Abstract
Plasmid conjugation systems are composed of two components, the DNA transfer and replication system, or Dtr, and the mating pair formation system, or Mpf. During conjugal transfer an essential factor, called the coupling protein, is thought to interface the Dtr, in the form of the relaxosome, with the Mpf, in the form of the mating bridge. These proteins, such as TraG from the IncP1 plasmid RP4 (TraGRP4) and TraG and VirD4 from the conjugal transfer and T-DNA transfer systems of Ti plasmids, are believed to dictate specificity of the interactions that can occur between different Dtr and Mpf components. The Ti plasmids of Agrobacterium tumefaciens do not mobilize vectors containing the oriT of RP4, but these IncP1 plasmid derivatives lack the trans-acting Dtr functions and TraGRP4. A. tumefaciens donors transferred a chimeric plasmid that contains the oriT and Dtr genes of RP4 and the Mpf genes of pTiC58, indicating that the Ti plasmid mating bridge can interact with the RP4 relaxosome. However, the Ti plasmid did not mobilize transfer from an IncQ relaxosome. The Ti plasmid did mobilize such plasmids if TraGRP4 was expressed in the donors. Mutations in traGRP4 with defined effects on the RP4 transfer system exhibited similar phenotypes for Ti plasmid-mediated mobilization of the IncQ vector. When provided with VirD4, the tra system of pTiC58 mobilized plasmids from the IncQ relaxosome. However, neither TraGRP4 nor VirD4 restored transfer to a traG mutant of the Ti plasmid. VirD4 also failed to complement a traGRP4 mutant for transfer from the RP4 relaxosome or for RP4-mediated mobilization from the IncQ relaxosome. TraGRP4-mediated mobilization of the IncQ plasmid by pTiC58 did not inhibit Ti plasmid transfer, suggesting that the relaxosomes of the two plasmids do not compete for the same mating bridge. We conclude that TraGRP4 and VirD4 couples the IncQ but not the Ti plasmid relaxosome to the Ti plasmid mating bridge. However, VirD4 cannot couple the IncP1 or the IncQ relaxosome to the RP4 mating bridge. These results support a model in which the coupling proteins specify the interactions between Dtr and Mpf components of mating systems.
Plasmid conjugation conceptually can be divided into two functions. In the first, the DNA is processed by a complex of proteins, one of which introduces a single-strand nick at the nic site within the oriT recognition sequence. Called the relaxosome, the proteins of this complex are coded for by genes of the Dtr (DNA transfer and replication) component of the transfer system. In the second, the nucleoprotein transfer intermediate comprised of the nicked strand covalently linked at the 5′ end to the relaxase is secreted from the donor directly into the recipient via a bridge that forms between the mating pair. This translocation apparatus is a complex membrane-associated structure coded for by the Mpf (mating pair formation) genes.
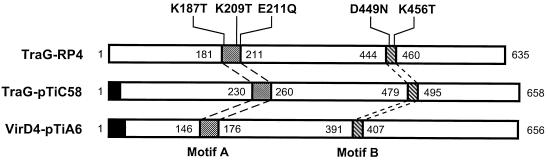
The relaxosome of one conjugal plasmid may or may not be transferrable by the Mpf system of another. Specificity is conferred, in part, by a single protein which is thought to couple the relaxosome with the mating bridge (8, 29). These specificity determinants, exemplified by TraG of the IncP plasmid RP4 (TraGRP4), comprise a family of related proteins (29). All contain two conserved regions, and many contain N-terminal secretion signals (Fig. 1 and reference 29). While essential for conjugal transfer, where examined, these proteins are not required for construction of the transport complex. For example, TraGRP4, encoded by the Tra1 region (Fig. 2 and reference 58), is required for conjugal transfer but not for Mpf-dependent pilus production or sensitivity to Mpf-specific bacteriophages such as PRD1, pf3, and PRR1 (24, 30, 53).
FIG. 1.
Structure and relatedness of coupling proteins from the RP4 and Ti plasmid transfer systems. The length of each protein in amino acid residues is indicated by the numbers at each end. Filled regions indicate amino acid sequences with properties of N-terminal secretion signals. The stippled and cross-hatched regions and their coordinates delimit the two motifs conserved among the members of the TraG family (19, 29). The amino acid substitution mutants of TraGRP4 are indicated by the single-letter designation for the wild-type residue followed by the position number and the mutant residue.
FIG. 2.
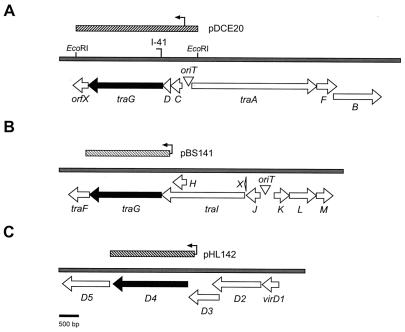
Gene organization of the Dtr regions of the transfer systems from RP4 and pTiC58. The black-filled arrows represent genes coding for the coupling proteins of the three transfer systems. (A) tra locus of the Ti plasmid tra system. Locations of genes within the two tra operons, as well as the oriT sequence, are indicated by the large arrows. The flagstaff represents the site of the Tn3HoHo1 insertion located in traG of the mutant Ti plasmid pDCKI41. The diagonally hatched bar represents the EcoRI fragment from pTiC58 containing traG cloned in pDCE20. The small arrow represents the location and direction of transcription of the native TraR-dependent promoter responsible for expression of the traCDG operon. (B) Tra1 core region of RP4. The locations of the essential Dtr genes and the oriT are shown by large arrows. The diagonally hatched bar depicts the fragment containing the traG gene cloned in pBS141. The arrow represents the Tac promoter, provided by the vector, that drives expression of the gene. (C) virD operon of the Ti plasmid. The five genes of the virD operon are shown by the large arrows. The diagonally hatched bar depicts the fragment containing the virD4 gene cloned in pHL142. The arrow represents the lac promoter, provided by the vector, that drives expression of the gene.
