Abstract
Background:
Current recommendations regarding radiotherapy treatment for unfavorable intermediate-risk prostate cancer (UIR-PCa) include external beam radiotherapy (EBRT) ± brachytherapy boost (BT) ± androgen deprivation therapy (ADT). The ideal radiotherapy treatment approach for UIR-PCa has not been well-defined. We hypothesized that EBRT+BT±ADT is associated with improved overall survival (OS) relative to EBRT±ADT in men with UIR-PCa.
Materials and Methods:
The National Cancer Database (NCDB) was used to retrospectively identify 32,246 men diagnosed between 2004–2015 with UIR-PCa who received EBRT (n=13,265), EBRT+ADT (n=13,123), EBRT+BT (n=3,440), or EBRT+BT+ADT (n=2,418). OS was the primary outcome. Inverse probability of treatment weighting was used to adjust for covariable imbalances and weight-adjusted multivariable analysis (MVA) using Cox regression modeling was used to compare OS hazard ratios.
Results:
Median follow-up was 60 months (range: 3–168 months). EBRT+ADT correlated with improved OS relative to EBRT alone on MVA (Hazard Ratio (HR): 0.92, [95% Confidence Interval: 0.87–0.98], p=.005). Compared to EBRT+ADT, EBRT+BT (HR: 0.77 [0.69–0.85], p=3×10−7) and EBRT+BT+ADT (HR: 0.75 [0.67–0.83], p=6×10−8) were associated with improved OS. 8-year OS for the EBRT+ADT versus EBRT+BT+ADT was 70% and 78% (p<.0001), which is similar to historical clinical trials (ASCENDE-RT 9-yr OS: 74% vs 78%, p=.29). Relative to EBRT+BT, EBRT+BT+ADT was not associated with improved OS (HR: 0.99 [0.87–1.11], p=.82).
Conclusion:
In a large retrospective cohort, the addition of brachytherapy to EBRT correlated with improved survival in men with UIR-PCa. Men receiving EBRT+ADT+BT had improved OS relative to EBRT+ADT. The addition of ADT to EBRT, but not to EBRT+BT, correlated with improved OS.
Keywords: prostate cancer, brachytherapy, radiotherapy, radiation treatment, unfavorable intermediate-risk, intermediate-risk
INTRODUCTION
Intermediate-risk prostate cancer is the largest and most heterogenous class of prostate cancer and developing optimal treatment strategies remains challenging. Men with intermediate-risk cancer are further stratified into favorable or unfavorable intermediate-risk (UIR) disease, with UIR patients defined as men with primary Gleason 4 cancer, higher-volume disease (≥ 50% positive biopsy cores) and one intermediate-risk factor, or more than one intermediate-risk factor. Men with UIR cancers often having outcomes more similar to high-risk disease than favorable intermediate-risk disease (1,2). Clinical trials have rarely addressed treatment decisions in this cohort, with most trials grouping UIR patients with either favorable intermediate-risk or high-risk disease. The NCCN currently recommends three definitive treatment options for men with UIR prostate cancer: radical prostatectomy ± pelvic lymph node dissection; external beam radiation therapy (EBRT) plus 4–6 months of androgen deprivation therapy (ADT); or combination EBRT with a brachytherapy boost (EBRT+BT) ± ADT (1).
Modern advances in EBRT precision have reduced toxicity and permitted dose-escalation that is associated with improved disease control, but patients still frequently recur (3–5). The addition of short-term ADT to EBRT is associated with improved overall survival and is often recommended (6–9). In UIR and high-risk prostate cancer, low-dose rate (LDR) or high-dose rate (HDR) brachytherapy is often added as a boost to EBRT, which permits further dose escalation beyond doses that can be delivered routinely with EBRT. Three randomized clinical trials have shown that adding BT to EBRT improves biochemical progression-free survival, but none of these studies were able to show a metastasis-free survival, cancer-specific survival, or overall survival benefit (10–12). The largest study was the ASCENDE-RT trial, which showed improved biochemical control in men with UIR or high-risk prostate cancer receiving EBRT+BT+ADT versus dose-escalated EBRT+ADT (11). On an unplanned subset analysis, EBRT+BT improved biochemical control versus EBRT in the UIR cohort, but the addition of brachytherapy boost did not reduce distant metastases or mortality. However, metastasis-free survival and overall survival were not the primary endpoints of ASCENDE-RT, and the trial was not powered to detect any difference in these endpoints. While there is data for high-risk patients that EBRT+BT is associated with reduced distant metastases and improved prostate cancer-specific survival compared to EBRT, the benefit of EBRT+BT for UIR patients is less well-established. It is also unclear whether the addition of ADT to EBRT+BT improves oncologic outcomes for men with UIR disease. To address these questions, we investigated survival rates associated with EBRT+BT versus EBRT alone in men with UIR prostate cancer using the National Cancer Database (NCDB). We hypothesized that the addition of a brachytherapy boost would be associated with an improvement in overall survival versus EBRT without brachytherapy boost for men with UIR prostate cancer. We also hypothesized that the addition of ADT to EBRT+BT would not be associated with an overall survival benefit in the setting of dose-escalation with EBRT and brachytherapy boost for UIR disease.
