Abstract
Background:
Smartphones present a near-ubiquitous channel through which structured lifestyle change can reduce risk or progression of the most common noncommunicable diseases. We explored whether a digital structured lifestyle program enhances weight loss.
Methods:
We randomized overweight and obese participants attending a four-month lifestyle change program to either standard weekly coaching sessions (controls), or standard treatment supplemented with a digital therapeutic mobile application (intervention). Changes in body mass index after four months were the main outcome measure. Odds ratios of achieving 5% weight loss were estimated with unconditional logistic regression.
Results:
Of 234 eligible persons, 146 (62%) agreed to participate, were block-randomized, showed up for the baseline measures, and constituted the intention-to-treat (ITT) sample (n = 95 intervention group, n = 51 control group). In the intervention group, 70 (74%) downloaded the mobile application and completed the program (intervention per-protocol). Significant weight loss and BMI reduction were observed for both the intention-to-treat intervention group (P < 0.05, P = 0.01) and the per-protocol intervention group (P < 0.0001, P < 0.0001). For the intervention per-protocol group, the odds ratio of achieving 5% weight loss, compared to not treated per-protocol, was 3.3 (95% CI 1.3-8.2), adjusting for age and weight at baseline.Attendance to weekly coaching sessions decreased by 18% during the program in the control group while it increased by 3% amongst the per-protocol group (P = 0.004).
Conclusions:
These preliminary findings support the benefit of a digital therapeutic to enhance weight reduction and attendance in a structured lifestyle change program. Larger trials of longer duration are needed to confirm these findings.
Keywords: digital therapeutic, intervention study, mobile application, obesity, prediabetes, weight loss
Introduction
In the past 50 years, 1 obesity has become a global epidemic associated with major noncommunicable diseases, including cardiovascular diseases, diabetes, and cancers. 2 In high-income countries such as the United States and the United Kingdom approximately one-third of adults have prediabetes,3,4 but this condition has also become prevalent in Asia and sub-Saharan Africa. 5
Structured lifestyle change programs including weight loss goals of 5% to 7%, have been shown to prevent type 2 diabetes and significantly reduce risk factors for cardiovascular disease in populations with obesity and prediabetes.6,7Unfortunately, sustainable lifestyle changes—and weight reduction in particular—are difficult to achieve. Hence, there is a global need for new, cost-effective solutions and smartphones have been identified as a “particularly attractive avenue for delivering health interventions”. 8
The rapid worldwide growth in smartphone ownership provides novel opportunities for large-scale, effective and economically viable lifestyle interventions. In the United States, for instance, smartphone ownership among adults was estimated to 82% in 2016, while overall in advanced economies the median smartphone ownership was 76% in 2018.9,10 Smartphones are widely distributed and people tend to carry them at all times, spending several hours per day using smartphone applications. 9 While the reach and possible health applications of mobile technologies are increasingly apparent, use for health interventions is still limited and has some limitations. Many commercial mobile apps, for example, rely on a narrow range of strategies, impart few weight loss benefits, and require tracking and self-monitoring efforts that can be tedious and difficult to sustain.11-14 Indeed, a gap exists in mobile technologies supported by evidence-based theories and advanced technologies such as machine learning. To this end, we tested a structured, four-month-long lifestyle change program with a mobile application, in an adult population classified as overweight or obese.
Methods
Participants
We conducted a randomized trial involving participants at a private health clinic (Heilsuborg Health Clinic) in Reykjavik, Iceland, providing structured lifestyle modification for overweight or obese individuals. The protocol was approved by the National Bioethics Committee (VSN 15-110-S1), and all participants provided written informed consent. Study participants were enrolled from individuals who signed up for a four-month lifestyle change program between September 2015 and January 2016. Eligibility criteria: ≥18 years of age; body mass index (BMI) of ≥25 kg/m2; females should not be pregnant at enrollment.
Intervention and Outcome
Participants in the trial met in groups of 15 to 20 persons, at different times during the week. Each group was randomly assigned through block randomization to one of two study arms by the clinic staff. The control group participated in one-hour-long physical workouts once to twice a week with coaching on strength and endurance, along with a monthly one-hour-long nutrition and health education class. Participants in the intervention group additionally received an offer to supplement their standard treatment with a mobile application (Sidekick; www.sidekickhealth.com). Participants were not on a specific diet but received recommendations for making lifestyle changes that followed the Icelandic public health guidelines. Both interventions lasted four months.
The primary outcome was weight loss, as measured by the health care clinic staff at baseline and study-end, blinded to treatment assignment. Additionally, clinic staff collected background demographic information such as education level and marital status, measured participant height, and registered class attendance. Missing values on age for three participants in the study were replaced with the mean age for the total group. For intention-to-treat analyses, missing weight at study-end for participants who did not complete the program was replaced by weight at baseline (or using the last observation carried forward).