The IncRh1 Ti plasmids of Agrobacterium tumefaciens contain two transfer systems. One, coded for by vir, transfers a discrete portion of the plasmid, called the T region, from the bacterium to the plant (14). The Dtr functions of this system are coded for by the virD and virC operons (23, 48, 50) located in the vir regulon of the Ti plasmid (41, 47). The nicking sites, called borders, flank and define the T region, and are closely related at the nucleotide sequence level to the nic site within oriT of RP4 (37). Furthermore, VirD2, the border-specific strand transferase, is related to TraI, the oriT-specific relaxase of RP4 (37, 52). On the other hand, the Ti plasmid vir mating bridge coded for by the virB operon is only distantly related to Tra2, the locus coding for the Mpf functions of RP4. Components of the VirB Mpf system are most closely related to those of the IncN plasmid pKM101 (39) and to vir and ptl, which code for transporters known or thought to be required for the secretion of virulence factors by Brucella suis and Bordetella pertussis, respectively (35, 55; reviewed in reference 57; see also reference 12).
The second Ti plasmid conjugation system mediates transfer of the entire plasmid from donors to bacterial recipients (reviewed in reference 18). This system is composed of two distantly linked units; tra, a set of two operons divergently transcribed from an intergenic region that contains the oriT (1, 11, 19); and trb, an operon of 12 genes that codes for the conjugal mating bridge (1, 31). The tra system also is chimeric; the oriT and Dtr genes encoded by tra are related to those of the IncQ plasmid RSF1010 (1, 11, 19), while the Mpf functions, coded for by the tra and trb operons, are closely related to those of the Tra2 genes of RP4 (1, 31).
The two Ti plasmid transfer systems each contain a coupling component related to TraGRP4 (Fig. 1). The vir element, called VirD4, is coded for by the virD operon (Fig. 2) and is essential for transfer of the T strand, the processed form of the T region, to plant cells (33, 40). Like TraGRP4, the protein localizes to the cell membrane complex, although both lack canonical membrane-spanning domains (13, 36; V. L. Waters, E. Lanka, and D. G. Guiney, Abstr. 95th Gen. Meet. Am. Soc. Microbiol. 1995, abstr. H-123, p. 513, 1995). The tra component, also called TraG (Fig. 1), has not been localized but is essential for conjugal transfer (19). The gene that codes for this component is located in the traCDG operon which flanks the Ti plasmid oriT (Fig. 2 and reference 19).
The IncQ plasmid RSF1010 contains an oriT (16), a site-specific relaxase, MobA (15), and additional relaxosome proteins, MobB and MobC (45, 46). However, the plasmid lacks genes for Mpf functions and a coupling protein (46) and, while mobilizable by other plasmids (56), is not self-conjugal. Mobilization requires the Mpf functions and the coupling protein of the conjugal helper plasmid. For example, mobilization of RSF1010 by RP4 requires the Tra2 locus, traG, and traF, the Tra1 gene coding for the pilin processing enzyme (17), but not traI or other components of the RP4 relaxosome (24, 30, 53). Similarly, the Ti plasmid vir system mobilizes RSF1010 to plant cells (6) and also to bacterial recipients (3). Both transfer processes require the virB-encoded Mpf system and VirD4 (20, 21) as well as the RSF1010 oriT and relaxosome components (6). On the other hand, although the Ti plasmid oriT and its associated relaxase, TraA, are related to the analogous components of the RSF1010 mob system (11, 19), the Ti plasmid tra system does not transfer the IncQ plasmid (11). This failure to transfer is surprising considering that TraG and the Mpf system of the Ti plasmid are closely related to their homologs of RP4, which mobilizes RSF1010 at high frequency. Thus, we undertook a study to determine what factors account for the inability of the Ti plasmid conjugal transfer system to mobilize RSF1010.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains of A. tumefaciens and Escherichia coli and the plasmids used in this study are listed in Table 1. pDB127, which is derived from pDB126, contains an in-frame deletion in traG and is nonconjugal (2). Transfer of this plasmid is restored by providing traG of RP4 in trans (2). pDCKI41, which contains a Tn3HoHo1 insertion in traG, is a transfer-minus (Tra−) derivative of the transfer-constitutive (Trac) Ti plasmid pTiC58ΔaccR (19). pDCE20 is a recombinant plasmid in which EcoRI fragment 20 of pTiC58 is inserted in the IncP1 vector pRK415 (11, 27). This clone contains the entire traCDG operon and its native TraR-dependent promoter (19). pDSK519, which codes for resistance to kanamycin (27), and pMMB67HE, which codes for resistance to ampicillin and carbenicillin (22), are mobilizable vectors derived from RSF1010. Both retain oriT and mobA, mobB, and mobC, the three genes required to form the RSF1010 relaxosome.
TABLE 1.
Bacteria and plasmids used in this study
| Bacterial strain or plasmid | Relevant genotype, phenotype, or characteristica | Source or reference |
|---|---|---|
| A. tumefaciens | ||
| NT1 | Ti plasmid-cured derivative of C58, contains pAtC58 | 54 |
| C58C1RS | Ti plasmid-cured derivative of C58; Rifr Strr; contains pAtC58 | 49 |
| C58C1EC | Ti plasmid-cured derivative of C58; Eryr Chlr; contains pAtC58 | 32 |
| UIA5 | Ti plasmid-cured derivative of GMI9017; Rifr Strr; lacks pAtC58 | 11, 42 |
| NT1(pZLR4) | AAI bioindicator strain; Cbr | 9 |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 43 |
| HB101 | hsdS20 recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 supE44 Strr | 43 |
| HB101Nx | hsdS20 recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 supE44 Strr Nalr | 2 |
| HB101RifR | hsdS20 recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 supE44 Strr Rifr | 11 |
| S17-1 | pro (r− m+) Mob+ Ampr Chlr Tmpr Strr | 19 |
| Plasmids | ||
| pTiC58ΔaccR | accR deletion mutant of pTiC58; Tracaccc | 11 |
| pDCKI41 | traG::Tn3HoHo1 insertion mutant of pTiC58ΔaccR; Tra− | 19 |
| pJB3 | IncP1 cloning vector; Mob+ Apr Cbr | 5 |
| pRK415 | IncP1 cloning vector; Mob+ Tcr | 27 |
| pDSK519 | IncQ cloning vector; Mob+ Kmr | 27 |
| pMMB67HE | IncQ cloning vector; Mob+ Apr Cbr | 22 |
| pVK225 | Cosmid clone of virCDE region from pTiA6NC | 26 |
| pDB126 | Tra1 core region and Tra2 region of RP4 cloned in ColD vector; Tra+ Cmr | 2 |
| pDB127 | traG deletion mutant of pDB126; Tra− Cmr | 2 |
| pBS141 | traG of RP4 cloned in pMMB67HE; IncQ Apr Cbr | 2 |
| pBS141K187T | K187→T mutant of traG in pBS141; IncQ Apr Cbr | 2 |
| pBS141K209T | K209→T mutant of traG in pBS141; IncQ Apr Cbr | |
| pBS141E211Q | E211→Q mutant of traG in pBS141; IncQ Apr Cbr | 2 |
| pBS141D449N | D449→N mutant of traG in pBS141; IncQ Apr Cbr | 2 |
| pBS141K456T | K456→T mutant of traG in pBS141; IncQ Apr Cbr | 2 |
| pDCE20 | traCDG operon of pTiC58 cloned in pRK415; IncP1 Tcr | 19 |
| pHL142 | virD4 of pTiA6NC cloned in pDSK519; IncQ Kmr | This study |
| pPLtrb | traI/trb region of pTiC58 cloned in pJB3; IncP1 Apr Cbr | This study |
| pPLtrb-DB | Tra1 core region of RP4 cloned into pPLtrb; IncP1 Apr Cbr | This study |
| pZLQR | traR of pTiC58 cloned in pBBR1MCS2; Inc? Kmr | 34 |
| pZLQRF | traF of pTiC58 cloned into pZLQR; Inc? Kmr | This study |
Antibiotics: Amp and Ap, ampicillin; Cb, carbenicillin; Chl and Cm, chloramphenicol; Ery, erythromycin; Km, kanamycin; Nal, nalidixic acid; Rif, rifampin; Str, streptomycin; Tc, tetracycline; Tmp, trimethoprim. Other symbols: accc, constitutive expression of acc; Inc?, unknown incompatibility group; Mob, mobilizable; Tra+, conjugation proficient; Tra−, conjugation deficient; Trac, constitutive for conjugation.
