Abstract
Background:
To examine whether lower neighborhood-level and individual-level indicators of socioeconomic status (SES) are associated with subsequently worse neurological disability in people with MS (pwMS).
Methods:
In a multi-center study using prospectively collected data from discovery cohorts (University of Pittsburgh, N=1316) and replication cohorts (Columbia University, N=488), we calculated a neighborhood SES indicator, area deprivation index (ADI), based on participants’ residence at enrollment, and we derived an individual SES indicator based on participants’ household income. Patient-reported neurological outcomes included the Multiple Sclerosis Rating Scale–Revised (MSRS-R), Patient-Determined Disease Steps (PDDS), and Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function scores from 2018 to 2020. We performed covariate-adjusted regression analyses in each cohort and then random-effects meta-analyses.
Results:
Higher ADI (lower SES) in 2015 was associated with subsequently worse neurological outcomes during 2018–2020 (discovery: MSRS-R, β=0.62, 95%CI [0.36,0.89], p<0.001; PDDS, β=0.11, 95%CI [0.02,0.20], p=0.02 | replication: MSRS-R, β=0.46, 95%CI [0.21,0.72], p<0.001; PDDS, β=0.12, 95%CI [0.03,0.21], p=0.009, PROMIS, β=−0.60, 95%CI [−1.12,−0.08], p=0.025). Lower neighborhood percent with college education (MSRS-R, β=−7.31, 95%CI [−8.99,−5.64], p<0.001; PDDS, β=−1.62, 95%CI [−2.20,−1.05], p<0.001; PROMIS, β=9.31, 95%CI [5.73,12.89], p<0.001), neighborhood median household income (MSRS-R, β=−3.80e-05, 95%CI [−5.05e-05,−2.56e-05], p<0.001; PDDS, β=−8.58e-06, 95%CI [−1.28e-05,−4.32e-06], p<0.001; PROMIS, β=2.55e-05, 95%CI [5.96e-07,5.05e-05], p=0.045), and neighborhood median home value (MSRS-R, β=−6.50e-06, 95%CI [−8.16e-06,−4.84e-06], p<0.001; PDDS, β=−1.54e-06, 95%CI [−2.11e-06,−9.65e-07], p<0.001; PROMIS, β=4.98e-06, 95%CI [1.81e-06,8.14e-06], p=0.002) drove the association between higher ADI and subsequently worse neurological disability (in joint analyses). Neighborhood percent of population with Medicaid (but not private insurance) significantly mediated the observed covariate-adjusted associations. Higher individual-level household income bracket was associated with better neurological outcomes (MSRS-R: R=−0.39, p<0.001; PDDS: R=−0.35, p<0.001; PROMIS: R=0.37, p<0.001), independent of ADI.
Conclusions:
Lower neighborhood SES is associated with subsequently worse neurological outcomes in pwMS. Future testing of targeted intervention through public policies that improve SES are warranted.
Keywords: multiple sclerosis, socioeconomic status, area deprivation index, insurance, disability, neurological outcome
1.0. Introduction
Socioeconomic status (SES) is complex but a key determinant of health outcomes (Mackenbach et al., 2008). SES is a dynamic measure influenced by politics, geography, and other temporal changes over a person’s lifetime. As healthcare moves towards a more equitable and patient-centered approach, it is crucial to gain a nuanced understanding of the impact of SES on health outcomes.
This need is particularly urgent in chronic neurological diseases such as multiple sclerosis (MS) given their disproportionally high socioeconomic burden (Fattore et al., 2012; Hartung, 2021; Jennum et al., 2012) and modifiable disease course (Giovannoni, 2018; Goldschmidt and McGinley, 2020). Low SES or proxy measures of SES in people with MS (pwMS) is associated with increased risk of MS (Bjørnevik et al., 2016a; Briggs et al., 2015, 2014; Dobson et al., 2020), worsening disability (Briggs et al., 2019; Calocer et al., 2020; D’hooghe et al., 2016; Harding et al., 2019), decreased access to neurological care (Contentti et al., 2019; Minden et al., 2008; Moss et al., 2020; Roddam et al., 2019), and lower quality of life (Boogar et al., 2018; Wang et al., 2020).
Few prior studies have examined the temporal association between SES, both at neighborhood and individual level, and subsequent neurological and physical function following MS diagnosis using multiple distinct clinically relevant patient-reported outcomes (PROs). PROs provide real-world evidence of neurological function. In this study, we assessed whether low SES was associated with subsequent accumulation of neurological and physical disability in pwMS, leveraging multiple cohorts at the neighborhood and individual level and using three distinct PROs that assess multiple domains of MS disability. We hypothesize that lower SES led to subsequently worse PROs of neurological and physical function in pwMS.
2.0. Methods
2.1. Study Design and Participants
The study inclusion criteria were adults 18 years or older with a neurologist-confirmed MS diagnosis. This study included 1,316 pwMS for the discovery analysis and 488 pwMS for replication (Figure 1). Discovery cohorts included: (1) the Prospective Investigation of Multiple Sclerosis in the Three Rivers Region (PROMOTE) study, a clinic-based MS cohort at the University of Pittsburgh Medical Center (UPMC) that began enrollment in January 2017 (NCT02994121) (Epstein et al., 2022; Kever et al., 2022; Levin et al., 2021, 2020; Levit et al., 2022; Mani et al., 2021); (2) the Pittsburgh COVID-19 and MS population study, an online survey study that began enrollment in April 2020 (Epstein et al., 2022; Kever et al., 2022; Levin et al., 2021, 2020; Levit et al., 2022; Mani et al., 2021). Replication cohorts included: (1) the Columbia University Irving Medical Center (CUIMC) MS genetics study, a clinic-based cohort that began enrollment in April 2018; (2) The CUIMC COVID-19 and MS population study, an online survey study that began enrollment in April 2020 (Epstein et al., 2022; Kever et al., 2022; Levin et al., 2021, 2020; Levit et al., 2022; Mani et al., 2021); (3) the Genes and Environment in Multiple Sclerosis (GEMS) study of people with first-degree family history of MS that began enrollment in July 2010 (NCT01353547) (Dhand et al., 2018; Xia et al., 2017, 2016). We reported detailed eligibility criteria for each source study in eTable 1. Data freeze occurred at the end of July 2020 for all cohorts. We acquired data through questionnaires and electronic health records data review. Questionnaires were deployed using the Research Electronic Data Capture (REDCap), an institution-approved, confidentiality-preserving, and secure web platform.
Figure 1. Overview of the Study Cohorts and Design.
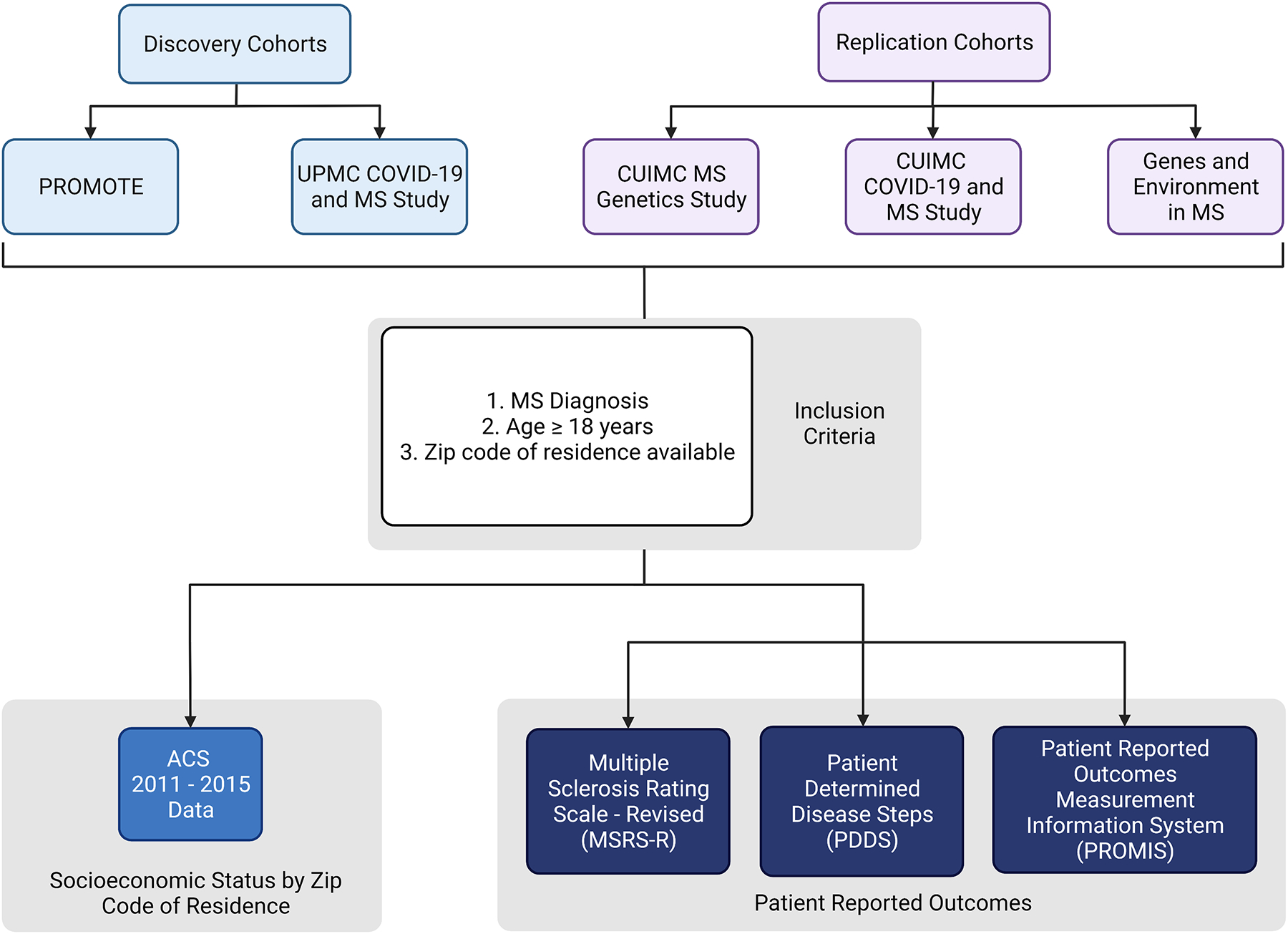
American Community Survey data from 2011–2015 (referred as 2015) provided neighborhood socioeconomic status as the exposure. Patient-reported outcomes (MSRS-R, PDDS, PROMIS-physical function) surveyed during the most recent year between 2018 and 2020 provided the clinically relevant neurological and physical functional assessment. CUIMC, Columbia University Irving Medical Center. PROMOTE, Prospective Investigation of Multiple Sclerosis in the Three Rivers Region. This figure was created with BioRender.com.
