Abstract
Small vasculature, venous obstruction, or congenital anomalies can preclude transvenous access to the heart, often resulting in open chest surgery to implant cardiac therapy leads for pacing, defibrillation, or cardiac resynchronization. A minimally invasive approach under direct visualization could reduce tissue damage, minimize pain, shorten recovery time, and obviate the need for fluoroscopy. Therefore, PeriPath was designed as a single-use, low-cost pericardial access tool based on clinical requirements. Its mechanical design aids in safe placement of conductive leads to the pericardium using a modified Seldinger technique. The crossed working channels provide an optimal view of the surgical field under direct visualization. Finite element analysis (FEA) confirms that the device is likely not to fail under clinical working conditions. Mechanical testing demonstrates that the tensile strength of its components is sufficient for use, with minimal risk of fracture. The PeriPath procedure is also compatible with common lead implantation tools and can be readily adopted by interventional cardiologists and electrophysiologists, allowing for widespread implementation. Prior animal work and a physician preliminary validation study suggest that PeriPath functions effectively for minimally invasive lead implantation procedures.
Background
Infants and children that suffer from bradyarrhythmias, such as sinus node dysfunction or atrioventricular block, or tachyarrhythmias, such as ventricular tachycardia or fibrillation, may require implantation of a cardiovascular implantable electronic device (CIED), such as a pacemaker or implantable cardioverter defibrillator (ICD), to restore and/or maintain normal heart rate and rhythm. The implant procedure is intended to be percutaneous, with an interventional electrophysiologist accessing the heart via the subclavian vein to attach thin conductive leads that are as small as 1.5 mm in diameter into endocardial tissue [1]. Unfortunately, neonates, infants, children, and even some adults with congenital heart disease cannot undergo the standard implantation procedure due to small vasculature, potential venous obstruction, and/or congenital anomalies that can preclude transvenous access to the heart [2].
The current approach for these patients is to implant pacing or defibrillator leads via open chest surgery. Conductive pacing leads are sewn on or screwed into the epicardial surface of the heart via a sternal or thoracic incision. Instead of an outpatient procedure, the patient must now spend multiple postoperative days in the hospital, often requiring intensive care. They will also experience more pain and are at a higher risk of infection compared to a transvenous procedure [3]. Patients who have previously undergone cardiac or other thoracic surgeries may also develop intrathoracic adhesions, which can increase procedural complications and morbidity [4].
In addition to pacing for bradycardia, there are nearly 50,000 children and adults per year who require cardiac resynchronization therapy (CRT) for heart failure [5]. CRT requires placement of pacing leads (either endocardial or epicardial) on both ventricles to improve the synchrony of ventricular depolarization and contraction. In 30% of transvenous CRT cases, patients do not derive hemodynamic benefit. Limitations of transvenous access or insufficient coronary sinus branches on which to pace the left ventricle likely contribute to CRT nonresponsiveness [6]. Other patient populations who might benefit from a minimally invasive solution include those with tortuous vasculature, venous anomalies, or the inability to tolerate open surgical approaches due to blood disorders.
Pericardial access under direct thoracoscopic visualization is a promising alternative approach to open chest surgery that would revolutionize care for many of these patients. First, a minimally invasive approach reduces collateral tissue damage, minimizes pain, and shortens recovery times [7], even in pediatric populations when surgeons implant ICD leads using a smaller surgical incision and fluoroscopy [8]. Second, direct visualization clearly identifies coronary arteries and other key epicardial structures and eliminates the need for fluoroscopy, which has damaging ionizing radiation effects [9]. Finally, a minimally invasive approach can be readily learned by interventional cardiologists and electrophysiologists, enabling quick adoption of the technique to increase the number of patients treated.
Previous Work
Clinical Solutions.
There are a few clinical approaches that have been studied to assist with less invasive implantation of CIED devices. Novel techniques for minimally invasive pacemaker or ICD implantation include the use of a subcutaneous array for the shocking coil [10], placement of the shocking coil in the transverse sinus or the anterior portion of the left atrial appendage [11], video-assisted thoracoscopic-guided lead placement through the intercostal space [12], and lead placement through a mini-thoracotomy incision [13]. While these are less invasive than open sternotomy or thoracotomy, they still require a large incision, a cardiac surgeon, and longer recovery times with postoperative pain compared to a minimally invasive approach. Additionally, all these studies involve surgeons using approved medical devices off label indications they were not designed for, presenting even greater risks to patients. This highlights the need for an enabling technology to implant cardiac therapy leads for pacing, defibrillation, and CRT in patients of any age and size and regardless of the presence of structural or congenital heart defects.
Engineering Solutions.
There are three commercial devices that can be used to implant a medical device laparoscopically: multilumen ports, single lumen trocars, and force sensing needles. Commercial multilumen devices include the SILS Port (Medtronic/Covidien) and GelPort (Applied Medical). Notably, the SILS port was used in a single-incision thoracoscopic surgery to treat primary spontaneous pneumothorax [14], yet the device requires a 4–5 cm incision, which is comparable to an open surgical minithoracotomy [15]. Alternatively, multiport thoracic surgery with single channel trocars has been proposed for video-assisted thoracoscopic surgery (VATS); however, the technique requires more than one operator to manage the ports, which is not ideal for pediatric surgeries, and is less preferred compared to uniport VATS [16]. While multilumen ports and single lumen trocars have been used off label, EpiEP's pericardial access system is the only commercial device specifically indicated for pericardial access. EpiAccess consists of a pressure sensitive needle and visualization unit that provides feedback when a needle contacts pericardial tissue. Clinical studies have demonstrated safe pericardial access [17], but adoption may be hindered by system cost and size that limits integration within the hospital.
PeriPath Solution.
To overcome these limitations in existing surgical approaches and medical devices, we have designed PeriPath, a low profile and low-cost pericardial access tool that obviates the need for open chest surgery for the placement of conductive leads (Fig. 1). PeriPath has been used to implant pacing leads [18], defibrillation leads [19], and miniature pacemakers [20] in infant animal models using a 1 cm incision. In this work, we describe in detail the mechanical design and functionality of the PeriPath device in the context of clinical requirements for pericardial access. We then simulate mechanical failure using Finite Element Analysis (FEA) based on the clinical requirements. Standardized verification testing is performed on manufactured prototypes to demonstrate the tool performs as intended. We conclude with a preliminary validation study to assess Human Factors by comparing PeriPath with other techniques utilizing commercial access tools.