Media, chemicals, and growth conditions.
A. tumefaciens strains were grown at 28°C in Luria-Bertani (LB) broth (43), in ABM minimal medium (10), or on nutrient agar plates (Difco Laboratories, Detroit, Mich.). E. coli strains were grown at 28°C or 37°C in LB medium. All liquid cultures were grown with shaking to ensure aerobic conditions. Antibiotics were included in media at the following concentrations: for A. tumefaciens, carbenicillin, 100 or 200 μg/ml; chloramphenicol, 100 μg/ml; erythromycin, 150 μg/ml; kanamycin, 50 or 100 μg/ml; nalidixic acid, 50 μg/ml; rifampin, 50 μg/ml; and streptomycin, 200 μg/ml; for E. coli, ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; nalidixic acid, 30 μg/ml; and rifampin, 30 μg/ml. AT minimal medium containing nopaline (Sigma Chemical Co., St. Louis, Mo.) as the sole carbon source was prepared as described elsewhere (38).
Plasmid constructions.
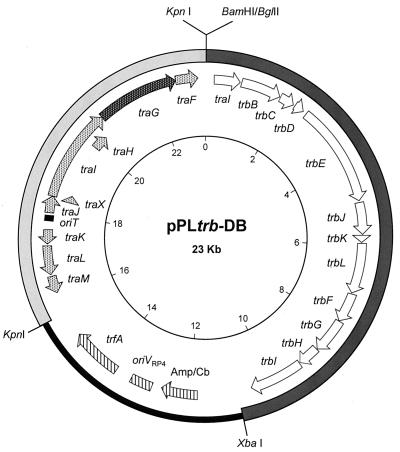
pHL142, which contains virD4 from pTiA6NC expressed from the lac promoter of pDSK519, was constructed as follows. The gene was amplified by PCR from pVK225 (26) using as the 5′ primer 5′-GGCTCTAGAGGTGAAGTCATGAATTCCAGC-3′, which contains an XbaI site (underlined) and the start codon of virD4 (italics), and as the 3′ primer 5′-CGGGGTACCTCATTTCGCAGGCTGTGCCGG-3′, which contains a KpnI site (underlined) and the termination codon of virD4 (italics). The PCR product was digested with the two enzymes and cloned into similarly digested pDSK519. pPLtrb was constructed by cloning the trb operon of pTiC58 as a BglII-XbaI fragment into the IncP1 vector pJB3 (5). The plasmid confers resistance to ampicillin and carbenicillin. pPLtrb-DB (Fig. 3) was constructed by cloning the core Tra1 region of RP4 as a KpnI fragment from pDB126 into pPLtrb. pPLQRF was constructed by cloning traF, which codes for the putative pilin-processing enzyme from pTiC58, into pZLQR (34) as an EcoRI-HindIII fragment. This clone, which also expresses traR, is derived from pBBR1MCS2, a vector that is compatible with IncP, IncQ, and IncRh1 plasmids (28).
FIG. 3.
Genetic structure of pPLtrb-DB. This plasmid, based on the IncP1 vector pJB3 (thin black line) (5), contains the entire trb region from pTiC58 (open arrows) and the Tra1 core region from RP4 (light shaded arrows). traG, which codes for the RP4-coupling protein, is indicated by the dark shaded arrow. oriV, origin of vegetative plasmid replication; trfA, the gene coding for the replication initiation protein of RP4; Amp/Cb, the bla gene coding for resistance to ampicillin and carbenicillin. See Materials and Methods for details.
Plasmids were introduced into A. tumefaciens strains by mobilization from E. coli S17-1 or by electroporation as described previously (19) and into E. coli strains by CaCl2-mediated transformation (43).
Matings.
Matings between A. tumefaciens donors and A. tumefaciens or E. coli recipients were conducted at 28°C, while those between E. coli donors and recipients were conducted at 37°C. All matings were performed on nitrocellulose filters placed on the surface of ABM agar, nutrient agar, or LB agar plates as described by Cook and Farrand (11). In all cases, donors and recipients were grown to late exponential phase, and matings were allowed to proceed for 2- to 24-h intervals before the mixes were recovered from the filters, diluted, and plated onto selective media. The titer of the donor population was determined by dilution plating just prior to mixing with recipients. Frequencies of transfer are expressed as transconjugants recovered per input donor. Coinheritance of pTiC58 or its derivatives was assessed by picking a minimum of 100 transconjugant colonies to AT nopaline plates containing antibiotics selective for the recipient. Coinheritance of the RSF1010-based plasmids was assessed by picking a similar number of transconjugant colonies to nutrient agar or LB agar plates supplemented with the appropriate antibiotics.
AAI assays.
Production of AAI [N-(3-oxo-octanoyl)-l-homoserine lactone] was assessed by a semiquantitative plate assay using NT1(pZLR4) as the bioreporter as described by Cha et al. (9). NT1(pTiC58ΔaccR) was used as the positive control.
