Abstract
Background:
In 2017, DNA mismatch repair/microsatellite instability (MMR/MSI) testing was nationally recommended for advanced colorectal cancers based on favorable immune checkpoint inhibitor responses among patients with MMR-deficient/MSI-high tumors.
Methods:
Patients ≥20-years-old presenting with stage IV colorectal adenocarcinoma from 2010-2017 were identified from the National Cancer Database. 2017 was the latest year with available testing utilization data. Patient, tumor, socioeconomic, and care setting characteristics were evaluated for association with upfront MMR/MSI testing in 2017 using multivariable logistic regression and average adjusted predicted probabilities (%AAP).
Results:
Among 72,830 stage IV colorectal cancers, upfront MMR/MSI testing levels increased from 16.4% in 2010 to 56.4% in 2017. For patients diagnosed in 2017 (i.e. following national recommendations, n=10,022), testing levels were lower for older patients (padj<0.001), and were independent of patients' race/ethnicity and insurance status. Patients from the poorest quartile of households received less testing (49.6%AAP, 99.9CI: 45.5-53.7) than patients from the 3rd (56.9%AAP, 99.9CI: 53.3-60.6, padj<0.001) or 4th quartiles (57.6%AAP, 99.9CI: 54.3-60.9, padj<0.001).
Although testing levels improved most at community programs, they remained lower in 2017 (46.6%AAP, 99.9CI: 41.0-52.1) compared to academic/NCI-designated comprehensive cancer centers (62.8%AAP, 99.9CI: 59.7-65.8, padj<0.001).
Conclusions:
Upfront MMR/MSI testing utilization for advanced colorectal cancer patients has increased but there is still substantial need for optimization. Testing utilization disproportionately lagged for patients who were older, from the poorest quartile of households, or managed at community cancer programs.
Impact:
Our findings indicate opportunities for improving rates of MMR/MSI testing and reporting, possibly through incorporation into quality control and accreditation metrics.
Keywords: Mismatch repair, Microsatellite instability, Colorectal cancer, Biomarker, Immune checkpoint inhibitor, Disparities
INTRODUCTION
In 2015, the KEYNOTE-016 trial first demonstrated the efficacy of PD-1 checkpoint inhibitors for patients with DNA mismatch repair deficient (dMMR)/microsatellite instability high (MSI-H) advanced colorectal cancer (CRC), in which 40% of the dMMR/MSI-H CRC patients experienced objective responses compared to none of the MMR-proficient cases.(1) dMMR cancer arises as a consequence of the loss of function of MMR genes (typically MLH1, MSH2, MSH6, or PMS2) – occurring either following acquisition of a second pathogenic mutation in patients with inherited mutations in MMR genes (“Lynch syndrome”) or, more commonly, through somatic silencing of MLH1 via promoter hypermethylation. dMMR causes a high tumor mutational burden in microsatellite repeat regions ("MSI-H"), resulting in expression of tumor neoantigens and consequent lymphocytic infiltration within the tumor.(2) Blockade of the T-cell inhibitory pathway bolsters native antitumoral responses in dMMR/MSI-H tumors.
The success of PD-1 checkpoint inhibitors for advanced dMMR/MSI-H cancers in KEYNOTE-016/-164/-012/-028/-158 and CheckMate-142 galvanized a change in National Comprehensive Cancer Network (NCCN) guidelines 1.2017 to recommend pembrolizumab or nivolumab as a second-line treatment option for advanced dMMR/MSI-H CRC.(1,3-5) Also, in early February of 2017, ASCP/CAP/AMP/ASCO jointly released a comprehensive practice guideline on molecular biomarker testing for CRC patients, recommending that MMR testing – previously only recommended for patients at increased risk for Lynch syndrome – be conducted universally.(6-8)
Those clinical trials’ successes across multiple cancer types culminated in the first cancer site-agnostic U.S. Food and Drug Administration (FDA) accelerated approval of pembrolizumab in May 2017 for advanced solid tumors with dMMR/MSI-H, including advanced CRCs that had progressed following fluoropyrimidine, oxaliplatin, and irinotecan chemotherapy; followed by nivolumab in July 2017 for the same indication.(9-11) These milestones marked a paradigm shift in the management of advanced CRC and cemented the utility of MMR/MSI status as a predictive biomarker. Since those landmark advances, the NCCN guidelines have been further updated to recommend universal MMR/MSI testing for all newly-diagnosed CRCs and in 2020 the FDA expanded approval of pembrolizumab to the first-line setting for advanced dMMR/MSI-H CRC patients.(12)
A previous study of CRC patients newly-diagnosed in 2010-2012 found that only 21% of stage IV patients received MMR/MSI testing, including only 36% of young patients for whom testing had been indicated at the time.(13) Given the recent expansion in guideline-recommended MMR screening criteria, we undertook an evaluation of the upfront MMR/MSI testing practice patterns in the U.S. for advanced CRC patients diagnosed in 2017 to determine whether testing utilization has improved.
