catherine Chen, RC; Erin Sullivan, RC; Courtney Merritt, RC; Deana Rich, RC; Julie McGalliard, AP; Wren Haaland, AP; Kathryn Whitlock, AP; Derek Salud, AP.
University of Utah (LAPSE Data Coordinating Center), Salt Lake City, UT: J Michael Dean, PI; Richard Holubkov, CI; Whit Coleman, RC; Samuel Sorenson, RC; Ron Reeder, AP; Russell Banks, AP; Angie Webster, AP; Jeri Burr, AP; Stephanie Bisping, AP; Teresa Liu, AP; Emily Stock, AP; Kristi Flick, AP.
Texas A&M University, College Station, TX: James Varni, AP
This investigation was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, R01HD073362, and was also supported, in part, by the following cooperative agreements: UG1HD050096, UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171, UG1HD083166, UG1HD083170, U10HD050012, U10HD063106, and U01HD049934.
Copyright Form Disclosure:
Drs. Banks, Carcillo, Sorenson, and Zimmerman’s institutions received funding from the National Institute of Child Health and Human Development. Drs. Banks, Reeder, Berg, Newth, Pollack, Meert, Carcillo, Mourani, Sorenson, Varni, Cengiz, and Zimmerman received support for article research from the National Institutes of Health (NIH). Drs. Reeder, Berg, Newth, Pollack, Meert, Mourani, and Varni’s isntittuions recevied funding from the NIH. Dr. Newth received funding from Philips Research North America, Hamilton Medical, and Nihon Kohden Orange Med. Dr. Zimmerman’s institution received funding from Immunexpress; he received funding from Elsevier Publishing; he disclosed the off-label product use of Corticosteroids as adjunctive treatment for pediatric septic shock. Dr. Kamps has disclosed that she does not have any potential conflicts of interest.
REFERENCES
- 1.Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, et al. : The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med 2018; 6:223–230 [DOI] [PubMed] [Google Scholar]
- 2.Weiss SL, Fitzgerald JC, Pappachan J, et al. : Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glass RI, Guttmacher AE, Black RE: Ending preventable child death in a generation. JAMA - J Am Med Assoc 2012; 308:141–142 [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Johnson HL, Cousens S, et al. : Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379:2151–2161 [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman JJ, Banks R, Berg RA, et al. : Trajectory of Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med 2020; 48:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Killien EY, Farris RWD, Watson RS, et al. : Health-Related Quality of Life Among Survivors of Pediatric Sepsis. Pediatr Crit Care Med 2019; 20:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerman JJ, Banks R, Berg RA, et al. : Critical Illness Factors Associated With Long-Term Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med 2020; 48:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson SJ, Cvijanovich NZ, Thomas NJ, et al. : Corticosteroids and pediatric septic shock outcomes: A risk stratified analysis. PLoS One 2014; 9:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markovitz BP, Goodman DM, Watson RS, et al. : A retrospective cohort study of prognostic factors associated with outcome in pediatric severe sepsis: What is the role of steroids? Pediatr Crit Care Med 2005; 6:270–274 [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman JJ, Williams MD: Adjunctive corticosteroid therapy in pediatric severe sepsis: Observations from the RESOLVE study. Pediatr Crit Care Med 2011; 12:2–8 [DOI] [PubMed] [Google Scholar]
- 11.Menon K, Dayre McNally J, Choong K, et al. : A cohort study of pediatric shock: Frequency of corticosteriod use and association with clinical outcomes. Shock 2015; 44:402–409 [DOI] [PubMed] [Google Scholar]
- 12.Wong HR, Atkinson SJ, Cvijanovich NZ, et al. : Combining Prognostic and Predictive Enrichment Strategies to Identify Children with Septic Shock Responsive to Corticosteroids. Crit Care Med 2016; 44:e1000–e1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon S, Hermayer KL: Glucocorticoid-induced hyperglycemia. Am J Med Sci 2013; 345:274–277 [DOI] [PubMed] [Google Scholar]