RESULTS
Ti plasmid trb can transfer the RP4 relaxosome.
Although the trb region of pTiC58 and Tra2 of RP4 clearly are of the same phylogenetic lineage (31), the Ti plasmid does not mobilize vectors that contain the RP4 oriT but lack the Tra1 genes required for a functional relaxosome (11). This is not surprising; the oriT of RP4 is unrelated to that of the nopaline- and octopine-type Ti plasmids (1, 11), making it unlikely that the Ti plasmid Dtr functions recognize and process at the RP4 nic site. However, it is conceivable that if allowed to form, the RP4 relaxosome would be recognized by the Ti plasmid Mpf system. Thus, we determined whether a plasmid encoding the RP4 Tra1 Dtr system but lacking the IncP1 Mpf functions could be mobilized from an A. tumefaciens donor when provided with a copy of the Ti plasmid trb system. To do this, we constructed pPLtrb-DB, which contains the core Tra1 region, including oriT, of RP4 and the entire trb operon of pTiC58 (Fig. 3). Expression of trb requires TraR, the quorum-sensing transcriptional activator (25, 31). We provided this gene, as well as traF, the putative pilin-processing enzyme from pTiC58, in trans on pPLQRF. Donors harboring pPLtrb-DB and pPLQRF transferred the former plasmid at a frequency of 1.3 × 10−6 per input donor. Transfer was dependent on traR and traF; donors lacking pPLQRF failed to transfer pPLtrb-DB (data not shown). Thus, the RP4 relaxosome can interface with the Ti plasmid trb complex.
On the other hand, RSF1010 contains an oriT remarkably similar to that of the Ti plasmids (11) and encodes its own relaxosome components (46). One such component, MobA, is the cognate relaxase and is related to TraA of the Ti plasmid tra system (19, 45). RSF1010, while mobilized at high frequency by RP4 (56), is not mobilized at detectable levels by pTiC58 (11). Taken together, these results indicate that the Ti plasmid mating bridge can interact with the relaxosome of RP4 but not with that of RSF1010.
TraGRP4 allows pTiC58 to mobilize RSF1010.
Interaction between the relaxosome and the Mpf complex of RP4 is thought to be mediated by TraG (30). The TraG proteins of RP4 and pTiC58 are closely related (1, 19) (Fig. 1) and essential for conjugal transfer (2, 19), and that of RP4 is required for mobilization of RSF1010 (30). Based on these observations, we hypothesized that TraGRP4, but not TraGpTiC58, could couple the relaxosome of RSF1010 with the Ti plasmid Mpf complex. To test this hypothesis, we determined whether pTiC58ΔaccR can mobilize IncQ plasmids if provided with traGRP4. We also tested five single amino acid substitution mutants of traGRP4 (Fig. 1), as well as two RP4-mobilizable vectors based on the RSF1010 replicon. To exclude any influence of pAtC58 we used A. tumefaciens UIA5, which lacks this plasmid (Table 1), as the donor in this set of matings. As shown in Table 2, pTiC58ΔaccR failed to mobilize pDSK519 and pMMB67HE, the two RSF1010-derived vectors. However, the Trac Ti plasmid mobilized pBS141, the pMMB67HE clone that contains wild-type traGRP4, at a low but easily detectable frequency (Table 2).
TABLE 2.
TraG of RP4 allows pTiC58 to mobilize IncQ plasmidsa
| IncQ plasmidb | traG allelec | Mobilization frequencyd | Ti plasmid coinheritancee |
|---|---|---|---|
| pDSK519 | None | <10−9 | NA |
| pMMB67HEf | None | <10−9 | NA |
| pBS141 | Wild type | 3 × 10−7 | 0.04 |
| pBS141K187T | K187T | <10−9 | NA |
| pBS141K209T | K209T | 1.6 × 10−8 | 0.14 |
| pBS141E211Q | E211Q | <10−9 | NA |
| pBS141D449N | D449N | <10−9 | NA |
| pBS141K456T | K456T | 5.5 × 10−7 | 0.28 |
A. tumefaciens UIA5(pTiC58ΔaccR) was used as the donor in filter matings with strain C58C1EC as described in Materials and Methods.
All plasmids are derivatives of RSF1010 and contain the IncQ oriT and mobilization genes mobA, mobB, and mobC.
See Fig. 2.
Expressed as transconjugants obtained per input donor. The full set of matings was repeated twice with indistinguishable patterns of results. The data from a single set of matings conducted in parallel on the same day are presented.
Assessed by picking 100 transconjugant colonies to AT minimal medium containing nopaline as the sole carbon source as described in Materials and Methods. NA, not applicable.
Vector used for construction of the pBS141 series of plasmids.
The Ti plasmid also mobilized one point mutant of TraGRP4, pBS141K456T, at frequencies similar to that of the wild-type plasmid, pBS141 (Table 2). This allele, with a K-to-T substitution at position 456, complements a traGRP4-null mutant, pDB127, to near-wild-type levels of transfer (2). Three mutants, pBS141K187T, pBS141E211Q, and pBS141D449N, were not mobilized by the Ti plasmid at detectable frequencies, while the remaining mutant, pBS141K209T, was mobilized but at a frequency approximately 20-fold lower than the wild-type parent (Table 2). The K187T, E211Q, and D449N mutations do not complement the traGRP4 null mutation while the K209T mutation results in a partially active protein (2). Thus, TraGRP4 conferred on the Ti plasmid the capacity to mobilize RSF1010. Moreover, mutations in the gene exhibited phenotypes for Ti plasmid-mediated mobilization of RSF1010 that mimicked those observed for complementation of a traG null mutation in the RP4 transfer system (2).
Mobilization of RSF1010 may restrict cotransfer of the Ti plasmid to the same recipient.
We assessed transconjugants selected for acquisition of pBS141 or its derivatives for coinheritance of the Ti plasmid. Frequencies of cotransfer ranged from 4 to 28% (Table 2). However, we previously reported that RSF1010 has no effect on the frequency of tra-mediated conjugal transfer of pTiC58 (11). We considered the possibility that by allowing the Ti plasmid to mobilize RSF1010, traGRP4 influences the conjugal transfer of the Ti plasmid itself. To test this, we mated UIA5(pTiC58ΔaccR) harboring either pDSK519 or pBS141 with C58C1EC and selected independently for transfer of the Ti plasmid and the IncQ plasmid. As before, the Ti plasmid mobilized pBS141 but not the empty vector (Table 3). However, neither pDSK519 nor pBS141 detectably inhibited transfer of the Ti plasmid itself. Transconjugants selected for inheritance of pBS141 again showed low coinheritance of pTiC58ΔaccR (Table 3). As expected from the 104-fold differences between the transfer and mobilization frequencies, none of the tested transconjugants selected for acquiring the Ti plasmid had coinherited pDSK519 or pBS141 (Table 3).