Methods
Patient Cohort
The NCDB cancer registry was queried for men diagnosed from 2004 and 2015 with adenocarcinoma of the prostate treated with definitive radiotherapy with curative intent. Patients had to meet criteria for unfavorable intermediate-risk prostate cancer, defined as intermediate-risk disease and either: primary Gleason 4 disease, 2 of 3 intermediate-risk factors (cT2b-T2c, PSA: 10 ≤ × ≤ 20, Gleason 3+4 disease), or higher-volume disease (≥ 50% positive biopsy cores). Men meeting any of the following criteria were excluded: (i) nodal or metastatic disease, (ii) receipt of surgery, chemotherapy, or immunotherapy; (iii) unknown ADT status or missing information on the number of days after diagnosis when ADT was initiated, (iv) ADT initiation > 180 days from diagnosis, (v) prior radiation to the prostate or pelvis, (vi) missing information on the cumulative radiation dose, number of fractions, or the numbers of days after diagnosis when radiotherapy was initiated; (vii) radiotherapy initiation > 180 days after diagnosis, and (viii) men with moderate-to-severe medical comorbidities (Charlson-Deyo comorbidity index (CDCI) scores > 1) (Supplemental Figure 1).
Radiotherapy and Covariables
Men were stratified into four treatment groups: (i) EBRT, (ii) EBRT+ADT, (iii) EBRT+BT, (iv) EBRT+ADT+BT. EBRT alone was delivered with conventional fractionation (≥ 72 Gy in 1.8–2.0 Gy per fraction) or moderate hypofractionation (2.4–3.2 Gy per fraction with a biologically equivalent dose (BED) > 120 Gy). Among EBRT alone patients, 98% received conventional fractionated radiotherapy. EBRT plus brachytherapy boost was defined as patients receiving EBRT to a total dose of 40–50.4 Gy, followed by high-dose rate (HDR) or low-dose rate (LDR) brachytherapy. Information on RT fields was included in the analysis and men were stratified into three groups based on NCDB coding: prostate only (code 41), prostate + pelvic lymph nodes (Code 35 or 29), or unknown. Covariables included in the analysis included age, race, ethnicity, CDCI score, insurance status, educational attainment (divided into quartiles on the basis of residents in the patient’s zip code who did not graduate high school), median income quartiles (divided into quartiles on the basis of zip code of residence), treatment at an academic center, PSA as diagnosis, Gleason score, clinical T stage, and year of diagnosis.
Statistical Analysis
Overall survival (OS) was the primary endpoint. Chi square and Student t-tests were used to detect significant differences among categorical and continuous variables, respectively. The Kaplan-Meier method was used to estimate OS and log-rank tests were used to compare treatment arms. Multivariable (MVA) overall survival hazard ratios were estimated using Cox regression analysis.
The inverse probability of treatment weighting (IPTW) was used to adjust for covariable imbalance. The probability of receiving a treatment was estimated using a binomial logistic regression model to generate propensity scores that included: age, CDCI score, PSA, clinical T stage, Gleason score, year of diagnosis, insurance status, treatment at an academic center, race, and ethnicity, educational attainment within the patient’s area of residence, and median household income within the patient’s area of residence. Inverse propensity weights were generated, with truncation of the most extreme weights as previously described (α = 0.0001) (13), and a pseudo-sample population in which measured baseline covariables were balanced between treatment groups was generated. Acceptable covariable balance among treatment groups was verified using the standardized mean difference, with a standardized mean difference less than 0.1 (10%) considered negligible (14). MVA was performed with weights applied to the time-dependent Cox proportional hazard model to compare the effects of EBRT plus brachy boost versus EBRT alone. Inverse propensity treatment weighted Kaplan-Meier curves were generated as the weighted product limit estimator. All analysis was performed in R software (Vienna, Austria). All tests were two-sided. P values < 0.05 were considered statistically significant.
Results
The study cohort included 32,246 UIR prostate cancer patients treated with: (i) EBRT (n = 13,265), (ii) EBRT+ADT (n = 13,123), (iii) EBRT+BT (n = 3,440), or (iv) EBRT+BT+ADT (n = 2,418). Radiation dose and fractionation regimens for EBRT alone and EBRT+BT are summarized in Supplemental Table 1. In the EBRT alone group, 98% of patients received conventional fractionation. Among patients receiving a brachy boost, 2,807 patients (48%) received LDR, 1,834 patients (31%) received HDR, and 1,217 patients (21%) received brachytherapy, not otherwise specified (Supplemental Table 1). A total of 39% and 46% of patients within the EBRT alone and EBRT+BT groups received prophylactic nodal irradiation, respectively (Table 1). The distribution of baseline characteristics among men stratified by treatment with EBRT alone or EBRT+BT is summarized in Table 1. The median age was lower and the proportion of patients receiving ADT was higher in patients treated with EBRT+BT. The median time to follow-up was 60 months (range: 3–168 months), and the average time to follow-up was 62 months (standard deviation ± 39 months).
Table 1.