Fig. 1.
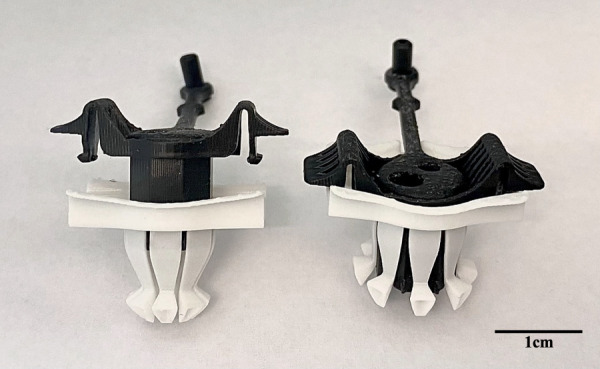
PeriPath in the unlocked (left) and locked (right) positions. The scale bar represents 1 cm.
Device Design
Clinical Requirements for Design.
The PeriPath device is an innovative technology that aids minimally invasive implantation of pacing and defibrillator leads in the hopes of decreasing patient pain, recovery times, and risk of infection. The device's constrained working channels promote direct visualization and eliminate tool clashing, minimizing risk of blind heart perforation or damage to coronary vasculature. PeriPath's design was based on clinically motivated requirements from discussions with experienced surgeons, interventional cardiologists, and electrophysiologists (Table 1).
Table 1.
Detailed design specifications for the PeriPath device
| Category | Requirement | Value | Rationale |
|---|---|---|---|
| Size | Diameter | <1 cm | The 1 cm window is essential to deliver larger cardiac therapies to the heart |
| Mechanical | Angles | 25 deg | Optimal surgical field of view prevents risk of ventricular punctures |
| Forces | Insertion | <1 N | Device insertion should not require excess force, demonstrating its ease of use |
| Forces | Deployment | <10 N | Device deployment should not require excess force, demonstrating its ease of use |
| Forces | Working channel drag forces | <2 N | Manipulation of instrumentation within the working channel should require minimal effort |
| Forces | Visualization channel drag forces | >2 N and <10 N | Endoscope insertion should not require excess force and should not dislodge during a procedure |
| Forces | Retention | >10 N | The device should not be easily dislodged when locked into the incision during a procedure |
| Forces | Torque | <0.01 N·m | The device should not fracture when twisted at a usable torque. |
| Operation | Mode of operation | Single User | One user should have the ability to hold the endoscope and manipulate instrumentation simultaneously |
Device Components
Elastic Core With Tethered Cannulated Plug.
The elastic core, made from ethylene vinyl acetate copolymer (EPU40, Carbon, Redwood City, CA), contains a visualization (5 mm) and working (3.5 mm) channel (Fig. 2). The 25 deg of separation between the working channels optimizes the surgical field so that the delivery tools do not clash with each other and remain within the field of view for the entirety of the procedure. Only a single operator is needed due to the fixed working channels, and PeriPath's diameter is only 1 cm. The visualization channel is used to accommodate a trocar for thoracic insufflation and an endoscope for imaging. The 3.5 mm working channel is used to accommodate implantation instruments, such as a guidewire, dilator, and sheath. A rigid polyurethane (RPU70, Carbon, Redwood City, CA) plug with a 1.27 mm cannulation is tethered to the elastic core and used to reduce the smaller port for a needle to be used for incising the pericardium (Fig. 2) while maintaining adequate insufflation. PeriPath's core can be removed to create a 1 cm surgical window that permits implantation of devices larger than the working channel.
Fig. 2.
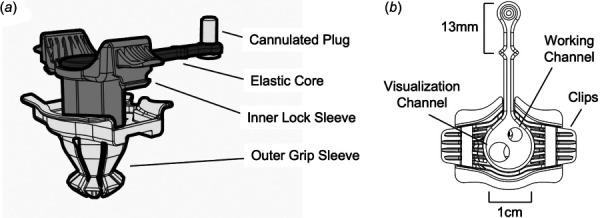
PeriPath with labeled device components in (a) isometric and (b) top views
Inner Lock Sleeve.
The inner lock sleeve is also made up of RPU70, which is known for its high strength, functional toughness, and high ductility. The component's biocompatibility and ultimate tensile strength of 40 MPa makes it a good option to hold the elastic core. It also includes clips that function to snap the inner lock sleeve into the outer grip sleeve to secure the components together (Fig. 2).
Outer Grip Sleeve.
The last component of the PeriPath device is its outer grip sleeve, which is required to secure PeriPath within the skin incision (Fig. 2). This component is composed of a styrene material known as MPU100 (Carbon, Redwood City, CA), a material comparable to medical-grade acrylonitrile butadiene styrene. Not only is this material biocompatible, sterilizable, and durable, but it also has engineering-grade mechanical properties, such as a high-tensile strength of 38 MPa.
The outer grip sleeve is the first component to enter the incision, so its design is slightly tapered for a less traumatic insertion. This part also has collapsible distal flange that deploys and anchors the device across the chest wall (Fig. 1). The novel flange makes PeriPath shorter than competing trocars, providing access to the thoracic space without crossing the diaphragm or risking injury to the heart or lungs on insertion. Finally, the outer grip sleeve includes wings that help to release the inner lock sleeve clips when squeezed. Overall, the design characteristics of the outer grip sleeve aid in easy insertion, use, and removal of the device.
Procedure Workflow
Setup.
Figure 3 details the surgical workflow for using the PeriPath access tool. First, PeriPath is given to the user in the assembled state, and the patient is marked below the xiphoid process with a 13 mm line using the distance between the base and the diamond on the core's tether as a guide (labeled in Fig. 2(b)). An incision is made along the mark using the standard surgical technique. The device is placed in the incision and oriented so that the tether points toward the patient's head (Fig. 3(a)). PeriPath is locked into the incision by firmly pressing down on the center of the inner locking sleeve. This locking motion deploys the flanges and grips the feet to the underside of the skin.
Fig. 3.