TABLE 3.
TraGRP4-mediated mobilization from the IncQ relaxosome does not interfere with Ti plasmid transfer but may restrict transfer to the same recipient
| IncQ plasmid | Transfer frequencya of:
|
|||
|---|---|---|---|---|
| pTiC58ΔaccRb | Coinheritance of IncQ plasmidc | IncQ derivatived | Coinheritance of Ti plasmide | |
| None | 7.1 × 10−3 | NA | NA | NA |
| pDSK519 | 6.7 × 10−2 | <0.01 | <10−8 | NA |
| pBS141 | 1.2 × 10−2 | <0.01 | 3 × 10−6 | 0.12 |
Expressed as transconjugants per input donor. The experiment was repeated once with similar patterns of results. NA, not applicable.
Transconjugants were selected on AT minimal medium supplemented with chloramphenicol and erythromycin and containing nopaline as the sole carbon source.
Assessed by picking 100 transconjugant colonies to nutrient agar containing kanamycin (pDSK519) or carbenicillin (pBS141) in addition to chloramphenicol and erythromycin.
Transconjugants were selected on nutrient agar containing kanamycin (pDSK519) or carbenicillin (pBS141) in addition to chloramphenicol and erythromycin.
Assessed by picking 100 colonies to AT minimal agar containing nopaline as the sole carbon source in addition to chloramphenicol and erythromycin.
TraGRP4 cannot substitute for TraGpTiC58 for Ti plasmid transfer.
TraGRP4 allows pTiC58 to mobilize RSF1010, suggesting that this protein and TraGC58 are functionally interchangeable. We assessed this possibility by determining whether traGRP4 could restore transfer to pDCKI41, a Tra− traG mutant of pTiC58ΔaccR (Fig. 2 and reference 19). We first determined if the mutation in this plasmid is complementable by traGC58. pDCE20, which codes for this gene as well as the upstream TraR-dependent promoter region (Fig. 2), restored conjugal transfer of pDCKI41 to wild-type levels (data not shown). On the other hand, neither the vector pMMB67HE nor pBS141, the traGRP4-containing recombinant clone, detectably complemented the traG mutation in the Ti plasmid (data not shown). However, while pMMB67HE was not transferred at a detectable level, pBS141, was mobilized by the traG mutant Ti plasmid at a frequency of 2.2 × 10−6 per input donor. Thus, while TraGRP4 will not substitute for TraGpTiC58 for Ti plasmid transfer, it will substitute for mobilization of the IncQ vector.
VirD4 can replace TraGpTiC58 for RSF1010 mobilization but not for Ti plasmid transfer.
Given the relatedness between VirD4 and the TraG family (Fig. 1 and reference 29), we determined whether this vir component can restore transfer to pDCKI41, the traG mutant of pTiC58. To test this possibility, we introduced pHL142, which contains virD4 expressed from the lac promoter of pDSK519 (Fig. 2), into NT1(pDCKI41). The A. tumefaciens donor mobilized pHL142 to A. tumefaciens and E. coli recipients but failed to transfer the mutant Ti plasmid to the A. tumefaciens recipient at a detectable frequency (Table 4). Donors harboring the empty vector, pDSK519 failed to transfer either pDCKI41 or the IncQ plasmid. VirD4 also failed to complement the traGRP4 mutation in pDB127; E. coli donors harboring the mutant ΔtraGRP4 plasmid and pHL142 failed to transfer either element at a detectable frequency to E. coli recipients (data not shown).
TABLE 4.
VirD4 couples transfer of an IncQ plasmid but not the Ti plasmid to the Ti plasmid mating bridge
| Source of coupling proteina | Coupling protein | Frequency of transferb of:
|
||
|---|---|---|---|---|
| pDCKI41 to A. tumefaciens | IncQ plasmid to:
|
|||
| A. tumefaciens | E. coli | |||
| None | None | <10−8 | NA | NA |
| pDCE20 | TraGpTiC58 | 2.3 × 10−3 | NA | NA |
| pDSK519 | None | <10−8 | <10−8 | <10−8 |
| pHL142 | VirD4 | <10−8 | 1.9 × 10−5 | 1.4 × 10−6 |
All A. tumefaciens donors harbored pDCKI41 (traG::Tn3HoHo1) in addition to the recombinant plasmid providing the coupling protein. NA, not applicable.
Expressed as transconjugants obtained per input donor. The experiment was repeated three times with indistinguishable patterns of results.
DISCUSSION
We conclude from our studies that the failure of pTiC58 to mobilize RSF1010 results from the inability of the Ti plasmid TraG protein to recognize the relaxosome of the IncQ plasmid. The IncQ Dtr complex can be transferred by the Ti plasmid mating bridge but requires TraGRP4 or VirD4 as the coupling protein (Tables 2 and 4). Furthermore, mutations in TraGRP4 that affect function in the RP4 transfer system exert a similar effect on Ti plasmid-mediated mobilization from the IncQ relaxosome (Table 2). This observation suggests that TraGRP4 interacts with the transfer systems of RP4 and pTiC58.
The Ti plasmid mating bridge will transfer the RP4 relaxosome, albeit at a low frequency. Transfer is dependent on one or more trans-acting components of the RP4 Dtr system; the Ti plasmid will not mobilize plasmids that contain only the RP4 oriT (11). Furthermore, transfer is dependent on TraR and AAI, which are required for expression of the Ti plasmid trb operon (31). Dependence on the quorum-sensing regulatory components rules out the possibility that transfer of the RP4 relaxosome is mediated by some other conjugal system in the A. tumefaciens donor. Transfer of the RP4 Dtr complex by the Ti plasmid Mpf system is not surprising considering the degree of relatedness between the Mpf proteins of the two conjugation systems (31). However, the components of the two Mpf systems are not interchangeable; the IncP1 Tra2 system will not restore transfer to Ti plasmids with mutations in any one of the nine essential trb genes (32). Furthermore, failure of such Ti plasmid trb mutants to transfer from donors that also harbor an IncP1 plasmid indicates that neither TraGIncP1 nor TraGpTiC58 can interface the Ti plasmid relaxosome with the IncP1 mating bridge. Our studies show that the IncP1-coupling protein will not restore conjugation to a Ti plasmid traG mutant. Thus, while TraGRP4 productively interfaces with the Ti plasmid mating bridge, it cannot interact with the Ti plasmid relaxosome.