2.2. Standard Protocol Approvals, Registrations, and Patient Consents
The institutional review boards of University of Pittsburgh (19080007) and CUIMC (AAAR4456) approved the study protocols. All participants provided written informed consent.
2.3. Neighborhood and Individual Indicators of Socioeconomic Status (SES)
Using the zip codes of the home residence of each participant at enrollment to query the American Community Survey SES datasets from 2011 to 2015 (abbreviated as 2015), we derived components of the neighborhood SES to calculate the area deprivation index (ADI). ADI for each participant represents the whole population of his or her home zip code. We excluded 8.5% of participants in discovery cohorts and 6.8% of participants in replication cohorts who reported zip code changes during the study duration. ADI is a well-accepted measure of SES, e.g., used by the United States Department of Health and Human Services for Medicare population analyses (Singh, 2003). To calculate ADI, we performed principal component (PC) analysis of seven zip code-level variables (percent unemployed, percent below United States poverty line, median household income, median home value, percent with no high school education, percent with college education, and percent living in households with more than one person per room), and computed the first PC score as the ADI for each zip code to indicate its neighborhood SES (Lakhani et al., 2019). Higher ADI indicated lower SES in a participant’s neighborhood zip code. We deployed a structured questionnaire from our prior studies (Dhand et al., 2018; Levin et al., 2020) in 2020 to obtain individual household income brackets to indicate the individual-level SES. Individual household income bracket data were available in a subgroup of the discovery (N=238, 18%) and replication (N=148, 30%) cohorts.
2.4. Patient Reported Outcomes
We assessed neurological and physical function using three clinically relevant, interrelated but distinct patient-reported outcomes (PROs): one general and two MS-specific. First, the National Institute of Health Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function (version 1.2) quantifies the general physical function. Nationally validated, PROMIS is a computer adaptive test to measure patient-reported health in patients across health and diseases (Cella et al., 2010), including MS (Senders et al., 2014). This measure is reported as a T-score and a standard deviation, with higher scores indicating better physical function or lower physical disability. The general US population has a normal distribution with a mean T-score of 50 and a standard deviation of 10. Second, the MS symptom burden using the Multiple Sclerosis Rating Scale-Revised (MSRS-R) measures the global neurological symptom burden, including eight neurological domains (walking, upper limb function, vision, speech, swallowing, cognition, sensory, bladder and bowel function; each domain scored as 0 to 4, with 0 indicating absent symptom and 4 indicating severe disability). MSRS-R correlates with clinical measures of neurological disability and is validated for pwMS (Bove et al., 2013; Wicks et al., 2012). Finally, Patient Determined Disease Steps (PDDS) evaluate gait impairment, on an ordinal scale from 0 to 8, with 0 indicating no gait impairment and 8 representing bed-bound status. PDDS approximates the rater-performed metrics of neurological function such as the Extended Disability Status Scale (Learmonth et al., 2013). We also dichotomized PDDS according to the requirement for full-time ambulatory assistance (≥4 versus <4). While MSRS-R and PDDS correlate well, the former includes more granular subscales of neurological symptoms, and the latter better defines gait function (Wicks et al., 2012). Participants may have multiple reports of PRO per year. For quality control to address clinically implausible fluctuations in PRO responses, we calculated the mean scores for each year (2018, 2019, 2020) and removed outlier PRO values that were at least 0.75 interquartile range above or below the mean. For each PRO, we then calculated the median scores for each year for each participant for downstream analyses.
2.5. Covariates
In all analyses, we accounted for the following confounding variables that may influence PROs: age of the latest PRO (for each measure), age of the first neurological symptom onset, disease duration, race and ethnicity, and disease modifying therapy (DMT) type. Disease duration was the year elapsed between age of the first neurological symptom onset and the age of the latest PRO response. Race and ethnicity were analyzed as a combined dichotomized race-ethnicity variable as Non-Hispanic White versus otherwise due to the size and distribution of the study population. The dichotomization was required as the small size of racial and ethnic groups did not make meaningful differences when adjusted as individual groups. DMTs were categorized as standard-efficacy (glatiramer acetate, interferon-beta, S1P receptor modulators such as fingolimod / siponimod, fumarates such as dimethyl fumarate, teriflunomide), higher-efficacy (B-cell depleting agents such as rituximab or ocrelizumab, natalizumab) or none (McGinley et al., 2021). When assessing the association between neighborhood health insurance coverage and between individual household income with PROs, we additionally adjusted for ADI in 2015.
2.6. Statistical Analyses
To assess the demographic and clinical characteristics between the discovery and replication cohorts, we used paired t-tests for comparing continuous variables and chi-squared tests for comparing dichotomous or categorical variables.
To assess the association between SES (ADI and its components) and PROs, we performed both Pearson correlations and covariate-adjusted regressions independently in discovery and replication cohorts. We then conducted random-effects meta-analyses to assess effect size distributions across cohorts. For assessing the association between SES and subsequent PROs, we also performed joint analyses, combining the discovery and replication cohorts. We used covariate-adjusted mediation analyses to examine whether the contribution of neighborhood-level insurance coverage mediates the associations between ADI and PROs (Bolin, 2014). To correct for multiple testing, the threshold for significance was p=0.05 divided by the number of independent tests for each covariate-adjusted regression model.
All statistical analyses were performed in the R programming environment (Team, 2021) except for the mediation analyses, which were performed using IBM SPSS 28.1 with PROCESS 4.0.
2.7. Data and Code Availability Statement
Code for analysis and figure generation is available at <https://github.com/kaboorgu/SESvsMS>. Anonymous data that support the findings of this study are available upon request to the corresponding author.
3.0. Results
3.1. Patient Characteristics
Discovery cohorts (n=1,316) and replication cohorts (n=488) shared similar proportions of women (Table 1). Compared to replication cohorts, participants in discovery cohorts were older at neurological symptom onset and at MS diagnosis, were less diverse in race and ethnicity, were more likely untreated, and had longer disease duration and greater self-reported disability.
Table 1.