MATERIALS AND METHODS
Patient Population
This IRB-approved retrospective cohort study examined the National Cancer Database (NCDB), a nationwide observational database containing 71% and 76% of newly-diagnosed colon and rectal cancers, respectively, in the U.S.(14) NCDB data are abstracted from medical records by certified tumor registrars from pathology reports, laboratory reports, and physician notes for patients managed at Commission on Cancer (CoC)-accredited hospitals.(15) Adult patients (≥20-years-old) who presented between 2010 and 2017 with stage IV histologically-confirmed colorectal invasive adenocarcinoma were identified using ICD-O-3 histological codes and primary colorectal site codes (C18.0, C18.2-18.9, C19.9, C20.9). Patients with unknown MMR/MSI testing status (38.2% of patients herein) or previous cancer diagnoses were excluded. 2010 was the first year that MMR/MSI testing data were reported in the NCDB, and 2017 was the latest available year for which “test not done” and unknown if tested were distinctly coded. The NCDB releases data approximately 3 years after a patient’s diagnosis.
Study Variables
The outcome of interest was the receipt of MMR/MSI testing. A lack of testing was defined as “test not done (test not ordered and not performed)” from the site-specific collaborative stage variables for CRCs, as previously reported.(13) The NCDB does not specifically distinguish between MMR immunohistochemical and MSI molecular testing modalities.(16) Additionally, the NCDB lacks details about testing strategy, sample details, or timing. MMR/MSI testing data are abstracted ≥4 months after initial diagnosis and registries continuously update the reported data for patients; however, it is possible that for some newly-diagnosed stage IV CRC patients, that their MMR/MSI testing occurred after registry abstraction and so was not captured.(16)
Independent variables of interest were patient age at diagnosis, sex, race/ethnicity, Charlson-Deyo comorbidity index, and tumor features including primary site, histological subtype (adenocarcinoma, mucinous adenocarcinoma – including “mucin-producing adenocarcinoma”, or signet ring cell carcinoma), metastatic site(s) (liver, lung, bone, brain, distant lymph node, and/or other site including carcinomatosis), surgery type at the primary site (none, diagnostic or excisional biopsy, or resection), and biopsy/resection of a regional or distant site (binary). Histologic types incompatible with a colorectal primary site were excluded (n<10; sebaceous adenocarcinoma, serous cystadenocarcinoma). Socioeconomic variables were patient’s insurance status, median household income for the patient’s ZIP code of residence, and population size of the patient’s county of residence. Care setting variables were the location (by U.S. Census Bureau-designated geographic divisions as follows: New England, Middle Atlantic, South Atlantic, East North Central, East South Central, West North Central, West South Central, Mountain, Pacific) and CoC category of the reporting facility.
Statistical Analysis
To evaluate the practice patterns of testing following the updated guidelines in 2017, the analysis was restricted to those patients diagnosed in 2017. Univariable associations were assessed by χ2 test. Features independently associated with MMR/MSI testing utilization were identified with multivariable logistic regression. Average adjusted predicted probabilities (%AAP) of MMR/MSI testing were estimated from the regression models.(17) Analyses were conducted using Stata (SEv15.1, StataCorp). Two-sided p values <0.001 were stipulated as significant.
Data Availability
The data analyzed in this study were obtained from the NCDB.