Baseline characteristics of patients treated with EBRT alone versus EBRT plus brachytherapy boost.
| Total (n = 32,246) |
EBRT Alone (n = 26,388) |
EBRT+BT (n = 5,858) |
P | |
|---|---|---|---|---|
| Average Follow-up, months (SD) | 62 (39) | 59 (38) | 72 (43) | |
| Age, Mean (SD) | 69 (7.5) | 70 (7.4) | 67 (7.4) | < 1 ×10−16 |
| Race | 0.16 | |||
| White | 25,626 (79) | 20,985 (79) | 4641 (79) | |
| Black | 5,736 (18) | 4,662 (18) | 1074 (18) | |
| Other | 884 (3) | 741 (3) | 143 (3) | |
| Spanish or Hispanic Origin | 0.02 | |||
| Non-Spanish, Non-Hispanic | 29,218 (91) | 23,862 (90) | 5,356 (91) | |
| Spanish or Hispanic | 3,028 (9) | 2,526 (10) | 502 (9) | |
| Insurance | < 2 × 10−16 | |||
| Uninsured | 435 (1) | 375 (1) | 60 (1) | |
| Private Insurance | 8,854 (28) | 6,602 (25) | 2,252 (38) | |
| Medicare | 20,849 (65) | 17,583 (67) | 3,266 (56) | |
| Medicaid/Other Government | 1,641 (5) | 1,425 (5) | 216 (4) | |
| Other | 467 (1) | 403 (2) | 64 (1) | |
| Income Level | 1.5 × 10−5 | |||
| < 38,000 | 6,207 (19) | 5,118 (20) | 1,089 (19) | |
| 38,000–47,999 | 7,602 (24) | 6,162 (23) | 1,440 (25) | |
| 48,000–62,999 | 8,388 (26) | 6,997 (27) | 1,391 (24) | |
| > 63,000 | 9,918 (31) | 8,003 (30) | 1,915 (33) | |
| Unknown | 131 (0) | 108 (0) | 23 (0) | |
| Education † | 8.7 × 10−4 | |||
| < 7% | 7,752 (24) | 6,262 (24) | 1,490 (25) | |
| 7–12.9% | 10,724 (33) | 8,874 (34) | 1,850 (32) | |
| 13–20.9% | 8,500 (27) | 7,003 (26) | 1,497 (26) | |
| ≥ 21% | 5,156 (16) | 4,157 (16) | 999 (17) | |
| Unknown | 114 (0) | 92 (0) | 22 (0) | |
| Treatment at Academic Center | ||||
| No | 23,152 (72) | 18,885 (72) | 4,267 (73) | 0.052 |
| Yes | 9,094 (28) | 7,503 (28) | 1,591 (27) | |
| Year of Diagnosis | <2.2 × 10−16 | |||
| 2004–2007 | 8,690 (28) | 6,611 (25) | 2,349 (40) | |
| 2008–2010 | 14,171 (44) | 11,858 (45) | 2,313 (40) | |
| 2011–2015 | 9,115 (28) | 7,919 (30) | 1,196 (20) | |
| CDCI (Comorbidity) | 0.55 | |||
| 0 | 28,052 (87) | 22,970 (87) | 5,082 (87) | |
| 1 ‡ | 4,194 (13) | 3,418 (13) | 776 (13) | |
| PSA, Mean (SD) | 8.4 (4.3) | 8.5 (4.3) | 7.9 (4.2) | < 1 ×10−16 |
| Gleason Score | 2.0 × 10−6 | |||
| 3+3 | 1,367 (4) | 1,184 (4) | 183 (3) | |
| 3+4 | 14,057 (44) | 11,544 (44) | 2,513 (43) | |
| 4+3 | 16,822 (52) | 13,660 (52) | 3,162 (54) | |
| Clinical T Stage | 2.6 × 10−9 | |||
| ≤ cT2a | 22,591 (70) | 18,638 (70) | 3,953 (67) | |
| T2b-T2c | 8,609 (27) | 6,862 (26) | 1,747 (30) | |
| T2, NOS | 1,046 (3) | 888 (4) | 158 (3) | |
| ADT | <2.2 × 10−16 | |||
| No | 16,705 (52) | 13,265 (50) | 3,440 (59) | |
| Yes | 15,541 (48) | 13,123 (50) | 2,418 (41) | |
| Radiation Fields | <2.2 × 10−16 | |||
| Pelvic | 18,921 (59) | 15,786 (60) | 3,135 (53) | |
| Pelvis + Pelvic LNs | 13,070 (40) | 10,390 (39) | 2,680 (46) | |
| Unknown | 255 (1) | 212 (1) | 43 (1) |
Summary statistics are represented as mean (standard deviation [SD]) for continuous variables and No. (%) for categorical variables.
Proportion of adults in the patient’s zip code who did not graduate from high school.
A CDCI score of 1 means that an individual has a history of one of the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, diabetes.
Abbreviations: ADT, androgen deprivation therapy; BT, brachytherapy; CDCI, Charlson-Deyo Comorbidity Index; EBRT; external beam radiotherapy; LN, lymph nodes; PSA, prostate specific antigen.