Participant characteristics
| Discovery Cohorts N = 1316 |
Replication Cohorts N = 488 |
P-Value2 | |
|---|---|---|---|
| Sex [N (%)] | 0.206 | ||
| Female | 951 (72.3) | 372 (76.2) | |
| Male | 364 (27.7) | 116 (23.8) | |
| Other | 1 (0.0) | -- | |
| Age1 [Mean Years (SD)] | 51.3 (12.7) | 47.3 (11.6) | <0.001 |
| Race [N (%)] | <0.001 | ||
| Black or African American | 100 (7.6) | 34 (7.0) | |
| Native American, Alaska Native or Native Hawaiian | 1 (0.1) | 4 (0.8) | |
| Asian | 13 (1.0) | 6 (1.2) | |
| White | 1199 (91.2) | 402 (83.1) | |
| Multi-Racial | 0 (0.0) | 11 (2.3) | |
| Other | 2 (0.2) | 27 (5.6) | |
| Ethnicity | <0.001 | ||
| Hispanic or Latino | 17 (1.3) | 46 (9.5) | |
| Not Hispanic or Latino | 1303 (98.3) | 438 (90.3) | |
| Not Sure | 6 (0.5) | 1 (0.2) | |
| Age of First Symptom Onset [Mean Years (SD)] | 32.9 (10.7) | 31.6 (11.2) | 0.017 |
| Age of MS Diagnosis [Mean Years (SD)] | 36.9 (10.9) | 34.9 (10.5) | 0.001 |
| Disease Duration3 [Mean Years (SD)] | 14.5 (10.4) | 12.4 (8.6) | <0.001 |
| Type of DMT [N (%)] | <0.001 | ||
| High Efficacy | 408 (31.4) | 140 (32.5) | |
| Standard Efficacy | 641 (49.3) | 258 (59.9) | |
| None | 252 (19.4) | 33 (7.7) | |
| Duration on Current DMT [N (%)] | <0.001 | ||
| <6 Months | 150 (12.0) | 27 (5.9) | |
| 6 Months to 5 Years | 668 (53.4) | 304 (66.5) | |
| >5 Years | 433 (34.6) | 126 (27.6) | |
| MSRS-R Composite Score [N, Median, (minimum, maximum)] | 1272, 8.5 (0.0, 30.0) |
488, 5.0 (0.0, 24.0) |
|
| PDDS [N, Median, (minimum, maximum)] | 1258, 2.0 (0.0, 9.0) |
482, 1.0 (0.0, 9.0) |
|
| Ambulation Assistance Required [N (%)] | 394 (31.3%) | 79 (16.4%) | |
| No Ambulation Assistance Required [N (%)] | 864 (68.7%) | 403 (83.6%) | |
| PROMIS Physical Function T-Score [N, Median, (minimum, maximum)] | 667, 43.3 (15.4, 73.3) |
434, 47.4 (15.4, 73.3) |
Note:
Participant’s age at the time of latest survey response for any of the three patient-reported outcomes.
P-value calculated from paired t-test for continuous variables and chi-squared test for dichotomous or categorical variables.
Disease duration is the time interval between age of the first MS-related symptom onset and age of the latest measured patient reported outcome.
3.2. Association between ADI and Subsequent Patient Outcomes
We first examined the relationship between neighborhood SES (i.e., computed as ADI based on the zip code of a participant’s home residence) in 2015 and subsequent self-reported neurological outcomes (i.e., the participant’s median PRO scores from the latest measured year between 2018 and 2020). In our dataset, higher ADI correlated with lower neighborhood median household income, indicating lower SES (R=−0.93, p<0.001) (eFigure 1). In Pearson correlation analyses, higher ADI in 2015 was significantly associated with subsequently greater global MS symptom burden (i.e., the total MSRS-R score) in both discovery (R=0.11, p<0.001) and replication cohorts (R=0.19, p<0.001) (Figure 2). In covariate-adjusted regression analyses, we not only confirmed the association between ADI and subsequently greater global MS symptom burden (MSRS-R) in both cohorts (discovery: β=0.62, 95%CI [0.36, 0.89], p<0.001; replication: β=0.46, 95%CI [0.21, 0.72], p<0.001), but also found that higher ADI in 2015 was significantly associated with subsequently greater gait impairment (based on the PDDS score) (discovery: β=0.11, 95%CI [0.02, 0.20], p=0.02; replication: β=0.12, 95%CI [0.03, 0.21], p=0.009) (Table 2, eTable 2). It is reassuring to observe the same direction and similar magnitude of association with PROs when using the neighborhood SES data from a later time frame (i.e., 2014–2018) for participants whose residential addresses stayed the same during the study duration (eTable 3). In random-effects meta-analyses of the standardized β coefficients from the covariate-adjusted regression analyses of the discovery and replication data, higher ADI in 2015 was significantly and consistently associated with the subsequent scores of all three PROs: greater MS symptom burden (MSRS-R: βmeta=0.54, 95%CI [0.36, 0.72], p<0.0001), greater gait impairment (PDDS: βmeta=0.11, 95%CI [0.05, 0.18], p=0.0005) and worse physical function (PROMIS: βmeta=−0.55, 95%CI [−0.94, −0.16], p=0.00058) (Figure 3).
Figure 2. Pearson correlation between area deprivation index in 2015 and subsequent patient-reported outcomes (PROs).
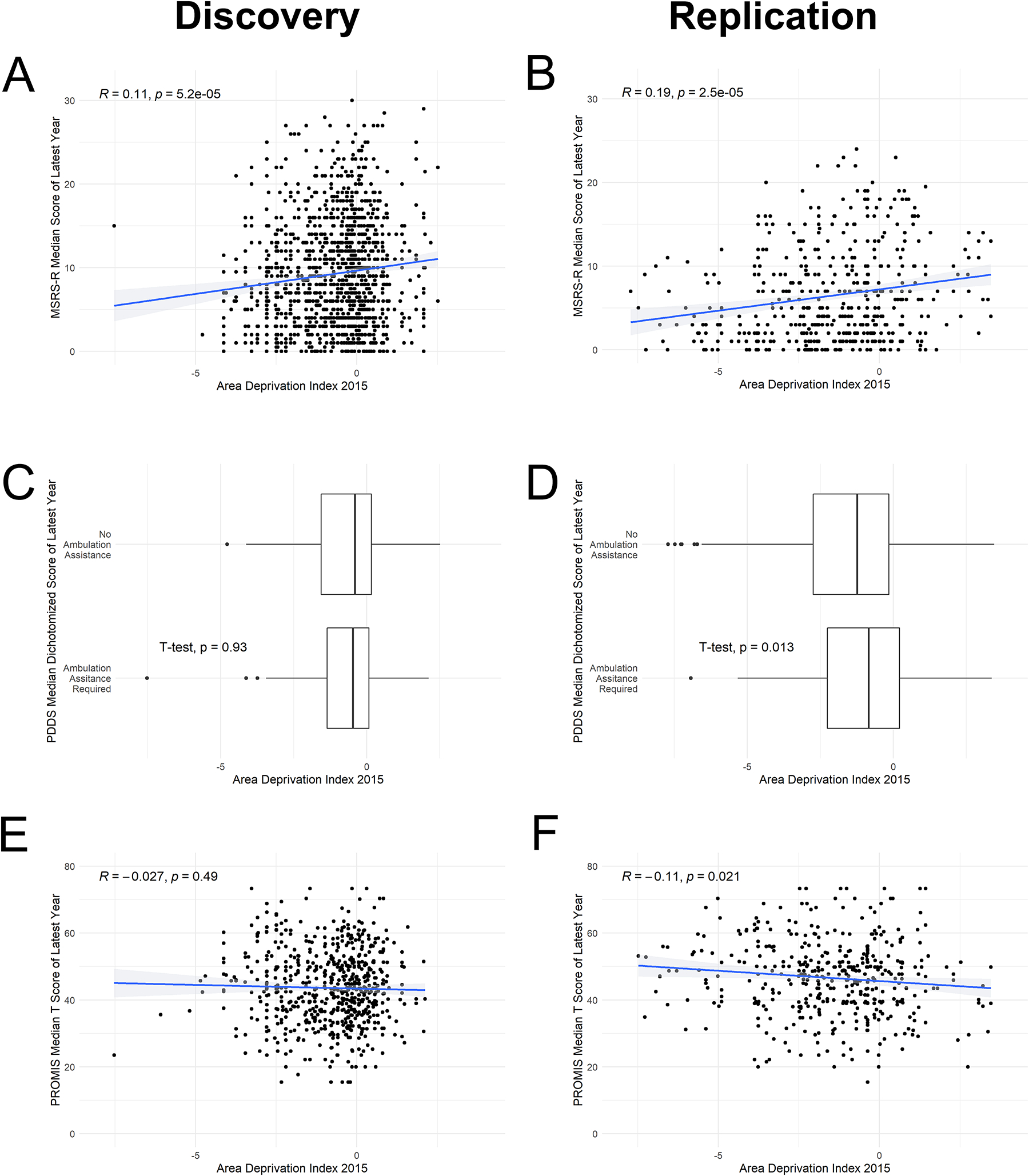
PROs included MSRS-R median score, PROMIS-physical function median T-scores, and proportion of people with PDDS median score dichotomized according to full-time requirement for ambulation assistance versus otherwise (PDDS≥4 versus <4), all from the latest year between 2018 and 2020.
Table 2.
Covariate-adjusted regression analysis using area deprivation index in 2015 as the exposure and the median scores of patient-reported outcomes of neurological and physical function from the latest year between 2018 and 2020.
| Discovery Cohorts | Replication Cohorts | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate 1 | 95% CI 2 | P-Value 3 | N 4 | Estimate 1 | 95% CI 2 | P-Value 3 | N 4 | |
| MSRS-R | 0.62 | 0.36, 0.89 | <0.001 | 1248 | 0.46 | 0.21, 0.72 | <0.001 | 421 |
| PDDS | 0.11 | 0.02, 0.20 | 0.020 | 1234 | 0.12 | 0.03, 0.21 | 0.009 | 416 |
| PROMIS | −0.49 | −1.08, 0.10 | 0.106 | 645 | −0.60 | −1.12, −0.08 | 0.025 | 378 |
Beta-coefficient from regression analysis adjusting for covariates as described in the Methods.
95% confidence intervals
The threshold for significance for each covariate-adjusted regression model with three separate outcomes was p=0.0167 after correction for multiple testing (in either discovery cohorts or in replication cohorts).