RESULTS
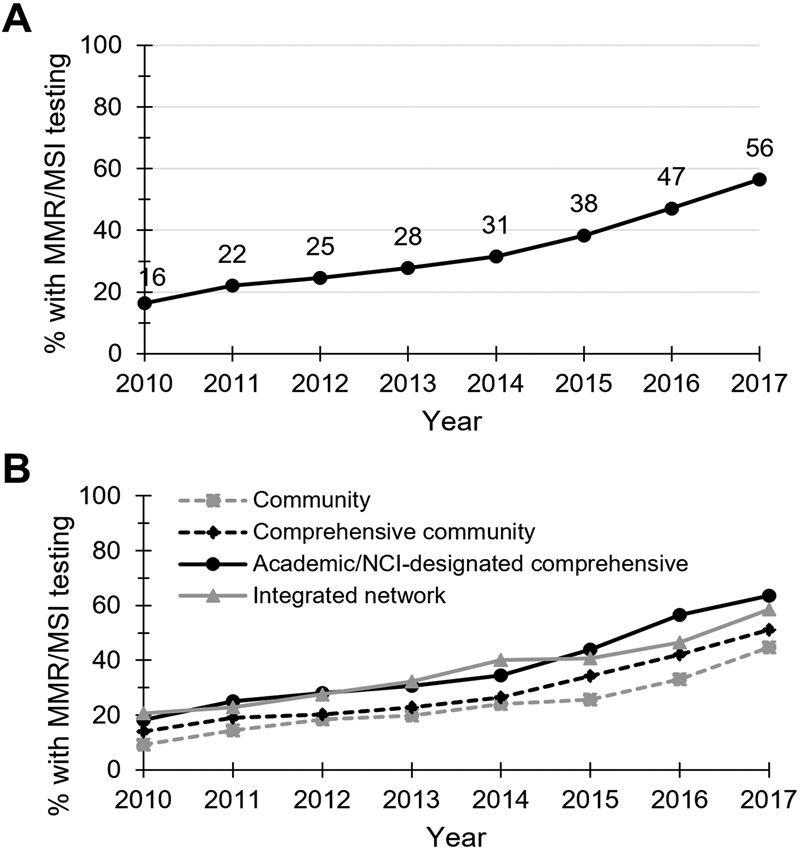
From 2010 to 2017, 72,830 adults with newly diagnosed advanced colorectal adenocarcinoma were identified. MMR/MSI testing levels increased from 16.4% (n=1,342/8,185) of patients in 2010 to 56.4% (n=5,657/10,022) in 2017 (Figure 1A). By contrast, MMR/MSI testing levels for all <50-year-old CRC patients rose from 35.3% in 2010 to 69.2% in 2017.
Figure 1. Temporal trends in MMR/MSI testing levels for stage IV colorectal cancer patients from 2010-2017.
Percent of newly-diagnosed stage IV colorectal adenocarcinoma cancer patients that received upfront MMR/MSI testing from 2010-2017, (A) overall and (B) categorized by cancer program type.
In order to examine the MMR/MSI testing patterns following the updated national guidelines in early 2017, predictors of testing were evaluated using multivariable logistic regression for those patients diagnosed in 2017 (n=10,022, of whom n=7,408 had complete data for multivariable analysis) and described using average adjusted predicted probabilities (%AAP). MMR/MSI testing in 2017 was associated with patient age at diagnosis (Supplemental Table 1), ranging from 64.4%AAP of patients <50 years old (99.9% confidence interval [CI]: 59.3-69.5) to 45.9%AAP of those 80 years and older (99.9CI: 40.2-51.5), with patients <50 years more likely to be tested than any other age group (all p<0.001). Testing was also associated with the extent of primary site surgery, with 71.0%AAP of resected patients receiving testing (99.9CI: 68.4-73.7), compared to just 44.5%AAP of biopsy-only (99.9CI: 40.9-48.0) and 35.2%AAP of no primary site surgery (99.9CI: 30.4-40.0) cases (both p<0.001). MMR/MSI testing was not associated with patient sex, race/ethnicity, Charlson-Deyo comorbidity index, tumor histology, or metastatic sites.
The relationships between testing and patient socioeconomic status were evaluated. Patient insurance status was not associated with MMR/MSI testing following multivariable adjustment. In unadjusted analysis, 63.7% of privately insured patients received MMR/MSI testing, compared to 50.3% of uninsured and 58.8% of Medicaid-insured patients (p<0.001). Socioeconomic status (reported by the NCDB as the median household income of the ZIP code of patient’s residence) was independently associated with testing in adjusted multivariate analysis: patients from the poorest quartile of households received less testing (49.6%AAP, 99.9CI: 45.5-53.7) than patients from the 3rd (56.9%AAP, 99.9CI: 53.3-60.6, p<0.001) or 4th quartiles (57.6%AAP, 99.9CI: 54.3-60.9, p<0.001).