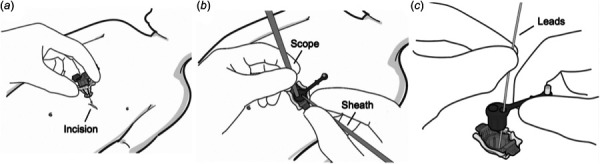
PeriPath procedure workflow overview. (a) Inserting the PeriPath device into the incision. (b) Utilizing the access ports of the PeriPath device. (c) Removing the core of the device.
Operation.
With PeriPath locked into the incision, the access port can be used to conduct the surgical procedure (Fig. 3(b)). The trocar is positioned in the larger working channel, and the left lung is partially collapsed by insufflation. The deflectable scope is passed through the trocar, and its viewing angle is adjusted to visualize the heart. The tethered plug is inserted into the smaller port to reduce the diameter and limit insufflation gas leakage. A needle is placed through the plug in the working channel and advanced into the surgical field of view to pierce the pericardial sac under direct visualization. The needle is replaced by a flexible guide wire to maintain pericardial access. A sheath with a dilator is advanced in the pericardial space using the modified Seldinger technique. Finally, a cardiac lead is inserted through the sheath and fixated directly to the epicardium [21].
Removal.
To remove PeriPath, the elastic core is first pulled out from the inner grip sleeve by pinching the tether close to the core and gently pulling in an upward direction, while the opposite hand resists the pull on the outer sleeve flange (Fig. 3(c)). A scalpel is used to cut along the vertical axis of the port and separate the elastic core from the lead. The inner lock sleeve is unlocked from the outer grip sleeve by pressing on the ends of the locking tabs in an inward direction. The PeriPath device is removed from the incision by pulling upward on the lock sleeve tabs. The leads are held in place to prevent them from pulling out with the port.
Performance Testing
Finite Element Analysis.
Finite element analysis (FEA) using SolidWorks SimulationXpress (BIOVIA, Dassault Systèmes, San Diego, CA) was used to first simulate performance of PeriPath under the required clinical forces (Table 1) and then find the maximum force at which the device would fracture. For each test, the material was set to MPU100, which has an elastic modulus of 1200 MPA, Poisson's ratio of 0.35, a mass density of 1120 kg/m3, a tensile strength of 38 MPA, and an ultimate strength of 15 MPA. Fixtures were added to prescribe zero displacements on certain faces during static simulation use (as shown with blunted arrows in Fig. 4), whereas external loads were added to demonstrate forces simulated (as shown with long arrows in Fig. 4). A curvature-based mesh was selected, and both stress and factor of safety (FOS) were analyzed for each test (Fig. 4). Three simulations were run per test to confirm repeatability and reproducibility of the FEA, with marginal differences in maximum stress (±0.02 MPa) and minimum FOS (±0.01) observed between simulations. The most critical values (highest stress and lowest FOS) were recorded.
Fig. 4.
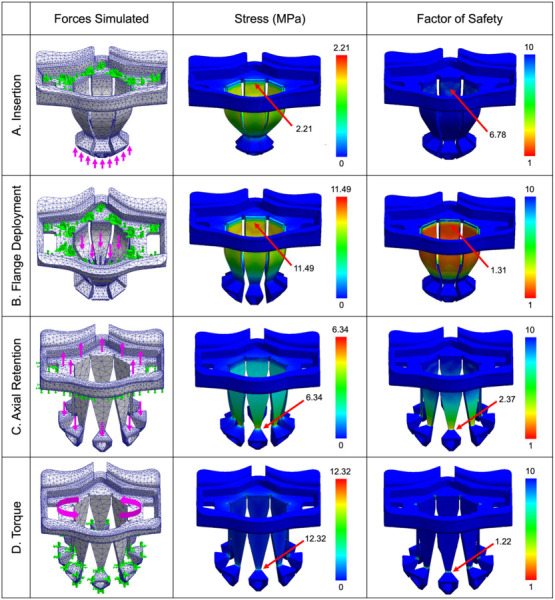
FEA of PeriPath demonstrating forces simulated, stress, and factor of safety under (a) insertion forces, (b) flange deployment forces, (c) axial retention forces, and (d) torque. Fixtures are indicated by blunted arrows, whereas external forces are indicated by long arrows. Maximum stress and minimum factor of safety are labeled.
Forces used during FEA simulation were the maximum expected clinical force as reported in Table 1. A maximum stress less than MPU100's ultimate strength of 15 MPa and a FOS greater than 1 was considered clinically acceptable. When determining the conditions for device failure, the simulated use force was increased until the FEA resulted in a FOS less than 1. Device failure forces are reported to the nearest Newton.
Insertion.
The first experiment simulated PeriPath's response when placed into a skin incision. A fixture was placed along the top of the device to emulate the physician's hand, and a 1 N upward external force was placed on the feet of the device to show the pressure of the skin on the device during insertion. Under clinically expected forces, FEA demonstrated a maximum insertion stress of 2.21 MPa and a minimum FOS of 6.78, which confirmed PeriPath would withstand clinical insertion (Fig. 4(a)). The maximum force to device failure was 14 N.
Flange Deployment.
Similarly, FEA was used to emulate the clinical forces needed to deploy the flanges of the device and lock it into the incision. A fixture was placed along the top of the device, with external loads pointing downward onto the flanges to mimic the inner lock sleeve sliding downward. From the FEA, a downward axial force of 10 N total within the outer grip sleeve generated a maximum stress of 11.49 MPa and a minimum FOS of 1.31. These results indicated that PeriPath would not break when being locked in the incision (Fig. 4(b)). The maximum force until failure was 15 N.
Axial Retention.
Another risk during a cardiac procedure is dislodgement of the device, so the effects of retention were investigated. FEA was performed by first adding a fixture to the bottom of the device and applying 10 N of upward force on the top of the device to represent pulling out of an incision. In addition, 1 N downward forces were applied to the feet of the device to simulate the effects of the underside of the skin on the feet. The results of the FEA under clinical forces show a maximum stress of 6.34 MPa, which produced a minimum FOS of 2.37, supporting the clinical design specification that PeriPath's remains locked during the procedure (Fig. 4(c)). The maximum force to device failure was 22 N.
Torque.
