Abstract
Cutaneous involvement in multiple myeloma with extramedullary disease is rare. We report the case of a refractory multiple myeloma patient who developed a cutaneous lesion. Histopathology revealed dermal immature plasma cell infiltrate with a lack of CD138 expression. This cutaneous location was associated with an aggressive clinical course and short survival.
Keywords: cutaneous involvement, extramedullary disease, immature plasma cells, multiple myeloma
Cutaneous infiltration is a rare manifestation of multiple myeloma. It generally occurs at a late stage of the disease and is an ominous clinical sign. Skin involvement is usually associated with poor prognosis and requires aggressive therapies.
1. INTRODUCTION
Multiple myeloma (MM) is characterized by clonal expansion of plasma cells in bone marrow. In some MM patients, myeloma cells can lose their dependence on the bone marrow microenvironment and then infiltrate soft tissues, resulting in extramedullary multiple myeloma (EMM). EMM may be present at the time of MM diagnosis (primary EMM) or during follow‐up of the disease (secondary EMM). 1
2. CASE REPORT
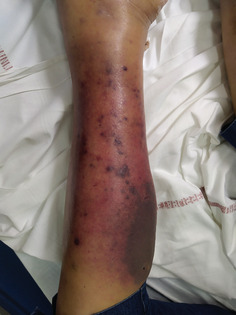
A 61‐year‐old woman was being followed up in the department of Internal Medicine for non‐secretory MM diagnosed in 2008 and revealed by a lytic lesion at T11. Her past medical history included sarcoidosis. She underwent local radiotherapy. First‐line therapy consisted of four cycles of bortezomib and dexamethasone followed by high‐dose melphalan and autologous stem cell transplantation (ASCT). The response was good. She relapsed in 2012 and was treated initially with lenalidomide and dexamethasone followed by a second high‐dose course of melphalan and ASCT and then lenalidomide maintenance. In 2016, she presented with disease progression and received the triplet combination bortezomib‐cyclophosphamide‐dexamethasone. This regimen was rapidly switched to daratumumab and dexamethasone because of thrombopenia related to massive bone marrow invasion of plasma cells. A very good response was achieved. Unfortunately, she relapsed in February 2018, presenting with bone pain and diffuse progressive bone lesions. She started a new line of therapy comprising ixazomib, pomalidomide, dexamethasone, and radiotherapy in the lumbar spine and left tibia. In December 2018, due to progressive bone lesions, particularly epidural posterior infiltration at T6‐T7, she underwent local radiotherapy in the thoracic spine and femurs. A bone marrow biopsy showed a massive invasion of atypical plasma cells with KRAS mutation. A treatment of nine cycles of carfilzomib, cyclophosphamide, and dexamethasone was started in March 2019. She subsequently developed two costal plasmacytomas and underwent radiotherapy. Salvage therapy by selinexor was initiated in November 2019. In March 2020, multiple violaceous nodules appeared on the left leg, some of them tend to become confluent and form an extensive infiltrated plaque (Figure 1). Serum soluble vascular epithelial growth factor (sVEGF) was normal. The histopathologic study of these lesions showed focal and perivascular infiltrate composed of immature plasma cells within the dermis. Tumor cells are poorly cohesive and characterized by increase in cell size, eosinophilic cytoplasm, ovoid nucleus, thin chromatin, and one or multiple nucleoli. On immunohistochemistry, the cells were positive for MUM1 and lambda light chains (Figure 2). They were negative for CD138. We observed that malignant cells changed their shape when invading the skin. Indeed, many years before skin involvement, a bone marrow biopsy showed smaller malignant cells exhibiting fewer nucleoli and a chromatin in a more lumpy pattern. Plasma cells showed positivity for CD138 and monoclonal expression for lambda light chain (Figure 3). Unfortunately, she died shortly after the diagnosis of cutaneous involvement.
FIGURE 1.