Although testing levels improved the most over time at community cancer programs (rising from 9.3% in 2010 to 44.7% in 2017; Figure 1B), they remained significantly lower in 2017 (46.6%AAP, 99.9CI: 41.0-52.1) than those at integrated network programs (55.3%AAP, 99.9CI: 50.5-60.2, p<0.001) and academic/NCI-designated comprehensive cancer centers (62.8%AAP, 99.9CI: 59.7-65.8, p<0.001; Supplemental Table 1). Patients managed at academic/NCI-designated comprehensive cancer centers were independently more likely to receive MMR/MSI testing than those at any other cancer program type (all p<0.001). In 2017, MMR/MSI testing was reported as unknown (code 999) for 24.2% of stage IV CRC patients – a rate which did not significantly vary by hospital type (Supplemental Table 2).
Regarding geographic practice patterns in 2017, whereas the fewest patients (n=443) presented to hospitals in New England (i.e. CT, MA, ME, NH, RI, VT), they were most likely to receive MMR/MSI testing (67.4%AAP, 99.9CI: 59.7-75.3) compared to patients diagnosed elsewhere in the U.S. (all p≤0.001) except the Pacific division (p=0.05; i.e. AK, CA, HI, OR, WA) (Supplemental Table 1). Patients managed at hospitals in East North Central (49.3%AAP, 99.9CI: 44.9-53.7; i.e. IL, IN, MI, OH, WI) and East South Central (52.4%AAP, 99.9CI: 45.5-59.3; i.e. AL, KY, MS, TN) regions were among the least likely to be tested. This was despite New England being tied for having the fewest NCI-designated Comprehensive Cancer Centers (n=3), while East North Central is tied for second most (n=8). Additionally, although New England and East North Central had the highest proportions of advanced CRC patients who were managed at community cancer programs (14.2% and 14.9%, respectively; with the lowest being 3.5% in Mountain [i.e. AZ, CO, ID, MT, NM, NV, UT, WY] and 5.6% in Middle Atlantic [i.e. NJ, NY, PA] divisions), 57.0% of patients at New England cancer programs came from the richest quartile of ZIP codes, compared to 25.4% in East North Central and 12.8% in East South Central (p<0.001).
DISCUSSION
With the approval of checkpoint blockade immunotherapy for the treatment of dMMR/MSI-H advanced CRC, universal screening became the recommended standard of care in early 2017.(5,6,9,10) Although testing levels more than tripled between 2010 and 2017 – with a ~20% increase from 2016 to 2017 – after the guidelines were updated in early 2017, >43% of advanced CRC patients in the U.S. were still not receiving MMR/MSI testing within 4 months of presenting with stage IV disease. Because the NCDB reports data from initial presentation, it is possible that some patients received MMR/MSI testing more than 4 months after their initial diagnosis and were not captured by continuing review by registries. Our results are corroborated by those from a recent analysis of 23 oncology practices from across the eastern U.S., in which 43% of their metastatic colon cancer cases (both synchronous and metasynchronous) in 2017 had not received MMR/MSI testing.(18) We found that advanced CRC patients who were older, from the poorest quartile of households, or managed at community cancer programs were independently less likely to receive guideline-aligned MMR/MSI testing.