To simulate the performance of PeriPath under torque from tools in the working and visualization channels, FEA was used to elucidate the effects of twisting along the top surface of the device while the feet remained fixed in the locked position. When a clinically relevant torque of 0.01 N·m was applied, the maximum stress observed was 12.32 MPa, which produced a minimum FOS of 1.32 (Fig. 4(d)). This FOS is common for use with highly reliable materials where the loading and environmental conditions are not severe. This simulation emphasized that the PeriPath device is compatible with instrument manipulation per clinical requirements. The maximum torque until failure was 0.012 N·m. In practice, there are preventative measures against failure under torsion, which include proper flange deployment and deformation of struts on the feet of the device, which allows the feet to bend without shearing when the device is twisted 360 deg in the skin.
Device Mechanical Testing.
To confirm PeriPath's durability and validate the FEA, testing was performed on manufactured PeriPath devices using an Instron machine (model 5965, Norwood, MA). Experiments, excluding drag force, used synthetic tissue plates (Basic Tissue Plate, SynDaver, Tampa, FL) to mimic in vivo tissues. A 5 mm thick tissue was chosen since this tissue is marked for device testing and has similar mechanical properties to human tissue [22]. The skin was cut into 50 × 50 mm pieces, and a scalpel was used to create a 13 mm incision in each piece. The synthetic skin was held taut in a three-dimensional printed clamp that attached to the bottom rig of the Instron. A representative testing setup is shown in Fig. 5(a). Results from testing are plotted in Figs. 5(b)–5(f), with the dashed lines representing the maximum force to failure as determined from FEA.
Fig. 5.
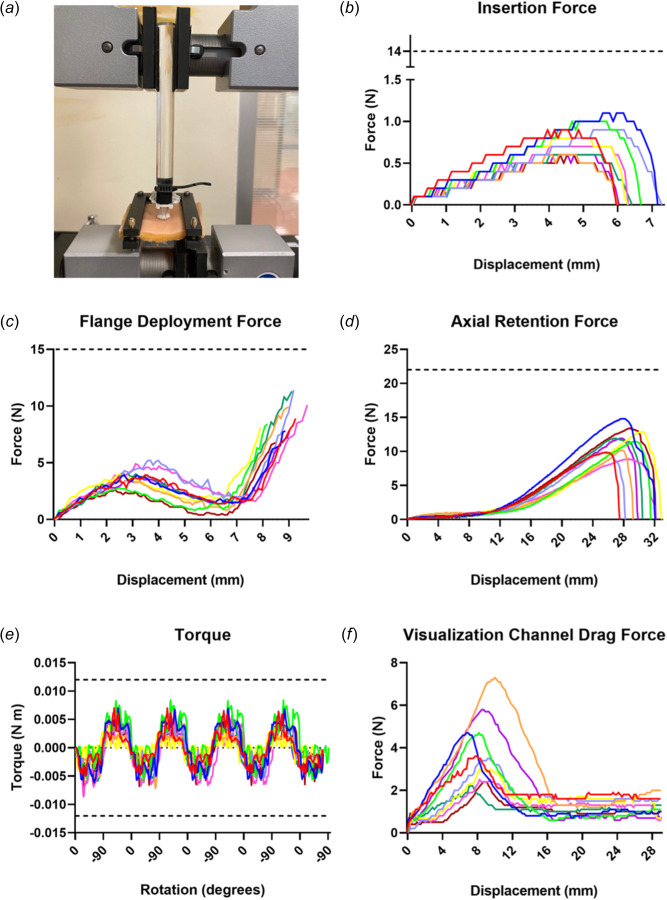
Device verification testing. (a) Example setup for testing insertion using the Instron. (b) Force required for insertion of PeriPath into an incision. This passes the clinically relevant acceptance criteria of <1 N stated in Table 1. (c) Forces required for flange deployment of the device. This passes the clinically relevant acceptance criteria of <10 N stated in Table 1. (d) Force required to dislodge the device axially. This passes the clinically relevant acceptance criteria of >10 N stated in Table 1. (e) Torque produced during cyclic rotations. This passes the clinically relevant torque acceptance criteria of <0.01 N·m stated in Table 1. (f) Drag forces required to insert tools within the larger visualization channel. This passes the clinically relevant acceptance criteria of <10 N and >2 N stated in Table 1. Each line represents a different specimen for each test. Dotted lines represent force or torque threshold rounded to the nearest Newton that would result in fracture.
Insertion.
Insertion forces were verified through compression testing at 1 mm/s using the Instron. A clear rod with a diameter of 0.5 in. was clamped to the top rig of the Instron to push on the core of PeriPath. Insertion testing began with PeriPath's feet resting on top of the synthetic skin incision and data collection stopped when the top of the outer grip sleeve touched the skin. Figure 5(b) shows that the maximum insertion force, defined as the maximum force it took to push the device through the synthetic skin incision, was 0.78 ± 0.19 N. This passes the clinically relevant insertion acceptance criteria of <1 N stated in Table 1.
Flange Deployment.
Flange deployment force, or the force required to insert the inner lock sleeve into the outer grip sleeve, was also determined using ten PeriPath devices and synthetic skins. The device was inserted into the skin in the unlocked position, with wooden dowels providing an upward force for the inner lock sleeve to sit. The same clear rod provided compression testing at a rate of 1 mm/s. Data collection stopped when the inner lock sleeve clicked into the outer grip sleeve. Figure 5(c) reveals an initial peak averaging to 3.86 ± 0.80 N during flange deployment, which represents overcoming sliding friction of the inner lock sleeve. The subsequent increase in force to 9.06 ± 1.55 N reflects the maximum force it takes the device to click into locked position. This passes the clinically relevant flange deployment acceptance criteria of <10 N stated in Table 1.
Axial Retention.
To confirm that the device will not fracture or dislodge under clinical use, retention testing was performed. Axial retention, or the ability for the device to stay locked into the skin while being pulled upward, was investigated using tension settings at a rate of 1 mm/s. Ten PeriPath devices and synthetic skin tissues were used. A piece of fishing wire (0.25 mm, 6 lb break, nylon monofilament) was looped around PeriPath as it sat into the skin to provide four points of contact for upward force. Data collection was stopped when the device exited the skin. The results in Fig. 5(d) show that axial retention reached an average peak force of 11.51 ± 1.85 N, which represents the force required to pull PeriPath out of the incision without disengaging the inner locking sleeve. Notably, PeriPath did not visibly fracture. This passes the clinically relevant axial retention acceptance criteria of >10 N stated in Table 1.