Unadjusted MVA and weight-adjusted MVA of each treatment group is summarized in Table 2. Unadjusted Kaplan Meier and weight-adjusted Kaplan Meier curves are shown in Figure 1. Inverse probability of treatment weighting was used to balance covariables that influence both treatment assignment and outcomes, and weight-adjusted Cox regression was used to determine the effect of brachy boost on groups with balanced cofounders. EBRT+ADT was associated with better OS than EBRT alone on MVA (Hazard Ratio (HR): 0.92, 95% Confidence Interval (95% CI): 0.87–0.98, p = 0.005) (Table 2). Eight years after treatment, 70% of patients receiving EBRT+ADT were alive compared to 67% of patients receiving EBRT+ADT (p < 0.0001) (Figure 1A and 1B). Baseline characteristics of the EBRT+ADT vs. EBRT+BT+ADT cohorts are summarized in Supplemental Table 2. Compared to EBRT+ADT, tri-modality therapy (EBRT+BT+ADT) was associated with improved OS (HR: 0.75, 95% CI: 0.67–0.83, p = 5.6 × 10−8) on MVA (Supplemental Table 3). Eight years after treatment, 78% of patients receiving tri-modality therapy were alive compared to 70% of patients receiving EBRT+ADT (p < 0.0001) (Figure 1A and 1B). Baseline differences between men treated with EBRT+BT without ADT vs. EBRT+BT+ADT are shown in Supplemental Table 4. Trimodality therapy was not associated with improved OS compared to EBRT plus brachytherapy without ADT (HR: 0.99, 95% CI: 0.87–1.11, p = 0.82) (Supplemental Table 5). EBRT plus brachytherapy boost without ADT had improved OS relative to patients treated with EBRT+ADT (HR: 0.77, 95% CI: 0.69–0.85, p = 2.9 × 10−7) (Table 3). Eight years after treatment, 70% of patients receiving EBRT+ADT were alive compared to 78% of patients receiving EBRT+BT without ADT (p < 0.0001) (Figure 1A and 1B). We further evaluated whether patients receiving EBRT alone with moderately hypofractionated radiotherapy versus EBRT+BT and found that brachy boost was not associated with improved OS (HR: 0.91, CI: 0.69–1.20, p = 0.50), though the analysis was underpowered to detect a statistical difference.
Table 2.
Multivariable analysis comparing EBRT alone to EBRT+ADT, EBRT+BT, and EBRT+BT+ADT using unweighted Cox regression model (left) or using inverse probability of treatment weighted hazard ratios (right)
| Unweighted MVA | IPTW-Weighted MVA | |||
|---|---|---|---|---|
| HR [95% CI] | P | HR [95% CI] | P | |
| Age ψ | 1.05 [1.04–1.05] | < 2 × 10−16 | 1.05 [1.04–1.06] | < 2 × 10−16 |
| Race | ||||
| White | 1.0 | - | 1.0 | - |
| Black | 0.98 [0.91–1.06] | 0.68 | 0.96 [0.86–1.06] | 0.40 |
| Other | 0.72 [0.60–0.86] | 0.0004 | 0.75 [0.58–0.99] | 0.04 |
| Spanish or Hispanic Origin | ||||
| Non-Spanish, Non-Hispanic | 1.0 | - | 1.0 | - |
| Spanish or Hispanic | 0.94 [0.87–1.02] | 0.12 | 0.86 [0.77–0.97] | 0.01 |
| Insurance | ||||
| Uninsured | 1.0 | - | 1.0 | - |
| Private Insurance | 0.97 [0.75–1.27] | 0.84 | 1.03 [0.77–1.37] | 0.83 |
| Medicare | 1.21 [0.93–1.57] | 0.15 | 1.17 [0.88–1.56] | 0.27 |
| Medicaid/Other Government | 1.29 [0.97–1.72] | 0.08 | 1.23 [0.89–1.69] | 0.21 |
| Income Level | ||||
| < 38,000 | 1.0 | - | 1.0 | - |
| 38,000–47,999 | 0.93 [0.86–1.00] | 0.06 | 0.96 [0.86–1.08] | 0.54 |
| 48,000–62,999 | 0.87 [0.80–0.94] | 0.001 | 0.88 [0.78–1.00] | 0.04 |
| > 63,000 | 0.83 [0.75–0.91] | 1.1 × 10−4 | 0.82 [0.71–0.94] | 0.005 |
| Education † | ||||
| < 7% | 1.0 | - | 1.0 | - |
| 7–12.9% | 1.14 [1.06–1.23] | 5.8 × 10−4 | 1.17 [1.06–1.29] | 0.002 |
| 13–20.9% | 1.12 [1.03–1.22] | 0.01 | 1.13 [1.01–1.28] | 0.046 |
| ≥ 21% | 1.17 [1.06–1.31] | 0.003 | 1.21 [1.04–1.42] | 0.01 |
| Treatment at Academic Center | 0.97 [0.92–1.03] | 0.32 | 1.0 [0.92–1.07] | 0.93 |
| Year of Diagnosis | ||||
| 2004–2007 | 1.0 | - | 1.0 | - |
| 2008–2010 | 1.04 [0.99–1.11] | 0.11 | 1.02 [0.95–1.11] | 0.58 |
| 2011–2015 | 0.96 [0.86–1.08] | 0.50 | 0.97 [0.81–1.16] | 0.75 |
| CDCI (Comorbidity) | ||||
| 0 | 1.0 | - | 1.0 | - |
| 1 ‡ | 1.43 [1.33–1.53] | < 2 × 10−16 | 1.40 [1.28–1.54] | 5.9 × 10−13 |
| PSA ψ | 1.02 [1.01–1.03] | 6.4 × 10−12 | 1.02 [1.01–1.03] | 4.3 × 10−6 |
| Gleason Score | ||||
| 3+3 | 1.0 | - | 1.0 | - |
| 3+4 | 0.95 [0.84–1.08] | 0.46 | 0.98 [0.83–1.17] | 0.73 |
| 4+3 | 0.98 [0.86–1.11] | 0.73 | 1.02 [0.85–1.22] | 0.99 |
| Clinical T Stage | ||||
| ≤ cT2a | 1.0 | - | 1.0 | - |
| T2b-T2c | 1.13 [1.07–1.20] | 1.6 × 10−5 | 1.09 [1.01–1.18] | 0.03 |
| T2, NOS | 1.18 [1.04–1.34] | 0.01 | 1.12 [0.94–1.32] | 0.20 |
| Radiation Fields | ||||
| Pelvic | 1.0 | - | 1.0 | - |
| Pelvic + Pelvic LN | 1.03 [0.98–1.09] | 0.15 | 1.09 [1.02–1.16] | 0.009 |
| Treatment | ||||
| EBRT | 1.0 | - | 1.0 | - |
| EBRT + ADT | 0.92 [0.87–0.97] | 0.003 | 0.92 [0.87–0.98] | 0.005 |
| EBRT + BT | 0.71 [0.64–0.77] | 7.6 × 10−14 | 0.71 [0.64–0.78] | 8.7 × 10−12 |
| EBRT + BT + ADT | 0.69 [0.63–0.76] | 1.0 × 10−13 | 0.69 [0.62–0.76] | 2.9 × 10−12 |
Variable evaluated as a continuous variable.