N, the number of observations for each covariate-adjusted regression model.
Figure 3. Forest plots and meta-analysis of the covariate-adjusted regression analyses.
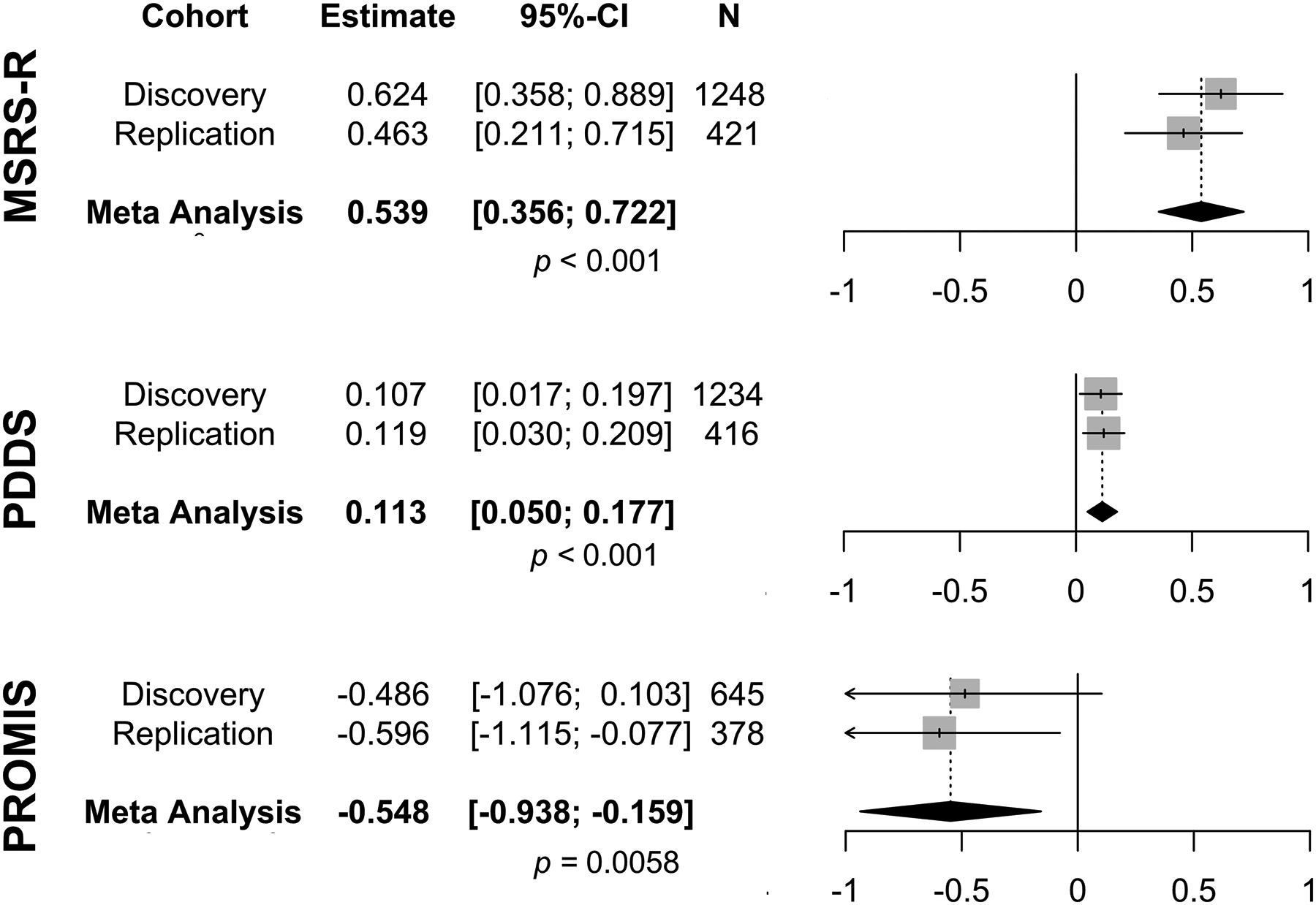
Area deprivation index in 2015 was the exposure for the median scores of the patient-reported outcomes of neurological and physical function from the latest year between 2018 and 2020. Higher scores of MS symptom burden (MSRS-R), higher scores of gait impairment (PDDS) and lower T-scores of PROMIS-physical function indicated greater neurological and/or physical disability. Each square was the standardized β coefficient, and each line was the 95% confidence interval (95%-CI). The diamond represented the meta-analyzed β coefficient and 95% CI in a random-effects meta-analysis of the two cohorts. Higher ADI was associated with subsequently greater MSRS-R and PDDS. The significance threshold for multiple testing was p=0.0167.
3.3. Association between ADI Components and Subsequent Patient Outcomes
To better understand the driving factors of the observed associations between ADI and subsequent PROs, we next examined the role of the seven component factors that together form the ADI (eTables 4 – 10). We performed Pearson correlation analysis on the joint cohorts since both cohorts showed the same directions and comparable effect sizes when we examined the association between ADI and PROs. Three ADI component factors in 2015 consistently showed significant associations with subsequent scores from the latest measured year between 2018 and 2020 for all three PROs (Figure 4). Specifically, a lower percentage of the neighborhood population with college education (MSRS-R: R=−0.2, p<0.0001; PDDS: R=−0.12, p<0.0001; PROMIS: R=0.13 p<0.0001), lower neighborhood median household income (MSRS-R: R=−0.17, p<0.0001; PDDS: R=−0.1, p<0.0001; PROMIS: R=0.09 p=0.0048), and lower neighborhood median home value (MSRS-R: R=−0.21, p<0.0001; PDDS: R=−0.15, p<0.0001; PROMIS: R=0.12 p<0.0001) were associated with subsequently worse scores for all three PROs. The observed relationships largely persisted after adjusting for covariates in regression analyses (neighborhood percent with college education: MSRS-R, β=−7.31, 95%CI [−8.99,−5.64], p<0.001; PDDS, β=−1.62, 95%CI [−2.20,−1.05], p<0.001; PROMIS, β=9.31, 95%CI [5.73,12.89], p<0.001 | neighborhood median household income: MSRS-R, β=−3.80e-05, 95%CI [−5.05e-05,−2.56e-05], p<0.001; PDDS, β=−8.58e-06, 95%CI [−1.28e-05,−4.32e-06], p<0.001 | neighborhood median home value | MSRS-R, β=−6.50e-06, 95%CI [−8.16e-06,−4.84e-06], p<0.001; PDDS, β=−1.54e-06, 95%CI [−2.11e-06,−9.65e-07], p<0.001; PROMIS, β=4.98e-06, 95%CI [1.81e-06, 8.14e-06], p=0.002) (Table 3, eTables 3 – 5). However, the association between median household income and PROMIS (β=2.55e-05, 95%CI [5.96e-07,5.05e-05], p=0.045) did not meet the significance threshold for multiple testing. Interestingly, a lower percentage of the neighborhood population living in crowded households was associated with subsequently worse global symptom burden (MSRS-R: R=−0.083 p=0.00052) and greater gait impairment (PDDS: R=−0.09, p=0.00018) (eFigure 2), but only the association with MSRS-R persisted after covariate adjustment (β=−16.83, 95%CI [−28.78, −4.89], p=0.006) (eTable 7).
Figure 4. Pearson correlation between the driving components of the area deprivation index (ADI) and subsequent patient-reported outcomes (PROs) of neurological and physical function.