These results, as summarized in Table 5, are reminiscent of those reported by Cabezón et al. (7), who concluded that specificities between the relaxosomes and mating bridges of RP4 and the IncW plasmid R388 are conferred by the respective coupling proteins, TraG and TrwB. TraGRP4 can function with the Mpf system but not the relaxosome of R388 (7). Similarly, TraGRP4 allowed pTiC58 to mobilize an IncQ plasmid but did not complement a traGpTiC58 mutant for transfer of the Ti plasmid. Furthermore, Cabezón et al. reported that maximum transfer frequencies occurred only in combinations that included cognate Dtr, Mpf, and coupling proteins (7). Similarly, while RP4 mobilizes RSF1010 at high frequency (30, 56), TraGRP4-mediated mobilization of the IncQ plasmid via the Ti plasmid mating bridge occurs at a low frequency (Table 2). This difference in frequency suggests that the IncP1-coupling protein does not efficiently interface the RSF1010 relaxosome to the Ti plasmid Mpf system.
TABLE 5.
Interactions between Mpf and Dtr systems mediated by IncRh1 and IncP coupling proteins
| Coupling protein | Mating bridgea (commentb)
|
Relaxosome | ||
|---|---|---|---|---|
| IncRh1 trb | IncP | IncRh1 vir | ||
| TraGpTiC58 | + (C) | − (32) | IncRh1 tra | |
| IncP | ||||
| − (11) | IncQ | |||
| − (33) | IncRh1 vir | |||
| TraGRP4 | − (TS) | − (32) | IncRh1 tra | |
| + (TS) | + (C) | IncP | ||
| + (TS) | + (56) | IncQ | ||
| − (33, 47) | IncRh1 vir | |||
| VirD4 | − (TS) | IncRh1 tra | ||
| − (TS) | IncP | |||
| + (TS) | − (TS) | + (3, 6, 20, 21) | IncQ | |
| + (C) | IncRh1 vir | |||
+, effective interface; −, ineffective interface.
C, cognate system; TS, this study. Numbers denote references in which interactions were observed or can be inferred from the data.
Remarkably, VirD4, the TraG homolog from the Ti plasmid vir system, can couple relaxosomes to the Ti plasmid conjugal mating bridge. Like TraGRP4, VirD4 exhibits specificity in this interaction, coupling the relaxosome of RSF1010 but not that of the Ti plasmid to the Ti plasmid Mpf system (Table 4). Furthermore, VirD4 failed to complement an RP4 traG mutation for transfer from the RP4 relaxosome. We conclude from these results that VirD4 can productively recognize the Mpf complex of the Ti plasmid conjugal transfer system, and also the RSF1010 relaxosome, but not the relaxosomes of the Ti plasmid or RP4 (Table 5). Similarly, VirD4 will couple the IncQ relaxosome to the Ti plasmid mating bridge but not to that of RP4 (Table 5). This observation is surprising given the close phylogenetic relationships between the Mpf systems of the two plasmids. On the other hand, neither TraG from an IncP plasmid nor that from pTiC58 will complement mutations in VirD4 for VirB-mediated transfer of T strands to plant cells (33, 47). Whether either TraG protein can interact with VirD2-border complexes, the T-strand equivalent of the relaxosome, remains to be determined.
Taken together, the results point to these coupling proteins as determinants of recognition and specificity. Thus, TraG of the Ti plasmid does not recognize the relaxosomes of RSF1010 or RP4. TraGRP4, on the other hand, can couple its own relaxosome and that of RSF1010 to the Ti plasmid mating bridge (Table 5). But, based on its inability to restore transfer to a traG mutant of pTiC58, TraGRP4 does not recognize the relaxosome of the Ti plasmid. In this regard, most of the available data indicate that specificity is conferred through interactions between the coupling protein and the relaxosome. However, two experimental observations point to specificities with respect to the coupling protein and the mating bridge. First, IncP1 plasmids do not restore transfer to trb mutants of pTiC58ΔaccR (31, 32). Thus, the Ti plasmid TraG protein apparently cannot couple the Ti plasmid relaxosome to the IncP1 Mpf complex. Second, VirD4 can interface the RSF1010 relaxosome to the Ti plasmid mating bridge (Table 4) and also to the vir mating bridge (6), but VirD4 does not allow a traG mutant of the RP4 system to mobilize the IncQ plasmid (data not shown). This observation suggests that VirD4 can couple the IncQ relaxosome to the Ti plasmid Mpf but cannot couple this relaxosome to the RP4 mating bridge.
TraGRP4-mediated mobilization of the IncQ plasmid via the Ti plasmid tra system does not interfere with conjugal transfer of pTiC58 (Table 3). However, although pTiC58 transfers at frequencies 3 to 4 orders of magnitude higher than pBS141, transconjugants selected for receiving the IncQ plasmid coinherit the Ti plasmid at relatively low frequencies (Tables 2 and 3). These observations suggest that Ti plasmid mating bridges catalyzing TraGRP4-mediated transfer of the IncQ plasmid cannot also transfer the Ti plasmid. Thus, we suggest that relaxosome recognition by any given mating bridge is determined by the coupling protein involved in the interaction. This hypothesis could explain why RSF1010 inhibits VirB-mediated T-strand transfer to plants (4, 51) but not trb-mediated Ti plasmid transfer to bacteria (11). In the first case, transfer of both nucleoprotein intermediates is mediated by VirD4 only, and the RSF1010 relaxosome competes with the VirD2-T strand complex for VirB mating bridges (4), perhaps through VirD4. In the latter case, we propose that any given mating bridge is associated with TraGpTiC58 or TraGRP4 but not with both. The former recognizes the relaxosome of the Ti plasmid but not that of RSF1010, while the latter recognizes the relaxosome of RSF1010 but not that of the Ti plasmid. Thus, when both coupling proteins are available, there is no competition between the two relaxosomes for a single mating bridge.
Although there is no direct evidence that the coupling proteins physically interact with the mating bridge, our genetic evidence, as well as that of Cabezón et al. (8), supports this hypothesis. Moreover, should they exist, it is not clear if these interactions are transient, with the coupling protein moving into and out of the complex, or permanent, in which the coupling protein is an integral part of the apparatus itself. However, TraGRP4 is not required for Mpf-associated pilus production or for sensitivity to infection by Mpf-dependent bacteriophages (24, 30, 53), suggesting that the coupling protein is not essential for the construction or structural integrity of the mating bridge itself.