Proportion of adults in the patient’s zip code who did not graduate from high school.
A CDCI score of 1 means that an individual has a history of one of the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, diabetes.
Abbreviations: 95% CI, 95% confidence interval; ADT, androgen deprivation therapy; BT, brachytherapy; CDCI, Charlson-Deyo Comorbidity Index; EBRT; external beam radiotherapy; HR, hazard ratio; LN, lymph nodes; PSA, prostate specific antigen.
Figure. 1:
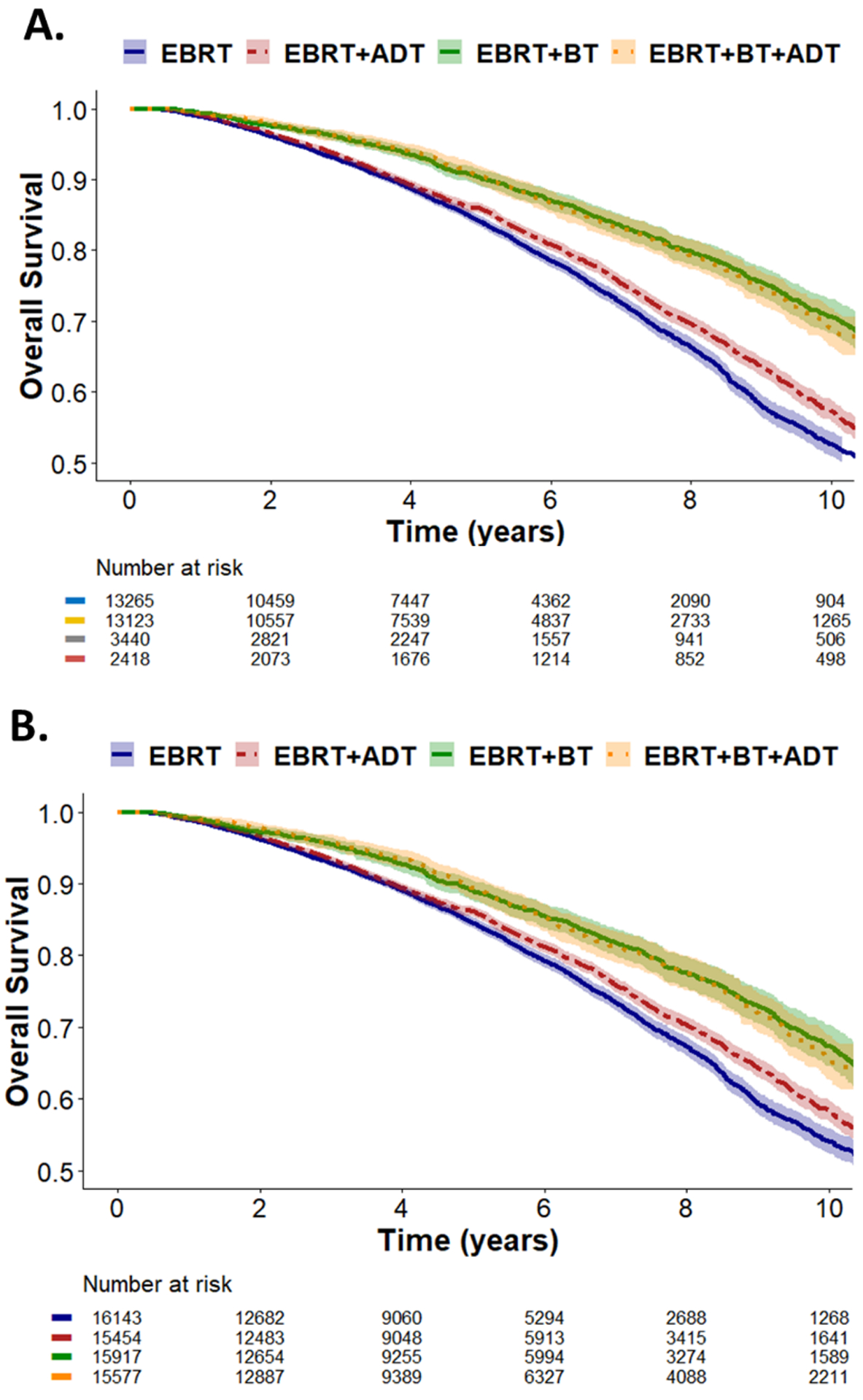
(A) Unweighted and (B) IPTW weight-adjusted Kaplan-Meier curves stratified by treatment with EBRT, EBRT+ADT, EBRT+BT, and EBRT+BT+ADT. Shading corresponds to 95% confidence intervals. Abbreviations: ADT, androgen deprivation therapy; BT, brachytherapy; EBRT, external beam radiation therapy.