Torque.
Twisting of the device within the incision was investigated to determine the torque required to dislodge PeriPath. The device was placed upside down in a manual chuck lower rig attached to a torque sensor (Instron, model 5965, Norwood, MA) and inserted into a piece of synthetic skin that was clamped to the upper rig. Torsional testing at 0.5 rev/s was selected, and four cycles of 90 deg rotations clockwise and counterclockwise were observed for ten PeriPath devices and synthetic skins. Data collection was stopped after four cycles occurred. Figure 5(e) shows a torque with a maximum magnitude of 0.007 ± 0.001 N·m was experienced. Again, PeriPath did not visibly fracture, even despite efforts to demonstrate cyclical fatigue. This passes the clinically relevant torque acceptance criteria of <0.01 N · m stated in Table 1.
Drag Force.
The drag forces of both ports within PeriPath's core component were tested to investigate the force needed to insert instrumentation. A 4.7 mm diameter stainless steel rod, in place of a standard trocar, and a 7 F sheath (Merit Medical, South Jordan, UT) were tested in the larger visualization channel and smaller working channel, respectively. The tools were doused in saline water to mimic the lubrication techniques used in surgeries. The device's inner lock sleeve was housed in a ball and swivel joint (0.5 in. ID, 1 in. OD) so the core could be inserted and easily oriented. Compression testing at 2 mm/s was performed using ten PeriPath core components.
Figure 5(f) shows that the drag forces for the larger visualization channel generate a maximum of 3.97 ± 1.65 N, which represents the force needed for the tool to overcome static friction and slide one of its ends through the port. The subsequent constant slope of 0.91 ± 0.48 N represents the general sliding friction once the tool is in the channel. Conversely, the drag forces for the smaller working channel reached a maximum force of 0.23 ± 0.03 N. Both results pass the clinically relevant drag force acceptance criteria of <10 N and <2 N stated in Table 1 for the visualization and working channel, respectively.
Preliminary Validation Study.
After verification testing, we conducted an experimental validation study with three participants to initially assess Human Factors such as ergonomics and workflow compatibility. This study was exempt from IRB approval as it was an initial feasibility test assessed by an anonymous performance survey involving less than five participants. The use of PeriPath was compared to an unconstrained subxiphoid two-trocar approach and a subxiphoid-left lateral two-trocar approach using commercial access tools. Each participant was asked to enter the thoracic cavity of a simulator doll [23] with a scope and needle using each of the three different approaches. They were tasked to obtain sheath access to the pericardial space using a modified Seldinger technique under direct visualization by the scope (Fig. 6). After each procedure, a NASA Task Load Index (TLX) and Usability Questionnaire were administered. Additionally, an observer recorded total time, accidental needle punctures, and damage to skin for each procedure. After completing all three procedures, a Comparison Questionnaire was administered asking the participants to rank the approaches 1–3 from favorite to least favorite in four categories: compatibility, comfort, performance, and preference.
Fig. 6.
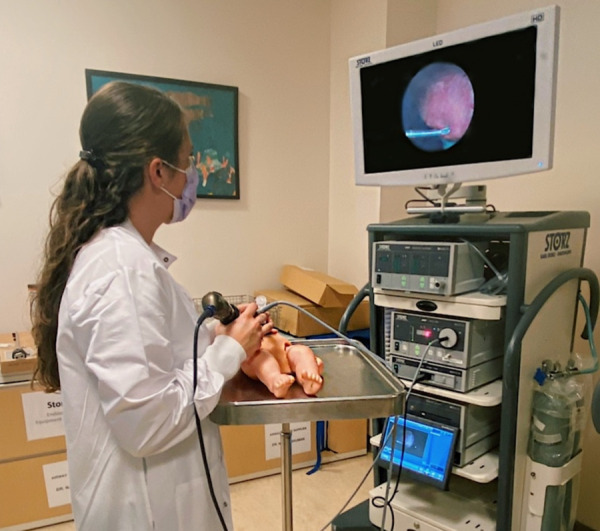
Demonstration of a minimally invasive pericardial access procedure using a simulator doll under direct visualization
Among the three participants, the PeriPath approach ranked as the overall favorite on the Comparison Questionnaire. Participants found it difficult to maneuver and visualize the needle with the scope in the subxiphoid-left lateral approach, often leading to more accidental needle punctures, underscoring the visualization improvement from a single incision approach. Additionally, the NASA TLX revealed that the PeriPath approach scored as well as or better than an unconstrained approach in terms of physical demand, temporal demand, performance, and frustration (Fig. 7) among the three participants.
Fig. 7.
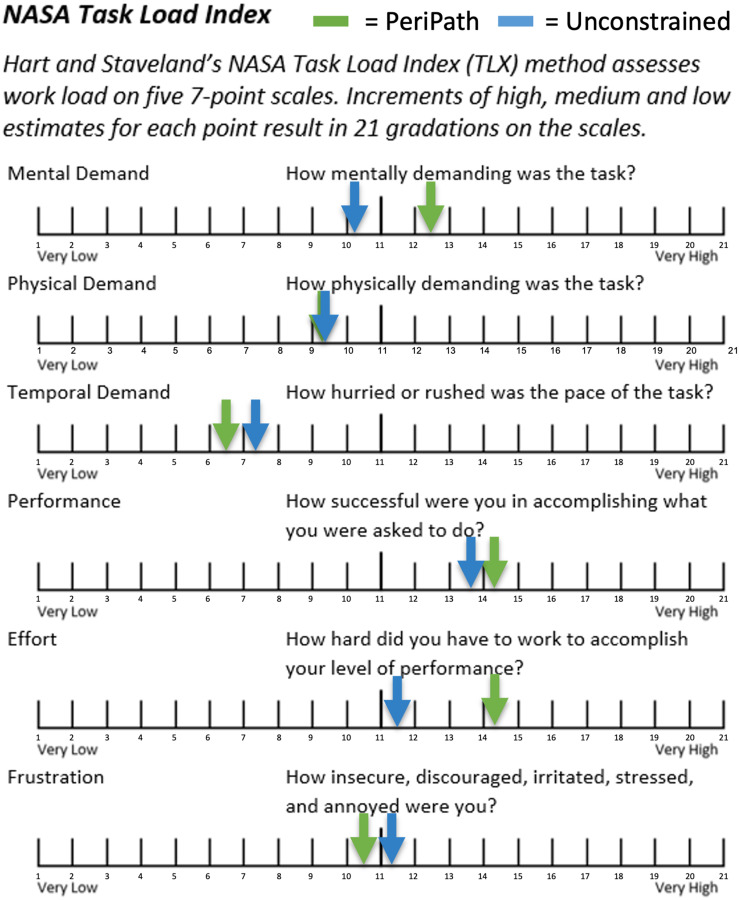
NASA task load index average results from the preliminary validation study (n = 3)
It should be noted that all participants requested an additional hand to help hold the endoscope at some point during each procedure. However, the participants mentioned that the PeriPath device resulted in less reliance on an assistant while gaining access to the pericardium and greater compatibility with the endoscope and access instruments. Finally, all three procedures resulted in similar damage to the skin based on the length of incisions made, and time of the procedure was more closely correlated to the experience of the participant and the learning curve using the simulator doll rather than the procedure itself.