Our results support a model in which the coupling protein interfaces the relaxosome with the mating bridge. The specificity of these proteins for any given component dictates whether the substrate, in the form of the nucleoprotein relaxosome, will be recognized and transported by the mating bridge to a recipient cell. Remarkably, there exists considerable latitude in such specificities (7, 8, 44). It remains to be determined which, if any, components of the relaxosome and of the mating bridge interact with the coupling protein. Similarly, although the C terminus of TraD, the F-coupling protein, confers some degree of specificity (44), the domains of these coupling proteins that are involved in these interactions have yet to be identified.
ACKNOWLEDGMENTS
We thank Ingyu Hwang, Fernando de la Cruz, and Stanton Gelvin for helpful discussions over the course of these studies.
This work was supported in part by grants R01 GM52465 from the NIH and AG92-3312-8231 from the USDA to S.K.F., 96-35301-3178 from the USDA to W.R., and SFB344/A8 from the Deutsche Forschungsgemeinschaft to E.L. C.M.H. was the recipient of an undergraduate research award from the Colgate-Palmolive company. P.-L.L. was supported in part by HATCH project 15-0326 to S.K.F.
REFERENCES
- 1.Alt-Mörbe J, Stryker J L, Fuqua C, Li P-L, Farrand S K, Winans S C. The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of IncP and distantly related to Ti plasmid vir genes. J Bacteriol. 1996;178:4248–4257. doi: 10.1128/jb.178.14.4248-4257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzer D, Pansegrau W, Lanka E. Essential motifs of relaxase (TraI) and TraG proteins involved in conjugative transfer of plasmid RP4. J Bacteriol. 1994;176:4285–4295. doi: 10.1128/jb.176.14.4285-4295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beijersbergen A, den Dulk-Ras A, Schilperoort R A, Hooykaas P J J. Conjugative transfer by the virulence system of Agrobacterium tumefaciens. Science. 1992;256:1324–1327. doi: 10.1126/science.256.5061.1324. [DOI] [PubMed] [Google Scholar]
- 4.Binns A N, Beaupré C E, Dale E M. Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J Bacteriol. 1995;177:4890–4899. doi: 10.1128/jb.177.17.4890-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blatny J M, Brautaset T, Winther-Larson H C, Haugan K, Valla S. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl Environ Microbiol. 1997;63:370–379. doi: 10.1128/aem.63.2.370-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan-Wollaston V, Passiatore J, Cannon F. The mob and oriT mobilization functions of a bacterial plasmid promote its transfer to plants. Nature (London) 1987;328:172–175. [Google Scholar]
- 7.Cabezón E, Lanka E, de la Cruz F. Requirements for mobilization of plasmids RSF1010 and ColE1 by the IncW plasmid R388: trwB and RP4 traG are interchangeable. J Bacteriol. 1994;176:4455–4558. doi: 10.1128/jb.176.14.4455-4458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabezón E, Sastre J I, de la Cruz F. Genetic evidence of a coupling role for the TraG protein family in bacterial conjugation. Mol Gen Genet. 1997;254:400–406. doi: 10.1007/s004380050432. [DOI] [PubMed] [Google Scholar]
- 9.Cha C, Gao P, Chen Y-C, Shaw P D, Farrand S K. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol Plant-Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- 10.Chilton M-D, Currier T C, Farrand S K, Bendich A J, Gordon M P, Nester E W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook D M, Farrand S K. The oriT region of the Agrobacterium tumefaciens Ti plasmid pTiC58 shares DNA sequence identity with the transfer origins of RSF1010 and RK2/RP4 and with the T-region borders. J Bacteriol. 1992;174:6238–6246. doi: 10.1128/jb.174.19.6238-6246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 13.Das A, Xie Y-H. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol Microbiol. 1998;27:405–414. doi: 10.1046/j.1365-2958.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 14.de la Cruz F, Lanka E. Function of the Ti-plasmid Vir proteins: T-complex formation and transfer to the plant cell. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae. Molecular biology of model plant-associated bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 281–301. [Google Scholar]
- 15.Derbeyshire K M, Hatfull G, Willetts N. Mobilization of nonconjugative plasmid RSF1010: a genetic and DNA sequence analysis of the mobilization region. Mol Gen Genet. 1987;206:161–168. doi: 10.1007/BF00326552. [DOI] [PubMed] [Google Scholar]
- 16.Derbyshire K M, Willetts N S. Mobilization of non-conjugative plasmid RSF1010; a genetic analysis of its origin of transfer. Mol Gen Genet. 1987;206:154–160. doi: 10.1007/BF00326551. [DOI] [PubMed] [Google Scholar]
- 17.Eisenbrandt R, Kalkum M, Lai E-M, Lurz R, Kado C I, Lanka E. Conjugative pili of IncP plasmids and the Ti plasmid T-pilus are composed of cyclic subunits. J Biol Chem. 1999;274:22548–22555. doi: 10.1074/jbc.274.32.22548. [DOI] [PubMed] [Google Scholar]
- 18.Farrand S K. Conjugation in the Rhizobiaceae. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae. Molecular biology of model plant-associated bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 199–233. [Google Scholar]
- 19.Farrand S K, Hwang I, Cook D M. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J Bacteriol. 1996;178:4233–4247. doi: 10.1128/jb.178.14.4233-4247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fullner K J. Role of Agrobacterium virB genes in transfer of T complexes and RSF1010. J Bacteriol. 1998;180:430–434. doi: 10.1128/jb.180.2.430-434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fullner K J, Nester E W. Environmental and genetic factors affecting RSF1010 mobilization between strains of Agrobacterium tumefaciens. In: Ream W, Gelvin S B, editors. Crown gall. Advances in understanding interkingdom gene transfer. St. Paul, Minn: APS Press; 1996. pp. 15–29. [Google Scholar]