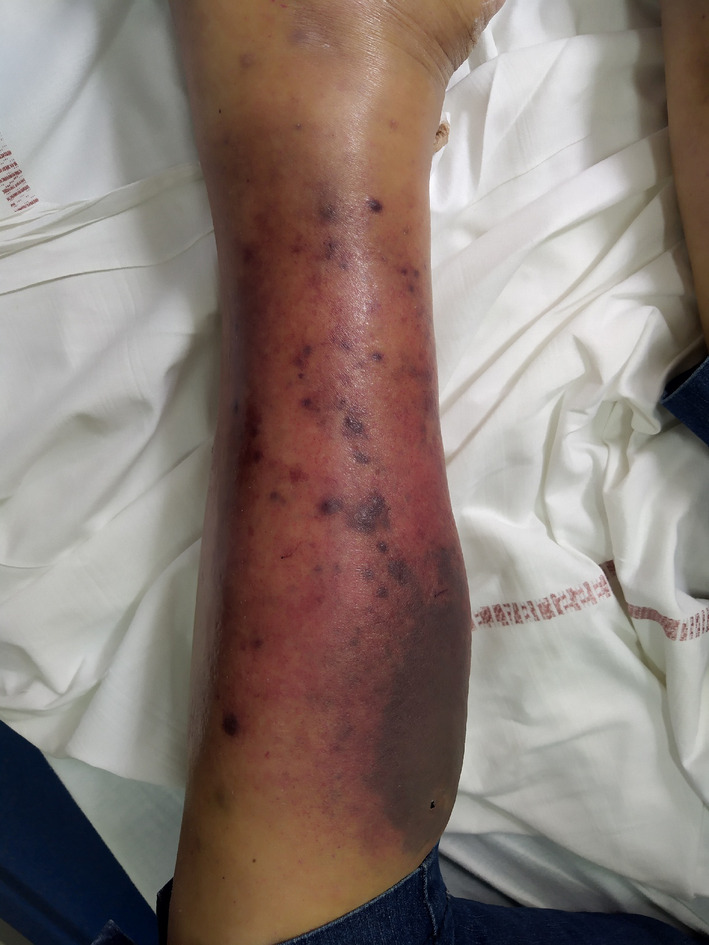
Numerous violaceous nodules with infiltrated plaque
FIGURE 2.
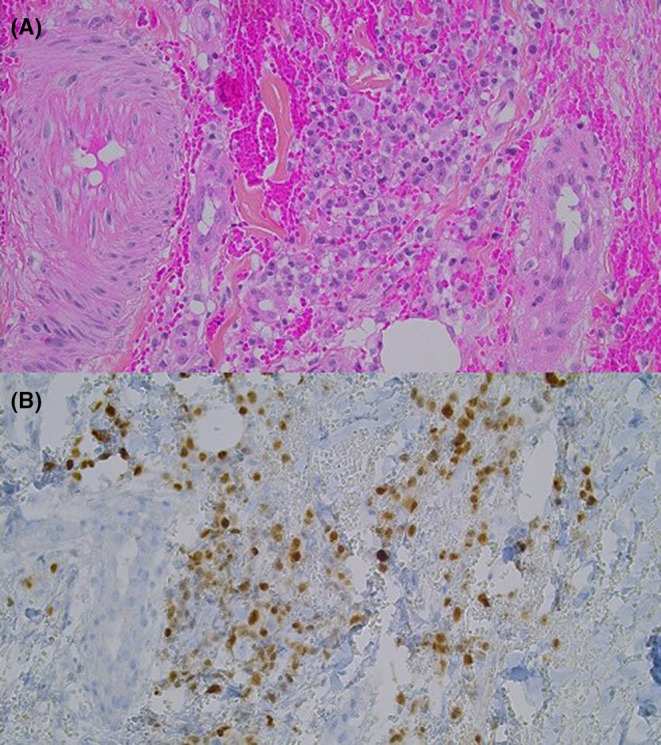
Morphological and immunohistochemical characteristics of the biopsed skin lesion. (A) Focal and perivascular dermal immature plasma cells infiltrate (HPS, 200×). (B) Plasma cells show positivity for MUM1 (IHC, 200×).
FIGURE 3.