Prior to the approval of checkpoint inhibitor therapy for dMMR/MSI-H advanced CRC, the underutilization of MMR testing for CRC patients at increased risk for Lynch syndrome—recommended by the NCCN in 2011—was well documented: just 48% of U.S. CRC patients younger than 50 (all stages) received MMR testing in 2012.(13,19,20) We found that testing levels increased to only 69% in 2017 for this high-risk population. Furthermore, for stage IV disease in particular, 69%AAP of <50-year-olds received upfront MMR/MSI testing, compared to 46%AAP of ≥80-year-olds. This age discrepancy might reflect persistent adherence to outdated Lynch syndrome screening criteria, which rarely triaged elderly patients to receive screening.(7,8) However, universal testing provides considerable clinical benefit in older patients because ~80-85% of dMMR/MSI-H CRCs are sporadic, with an average age at presentation of ≥75 years.(21)
A 2018 survey of 151 oncologists/pathologists/surgeons in the U.S. found that although 84% were aware of MMR/MSI testing guidelines (67% of NCCN, 13% of ASCP/CAP/AMP/ASCO); only 70% of physicians agreed that it is standard practice to perform universal testing for all CRC patients.(22) The largest barrier to testing endorsed by the physicians was insufficient tissue sample to run the test (48% of physicians); In our analyses, primary tumors that underwent diagnostic biopsy were substantially less likely to have upfront MMR/MSI testing than those that were resected, potentially reflecting insufficient tissue availability for molecular testing. While molecular testing may require more tissue than might be available in some biopsies, dMMR immunohistochemistry is more permissive in this regard, because only four 4-μm tissue sections are generally necessary. Furthermore, advances in plasma-based genotyping of circulating-tumor DNA may provide an alternative for MSI-H detection in cases of insufficient tissue.(23,24)
Another barrier to testing cited by surveyed physicians was worry regarding insurance coverage (31% of physicians)(22) – a concern that was shared by 27% of surveyed advanced CRC patients.(25) In our assessment, however, lack of insurance was not independently associated with upfront MMR/MSI testing in multivariable analysis when patients’ ZIP code-level household income was also included. By contrast, lower ZIP-code level household income was independently associated with lower testing levels, suggesting that broader socioeconomic considerations might affect access to biomarker testing in underappreciated ways.(26-31) Geographic discrepancies in upfront MMR/MSI testing utilization in the U.S. appeared, at least in part, to be associated with differences in the socioeconomic statuses of patient populations. Of note, although racial disparities have been previously identified in CRC patients’ outcomes, in our analyses, race/ethnicity was not independently associated with MMR/MSI testing utilization.(32-34) To assess for potential collinearity between race/ethnicity and socioeconomic status, we performed a sensitivity analysis, in which even after excluding ZIP code-level household income status or insurance status from our multivariable model, a significant association between race/ethnicity and MMR/MSI testing was not found.
Although upfront MMR/MSI testing levels improved across all cancer program types, in 2017 patients managed at community programs were still a third less likely to be tested than their counterparts at academic or NCI-designated comprehensive cancer programs. Approaches to MMR/MSI testing vary by institution, with some institutions reflexively performing testing on CRCs as initiated by pathologists and other institutions regarding MMR/MSI testing for Lynch syndrome screening as a genetic test, which require the treating physician to initiate testing with patient consent. it is conceivable that systematic differences in the approach to MMR/MSI testing could have contributed to the difference in testing rates in academic and community settings. Given the crucial role of MMR/MSI testing as a prognostic and predictive biomarker for CRC patients, one potential approach for encouraging more widespread adoption among community programs would be to use MMR/MSI testing as a performance metric for gauging the quality of an institution’s CRC care. Reflexive MMR/MSI testing at the time of initial diagnosis could join the National Quality Forum (NQF)’s currently endorsed quality measures for CRC patients. Additionally, MMR/MSI biomarker test reporting could be made a required part of the CAP Colon and Rectum Protocol and Laboratory Accreditation Program to encourage integration into pathology laboratory workflows nationwide.(35-37) An important element that the NCDB did not capture was the reason for a lack of testing and whether patients refused testing. Previous studies found that 36% of surveyed physicians cited patient refusal as a barrier to testing, with 29% of surveyed CRC patients reporting personal reservations about the benefits of MMR/MSI testing – together suggesting that improved patient education across institution types may be helpful in expanding testing.(22,25)
Underutilization of guideline-recommended biomarker testing for advanced CRC patients also extends beyond MMR/MSI: among metastatic colon cancer patients from 23 oncology practices across the U.S., overall, guideline-aligned biomarker (i.e. MMR/MSI, KRAS, NRAS, and BRAF) testing was utilized in only 40% of patients between 2013-2017.(18) Adoption of multigene next-generation sequencing assays may improve multi-biomarker testing rates, because these panels can also be used to infer MSI status by mutational signature; indeed, NGS is already supplanting MSI PCR testing in some academic institutions.(18,38) Overall, our results parallel those from other cancer contexts, in which utilization of biomarker testing lagged for patients from lower socioeconomic statuses or managed at community programs, indicating that more general barriers to molecular testing may exist.(39-42).