Table 3.
Multivariable Cox regression analysis of EBRT with ADT versus EBRT plus brachy boost using inverse probability of treatment weighted hazard ratios.
| HR [CI] | P | |
|---|---|---|
| Age ψ | 1.05 [1.04–1.06] | < 2 × 10−16 |
| Race | ||
| White | 1.0 | - |
| Black | 0.98 [0.84–1.13] | 0.74 |
| Other | 0.78 [0.55–1.10] | 0.15 |
| Spanish or Hispanic Origin | ||
| Non-Spanish, Non-Hispanic | 1.0 | - |
| Spanish or Hispanic | 0.88 [0.75–1.03] | 0.12 |
| Insurance | ||
| Uninsured | 1.0 | - |
| Private Insurance | 0.92 [0.62–1.37] | 0.68 |
| Medicare | 1.03 [0.70–1.53] | 0.86 |
| Medicaid/Other Government | 1.15 [0.72–1.83] | 0.55 |
| Income Level | ||
| < 38,000 | 1.0 | - |
| 38,000–47,999 | 0.87 [0.74–1.02] | 0.09 |
| 48,000–62,999 | 0.82 [0.69–0.97] | 0.02 |
| > 63,000 | 0.70 [0.57–0.85] | 3.3 × 10−4 |
| Education † | ||
| < 7% | 1.0 | - |
| 7–12.9% | 1.05 [0.91–1.21] | 0.99 |
| 13–20.9% | 1.00 [0.84–1.19] | 0.72 |
| ≥ 21% | 1.04 [0.84–1.29] | 0.57 |
| Treatment at Academic Center | 0.86 [0.77–0.98] | 0.02 |
| Year of Diagnosis | ||
| 2004–2007 | 1.0 | - |
| 2008–2010 | 1.07 [0.96–1.20] | 0.23 |
| 2011–2015 | 0.99 [0.78–1.27] | 0.96 |
| CDCI (Comorbidity) | ||
| 0 | 1.0 | - |
| 1 ‡ | 1.37 [1.20–1.57] | 5.1 × 10−6 |
| PSA ψ | 1.01 [0.99–1.02] | 0.11 |
| Gleason Score | ||
| 3+3 | 1.0 | - |
| 3+4 | 0.88 [0.68–1.14] | 0.34 |
| 4+3 | 0.88 [0.67–1.15] | 0.36 |
| Clinical T Stage | ||
| ≤ cT2a | 1.0 | - |
| T2b-T2c | 1.07 [0.96–1.20] | 0.21 |
| Radiation Fields | ||
| Pelvic | 1.0 | - |
| Pelvic + Pelvic LN | 1.06 [0.96–1.16] | 0.22 |
| Treatment | ||
| EBRT + ADT | 1.0 | - |
| EBRT + BT | 0.77 [0.69–0.85] | 2.9 × 10−7 |
Variable evaluated as a continuous variable.
Proportion of adults in the patient’s zip code who did not graduate from high school.
A CDCI score of 1 means that an individual has a history of one of the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, diabetes.
Abbreviations: 95% CI, 95% confidence interval; ADT, androgen deprivation therapy; BT, brachytherapy; CDCI, Charlson-Deyo Comorbidity Index; EBRT; external beam radiotherapy; HR, hazard ratio; LN, lymph nodes; PSA, prostate specific antigen.
Discussion
In this study of unfavorable intermediate-risk prostate cancer patients, we examined the comparative effectiveness of the preferred definitive radiotherapy treatment strategies for UIR disease listed in the NCCN guidelines. We confirmed that EBRT+ADT is associated with improved OS compared to EBRT alone. Furthermore, we found that the addition of brachytherapy to EBRT was associated with superior OS compared to both EBRT alone and EBRT+ADT. The addition of ADT to EBRT+BT had no impact on OS compared to EBRT+BT (HR = 0.99), though the limitations of the database do not allow us to assess whether the addition of ADT had an impact on biochemical recurrence or distant metastases in patients treated with EBRT+BT. This data provides important evidence that EBRT+BT is associated with a statistically significant improvement in OS compared to EBRT+ADT in a large cohort of patients with UIR, supplementing results from smaller prospective studies that reported only a biochemical control benefit for EBRT+BT (11). While a recent NCDB analysis examining UIR and high-risk patients treated as per the ASCENDE RT trial with EBRT+ADT versus EBRT+BT+ADT reported an OS benefit associated with brachytherapy, the study did not separately report results for UIR disease (15).