Discussion and Conclusion
This paper reported the design and functionality of a minimally invasive thoracic access port for percutaneous pericardial access. PeriPath is designed to eliminate the need for open-chest surgery, potentially reducing pain, decreasing procedure time, and minimizing operative complications. Furthermore, implantation using PeriPath may be performed by surgeons, interventional cardiologists, or electrophysiologists using existing hospital equipment and tools. Finally, the device enables insufflation and direct visualization without the need for radiation for improved safety and usability for pericardial access.
Potential risks for PeriPath under extreme use cases include dislodgement, device fracture, and flange shearing into the thoracic space during surgery. We sought to address these risks using FEA and mechanical testing to demonstrate the device's performance and failure mode under extreme loading for each risk. In future work, we will conduct expanded Human Factors testing to demonstrate device performance among multiple users in simulated use cases.
Our FEA verified that the device is likely to not fail under simulated working conditions. Specifically, all factor of safety measurements were greater than one, indicating that the structure's maximum strength or capacity is greater than its determined design load. Additionally, none of the simulations exceeded the material's stress ultimate strength. This highlights PeriPath's nominal chance of fracture for one-time clinical use in minimally invasive cardiac procedures.
Device mechanical testing demonstrated that the holding forces and tensile strength of PeriPath are sufficient for use. Insertion and deployment forces were below the maximum clinical requirement of 1 N and 10 N, respectively, reflecting the ease to setup the device during a surgical procedure. Tests observing axial retention and torsion exceeded clinical requirements as well, confirmed through lack of visible fracture at maximum usable forces. More than 10 N was required to dislodge the device from an incision in axial testing, and the feet of the device slipped instead of sheared during cyclical twisting.
Finally, the drag forces of the larger visualization channel and the smaller working channel also confirm clinical design specifications. Notably, the larger port was manufactured to have a slightly undersized port, as the viewing tools should not move as much during a single operator use, whereas the smaller port should allow the sheath to slide more easily. We used a 4 mm Karl Storz trocar and a 5 mm Xcel trocar, two commonly used brands, as references for the larger channel, which report drag forces of approximately 1.5—2 N and 0.25–0.5 N, respectively [24]. Our visualization port force of approximately 4 N is greater than the drag force of either of these brand trocars, indicating that the trocar will not become dislodged from PeriPath when an endoscope is used with the device. These findings support the use of PeriPath in clinical settings due to its safety, user-friendliness, and compatibility with common lead implantation tools.
The limited validation study suggests the effectiveness of the device to aid in obtaining sheath access inside the pericardium using a modified Seldinger technique with direct visualization provided by a deflectable endoscope. Among the three participants, PeriPath performed similarly to an unconstrained subxiphoid approach and was preferred over a subxiphoid-left lateral approach. These results may be limited due to the use of synthetic skin in both validation testing and the preliminary validation study, as it differs in elasticity and thickness of neonatal skin and obviates the chance of infection. However, the device is used temporarily during a 30 min procedure, and the incision is much smaller than alternative nonminimally invasive surgeries, reducing the risk of infection [25]. Additionally, there was lack of insufflation during performance testing, which does not accurately represent the opposing pressures of the thoracic space during a surgical procedure. Despite this, PeriPath has demonstrated successful insufflation and no wound infections in prior animal studies [26]. Finally, the preliminary validation study consisted of a small sample size (n = 3) so future work should aim to recruit more participants.
In conclusion, the PeriPath device was mechanically designed based on clinical requirements and demonstrates minimal risk of fracture. It also functions effectively for minimally invasive lead implantation procedures, evident through performance testing and a physician preliminary validation study. To move this technology forward, we plan to injection mold our devices and complete verification and validation testing against predicate devices for FDA approval. We will also conduct a full Human Factors study to illustrate failure modes when implanting devices in an animal model. A pivotal chronic pacing study in a juvenile porcine model will support an application for an Investigational Device Exemption from FDA for human use, which will be used to perform first-in-human pediatric clinical trials.
Acknowledgment
The authors would like to thank Vincent Cleveland for his help with the validation testing setups and Tyler Salvador for his help with the FEA. We would also like to thank the electrophysiologists and fellows that participated in our preliminary validation study, and design engineers Christopher Scholl and Dylan Paproski of Archimedic for their assistance in the design of the PeriPath tool.
Justin D. Opfermann, Paige N. Mass, Dr. Charles I. Berul, and Dr. Rohan N. Kumthekar are inventors of the device described in this paper. Justin D. Opfermann, Paige N. Mass, Dr. Charles I. Berul, and Dr. Rohan N. Kumthekar are also members of and hold Shares of Stock Options in PeriCor, LLC. The results of the study discussed in this publication could affect the value of PeriCor, LLC. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies.
Funding Data
National Heart, Lung, and Blood Institute (Award No. 1R43HL144352; Funder ID: 10.13039/100000002).