Limitations
Owing to NCDB design, this study has multiple limitations. NCDB data likely include coding errors and incomplete records.(15,43) 31% of patients herein were coded as MMR/MSI testing status unknown, and because a lack of testing could erroneously be coded as unknown testing status (code 999), we conducted a sensitivity analysis in which both unknown status and “test not done” (code 998) were considered to be a lack of testing. Using that definition, 43% of advanced CRCs in 2017 were tested, and our multivariable findings remained unchanged (Supplemental Table 3). Additionally, the rate of MMR/MSI testing status reported as unknown did not significantly vary by institution type in 2017, suggesting comparable cancer registry reporting rates across hospital settings.
Notably, although cancer registries report MMR/MSI testing status from at least 4 months after initial presentation and continuously update data for included patients, it is possible that some patients had subsequent testing following data abstraction for that patient that was not captured – thereby underestimating the MMR/MSI testing rates. Implementation of practice changes following the issuance of new guidelines takes time and because complete MMR/MSI test utilization data were not available in the NCDB for patients diagnosed after 2017, we were unable to measure how testing levels changed over time after 2017. Based on results from similar molecular testing contexts, practice changes can be slow to manifest nationally in the U.S. For instance, despite NCCN guideline recommendations in 2009 for KRAS testing to guide the use of anti-EGFR therapy in metastatic CRC patients, just 25% of patients were tested in 2010 – rising to only 35% by 2013.(42,44)
Conclusions
MMR/MSI testing utilization for stage IV CRC patients has improved in the last decade; however, we found that after consensus guidelines recommended universal testing in early 2017, 44% of newly-diagnosed stage IV CRC patients did not receive upfront testing. Testing utilization disproportionately lagged for patients who were older, from the poorest quartile of households, or managed at community cancer programs – less than half of these patients received upfront testing. Now that pembrolizumab has been approved for first-line management of stage IV CRC, upfront MMR/MSI testing should be incorporated into NQF performance metrics and CAP accreditation requirements.
Supplementary Material
Acknowledgements:
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC's NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Financial support:
J.B. Iorgulescu gratefully acknowledges funding support from the National Cancer Institute (K12CA090354) and Conquer Cancer Foundation.
Footnotes
Conflicts of interest: The authors report no conflicts of interest
This work was presented in part at the 2020 AACR Conference on The Science of Cancer Health Disparities in Racial/Ethnic Minorites and the Medically Underserved & 2020 Society for Immunotherapy of Cancer.
REFERENCES
- 1.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015. Jun 25;372(26):2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer—the stable evidence. Nat Rev Clin Oncol. 2010. Mar;7(3):153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overman MJ, Kopetz S, McDermott RS, Leach J, Lonardi S, Lenz HJ, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. JCO. 2016. May 20;34(15_suppl):3501–3501. [Google Scholar]
- 4.Diaz L, Marabelle A, Kim TW, Geva R, Cutsem EV, André T, et al. Efficacy of pembrolizumab in phase 2 KEYNOTE-164 and KEYNOTE-158 studies of microsatellite instability high cancers. Annals of Oncology. 2017. Sep 1;28:v128–9. [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Colon Cancer (Version 1.2017). [Google Scholar]
- 6.Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. JCO. 2017. Feb 6;35(13):1453–86. [DOI] [PubMed] [Google Scholar]
- 7.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999. Jun;116(6):1453–6. [DOI] [PubMed] [Google Scholar]
- 8.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004. Feb 18;96(4):261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. 2017. FDA. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication. Accessed Jun 9 2022. [Google Scholar]
- 10.FDA grants nivolumab accelerated approval for MSI-H or dMMR colorectal cancer. 2017. FDA. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-nivolumab-accelerated-approval-msi-h-or-dmmr-colorectal-cancer. Accessed Jun 9 2022. [Google Scholar]
- 11.Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin Cancer Res. 2019. Jul 1;25(13):3753–8. [DOI] [PubMed] [Google Scholar]
- 12.FDA approves pembrolizumab for first-line treatment of MSI-H/dMMR colorectal cancer. 2020. FDA. Available at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-first-line-treatment-msi-hdmmr-colorectal-cancer. Accessed Jun 9 2022. [Google Scholar]
- 13.Shaikh T, Handorf EA, Meyer JE, Hall MJ, Esnaola NF. Mismatch Repair Deficiency Testing in Patients With Colorectal Cancer and Nonadherence to Testing Guidelines in Young Adults. JAMA Oncol. 2018. Feb 8;4(2):e173580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallin K, Browner A, Palis B, Gay G, McCabe R, Nogueira L, et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012–2014. Ann Surg Oncol. 2019. Jun 1;26(6):1604–12. [DOI] [PubMed] [Google Scholar]
- 15.Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017. 01;3(12):1722–8. [DOI] [PubMed] [Google Scholar]
- 16.Collaborative Stage Work Group of the American Joint Committee on Cancer. Collaborative Stage Data Collection System Coding Instructions, version 02.