This study is consistent with and extends prior research on radiotherapy treatment for UIR. At 8-years, IPTW-adjusted OS was significantly different at 78% versus 70% in the EBRT+BT+ADT versus the EBRT+ADT alone arms. This is comparable to ASCENDE RT, which reported a 9-year OS of 78% vs 74% in the EBRT+BT+ADT and EBRT+ADT arms, but was not statistically significant (P=.29) (11). While ASCENDE-RT showed no OS benefit, the trial reported a statistical correlation between biochemical-BFS and increased all-cause mortality (HR: 6.30, P<.001). Taken together, this suggests that ASCENDE RT, which enrolled 398 men, was likely not powered for a 20–25% difference in biochemical-PFS to translate into an OS benefit. Thus, it is not unreasonable to assume that this large of an absolute improvement in biochemical control could ultimately translate into an OS improvement in a much larger patient cohort of 32,246 men drawn from the NCDB. In addition, prior studies have shown that the use of ADT with EBRT improves OS and DSS in men with intermediate-risk prostate cancer (6,7). Similarly, in patients with high-risk prostate cancer or Gleason 9–10 disease, prior retrospective studies have reported a disease-specific survival benefit associated with adding ADT to EBRT+BT (trimodality therapy) (6,16). Moreover, a recent clinical trial showed that men with predominantly high-risk disease receiving EBRT+BT derived a metastasis-free survival benefit from longer ADT duration (17). Since macroscopic disease often extends beyond the prostate capsule in men with high-risk prostate cancer (18,19), ADT can enhance radiation-induced cell death, thereby improving locoregional control. However, UIR disease is less aggressive and more likely to be confined to the prostate. To our knowledge, few studies have evaluated the role of ADT in UIR patients treated with EBRT+BT. The role of ADT for patients treated with EBRT+BT are largely extrapolated from studies using mixed cohorts of UIR and high-risk patients. A recent retrospective, single-institution study by Mendez et al. did investigate this question for UIR disease and reported that the addition of ADT to EBRT+BT was associated with a statistically significant improvement in biochemical failure-free survival with no improvement in metastasis-free survival in a matched cohort of 156 patients. A metanalysis using combined cohorts of high- and intermediate-risk patients drawn from 9 clinical trials reported a survival benefit associated with trimodality therapy (20). In this study by Jackson et al., the authors argued that ADT should not be omitted in patients treated with brachytherapy boost. The study was primarily in high-risk patients and included few patients with intermediate-risk disease treated with EBRT+BT without ADT [21 patients from a single-institution trial plus a minority of the 48 intermediate-risk disease patients treated with EBRT+BT on the Hoskin trial, since 77% received ADT] (10,12). The Jackson et al. study demonstrates the value of ADT for high-risk disease treated with EBRT+BT, but the small size of the UIR cohort (<50 patients) treated with EBRT+BT without ADT greatly limits the study’s ability to address the question for UIR disease. Other retrospective studies failed to show a benefit with adding ADT to EBRT+BT in these mixed patient populations (21,22). These interpretations are confounded by the inclusion of high-risk patients, making it difficult to determine if UIR patients receiving EBRT+BT benefit from ADT. We believe our study helps to address this question by limiting the analysis to UIR patients and with a sufficiently large sample size to detect a survival difference, if such a difference exists. While we show in this study that ADT provides a clinically meaningful and statistically significant survival advantage in patients receiving EBRT alone, which is consistent with prior studies (7,23), the addition of ADT to EBRT+BT was not associated with a survival advantage. Further research is warranted regarding the benefits of ADT in the setting of EBRT+BT, given the significant quality-of-life and toxicity considerations of ADT, which include vasomotor symptoms, weight gain, dyslipidemia, erectile dysfunction, osteoporosis, and depression, and increased risk of cardiovascular disease (24). Our results are consistent with current NCCN guidelines that lists EBRT + BT with or without ADT as acceptable options (1).
Our study has several important limitations. As a retrospective, non-randomized study, the results are hypothesis-generating and require confirmation in randomized trials. In addition, data on other important endpoints including biochemical control, metastasis-free survival, and cancer-specific survival are missing from the NCDB. It is very likely that the addition of ADT to EBRT+BT improves biochemical control to some degree and possibly even distant metastatic control for UIR patients. However, given the large size of the cohort, we would have expected at least a small trend toward improved OS for patients treated with EBRT+BT+ADT, if the addition of ADT had a significant impact on reducing distant disease, and not the observed HR of 0.99. While we adjusted for measured confounders using MVA and IPTW, we cannot account for unmeasured confounders (i.e., duration of ADT therapy, HDR dose, performance status). It is possible that ADT duration was very short for some patients treated with EBRT+BT and prescribed primarily to help shrink the size of the prostate prior to brachytherapy implantation, which could reduce the observed oncologic benefit of standard-duration ADT. The study is also limited by selection biases inherent to retrospective studies, most notably that patients selected to undergo brachytherapy, which is generally performed under anesthesia, may have better performance status than patients treated with EBRT without brachytherapy. We restricted the analysis to otherwise healthy patients with few medical comorbidities (CDCI of 1 or less with a CDCI score of 0 meaning no comorbidities) to help address the selection bias issue. While the comorbidity index (CDCI) was equally balanced between groups, CDCI scores are a surrogate for performance status and not a true measure of overall performance status. However, prior surgical series have shown that adding ASA/ECOG performance scores to models already containing the CDCI to address confounding did not yield improvements in risk adjustment models for comparative assessment of cancer outcomes (25). Even though the cohort treated with brachytherapy boost ± ADT is more homogeneous than comparisons of EBRT vs. EBRT+BT, there was still selection bias in the choice of whether to prescribe ADT, with physicians more likely to prescribe ADT to patients with more aggressive disease. We think that our model contains relevant clinical and pathologic variables to help account for such differences, but unmeasured confounders could still potentially impact on the overall results. Lastly, given that only a small subset of patients in the EBRT alone arm were treated with moderate hypofractionated radiotherapy, we cannot assess if EBRT+BT improves outcomes compared to patients treated with hypofractionated EBRT.