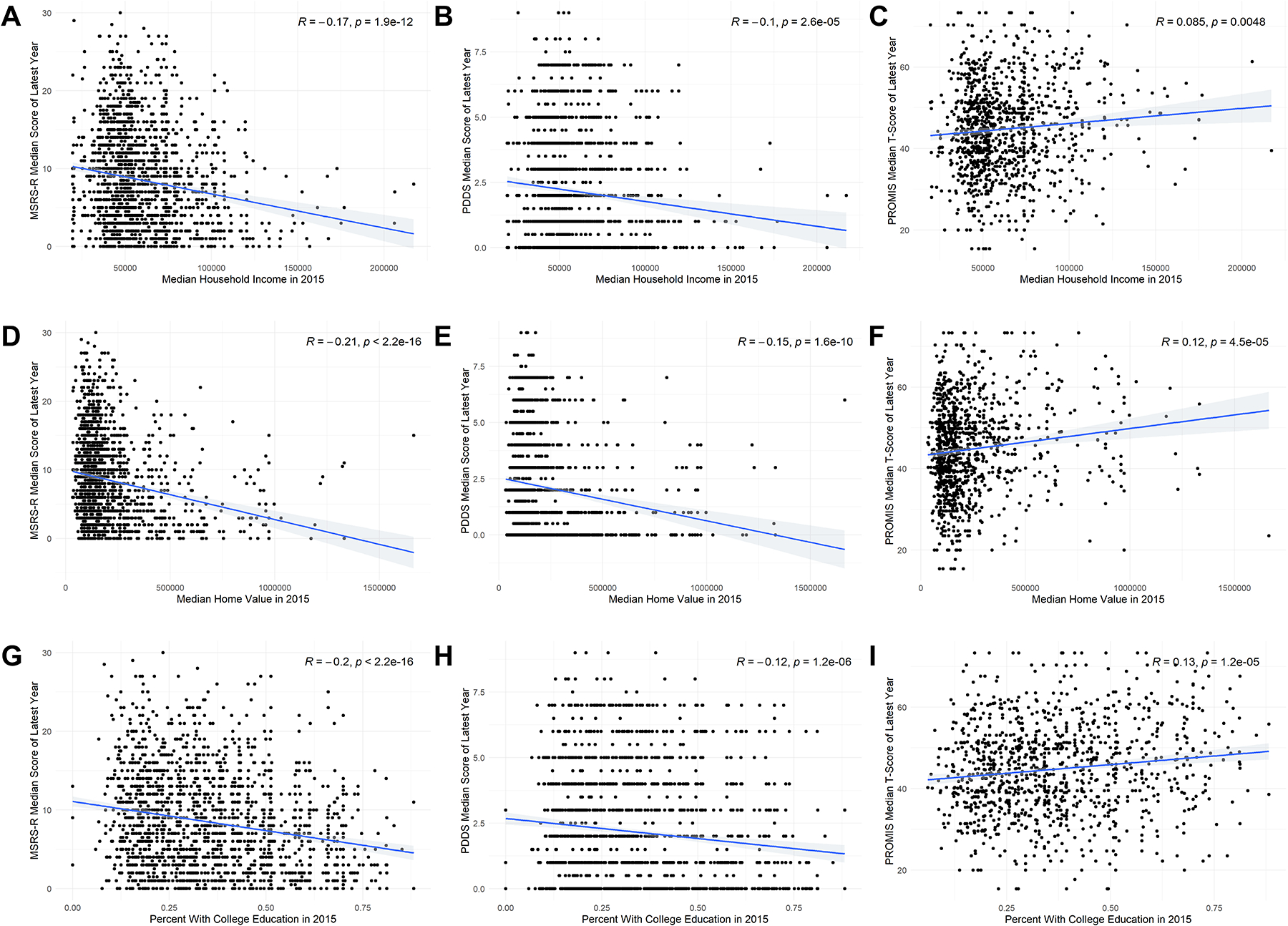
The significant ADI components in 2015 included neighborhood median household income (A-C), neighborhood median home value (D-F), and neighborhood percent with college education (G-I) within the zip code of a participant’s residential address. The PROs included the median scores of MSRS-R, PDDS, and PROMIS-physical function from the latest year between 2018 and 2020.
Table 3.
Covariate-adjusted regression analysis of individual driving components of the area deprivation index (ADI) in 2015 as the exposure and median scores of patient-reported outcomes of neurological function from the latest year between 2018 and 2020 from the joint cohorts.
| Estimate1 | 95% CI2 | P-Value3 | N4 | ||
|---|---|---|---|---|---|
| Neighborhood Median Household Income | MSRS-R | −3.80e-05 | −5.05e-05, −2.56e-05 | <0.001 | 1665 |
| PDDS | −8.58e-06 | −1.28e-05, −4.32e-06 | <0.001 | 1646 | |
| PROMIS | 2.55e-05 | 5.96e-07, 5.05e-05 | 0.044 | 1020 | |
| Neighborhood Median Home Value | MSRS-R | −6.50e-06 | −8.16e-06, −4.84e-06 | <0.001 | 1666 |
| PDDS | −1.54e-06 | −2.11e-06, −9.65e-07 | <0.001 | 1647 | |
| PROMIS | 4.98e-06 | 1.81e-06, 8.14e-06 | 0.00209 | 1021 | |
| Neighborhood Percent with College Education | MSRS-R | −7.31 | −8.99, −5.64 | <0.001 | 1668 |
| PDDS | −1.62 | −2.20, −1.05 | <0.001 | 1649 | |
| PROMIS | 9.31 | 5.73, 12.89 | <0.001 | 1022 | |
Beta-coefficient from regression analysis adjusting for covariates as described in the Methods.
95% confidence intervals
For each ADI component, the threshold for significance for each covariate-adjusted regression model with three separate outcomes was p=0.0167 after correction for multiple testing.
N, the number of observations for each covariate-adjusted regression model.
3.4. Association between Insurance Coverage and Subsequent Patient Outcomes
Healthcare access mediates health outcomes across a variety of diseases, including MS (Briggs et al., 2019; Contentti et al., 2019). However, ADI does not consider healthcare access such as insurance coverage. Using neighborhood percent of population with private or Medicaid insurance, we examined the role of neighborhood-level healthcare access. Specifically, we examined the association between percent of population with private insurance and percent of population with Medicaid insurance in the participants’ residential neighborhood and their subsequent PROs in joint cohort analysis. Medicaid is a federal and state insurance program for economically disadvantaged individuals in the United States. In Pearson correlation analyses, higher neighborhood percent of population with private insurance in 2015 was associated with subsequently lower global MS symptom burden in pwMS (MSRS-R, R=−0.12, p<0.001), whereas higher neighborhood percent of population with Medicaid in 2015 was associated with a subsequently higher global MS symptom burden (MSRS-R, R=0.056, p=0.02) (eFigure 3). Covariate-adjusted regression analyses that additionally accounted for ADI in 2015 found no significant association between neighborhood percent of population with private insurance and any of the subsequent PROs (eTable 11). In contrast, covariate-adjusted regression analyses that additionally accounted for ADI showed significant association between higher neighborhood percent of population with Medicaid insurance in 2015 and subsequently lower global MS symptom burden in pwMS (MSRS-R: β=−9.53, 95%CI [−15.39, −3.67], p=0.001) and lower gait impairment (PDDS: β=−2.37, 95%CI [−4.38, −0.36], p=0.021) (eTable 12).
To examine the interaction among neighborhood-level insurance coverage, ADI, and PROs, we performed covariate-adjusted mediation analyses (eFigure 4). When neighborhood percent of population with Medicaid insurance was included as a mediator, the association between ADI and MSRS-R (Indirect effect=−0.30, SE=.10, 95%CI [−.50, −0.11]) and the association between ADI and PDDS (Indirect effect=−0.07, SE=0.03, 95%CI [−0.13,−0.00]) but not the association between ADI and PROMIS (Indirect effect=0.17, SE=0.21, 95%CI [−0.24,0.58]) were significantly modified (Table 4). These findings suggest that neighborhood percent of population with Medicaid insurance mediates the association between ADI with global MS symptom burden and with gait function in pwMS. On the other hand, neighborhood percent of population with private insurance did not significantly mediate the association between ADI and any of the PROs.
Table 4.
Indirect effects of neighborhood-level health insurance coverage (percent of population with private or Medicaid insurance within the neighborhood of the participant’s home residence) in 2015 on mediating the covariate-adjusted association between area deprivation index in 2015 and the median scores of patient-reported outcomes of neurological and physical function from the latest year between 2018 and 2020 from the joint cohorts.
| Percent with Private Insurance | Percent with Medicaid Insurance | |||||||
|---|---|---|---|---|---|---|---|---|
| Indirect Effect 1 | 95% CI 2 | SE 3 | N 4 | Indirect Effect 1 | 95% CI 2 | SE 3 | N 4 | |
| MSRS-R | −0.06 | −0.30, 0.18 | 0.12 | 1675 | −0.30 | −0.50, −0.11 | 0.10 | 1675 |
| PDDS | −0.03 | −0.11, 0.05 | 0.04 | 1656 | −0.07 | −0.13, −0.00 | 0.03 | 1656 |
| PROMIS | 0.03 | −0.52, 0.57 | 0.27 | 1092 | 0.17 | −0.24, 0.58 | 0.21 | 1092 |
Indirect effect (in path c’) was calculated by multiplying path a, the independent variable (area deprivation index) to the mediator variable (percent with private or Medicaid insurance), by path b, the mediator variable to the dependent variable (patient reported outcome). Refer to eFigure 4 for data of each path.
95% confidence intervals (CI). The indirect effect due to the addition of the mediator is significant when the 95% CIs do not encompass 0.
Standard Error
N, the number of observations for each covariate-adjusted regression model.
3.5. Association between Individual Household Income and Patient Outcomes
Given that the neighborhood median household income was a key component of the ADI (eFigure 1), we finally examined the individual household income in a subgroup analysis. Specifically, we obtained individual household income brackets and PROs based on participants’ response to a single questionnaire deployed in 2020. In an ordinal regression, lower individual household income bracket was associated with greater MS symptom burden (MSRS-R, R=−0.39, p<0.001), worse gait impairment (PDDS, R=−0.35, p<0.001), and worse physical function (PROMIS, R=0.37, p<0.001) (eFigure 5). In covariate-adjusted regression analyses that additionally accounted for ADI, we confirmed the association between individual household income bracket and PROs (MSRS-R, β=−0.74, 95% CI [−0.94, −0.54], p<0.001; PDDS, β=−0.24, 95% CI [−0.32, −0.17], p<0.001; PROMIS, β=1.32, 95% CI [0.92, 1.72], p<0.001) as independent of neighborhood SES (Table 4, eTable 13).