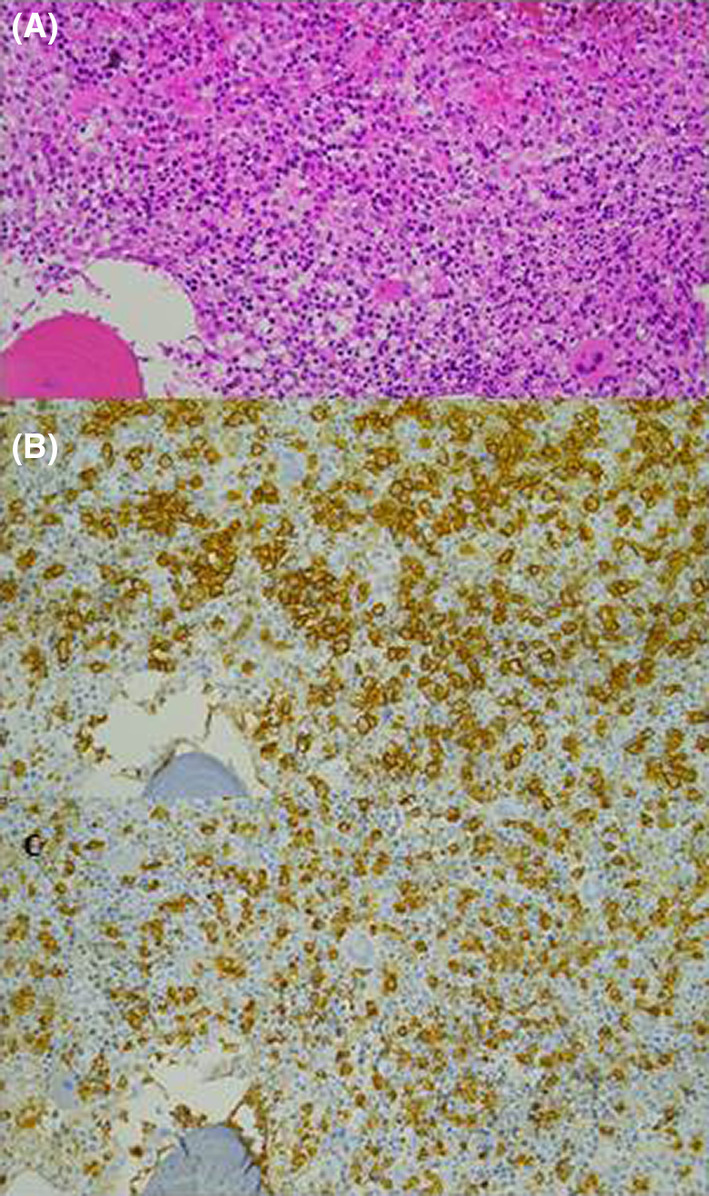
Bone marrow biopsy before skin involvement. (A) Abundant plasma cells infiltration (HE, 200×). (B) Tumor cells show positivity for CD138 (IHC, 200×). (C) The plasma cells predominantly express the lambda light chain (IHC, 200×).
3. DISCUSSION
Extramedullary disease (EM) varies among studies. In a longitudinal study of 1003 consecutive MM patients, Varettoni et al. 2 showed that 6% of them (56/1003) had EM involvement during follow‐up. In a cohort of 834 patients, Deng et al. found that 3.4% (28/834) of patients showed EM relapse and/or progression during follow‐up. 3 Among them, only two patients had skin involvement. Since the initial report of skin involvement in MM published by Bruno Bloch in 1910, several case reports and case series have been reported in the medical literature. 4 , 5 , 6 , 7 , 8 Therefore, cutaneous involvement is rare, usually occurring late in the course of the disease, and is associated with poor prognosis. This confirms the findings reported in our observation. We observed the lack of CD138 expression by the plasma cell lineage. In a different case series of MM located on the skin, cutaneous plasma cells were strongly stained with CD138. Interestingly, it has been demonstrated that the loss of CD138 may contribute to cell invasion and the EM spreading of MM. 9 Moreover, we observed KRAS mutation in the bone marrow sample of our patient. This result is in accordance with the high frequency of 69% of RAS mutations found in patients with EM relapse. 10 This mutation is usually present at the time of diagnosis. The mechanisms involved in skin homing of plasma cells are unknown. However, in a recent report of MM in a cutaneous location, the authors found high expression of CCR10 in plasma cells at the diagnosis and relapse, and a lack of CXCR4 surface expression during disease progression. 11
4. CONCLUSION
Cutaneous involvement in MM is uncommon and is associated with poor prognosis. It could be also a sign of the last stage of this disease. Further studies are warranted to understand underlying molecular mechanisms and to better define biological markers and genetic features as risk factors of transition to EMM. This could consequently lead to the development of new treatment strategies.