- 14.Wong HR, Cvijanovich NZ, Anas N, et al. : Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med 2015; 191:309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong HR, Cvijanovich NZ, Anas N, et al. : Pediatric Sepsis Biomarker Risk Model-II: Redefining the Pediatric Sepsis Biomarker Risk Model with Septic Shock Phenotype. Crit Care Med 2016; 44:2010–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yehya N, Vogiatzi MG, Thomas NJ, et al. : Cortisol Correlates with Severity of Illness and Poorly Reflects Adrenal Function in Pediatric Acute Respiratory Distress Syndrome. J Pediatr 2016; 177:212–218.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols B, Kubis S, Hewlett J, et al. : Hydrocortisone Therapy in Catecholamine-Resistant Pediatric Septic Shock: A Pragmatic Analysis of Clinician Practice and Association with Outcomes∗. Pediatr Crit Care Med 2017; 18:e406–e414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein B, Giroir B, Randolph A: International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 19.Pollack MM, Dean JM, Butler J, et al. : The ideal time interval for critical care severity-of-illness assessment. Pediatr Crit Care Med 2013; 14:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntosh AM, Tong S, Deakyne SJ, et al. : Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med 2017; 18:750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollack MM, Holubkov R, Glass P, et al. : Functional Status Scale: New pediatric outcome measure. Pediatrics 2009; 124:e18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein REK, Jessop DJ: Functional status ll(R): A measure of child health status. Med Care 1990; 28:1041–1055 [DOI] [PubMed] [Google Scholar]
- 23.Varni JW, Limbers CA, Neighbors K, et al. : The PedsQLTM Infant Scales: Feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res 2011; 20:45–55 [DOI] [PubMed] [Google Scholar]
- 24.Varni JW, Burwinkle TM, Seid M, et al. : The PedsQLTM* 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambul Pediatr 2003; 3:329–341 [DOI] [PubMed] [Google Scholar]
- 25.Czock D, Keller F, Rasche FM, et al. : Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 2005; 44:61–98 [DOI] [PubMed] [Google Scholar]
- 26.Vittinghoff E, Glitten D, Shiboski S, et al. : Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2nd ed. New York, NY: Springer; 2011. [Google Scholar]
- 27.Funk MJ, Westreich D, Wiesen C, et al. : Doubly robust estimation of causal effects. Am J Epidemiol 2011; 173:761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wehling M: Specific, nongenomic actions of steroid hormones. Annu Rev Physiol 1997; 59:365–393 [DOI] [PubMed] [Google Scholar]
- 29.Hadcock JR, Malbon CC: Regulation of β-adrenergic receptors by “permissive” hormones: Glucocorticoids increase steady-state levels of receptor mRNA. Proc Natl Acad Sci U S A 1988; 85:8415–8419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasidharan P: Role of corticosteroids in neonatal blood pressure homeostasis. Clin Perinatol 1998; 25:723–740 [PubMed] [Google Scholar]
- 31.Caprio M, Newfell BG, La Sala A, et al. : Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res 2008; 102:1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keh D, Boehnke T, Weber-Cartens S, et al. : Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med 2003; 167:512–520 [DOI] [PubMed] [Google Scholar]
- 33.Briegel J, Forst H, Haller M, et al. : Stress doses of hydrocortisone reverse hyperdynamic septic shock: A prospective, randomized, double-blind, single-center study. Crit Care Med 1999; 27:723–732 [DOI] [PubMed] [Google Scholar]
- 34.Venkatesh B, Finfer S, Cohen J, et al. : Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 2018; 378:797–808 [DOI] [PubMed] [Google Scholar]
- 35.Annane D, Renault A, Brun-Buisson C, et al. : Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 2018; 378:809–818 [DOI] [PubMed] [Google Scholar]
- 36.Annane D, Sébille V, Charpentier C, et al. : Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. J Am Med Assoc 2002; 288:862–871 [DOI] [PubMed] [Google Scholar]
- 37.Hebbar KB, Stockwell JA, Leong T, et al. : Incidence of adrenal insufficiency and impact of corticosteroid supplementation in critically ill children with systemic inflammatory syndrome and vasopressor-dependent shock. Crit Care Med 2011; 39:1145–1150 [DOI] [PubMed] [Google Scholar]
- 38.Hebbar KB, Petrillo T, Fortenberry JD: Adrenal insufficiency and response to corticosteroids in hypotensive critically ill children with cancer. J Crit Care 2012; 27:480–487 [DOI] [PubMed] [Google Scholar]
- 39.El-Nawawy A, Khater D, Omar H, et al. : Evaluation of early corticosteroid therapy in management of pediatric septic shock in pediatric intensive care patients: A randomized clinical study. Pediatr Infect Dis J 2017; 36:155–159 [DOI] [PubMed] [Google Scholar]
- 40.Sprung CL, Annane D, Keh D, et al. : Hydrocortisone therapy for patients with septic shock. N Engl J Med 2008; 358:111–124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.