4.0. Discussion
In this study of 1,804 adults with MS, lower SES at neighborhood level (i.e., high ADI) was associated with subsequently worse neurological outcomes and greater physical disability. The findings were replicated across clinically and demographically distinct cohorts, and further corroborated at the individual level, with findings consistent across three separate though interrelated, clinically relevant, and validated patient-reported outcomes in pwMS. Notably, we identified potential drivers and mediators of the associations between SES and MS outcomes as potential hypotheses for testing targeted interventions in the future.
Growing evidence implicates SES in influencing the course of chronic diseases (Minkler et al., 2006), including autoimmune diseases such as MS. However, there are conflicting findings in the MS literature (Goulden et al., 2015; Magyari, 2015; Wändell et al., 2020) with some reports of lack of association (Berg-Hansen and Celius, 2015; Goulden et al., 2016, 2015; Wändell et al., 2020). Many prior studies used individual components of SES rather than a composite indicator such as ADI (Bjørnevik et al., 2016a, 2016b; Briggs et al., 2015, 2019; D’hooghe et al., 2016; Goulden et al., 2016). Further, most studies assessed the risk of developing MS (Berg-Hansen and Celius, 2015; Bjørnevik et al., 2016a, 2016b; Briggs et al., 2015, 2014; Dobson et al., 2020; Goulden et al., 2015; Nielsen et al., 2013; Wändell et al., 2020), while others did not directly assess neurological function (Minden et al., 2008; Reyes et al., 2020; Wang et al., 2020). Few studies have examined the temporal association between a composite measure of SES and subsequent MS outcomes using clinically relevant PROs of neurological and physical dysfunction, particularly within the context of the socioeconomic and healthcare environment in the United States (Briggs et al., 2019).
Our study used ADI as a composite indicator of neighborhood SES based on the zip code of a participant’s home residence, with higher ADI indicating lower neighborhood SES. ADI accounts for multiple factors beyond household income: e.g., employment, living conditions (i.e., crowded household) and education level (Lakhani et al., 2019). In this study, we found that several individual components of ADI demonstrated consistently significant associations with subsequent PROs. Specifically, lower percentage of the neighborhood population with college education, lower neighborhood median household income, and lower neighborhood median home value might drive the observed association between lower neighborhood SES (i.e., high ADI) and subsequently worse PROs. Neighborhood household income and home values are well-known drivers of SES. Level of education has been used as an indicator for SES (Shavers, 2007), though the prior literature in MS has been conflicting. While some studies reported no association between education and MS (Goulden et al., 2015; Magyari, 2015), others reported higher (Bjørnevik et al., 2016a, 2016b; D’hooghe et al., 2016; Riise et al., 2011) or lower (Bjørnevik et al., 2016a, 2016b; Kurtzke and Page, 1997) levels of education in association with higher risk and/or disability progression in MS. Our study showed that higher neighborhood percent of population with college education was associated with better MS outcomes (for all three PROs), after accounting for confounders. Interestingly, higher neighborhood percent living in crowded households was associated with one of the three PROs (i.e., MSRS-R), a finding which could be attributable to protective social network factors (Levin et al., 2020; Yamout et al., 2013). Future investigations of crowded households in the context of social support are warranted. On the other hand, other components of the ADI such as neighborhood percent of unemployed, below poverty, or without high school education did not seem to be contributory. Taken together, we identified potential neighborhood SES component factors for future testing of targeted public policy interventions to improve neurological outcomes.
Beyond ADI, health insurance coverage could influence health outcomes across many diseases (Barker and Li, 2020; Diessner et al., 2020; Ghazi et al., 2021; Levy and Meltzer, 2008), including MS (Briggs et al., 2019; Contentti et al., 2019). Better insurance coverage likely represents wider access to healthcare, including preventative or specialty care (Levy and Meltzer, 2008). In our study, higher percent of neighborhood population with Medicaid insurance was associated with subsequently worse neurological outcomes (global symptom burden, gait impairment) in pwMS independent of ADI. In contrast, percent of neighborhood population with private insurance was not associated with PROs. Interestingly, percent of neighborhood population with Medicaid (but not percent of neighborhood population with private insurance) significantly mediated the observed covariate-adjusted associations between ADI and global MS symptom burden (MSRS-R) and between ADI and gait function (PDDS). Taken together, these findings suggest that broadly improving healthcare access might improve outcomes of pwMS living in economically disadvantaged communities. This interpretation requires some nuance. Most of our study participants received MS care at academic centers. It is likely that insurance coverage through Medicaid reflects a more limited extent of healthcare access since Medicaid often provides lesser coverage than private insurance. Future studies evaluating the role of health insurance at the individual level could validate the findings.
The study has several strengths. First, we validated the findings in multi-center, clinically and demographically distinct cohorts, increasing the generalizability of the findings to the MS population in the United States. Second, in analyzing the associations between SES and subsequent clinical outcomes of MS, we adjusted for potentially critical confounding covariates, including age of the latest PRO, age of the first neurological symptom onset, disease duration, race and ethnicity, and DMT type. Prior studies accounted for a smaller set of confounders (Calocer et al., 2020; Harding et al., 2019). Third, analysis of components of ADI enabled identification of key drivers of the association between SES and MS outcomes, generating hypothesis for future interventional studies. Fourth, we corroborated the neighborhood findings at the individual household income level. Finally, our study is unique in utilizing three clinically relevant, interrelated but distinct outcomes that broadly assessed multiple dimensions of neurological and physical function in pwMS. Prior studies of SES on MS progression primarily evaluated one single clinical outcome (Calocer et al., 2020; Crielaard et al., 2019; D’hooghe et al., 2016; Harding et al., 2019). The consistency in the association between SES and subsequent MS outcomes across all three PROs provided important real-world reassurance and enhanced the clinical interpretation of our findings.
This study also has limitations. First, we should interpret the findings as association rather than causation despite the temporal analysis given the observational study design. In the future, longitudinal prospective or interventional study design will help establish causality. Second, study outcomes are subjective assessments, though these clinically relevant PROs, which have all been validated against objective exams by clinician-raters, provide crucial real-world evidence of an individual’s neurological function (Bove et al., 2013; Cella et al., 2010; Learmonth et al., 2013; Senders et al., 2014; Wicks et al., 2012). Third, while we were able to examine the relatively long-term impact of neighborhood SES on subsequent neurological outcomes in pwMS, the study lacked the data or power to formally examine the impact of changing neighborhood SES due to relocation on subsequent PROs. Low SES is associated with high residential mobility (i.e., high frequency of relocation) (Clark, 2018). Participants in this study lived in their reported home addresses for an average of 14.05 years, while we excluded participants who moved during the study period as a trade-off to avoid potential confounding biases for which we could not accurately account (e.g., decreased participant engagement after relocation). Future studies with larger sample size and regular updates of residential address over longer timeframes may inform how residential mobility may mediate changes in SES and subsequent neurological outcomes in pwMS. Finally, while the study population is large and diverse with respect to clinical and demographic features, the sample size does not permit a well-powered subgroup analysis, e.g., comparing among race and ethnic subgroups (Gray-Roncal et al., 2021). Future studies with higher proportions of participants from diverse racial and ethnic backgrounds could help dissect whether social constructs (e.g., systemic racism) mediates the interactions between socioeconomic factors and MS outcomes. Also, future studies examining the differences among rural, suburban, and urban environments with respect to the association between SES and health outcomes in pwMS are warranted.
In summary, our study highlights SES as a potentially modifiable social environmental factor that could improve patient outcomes in pwMS. It will be critical to establish the causality in future prospective observational studies and possibly through interventional clinical trials based on hypotheses generated from this study. This study and future analyses could provide key evidence to guide public policy that impacts people with chronic neurological disorders such as MS.
Supplementary Material
Table 5.
Covariate-adjusted regression analysis using nine individual household income brackets ranging from “$0 to $19,999” to “$125,000 or higher” and the patient-reported outcomes of neurological and physical function for each participant in a subgroup analysis of joint cohorts that accounted for the area deprivation index.
| Estimate1 | 95% CI2 | P-Value3 | N4 | |
|---|---|---|---|---|
| MSRS-R | −0.74 | −0.94, −0.54 | <0.001 | 320 |
| PDDS | −0.24 | −0.32, −0.17 | <0.001 | 320 |
| PROMIS | 1.32 | 0.92, 1.72 | <0.001 | 284 |
Beta-coefficient from regression analysis adjusting for covariates as described in the Methods and additionally adjust for neighborhood area deprivation index.
95% confidence intervals
The threshold for significance for each covariate-adjusted regression model with three separate outcomes was 0.0167 after correction for multiple testing.
N, the number of observations for each covariate-adjusted regression model.
Acknowledgements
We would like to thank the study participants for their contribution. Zongqi Xia is funded by NINDS R01NS098023 and NINDS R01NS124882.
Abbreviations:
- MS
multiple sclerosis
- SES
socioeconomic status
- ADI
area deprivation index
- pwMS
people with Multiple Sclerosis
- PROs
patient-reported outcomes
- MSRS-R
multiple sclerosis rating scale-revised
- PDDS
patient determined disease steps
- PROMIS
patient-reported outcomes measurement information system, physical Function
Footnotes
Conflicts of Interest
None.