The Mobile Application
The Sidekick mobile application (https://sidekickhealth.com/) was designed to increase frequency of healthy behaviors through goal-setting, self-monitoring, and completion of health-related tasks (i.e., gamification of tasks) in three categories: nutrition, physical activity, and stress management. By completing gamified tasks, users accumulated points that provided virtual (e.g., badges) and altruistic rewards (e.g., charitable water donations). A visual representation of the users’ performance in different categories was provided, as well as a storyline, tracking individuals progress. Social support for adherence to the program was encouraged through the group members’ social interactions.
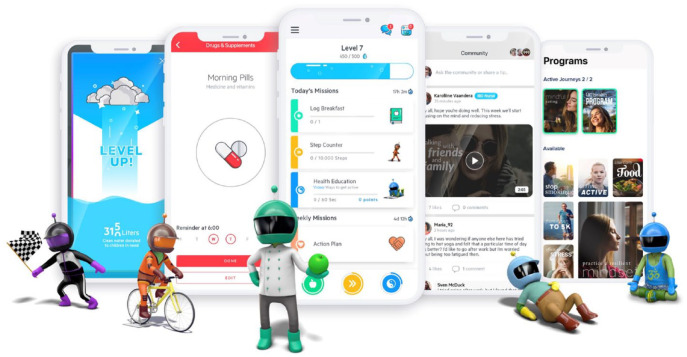
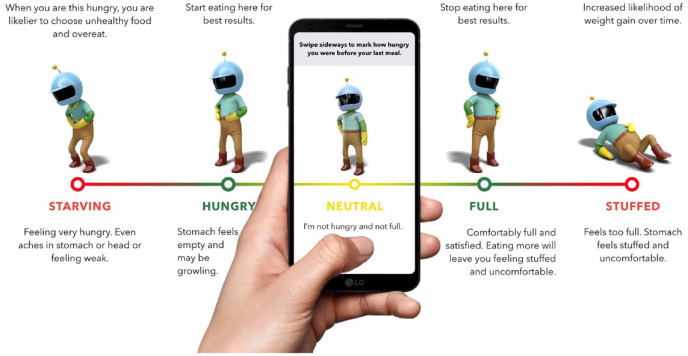
The application’s design was based on the field of behavioral economics, and related fields of behavioral research. Development of the application was guided by seven theoretical pillars as previously summarized. 15 First, the application employs compelling graphics, characters, competitions, and rewards to engage the “fast-thinking” or emotional centers in the brain, which are responsible for the majority of people’s lifestyle choices, as opposed to the “slow-thinking” or rational centers (Figure 1). 16 Second, to counter mental shortcuts associated with “mindless eating,” the application provides appetite awareness training 17 that encourages users to eat in response to internal hunger and satiety cues (Figure 2). Third, the application incorporates relaxation, meditation, and mindfulness exercises to alleviate decision-making deficits that can be caused by limits on cognitive capacity caused by stress.
Figure 1.
Screenshots from the mobile digital therapeutic, and characters that were developed to target impulsive, fast-thinking.
Figure 2.
Appetite awareness training exercise from the app.
Fourth, the application provides opportunities for instant gratification to counteract the tendency towards “present bias” in which people place a higher value on immediate gratification over long-term benefits. Fifth, health management is framed as an enjoyable task as opposed to an obligation; application messages emphasize personal achievement, social interaction, and having fun. Sixth, the application takes “channel factors” (small details that can have surprisingly large effects—either encouraging or inhibiting—on people’s behavior) into account in its design. Such factors lower the threshold for healthy choices by offering a wide variety of activities and accommodating users with low health literacy. Last, the application recognizes the influence of altruism on people’s behaviors, enabling users to earn points for rewards that benefit others in need, such as charitable donations.
Statistical Analysis
Group differences were tested using independent-sample t-tests or Mann–Whitney U tests as appropriate. Changes in weight, BMI, and attendance between the control and intervention groups were compared using an analysis of covariance model for a pre-post study design, controlling for age and baseline weight. 18 When comparing program attendance between the groups (average attendance during month 1 and 4), we additionally adjusted for average program attendance in the first month. We used binary logistic regression to estimate the odds ratio (OR) with 95% confidence interval (CI) of achieving 5% weight loss while controlling for age and baseline weight. All statistical tests were two-sided, and P-values <0.05 were considered statistically significant. For all statistical analyses, we used SPSS software, version 24.0 (SPSS Inc., IBM Chicago, IL, www.spss.com).