Nomenclature
- CIED =
cardiovascular implantable electronic device
- CRT =
cardiac resynchronization therapy
- FEA =
finite element analysis
- FOS =
factor of safety
- ICD =
implantable cardioverter defibrillator
- ID =
inner diameter
- OD =
outer diameter
- TLX =
task load index
References
- [1]. Burri, H. , 2015, “ Overcoming the Challenge of Venous Occlusion for Lead Implantation,” Indian Pacing Electrophysiol. J., 15(2), pp. 110–112. 10.1016/j.ipej.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Kuensting, L. L. , DeBoer, S. , Holleran, R. , Shultz, B. L. , Steinmann, R. A. , and Venella, J. , 2009, “ Difficult Venous Access in Children: Taking Control,” J. Emerg. Nurs., 35(5), pp. 419–424. 10.1016/j.jen.2009.01.014 [DOI] [PubMed] [Google Scholar]
- [3]. Romer, A. J. , Tabbutt, S. , Etheridge, S. P. , Fischbach, P. , Ghanayem, N. S. , Reddy, V. M. , Sahulee, R. , Tanel, R. E. , Tweddell, J. S. , Gaies, M. , Banerjee, M. , Retzloff, L. , Zhang, W. , and Patel, A. R. , 2019, “ Atrioventricular Block After Congenital Heart Surgery: Analysis From the Pediatric Cardiac Critical Care Consortium,” J. Thorac. Cardiovasc. Surg., 157(3), pp. 1168–1177. 10.1016/j.jtcvs.2018.09.142 [DOI] [PubMed] [Google Scholar]
- [4]. Rastan, A. , Mohr, F. W. , Rastan, A. , and Haensig, M. , 2011, “ Bioresorbable Adhesion Barrier for Reducing the Severity of Postoperative Cardiac Adhesions: Focus on REPEL-CV®,” Med. Devices, 4, pp. 17–25. 10.2147/MDER.S7957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Lindvall, C. , Chatterjee, N. A. , Chang, Y. , Chernack, B. , Jackson, V. A. , Singh, J. P. , and Metlay, J. P. , 2016, “ National Trends in the Use of Cardiac Resynchronization Therapy With or Without Implantable Cardioverter-Defibrillator,” Circulation, 133(3), pp. 273–281. 10.1161/CIRCULATIONAHA.115.018830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].European Society of Cardiology (ESC), Heart Rhythm Society, Heart Failure Society of America (HFSA), American Society of Echocardiography (ASE), American Heart Association (AHA), European Association of Echocardiography (EAE) of the ESC, and Heart Failure Association of the ESC (HFA), Daubert, J.-C. , Saxon, L. , Adamson, P. B. , Auricchio, A. , Berger, R. D. , Beshai, J. F. , Breithard, O. , Brignole, M. , Cleland, J. , DeLurgio, D. B. , Dickstein, K. , Exner, D. V. , Gold, M. , Grimm, R. A. , Hayes, D. L. , Israel, C. , Leclercq, C. , Linde, C. , Lindenfeld, J. , Merkely, B. , Mont, L. , Murgatroyd, F. , Prinzen, F. , Saba, S. F. , Shinbane, J. S. , Singh, J. , Tang, A. S. , Vardas, P. E. , Wilkoff, B. L. , Zamorano, J. L. , Anand, I. , Blomstrom-Lundqvist, C. , Boehmer, J. P. , Calkins, H. , Cazeau, S. , Delgado, V. , Estes, N. A. M. , Haines, D. , Kusumoto, F. , Leyva, P. , Ruschitzka, F. , Stevenson, L. W. , and Torp-Pedersen, C. T. , 2012, “ 2012 EHRA/HRS Expert Consensus Statement on Cardiac Resynchronization Therapy in Heart Failure: Implant and Follow-Up Recommendations and Management,” Europace, 14(9), pp. 1236–1286. 10.1093/europace/eus222 [DOI] [PubMed] [Google Scholar]
- [7]. Bové, T. , François, K. , De Caluwe, W. , Suys, B. , and De Wolf, D. , 2010, “ Effective Cardioverter Defibrillator Implantation in Children Without Thoracotomy: A Valid Alternative,” Ann. Thorac. Surg., 89(4), pp. 1307–1309. 10.1016/j.athoracsur.2009.06.066 [DOI] [PubMed] [Google Scholar]
- [8]. Haydin, S. , Saygi, M. , Ergul, Y. , Ozyilmaz, I. , Ozturk, E. , Akdeniz, C. , and Tuzcu, V. , 2013, “ Subxiphoid Approach to Epicardial Implantation of Implantable Cardioverter Defibrillators in Children: Subxiphoid epicardial ICD implantation,” Pacing Clin. Electrophysiol., 36(8), pp. 926–930. 10.1111/pace.12158 [DOI] [PubMed] [Google Scholar]
- [9]. Jaroszewski, D. E. , Altemose, G. T. , Scott, L. R. , Srivasthan, K. , DeValeria, P. A. , Lackey, J. , and Arabia, F. A. , 2009, “ Nontraditional Surgical Approaches for Implantation of Pacemaker and Cardioverter Defibrillator Systems in Patients With Limited Venous Access,” Ann. Thorac. Surg., 88(1), pp. 112–116. 10.1016/j.athoracsur.2009.04.006 [DOI] [PubMed] [Google Scholar]
- [10]. Schneider, A. E. , Burkhart, H. M. , Ackerman, M. J. , Dearani, J. A. , Wackel, P. , and Cannon, B. C. , 2016, “ Minimally Invasive Epicardial Implantable Cardioverter-Defibrillator Placement for Infants and Children: An Effective Alternative to the Transvenous Approach,” Heart Rhythm, 13(9), pp. 1905–1912. 10.1016/j.hrthm.2016.06.024 [DOI] [PubMed] [Google Scholar]
- [11]. Shaikh, A. R. , Sangrasi, A. K. , and Shaikh, G. A. , 2009, “ Clinical Outcomes of Laparoscopic Versus Open Appendectomy,” J. Soc. Laparoscopic Rob. Surg., 13(4), pp. 574–580. 10.4293/108680809X1258998404524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Hsia, T.-Y. , Bradley, S. M. , LaPage, M. J. , Whelan, S. , Saul, J. P. , Ringewald, J. M. , and Reed, J. H. , 2009, “ Novel Minimally Invasive, Intrapericardial Implantable Cardioverter Defibrillator Coil System: A Useful Approach to Arrhythmia Therapy in Children,” Ann. Thorac. Surg., 87(4), pp. 1234–1239. 10.1016/j.athoracsur.2009.01.015 [DOI] [PubMed] [Google Scholar]