In conclusion, our study of approximately 33,000 men with UIR prostate cancer found that EBRT+BT is associated with improved OS compared to EBRT alone, with the addition of ADT to EBRT+BT providing no additional survival benefit, though our study is not able to assess the impact of ADT on biochemical control or distant metastasis in the EBRT+BT cohort. These results provide additional data supporting the use of dose-escalated treatment with brachytherapy boost, an NCCN-approved treatment approach for UIR disease.
Supplementary Material
Acknowledgements:
This work was supported by institutional funds from the Departments of Radiation Oncology and Siteman Cancer Center at Washington University/Barnes Jewish Hospital in St Louis. BCB is supported by an NCI Cancer Clinical Investigator Team Leadership Award (CCITLA), P30 CA091842-20S2.
Disclosure/Conflicts of Interest:
Brian C. Baumann discloses service on a medical advisory panel for Sanofi/Regeneron, Boston Scientific, and Galera Therapeutics as well as honoraria from Mevion, all outside of the scope of the submitted work. He also discloses consulting work for Boston Scientific and Varian and research funding from Varian, all outside of the scope of the submitted work. The remaining authors have nothing to disclose. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Prostate Cancer (Version 2.2021) https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed March 2, 2021.
- 2.Dinh KT, Mahal BA, Ziehr DR, et al. Incidence and Predictors of Upgrading and Up Staging among 10,000 Contemporary Patients with Low Risk Prostate Cancer. J Urol 2015;194:343–9. [DOI] [PubMed] [Google Scholar]
- 3.Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 2014;15:464–73. [DOI] [PubMed] [Google Scholar]
- 4.Michalski JM, Moughan J, Purdy J, et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol 2018;4:e180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95–09. J Clin Oncol 2010;28:1106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amico AV, Moran BJ, Braccioforte MH, et al. Risk of death from prostate cancer after brachytherapy alone or with radiation, androgen suppression therapy, or both in men with high-risk disease. J Clin Oncol 2009;27:3923–8. [DOI] [PubMed] [Google Scholar]
- 7.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 2011;365:107–18. [DOI] [PubMed] [Google Scholar]
- 8.Zumsteg ZS, Spratt DE, Daskivich TJ, et al. Effect of Androgen Deprivation on Long-term Outcomes of Intermediate-Risk Prostate Cancer Stratified as Favorable or Unfavorable: A Secondary Analysis of the RTOG 9408 Randomized Clinical Trial. JAMA Netw Open 2020;3:e2015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol 2013;64:895–902. [DOI] [PubMed] [Google Scholar]
- 10.Hoskin PJ, Rojas AM, Bownes PJ, et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol 2012;103:217–22. [DOI] [PubMed] [Google Scholar]
- 11.Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys 2017;98:275–85. [DOI] [PubMed] [Google Scholar]
- 12.Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol 2005;23:1192–9. [DOI] [PubMed] [Google Scholar]
- 13.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson SB, Lester-Coll NH, Kelly JR, et al. Brachytherapy Boost Utilization and Survival in Unfavorable-risk Prostate Cancer. Eur Urol 2017;72:738–44. [DOI] [PubMed] [Google Scholar]
- 16.Kishan AU, Cook RR, Ciezki JP, et al. Radical Prostatectomy, External Beam Radiotherapy, or External Beam Radiotherapy With Brachytherapy Boost and Disease Progression and Mortality in Patients With Gleason Score 9–10 Prostate Cancer. JAMA 2018;319:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph D, Denham JW, Steigler A, et al. Radiation Dose Escalation or Longer Androgen Suppression to Prevent Distant Progression in Men With Locally Advanced Prostate Cancer: 10-Year Data From the TROG 03.04 RADAR Trial. Int J Radiat Oncol Biol Phys 2020;106:693–702. [DOI] [PubMed] [Google Scholar]
- 18.Davis BJ, Pisansky TM, Wilson TM, et al. The radial distance of extraprostatic extension of prostate carcinoma: implications for prostate brachytherapy. Cancer 1999;85:2630–7. [PubMed] [Google Scholar]
- 19.Sohayda C, Kupelian PA, Levin HS, et al. Extent of extracapsular extension in localized prostate cancer. Urology 2000;55:382–6. [DOI] [PubMed] [Google Scholar]
- 20.Jackson WC, Hartman HE, Dess RT, et al. Addition of Androgen-Deprivation Therapy or Brachytherapy Boost to External Beam Radiotherapy for Localized Prostate Cancer: A Network Meta-Analysis of Randomized Trials. J Clin Oncol 2020;38:3024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dattoli M, Wallner K, True L, et al. Long-term outcomes for patients with prostate cancer having intermediate and high-risk disease, treated with combination external beam irradiation and brachytherapy. J Oncol 2010;2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demanes DJ, Brandt D, Schour L, et al. Excellent results from high dose rate brachytherapy and external beam for prostate cancer are not improved by androgen deprivation. Am J Clin Oncol 2009;32:342–7. [DOI] [PubMed] [Google Scholar]
- 23.D’Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA 2008;299:289–95. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015;67:825–36. [DOI] [PubMed] [Google Scholar]
- 25.Dobbins TA, Badgery-Parker T, Currow DC, et al. Assessing measures of comorbidity and functional status for risk adjustment to compare hospital performance for colorectal cancer surgery: a retrospective data-linkage study. BMC Med Inform Decis Mak 2015;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