- 22.Fürste J P, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 23.Ghai J, Das A. The virD operon of Agrobacterium tumefaciens Ti plasmid encodes a DNA-relaxing enzyme. Proc Natl Acad Sci USA. 1989;86:9154–9158. doi: 10.1073/pnas.86.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haase J, Lurz R, Grahn A M, Bamford D H, Lanka E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J Bacteriol. 1995;177:4779–4791. doi: 10.1128/jb.177.16.4779-4791.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang I, Li P-L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knauf V C, Nester E W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982;8:45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- 27.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 28.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop II R M, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 29.Lessl M, Pansegrau W, Lanka E. Relationships of DNA-transfer-systems: essential transfer factors of plasmids RP4, Ti and F share common sequences. Nucleic Acids Res. 1992;20:6099–6100. doi: 10.1093/nar/20.22.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lessl M, Balzer D, Weyrauch K, Lanka E. The mating pair formation system of plasmid RP4 defined by RSF1010 mobilization and donor-specific phage propagation. J Bacteriol. 1993;175:6415–6425. doi: 10.1128/jb.175.20.6415-6425.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P-L, Everhart D M, Farrand S K. Genetic and sequence analysis of the pTiC58 trb locus, encoding a mating-pair formation system related to members of the type IV secretion family. J Bacteriol. 1998;180:6164–6172. doi: 10.1128/jb.180.23.6164-6172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P-L, Farrand S K. Essential components of the Ti plasmid trb system, a type IV macromolecular transporter. J Bacteriol. 1999;181:5033–5041. doi: 10.1128/jb.181.16.5033-5041.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin T-X, Kado C I. The virD4 gene is required for virulence while virD3 and orf5 are not required for virulence of Agrobacterium tumefaciens. Mol Microbiol. 1993;9:803–812. doi: 10.1111/j.1365-2958.1993.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 34.Luo Z-Q, Farrand S K. Signal-dependent DNA binding and functional domains of the quorum-sensing activator TraR as identified by repressor activity. Proc Natl Acad Sci USA. 1999;96:9009–9014. doi: 10.1073/pnas.96.16.9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli M L, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto S, Toyoda-Yamamoto A, Ito K, Takebe I, Machida Y. Localization and orientation of the VirD4 protein of Agrobacterium tumefaciens in the cell membrane. Mol Gen Genet. 1991;228:24–32. doi: 10.1007/BF00282443. [DOI] [PubMed] [Google Scholar]
- 37.Pansegrau W, Lanka E. Common sequence motifs in DNA relaxases and nick regions from a variety of DNA transfer systems. Nucleic Acids Res. 1991;19:3445. doi: 10.1093/nar/19.12.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petit A, Tempé J. Isolation of Agrobacterium Ti plasmid regulatory mutants. Mol Gen Genet. 1978;167:147–155. [Google Scholar]
- 39.Pohlman R F, Genetti H D, Winans S C. Common ancestry between IncN conjugal transfer genes and macromolecular export systems of plant and animal pathogens. Mol Microbiol. 1994;14:655–668. doi: 10.1111/j.1365-2958.1994.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 40.Porter S G, Yanofsky M F, Nester E W. Molecular characterization of the virD operon from Agrobacterium tumefaciens. Nucleic Acids Res. 1987;15:7503–7517. doi: 10.1093/nar/15.18.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogowsky P M, Powell B S, Shirasu K, Lin T-S, Morel P, Zykprian E M, Steck T R, Kado C I. Molecular characterization of the vir regulon of Agrobacterium tumefaciens: complete nucleotide sequence and gene organization of the 28.63-kbp regulon cloned as a single unit. Plasmid. 1990;23:85–106. doi: 10.1016/0147-619x(90)90028-b. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg C, Huguet T. The pAtC58 plasmid of Agrobacterium tumefaciens is not essential for tumor induction. Mol Gen Genet. 1984;196:533–536. [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Sastre J I, Cabezóne E, de la Cruz F. The carboxyl terminus of protein TraD adds specificity and efficiency to F-plasmid conjugative transfer. J Bacteriol. 1998;180:6039–6042. doi: 10.1128/jb.180.22.6039-6042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scherzinger E, Lurz R, Otto S, Dobrinski B. In vitro cleavage of double- and single-stranded DNA by plasmid RSF1010-encoded mobilization proteins. Nucleic Acids Res. 1992;20:41–48. doi: 10.1093/nar/20.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholz P, Haring V, Wittmann-Liebold B, Ashman K, Bagdasarian M, Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host range plasmid RSF1010. Gene. 1989;75:271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- 47.Stachel S E, Nester E W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986;5:1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toro N, Datta A, Carmi O A, Young C, Prusti R K, Nester E W. The Agrobacterium tumefaciens virC1 gene product binds to overdrive, a T-DNA transfer enhancer. J Bacteriol. 1989;171:6845–6849. doi: 10.1128/jb.171.12.6845-6849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Larebeke N, Genetello C, Schell J, Schilperoort R A, Hermans A K, Hernalsteens J P, van Montagu M. Acquisition of tumor-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature (London) 1975;255:742–743. doi: 10.1038/255742a0. [DOI] [PubMed] [Google Scholar]
- 50.Vogel A M, Das A. Mutational analysis of Agrobacterium tumefaciens pTiA6 virD1: identification of functionally important residues. Mol Microbiol. 1994;12:811–817. doi: 10.1111/j.1365-2958.1994.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 51.Ward J E, Dale E M, Binns A N. Activity of the Agrobacterium T-DNA transfer machinery is affected by virB gene products. Proc Natl Acad Sci USA. 1991;88:9350–9354. doi: 10.1073/pnas.88.20.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waters V L, Guiney D G. Processes at the nick region link conjugation, T-DNA transfer and rolling circle replication. Mol Microbiol. 1993;9:1123–1130. doi: 10.1111/j.1365-2958.1993.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 53.Waters V L, Strack B, Pansegrau W, Lanka E, Guiney D G. Mutational analysis of essential IncPα plasmid transfer genes traF and traG and involvement of traF in phage sensitivity. J Bacteriol. 1992;174:6666–6673. doi: 10.1128/jb.174.20.6666-6673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson B, Currier T C, Gordon M P, Chilton M-D, Nester E W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975;123:255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willetts N S, Crowther C. Mobilization of nonconjugative IncQ plasmid RSF1010. Genet Res. 1981;37:311–316. doi: 10.1017/s0016672300020310. [DOI] [PubMed] [Google Scholar]
- 57.Winans S C, Burns D L, Christie P J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. doi: 10.1016/0966-842X(96)81513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziegelin G, Pansegrau W, Strack B, Balzar D, Kröger M, Kruft V, Lanka E. Nucleotide sequence and organization of genes flanking the transfer origin of promiscuous plasmid RP4. J DNA Seq Map. 1991;1:303–327. doi: 10.3109/10425179109020786. [DOI] [PubMed] [Google Scholar]