- 17.Williams R Using the Margins Command to Estimate and Interpret Adjusted Predictions and Marginal Effects. The Stata Journal. 2012. Jun 1;12(2):308–31. [Google Scholar]
- 18.Gutierrez ME, Price KS, Lanman RB, Nagy RJ, Shah I, Mathura S, et al. Genomic Profiling for KRAS, NRAS, BRAF, Microsatellite Instability, and Mismatch Repair Deficiency Among Patients With Metastatic Colon Cancer. JCO Precision Oncology. 2019. Dec 1;(3):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benson AB, Arnoletti JP, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, et al. Colon Cancer. Journal of the National Comprehensive Cancer Network. 2011. Nov 1;9(11):1238–90. [DOI] [PubMed] [Google Scholar]
- 20.Mittal C, Dang D, Stoffel E, Menees S, Scott FI, Ahnen D, et al. Underutilization of Lynch Syndrome Screening at Two Large Veterans Affairs Medical Centers. Dig Dis Sci. 2020. Nov 1;65(11):3305–15. [DOI] [PubMed] [Google Scholar]
- 21.Young J, Simms LA, Biden KG, Wynter C, Whitehall V, Karamatic R, et al. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am J Pathol. 2001. Dec;159(6):2107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriksson J, Amonkar M, Al-Jassar G, Lambert J, Malmenäs M, Chase M, et al. Mismatch Repair/Microsatellite Instability Testing Practices among US Physicians Treating Patients with Advanced/Metastatic Colorectal Cancer. J Clin Med. 2019. Apr 24;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willis J, Lefterova MI, Artyomenko A, Kasi PM, Nakamura Y, Mody K, et al. Validation of Microsatellite Instability Detection Using a Comprehensive Plasma-Based Genotyping Panel. Clin Cancer Res. 2019. Dec 1;25(23):7035–45. [DOI] [PubMed] [Google Scholar]
- 24.Georgiadis A, Durham JN, Keefer LA, Bartlett BR, Zielonka M, Murphy D, et al. Non-invasive detection of microsatellite instability and high tumor mutation burden in cancer patients treated with PD-1 blockade. Clin Cancer Res. 2019. Dec 1;25(23):7024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eriksson J, Amonkar M, Al-Jassar G, Lambert J, Malmenäs M, Chase M, et al. Experience of mismatch repair/microsatellite instability (MMR/MSI) testing among patients with advanced/metastatic colorectal cancer in the US. Curr Med Res Opin. 2020. Aug;36(8):1355–61. [DOI] [PubMed] [Google Scholar]
- 26.Zafar SY, Peppercorn JM, Schrag D, Taylor DH, Goetzinger AM, Zhong X, et al. The Financial Toxicity of Cancer Treatment: A Pilot Study Assessing Out-of-Pocket Expenses and the Insured Cancer Patient’s Experience. Oncologist. 2013. Apr;18(4):381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Z, Han X, Guy GP, Davidoff AJ, Li C, Banegas MP, et al. Do cancer survivors change their prescription drug use for financial reasons? Findings from a nationally representative sample in the United States. Cancer. 2017. Apr 15;123(8):1453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yousuf Zafar S Financial Toxicity of Cancer Care: It’s Time to Intervene. J Natl Cancer Inst. 2016. May 1;108(5). [DOI] [PubMed] [Google Scholar]
- 29.Gilligan AM, Alberts DS, Roe DJ, Skrepnek GH. Death or Debt? National Estimates of Financial Toxicity in Persons with Newly-Diagnosed Cancer. Am J Med. 2018. Oct;131(10):1187–1199.e5. [DOI] [PubMed] [Google Scholar]