Role of the Funding Source
The study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Results
Demographics and Participation
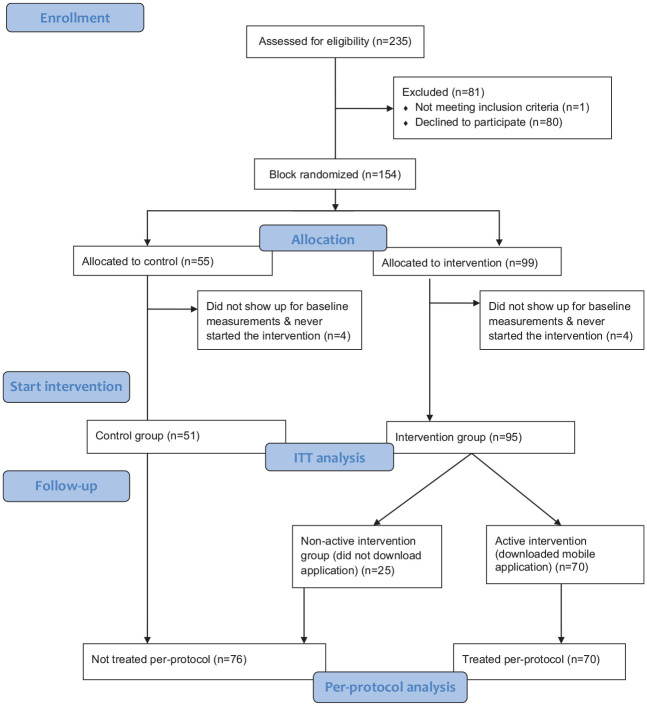
A total of 235 clients in the lifestyle change program at the health clinic were assessed for eligibility (92.8% women; average BMI 36.6 kg/m2; average age 49 years) (Figure 3) and invited to participate in the study. One participant was excluded due to pregnancy, and of the remaining 234 individuals, 146 (62%) agreed to participate, were block randomized, and showed up for baseline measurements (intervention, n = 95, control n = 51). Of the 95 who started the program in the intervention group, 70 (74%) downloaded the mobile application (the per-protocol intervention group).
Figure 3.
CONSORT flow diagram of participant recruitment and retention.
The baseline characteristics of the study participants (n = 146) are outlined in Table 1. The average age was 47 (standard deviation (SD) 12) years, average BMI was 36 (SD 5), and the majority were women (93%). Only eight participants had diabetes type 2, four in each group. No differences were observed at baseline between the intervention and control groups for weight, BMI, or age.
Table 1.
Baseline Characteristics of the Study Group.
| Characteristics | Total group | Allocation |
Treated per-protocol |
||
|---|---|---|---|---|---|
| Intervention | Control | Yes | No | ||
| Number | 146 | 95 | 51 | 70 | 76 |
| Age a (y) | 46.8 ± 11.7 | 46.8 ± 11.2 | 46.8 ± 12.1 | 45.8 ± 11.0 | 47.8 ± 12.4 |
| Weighta (kg) | 104.6 ± 17.4 | 104.7 ± 16.9 | 104.5 ± 18.4 | 103.6 ± 16.3 | 105.6 ± 18.4 |
| BMIa (kg/m2) | 36.3 ± 5.2 | 36.5 ± 4.8 | 36.1 ± 5.9 | 36.3 ± 4.7 | 36.3 ± 5.6 |
| Time of entry (%) | |||||
| September to December | 54.8 | 61.1 | 43.1 | 60.0 | 50.0 |
| January to April | 45.2 | 38.9 | 56.9 | 40.0 | 50.0 |
| Gender (%) | |||||
| Men | 7.5 | 7.1 | 9.3 | 4.3 | 10.5 |
| Women | 92.5 | 92.9 | 90.7 | 95.7 | 89.5 |
| University education b (%) | 46.2 | 47.5 | 44.4 | 50.0 | 43.3 |
| Married or cohabiting c (%) | 72.4 | 69.8 | 79.2 | 68.2 | 77.6 |
| Parent d (%) | 68.8 | 63.9 | 75.0 | 69.0 | 68.6 |
Mean ± SD.
Information available for 106 participants.
Information available for 105 participants.
Information available for 64 participants.
Abbreviations: BMI, body mass index; SD, standard deviation.