References
- Barke AR, Li L, 2020. The cumulative impact of health insurance on health status. Health Serv Res 55, 815–822. 10.1111/1475-6773.13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg-Hansen P, Celius EG, 2015. Socio-economic factors and immigrant population studies of multiple sclerosis. Acta Neurologica Scandinavica 132, 37–41. 10.1111/ane.12429 [DOI] [PubMed] [Google Scholar]
- Bjørnevik K, Riise T, Benjaminsen E, Celius EG, Dahl OP, Kampman MT, Løken-Amsrud KI, Midgard R, Myhr K-M, Torkildsen Ø, Vatne A, Grytten N, 2016a. Level of education and multiple sclerosis risk over a 50-year period: Registry-based sibling study. Mult Scler J 23, 213–219. 10.1177/1352458516646863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnevik K, Riise T, Cortese M, Holmøy T, Kampman MT, Magalhaes S, Myhr K-M, Wolfson C, Pugliatti M, 2016b. Level of education and multiple sclerosis risk after adjustment for known risk factors: The EnvIMS study. Multiple Scler Houndmills Basingstoke Engl 22, 104–111. 10.1177/1352458515579444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin JH, 2014. Hayes Andrew F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression‐Based Approach. New York, NY: The Guilford Press. J Educ Meas; 51, 335–337. 10.1111/jedm.12050 [DOI] [Google Scholar]
- BOOGAR IR, TALEPASAND S, JABARI M, 2018. Psychosocial and Medical Determinants of Health-related Quality of Life among Patients with Relapsing-Remitting Multiple Sclerosis. Archives Neuropsychiatry 55, 29–35. 10.29399/npa.16983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove R, Secor E, Healy BC, Musallam A, Vaughan T, Glanz BI, Greeke E, Weiner HL, Chitnis T, Wicks P, Jager PLD, 2013. Evaluation of an online platform for multiple sclerosis research: patient description, validation of severity scale, and exploration of BMI effects on disease course. PloS one 8, e59707–e59707. 10.1371/journal.pone.0059707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs FB, Green MC, Weintraub MLR, 2015. Role of socioeconomic position in multiple sclerosis etiology. Neurodegenerative disease management 5, 333–343. 10.2217/nmt.15.22 [DOI] [PubMed] [Google Scholar]
- Briggs FBS, Acuña BS, Shen L, Bellesis KH, Ramsay PP, Quach H, Bernstein A, Schaefer C, Barcellos LF, 2014. Adverse socioeconomic position during the life course is associated with multiple sclerosis. Journal of epidemiology and community health 68, 622–629. 10.1136/jech-2013-203184 [DOI] [PubMed] [Google Scholar]
- Briggs FBS, Thompson NR, Conway DS, 2019. Prognostic factors of disability in relapsing remitting multiple sclerosis. Mult Scler Relat Dis 30, 9–16. 10.1016/j.msard.2019.01.045 [DOI] [PubMed] [Google Scholar]
- Calocer F, Dejardin O, Kwiatkowski A, Bourre B, Vermersch P, Hautecoeur P, Launoy G, Defer G, 2020. Socioeconomic deprivation increases the risk of disability in multiple sclerosis patients. Multiple Sclerosis and Related Disorders 40, 101930. 10.1016/j.msard.2020.101930 [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, Group PC, 2010. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology 63, 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, 2018. The role of residential mobility in reproducing socioeconomic stratification during the transition to adulthood. Demogr Res 38, 169–196. 10.4054/demres.2018.38.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contentti EC, Giachello S, Correale J, 2019. Barriers to access and utilization of multiple sclerosis care services in a large cohort of Latin American patients. Mult Scler J 27, 117–129. 10.1177/1352458519898590 [DOI] [PubMed] [Google Scholar]
- Crielaard L, Kavaliunas A, Ramanujam R, Olsson T, Hillert J, Stridh P, Kockum I, Manouchehrinia A, 2019. Factors associated with and long-term outcome of benign multiple sclerosis: a nationwide cohort study. Journal of Neurology, Neurosurgery & Psychiatry 90, 761–767. 10.1136/jnnp-2018-319913 [DOI] [PubMed] [Google Scholar]
- Dhand A, White CC, Johnson C, Xia Z, Jager PLD, 2018. A scalable online tool for quantitative social network assessment reveals potentially modifiable social environmental risks. Nature communications 9, 3930. 10.1038/s41467-018-06408-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’hooghe MB, Haentjens P, Remoortel AV, Keyser JD, Nagels G, 2016. Self‐reported levels of education and disability progression in multiple sclerosis. Acta Neurol Scand 134, 414–419. 10.1111/ane.12555 [DOI] [PubMed] [Google Scholar]
- Diessner BJ, Weigel BJ, Murugan P, Zhang L, Poynter JN, Spector LG, 2020. Associations of Socioeconomic Status, Public vs Private Insurance, and Race/Ethnicity With Metastatic Sarcoma at Diagnosis. Jama Netw Open 3, e2011087. 10.1001/jamanetworkopen.2020.11087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson R, Jitlal M, Marshall CR, Noyce AJ, Robson J, Cuzick J, Giovannoni G, 2020. Ethnic and Socioeconomic Associations with Multiple Sclerosis Risk. Annals of Neurology 87, 599–608. 10.1002/ana.25688 [DOI] [PubMed] [Google Scholar]
- Epstein S, Xia Z, Lee AJ, Dahl M, Edwards K, Levit E, Longbrake EE, Perrone C, Kavak K, Weinstock-Guttman B, Diallo F, Ricci A, Riley CS, Jager PLD, Farber R, Wesley SF, Collaborative, M.S.R. to C.-19 (MSReCOV), 2022. Vaccination against SARS-CoV-2 in neuroinflammatory disease: early safety/tolerability data. Multiple Sclerosis and Related Disorders 57, 103433. 10.1016/j.msard.2021.103433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore G, Lang M, Pugliatti M, 2012. The Treatment experience, burden, and unmet needs (TRIBUNE) study – measuring the socioeconomic consequences of Multiple Sclerosis. Mult Scler J 18, 5–6. 10.1177/1352458512447262 [DOI] [PubMed] [Google Scholar]
- Ghazi L, Osypuk TL, MacLehose RF, Luepker RV, Drawz PE, 2021. Neighborhood Socioeconomic Status, Health Insurance, and CKD Prevalence: Findings From a Large Health Care System. Kidney Medicine 3, 555–564.e1. 10.1016/j.xkme.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G, 2018. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Current Opinion in Neurology 31, 233–243. 10.1097/wco.0000000000000561 [DOI] [PubMed] [Google Scholar]
- Goldschmidt C, McGinley MP, 2020. Advances in the Treatment of Multiple Sclerosis. Neurol Clin 39, 21–33. 10.1016/j.ncl.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden R, Ibrahim T, Wolfson C, 2015. Is high socioeconomic status a risk factor for multiple sclerosis? A systematic review. Eur J Neurol 22, 899–911. 10.1111/ene.12586 [DOI] [PubMed] [Google Scholar]
- Goulden R, Riise T, Myhr K-M, Pugliatti M, Wolfson C, 2016. Does low socioeconomic status in early life protect against multiple sclerosis? A multinational, case–control study. Eur J Neurol 23, 168–174. 10.1111/ene.12830 [DOI] [PubMed] [Google Scholar]
- Gray-Roncal K, Fitzgerald K, Ryerson LZ, Charvet L, Cassard SD, Naismith R, Ontaneda D, Mahajan K, Castro-Borrero W, Mowry E, 2021. Association of Disease Severity and Socioeconomic Status in Black and White Americans With Multiple Sclerosis. Neurology 97, e881–e889. 10.1212/wnl.0000000000012362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding KE, Wardle M, Carruthers R, Robertson N, Zhu F, Kingwell E, Tremlett H, 2019. Socioeconomic status and disability progression in multiple sclerosis: A multinational study. Neurology 92, e1497–e1506. 10.1212/wnl.0000000000007190 [DOI] [PubMed] [Google Scholar]
- Hartung DM, 2021. Health economics of disease-modifying therapy for multiple sclerosis in the United States. Therapeutic Advances in Neurological Disorders 14, 175628642098703. 10.1177/1756286420987031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennum P, Wanscher B, Frederiksen J, Kjellberg J, 2012. The socioeconomic consequences of multiple sclerosis: a controlled national study. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 22, 36–43. 10.1016/j.euroneuro.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Kever A, Walker ELS, Riley CS, Heyman RA, Xia Z, Leavitt VM, 2022. Association of personality traits with physical function, cognition, and mood in multiple sclerosis. Multiple Sclerosis and Related Disorders 103648. 10.1016/j.msard.2022.103648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke JF, Page WF, 1997. Epidemiology of multiple sclerosis in US veterans: VII. Risk factors for MS. Neurology 48, 204–213. 10.1212/wnl.48.1.204 [DOI] [PubMed] [Google Scholar]