- 30.Narang AK, Nicholas LH. Out-of-Pocket Spending and Financial Burden Among Medicare Beneficiaries With Cancer. JAMA Oncol. 2017. Jun 1;3(6):757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong YN, Schluchter MD, Albrecht TL, Benson AB, Buzaglo J, Collins M, et al. Financial Concerns About Participation in Clinical Trials Among Patients With Cancer. J Clin Oncol. 2016. Feb 10;34(5):479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder RA, Hu CY, Zafar SN, Francescatti A, Chang GJ. Racial Disparities in Recurrence and Overall Survival in Patients With Locoregional Colorectal Cancer. J Natl Cancer Inst. 2021. Jun 1;113(6):770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manz CR, Schrag D. Racial Disparities in Colorectal Cancer Recurrence and Mortality: Equitable Care, Inequitable Outcomes? J Natl Cancer Inst. 2021. Jun 1;113(6):656–7. [DOI] [PubMed] [Google Scholar]
- 34.Yothers G, Sargent DJ, Wolmark N, Goldberg RM, O’Connell MJ, Benedetti JK, et al. Outcomes among black patients with stage II and III colon cancer receiving chemotherapy: an analysis of ACCENT adjuvant trials. J Natl Cancer Inst. 2011. Oct 19;103(20):1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torous VF, Simpson RW, Balani JP, Baras AS, Berman MA, Birdsong GG, et al. College of American Pathologists Cancer Protocols: From Optimizing Cancer Patient Care to Facilitating Interoperable Reporting and Downstream Data Use. JCO Clinical Cancer Informatics. 2021. Aug 1;(5):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson RW, Berman MA, Foulis PR, Divaris DXG, Birdsong GG, Mirza J, et al. Cancer biomarkers: the role of structured data reporting. Arch Pathol Lab Med. 2015. May;139(5):587–93. [DOI] [PubMed] [Google Scholar]
- 37.Sluijter CE, van Workum F, Wiggers T, van de Water C, Visser O, van Slooten HJ, et al. Improvement of Care in Patients With Colorectal Cancer: Influence of the Introduction of Standardized Structured Reporting for Pathology. JCO Clinical Cancer Informatics. 2019. Dec 1;(3):1–12. [DOI] [PubMed] [Google Scholar]
- 38.Papke DJ, Nowak JA, Yurgelun MB, Frieden A, Srivastava A, Lindeman NI, et al. Validation of a targeted next-generation sequencing approach to detect mismatch repair deficiency in colorectal adenocarcinoma. Mod Pathol. 2018. Dec;31(12):1882–90. [DOI] [PubMed] [Google Scholar]
- 39.Lamba N, Chukwueke UN, Smith TR, Ligon KL, Aizer A, Reardon DA, et al. Socioeconomic Disparities Associated With MGMT Promoter Methylation Testing for Patients With Glioblastoma. JAMA Oncol. 2020. Dec 1;6(12):1972–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts MC, Kurian AW, Petkov VI. Uptake of the 21-Gene Assay Among Women With Node-Positive, Hormone Receptor-Positive Breast Cancer. J Natl Compr Canc Netw. 2019. Jun 1;17(6):662–8. [DOI] [PubMed] [Google Scholar]
- 41.Iorgulescu JB, Freedman RA, Lester SC, Mittendorf EA, Brock JE. 21-Gene Recurrence Score Adds Significant Value for Grade 3 Breast Cancers: Results From a National Cohort. JCO Precis Oncol. 2019;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charlton ME, Karlitz JJ, Schlichting JA, Chen VW, Lynch CF. Factors Associated With Guideline-recommended KRAS Testing in Colorectal Cancer Patients: A Population-based Study. Am J Clin Oncol. 2017. Oct;40(5):498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iorgulescu JB, Torre M, Harary M, Smith TR, Aizer AA, Reardon DA, et al. The Misclassification of Diffuse Gliomas: Rates and Outcomes. Clin Cancer Res. 2019. Apr 15;25(8):2656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charlton ME, Kahl AR, Greenbaum AA, Karlitz JJ, Lin C, Lynch CF, et al. KRAS Testing, Tumor Location, and Survival in Patients With Stage IV Colorectal Cancer: SEER 2010-2013. J Natl Compr Canc Netw. 2017;15(12):1484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study were obtained from the NCDB.