AUTHOR CONTRIBUTIONS
VS wrote the manuscript. VS, XB, JDK, AS, JS PD, and AD cared for the patient during follow‐up of the disease. CA provided results of the histopathologic study and immunohistochemical analysis. LS performed the skin biopsy.
CONFLICT OF INTEREST
There are no conflicts of interest to declare from any author with regard to this publication.
CONSENT
Written informed consent was obtained from the husband's patient for the publication of this case report and associated images.
ACKNOWLEDGEMENT
None.
Salle V, Attencourt C, Chevalier M, et al. Cutaneous involvement in relapsed multiple myeloma. Clin Case Rep. 2022;10:e06282. doi: 10.1002/ccr3.6282
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sevcikova S, Minarik J, Stork M, Jelinek T, Pour L, Hajek R. Extramedullary disease in multiple myeloma ‐ Controversies and future directions. Blood Rev. 2019;36:32‐39. [DOI] [PubMed] [Google Scholar]
- 2. Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325‐330. [DOI] [PubMed] [Google Scholar]
- 3. Deng S, Xu Y, An G, et al. Features of extramedullary disease of multiple myeloma: high frequency of p53 deletion and poor survival: a retrospective single‐center study of 834 cases. Clin Lymphoma Myeloma Leuk. 2015;15:286‐291. [DOI] [PubMed] [Google Scholar]
- 4. Güvenç B, Canataroğlu A, Gümürdülü Y, et al. Multiple myeloma with skin involvement. J Eur Acad Dermatol Venereol. 2001;15:328‐329. [DOI] [PubMed] [Google Scholar]
- 5. Tomas JF, Garrido JA, Ramos FJ, González M, San Miguel JF. Skin involvement in non‐secretory myeloma. Am J Med. 1988;84:373‐374. [DOI] [PubMed] [Google Scholar]
- 6. Varricchio S, Pagliuca F, Travaglino A, Gallo L, Villa MR, Mascolo M. Cutaneous localization of plasmablastic multiple myeloma with heterotopic expression of CD3 and CD4: skin involvement revealing systemic disease. J Cutan Pathol. 2019;46:619‐622. [DOI] [PubMed] [Google Scholar]
- 7. Requena L, Kutzner H, Palmedo G, et al. Cutaneous involvement in multiple myeloma: a clinicopathologic, immunohistochemical, and cytogenetic study of 8 cases. Arch Dermatol. 2003;139:475‐486. [DOI] [PubMed] [Google Scholar]
- 8. Malysz J, Talamo G, Zhu J, et al. Cutaneous involvement in multiple myeloma (MM): A case series with clinicopathologic correlation. J Am Acad Dermatol. 2016;74:878‐884. [DOI] [PubMed] [Google Scholar]
- 9. Bayer‐Garner IB, Sanderson RD, Dhodapkar MV, Owens RB, Wilson CS. Syndecan‐1 (CD138) immunoreactivity in bone marrow biopsies of multiple myeloma: shed syndecan‐1 accumulates in fibrotic regions. Mod Pathol. 2001;14:1052‐1058. [DOI] [PubMed] [Google Scholar]
- 10. de Haart SJ, Willems SM, Mutis T, et al. Comparison of intramedullary myeloma and corresponding extramedullary soft tissue plasmacytomas using genetic mutational panel analyses. Blood Cancer J. 2016;6:e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marchica V, Accardi F, Storti P, et al. Cutaneous localization in multiple myeloma in the context of bortezomib‐based treatment: how do myeloma cells escape from the bone marrow to the skin? Int J Hematol. 2017;105:104‐108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