- Lakhani CM, Tierney BT, Manrai AK, Yang J, Visscher PM, Patel CJ, 2019. Repurposing large health insurance claims data to estimate genetic and environmental contributions in 560 phenotypes. Nature Genetics 372, 793. 10.1038/s41588-018-0313-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D, 2013. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurology 13, 37. 10.1186/1471-2377-13-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SN, Riley CS, Dhand A, White CC, Venkatesh S, Boehm B, Nassif C, Socia L, Onomichi K, Leavitt VM, Levine L, Heyman R, Farber RS, Vargas WS, Xia Z, Jager PLD, 2020. Association of social network structure and physical function in patients with multiple sclerosis. Neurology 95, e1565–e1574. 10.1212/wnl.0000000000010460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SN, Venkatesh S, Nelson KE, Li Y, Aguerre I, Zhu W, Masown K, Rimmer KT, Diaconu CI, Onomichi KB, Leavitt VM, Levine LL, Strauss‐Farber R, Vargas WS, Banwell B, Bar‐Or A, Berger JR, Goodman AD, Longbrake EE, Oh J, Weinstock‐Guttman B, Thakur KT, Edwards KR, Riley CS, Xia Z, Jager PLD, Collaborative, M.S.R. to C. (MSReCOV), 2021. Manifestations and impact of the COVID‐19 pandemic in neuroinflammatory diseases. Annals of Clinical and Translational Neurology 8, 918–928. 10.1002/acn3.51314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levit E, Cohen I, Dahl M, Edwards K, Weinstock-Guttman B, Ishikawa T, Kavak K, Leavitt V, Nelson K, Onomichi K, Bar-Or A, Perrone C, Riley C, Venkatesh S, Jager PLD, Xia Z, Longbrake EE, Collaborative, M.S.R. to C.-19 (MSReCOV), 2022. Worsening physical functioning in patients with neuroinflammatory disease during the COVID-19 pandemic. Multiple Sclerosis and Related Disorders 103482. 10.1016/j.msard.2021.103482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H, Meltzer D, 2008. The Impact of Health Insurance on Health. Annu Rev Publ Health 29, 399–409. 10.1146/annurev.publhealth.28.021406.144042 [DOI] [PubMed] [Google Scholar]
- Mackenbach JP, Stirbu I, Roskam A-JR, Schaap MM, Menvielle G, Leinsalu M, Kunst AE, Health, E.U.W.G. on S.I. in, 2008. Socioeconomic inequalities in health in 22 European countries. New Engl J Medicine 358, 2468–81. 10.1056/nejmsa0707519 [DOI] [PubMed] [Google Scholar]
- Magyari M, 2015. Role of socio‐economic and reproductive factors in the risk of multiple sclerosis. Acta Neurol Scand 132, 20–23. 10.1111/ane.12426 [DOI] [PubMed] [Google Scholar]
- Mani A, Santini T, Puppala R, Dahl M, Venkatesh S, Walker E, DeHaven M, Isitan C, Ibrahim TS, Wang L, Zhang T, Gong E, Barrios-Martinez J, Yeh F-C, Krafty R, Mettenburg JM, Xia Z, 2021. Applying deep learning to accelerated clinical brain magnetic resonance imaging for multiple sclerosis. Frontiers in Neurology 12, 685276. 10.3389/fneur.2021.685276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley MP, Goldschmidt CH, Rae-Grant AD, 2021. Diagnosis and Treatment of Multiple Sclerosis. Jama 325, 765–779. 10.1001/jama.2020.26858 [DOI] [PubMed] [Google Scholar]
- Minden SL, Hoaglin DC, Hadden L, Frankel D, Robbins T, Perloff J, 2008. Access to and utilization of neurologists by people with multiple sclerosis. Neurology 70, 1141–1149. 10.1212/01.wnl.0000306411.46934.ef [DOI] [PubMed] [Google Scholar]
- Minkler M, Fuller-Thomson E, Guralnik JM, 2006. Gradient of Disability across the Socioeconomic Spectrum in the United States. New Engl J Medicine 355, 695–703. 10.1056/nejmsa044316 [DOI] [PubMed] [Google Scholar]
- Moss BP, Mahajan KR, Bermel RA, Hellisz K, Hua LH, Hudec T, Husak S, McGinley MP, Ontaneda D, Wang Z, Weber M, Tagliani P, Cárdenas-Robledo S, Zabalza A, Arrambide G, Carbonell-Mirabent P, Rodríguez-Barranco M, Sastre-Garriga J, Tintore M, Montalban X, Douglas M, Ogbuokiri E, Aravidis B, Cohen JA, Mowry EM, Fitzgerald KC, 2020. Multiple sclerosis management during the COVID-19 pandemic. Mult Scler J 26, 1163–1171. 10.1177/1352458520948231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen NM, Jørgensen KT, Bager P, Stenager E, Pedersen BV, Hjalgrim H, Koch-Henriksen N, Frisch M, 2013. Socioeconomic factors in childhood and the risk of multiple sclerosis. American Journal of Epidemiology 177, 1289–1295. 10.1093/aje/kws350 [DOI] [PubMed] [Google Scholar]
- Reyes S, Suarez S, Allen-Philbey K, Yildiz Ö, Mathews J, Anjorin G, Edwards F, Jain C, Turner B, Marta M, Gnanapavan S, Schmierer K, Giovannoni G, 2020. Socioeconomic status and disease-modifying therapy prescribing patterns in people with multiple sclerosis. Mult Scler Relat Dis 41, 102024. 10.1016/j.msard.2020.102024 [DOI] [PubMed] [Google Scholar]
- Riise T, Kirkeleit J, Aarseth JH, Farbu E, Midgard R, Mygland Å, Eikeland R, Mørland TJ, Telstad W, Førland PT, Myhr K-M, 2011. Risk of MS is not associated with exposure to crude oil, but increases with low level of education. Multiple Scler Houndmills Basingstoke Engl 17, 780–7. 10.1177/1352458510397686 [DOI] [PubMed] [Google Scholar]
- Roddam H, Rog D, Janssen J, Wilson N, Cross L, Olajide O, Dey P, 2019. Inequalities in access to health and social care among adults with multiple sclerosis: a scoping review of the literature. Mult Scler Relat Dis 28, 290–304. 10.1016/j.msard.2018.12.043 [DOI] [PubMed] [Google Scholar]
- Senders A, Hanes D, Bourdette D, Whitham R, Shinto L, 2014. Reducing survey burden: feasibility and validity of PROMIS measures in multiple sclerosis. Multiple Sclerosis Journal 20, 1102–1111. 10.1177/1352458513517279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavers VL, 2007. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc 99, 1013–23. [PMC free article] [PubMed] [Google Scholar]
- Singh GK, 2003. Area Deprivation and Widening Inequalities in US Mortality, 1969–1998. Am J Public Health 93, 1137–1143. 10.2105/ajph.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R.C., 2021. R: A language and environment for statistical computing, R Foundation for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Wändell P, Fredrikson S, Carlsson AC, Li X, Sundquist J, Sundquist K, 2020. Multiple sclerosis among first‐ and second‐generation immigrant groups in Sweden. Acta Neurol Scand 142, 339–349. 10.1111/ane.13314 [DOI] [PubMed] [Google Scholar]
- Wang Y, Tian F, Fitzgerald KC, Bhattarai JJ, Naismith RT, Hyland M, Calabresi PA, Mowry EM, 2020. Socioeconomic status and race are correlated with affective symptoms in multiple sclerosis. Multiple Sclerosis and Related Disorders 41, 102010. 10.1016/j.msard.2020.102010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks P, Vaughan TE, Massagli MP, 2012. The multiple sclerosis rating scale, revised (MSRS-R): development, refinement, and psychometric validation using an online community. Health and Quality of Life Outcomes 10, 70. 10.1186/1477-7525-10-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Steele SU, Bakshi A, Clarkson SR, White CC, Schindler MK, Nair G, Dewey BE, Price LR, Ohayon J, Chibnik LB, Cortese ICM, Jager PLD, Reich DS, 2017. Assessment of Early Evidence of Multiple Sclerosis in a Prospective Study of Asymptomatic High-Risk Family Members. JAMA Neurology 74, 293–300. 10.1001/jamaneurol.2016.5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, White CC, Owen EK, Korff AV, Clarkson SR, McCabe CA, Cimpean M, Winn PA, Hoesing A, Steele SU, Cortese ICM, Chitnis T, Weiner HL, Reich DS, Chibnik LB, Jager PLD, 2016. Genes and Environment in Multiple Sclerosis project: A platform to investigate multiple sclerosis risk. Annals of Neurology 79, 178–189. 10.1002/ana.24560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamout B, Issa Z, Herlopian A, Bejjani ME, Khalifa A, Ghadieh AS, Habib RH, 2013. Predictors of quality of life among multiple sclerosis patients: a comprehensive analysis. Eur J Neurol 20, 756–764. 10.1111/ene.12046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code for analysis and figure generation is available at <https://github.com/kaboorgu/SESvsMS>. Anonymous data that support the findings of this study are available upon request to the corresponding author.