- [13]. Andreassi, M. G. , Ait-Ali, L. , Botto, N. , Manfredi, S. , Mottola, G. , and Picano, E. , 2006, “ Cardiac Catheterization and Long-Term Chromosomal Damage in Children With Congenital Heart Disease,” Eur. Heart J., 27(22), pp. 2703–2708. 10.1093/eurheartj/ehl014 [DOI] [PubMed] [Google Scholar]
- [14]. Yang, H. C. , Cho, S. , and Jheon, S. , 2013, “ Single-Incision Thoracoscopic Surgery for Primary Spontaneous Pneumothorax Using the SILS Port Compared With Conventional Three-Port Surgery,” Surg. Endosc., 27(1), pp. 139–145. 10.1007/s00464-012-2381-6 [DOI] [PubMed] [Google Scholar]
- [15]. Liao, Y. , Long, X. , Zhu, S. , Tu, J. , Wen, H. , Xu, J. , and Wu, Y. , 2017, “ Minimally Access Via Left Anterior Mini-Thoracotomy for Repair of Adult Subarterial Ventricular Septal Defects,” J. Cardiothorac. Surg., 12(1), p. 48. 10.1186/s13019-017-0611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Yang, X. , Li, M. , Yang, X. , Zhao, M. , Huang, Y. , Dai, X. , Jiang, T. , Feng, M. , Zhan, C. , and Wang, Q. , 2018, “ Uniport Versus Multiport Video-Assisted Thoracoscopic Surgery in the Perioperative Treatment of Patients With T1–3N0M0 Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis,” J. Thorac. Dis., 10(4), pp. 2186–2195. 10.21037/jtd.2018.03.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Di Biase, L. , Burkhardt, J. D. , Reddy, V. , Romero, J. , Neuzil, P. , Petru, J. , Sadiva, L. , Skoda, J. , Ventura, M. , Carbucicchio, C. , Dello Russo, A. , Csanadi, Z. , Casella, M. , Fassini, G. M. , Tondo, C. , Sacher, F. , Theran, M. , Dukkipati, S. , Koruth, J. , Jais, P. , and Natale, A. , 2017, “ Initial International Multicenter Human Experience With a Novel Epicardial Access Needle Embedded With a Real-Time Pressure/Frequency Monitoring to Facilitate Epicardial Access: Feasibility and Safety,” Heart Rhythm, 14(7), pp. 981–988. 10.1016/j.hrthm.2017.02.033 [DOI] [PubMed] [Google Scholar]
- [18]. Clark, B. C. , Kumthekar, R. , Mass, P. , Opfermann, J. D. , and Berul, C. I. , 2020, “ Chronic Performance of Subxiphoid Minimally Invasive Pericardial Model 20066 Pacemaker Lead Insertion in an Infant Animal Model,” J Interventional Card. Electrophysiol., 59(1), pp. 13–19. 10.1007/s10840-019-00626-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Kumthekar, R. , and Berul, C. I. , 2018, “ Implantable Cardioverter Defibrillators and Biventricular Pacing in Pediatric Dilated Cardiomyopathy: Preventing Death and Delaying Heart Transplant,” Prog. Pediatr. Cardiol., 49, pp. 38–42. 10.1016/j.ppedcard.2018.04.002 [DOI] [Google Scholar]
- [20]. Kumthekar, R. N. , Opfermann, J. D. , Mass, P. , Clark, B. C. , Moak, J. P. , Sherwin, E. D. , Whitman, T. , Marshall, M. , and Berul, C. I. , 2019, “ Minimally Invasive Percutaneous Epicardial Placement of a Prototype Miniature Pacemaker With a Leadlet Under Direct Visualization: A Feasibility Study in an Infant Porcine Model,” Heart Rhythm, 16(8), pp. 1261–1267. 10.1016/j.hrthm.2019.02.033 [DOI] [PubMed] [Google Scholar]
- [21]. Opfermann, J. D. , Clark, B. C. , Davis, T. D. , Berul, C. I. , and Krieger, A. , 2016, “ A Single-Incision Delivery Tool for Epicardial Pacing and Defibrillation1,” J. Med. Devices, 10(2), p. 020930. 10.1115/1.4033123 [DOI] [Google Scholar]
- [22]. Franklin, S. E. , Baranowska, J. , Hendriks, C. P. , Piwowarczyk, J. , and Nachman, M. , 2017, “ Comparison of the Friction Behavior of Occluded Human Skin and Synthetic Skin in Dry and Moist Conditions,” Tribol. Trans., 60(5), pp. 861–872. 10.1080/10402004.2016.1223388 [DOI] [Google Scholar]
- [23]. Mass, P. N. , Contento, J. M. , Opfermann, J. D. , Sumihara, K. , Kumthekar, R. N. , and Berul, C. I. , 2022, “ An Infant Phantom for Pediatric Pericardial Access and Electrophysiology Training,” Heart Rhythm O2, 3(3), pp. 295–301. 10.1016/j.hroo.2022.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. van den Dobbelsteen, J. J. , Schooleman, A. , and Dankelman, J. , 2007, “ Friction Dynamics of Trocars,” Surg. Endosc., 21(8), pp. 1338–1343. 10.1007/s00464-006-9105-8 [DOI] [PubMed] [Google Scholar]
- [25]. Doenst, T. , Diab, M. , Sponholz, C. , Bauer, M. , and Färber, G. , 2017, “ The Opportunities and Limitations of Minimally Invasive Cardiac Surgery,” Dtsch. Arztebl. Int., 114(46), pp. 777–784. 10.3238/arztebl.2017.0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Kumthekar, R. N. , Opfermann, J. D. , Mass, P. , Clark, B. C. , Moak, J. P. , Sherwin, E. D. , Whitman, T. , Marshall, M. , and Berul, C. I. , 2020, “ Percutaneous Epicardial Placement of a Prototype Miniature Pacemaker Under Direct Visualization: An Infant Porcine Chronic Survival Study,” Pacing Clin. Electrophysiol., 43(1), pp. 93–99. 10.1111/pace.13843 [DOI] [PubMed] [Google Scholar]