Weight Loss and BMI Reduction
Changes in body weight and BMI were analyzed after adjusting for weight and age at baseline. Intention-to-treat (ITT) analyses 19 were performed on the 146 individuals who started the intervention. In the ITT analyses the 95 individuals who started in the intervention group were compared with the 51 individuals in the control group. The mean weight loss achieved from baseline to program-end was 2.9% in the intervention (n = 95) and 1.7% in the control group (n = 51). This weight loss was statistically significantly different between the two groups (P < 0.05). Also, significant differences were observed between the groups in their respective BMI reduction with an average reduction of 1.1 kg/m2 for the intervention group versus 0.6 kg/m2 in the control group (P = 0.01) (Table 2). In a per-protocol analysis, from baseline to program-end, the weight loss was 3.6% among those treated per-protocol (n = 70), and 1.5% among those not treated per-protocol (n = 76) (Table 3). This weight loss was significantly different between the two groups (P < 0.0001). Accordingly, a BMI reduction was achieved of 1.4 kg/m2 (treated per-protocol) and 0.5 kg/m2 (not treated per-protocol) which was significantly different (P < 0.0001) (Table 3).
Table 2.
Intention to Treat Analysis of Mean Weight- and BMI Reduction Over a Four-Month Period.
| Groups | Number (n) | Weight loss a (kg) | P-value |
|---|---|---|---|
| Intervention | 95 | 3.1 | 0.027 |
| Control | 51 | 1.8 | |
| Groups | Number (n) | Weight loss a (%) | P-value |
| Intervention | 95 | 2.9 | 0.036 |
| Control | 51 | 1.7 | |
| Groups | Number (n) | Changes in BMI a (kg/m2) | P-value |
| Intervention | 95 | 1.1 | 0.010 |
| Control | 51 | 0.6 |
Adjusted for age and weight at baseline.
Abbreviations: BMI, body mass index; n, number; SD, standard deviation.
Table 3.
Per-Protocol Analyses of Mean Weight- and BMI Reduction Over a Four-Month Period.
| Groups | Number (n) | Weight loss a (kg) | P-value |
|---|---|---|---|
| Treated per-protocol | 70 | 3.8 | <0.0001 |
| Not treated per-protocol | 76 | 1.5 | |
| Groups | Number (n) | Weight loss a (%) | P-value |
| Treated per-protocol | 70 | 3.6 | <0.0001 |
| Not treated per-protocol | 76 | 1.5 | |
| Groups | Number (n) | Changes in BMI a (kg/m2) | P-value |
| Treated per-protocol | 70 | 1.4 | <0.0001 |
| Not treated per-protocol | 76 | 0.5 |
Adjusted for age and weight at baseline.
Abbreviations: BMI, body mass index; n, number; SD, standard deviation.
Participants Treated Per-Protocol Were More Likely to Achieve 5% Weight Loss
From baseline to program-end, ITT analyses showed that the percentage of participants achieving the common benchmark of 5% weight loss was 23% for the intervention group, and the OR of achieving 5% weight loss compared to controls, was 2.8 (1.0-7.9). However, the percentage of participants achieving 5% weight loss in the treated per-protocol group (n = 70) was 27%, and 11% in the not treated per-protocol group (n = 76) (Table 4). The OR of achieving 5% weight loss in the treated per-protocol group compared to not treated per-protocol, was 3.3 (95% CI 1.3-8.2), adjusting for age and weight at baseline (Table 4).
Table 4.
Achieving 5% Weight Loss.
| 5% Weight loss, n (%) | OR (95% CI) a | |
|---|---|---|
| Control (n = 51) | 5 (10) | Ref |
| Intervention (n = 95) | 22 (23) | 2.8 (1.0-7.9) |
| 5% Weight loss, n (%) | OR (95% CI) a | |
| Not treated per-protocol (n = 76) | 8 (11) | Ref |
| Treated per-protocol (n = 70) | 19 (27) | 3.3 (1.3-8.2) |
Adjusted for age and weight at baseline.
Abbreviations: n, number; ref, reference value.
Intervention Adherence Higher in the Intervention Group
Information on average attendance to the program’s weekly coaching sessions was available for 45 participants in the study. Out of those with data available, average attendance in the treated per-protocol group (n = 20) increased from baseline (71%, SD = 22%) to study-end (75%, SD = 18%), a proportional increase of 5.6%. Conversely, average class attendance in the not treated per-protocol group (n = 25) decreased from baseline (81%, SD = 18%) to study-end (62%, SD = 30%), a proportional decrease of 23.5%. Adjusting for age, weight at baseline, and average class attendance rate in the first month of the program, the attendance rate among the group not treated per-protocol-decreased by 18% from baseline, while it increased by 3% during the study period (P = 0.004) for those who were treated per-protocol.
Discussion
With a reservation due to small numbers, these results support the hypothesis that a digital therapeutic mobile application can significantly augment a structured lifestyle change program, measured by weight loss and program adherence. Those who downloaded the mobile platform lost, on average, more weight compared with other participants. Moreover, during the four months, the mobile platform users were three times more likely to achieve at least 5% weight loss from baseline compared with standard in-person treatment.
It is of great importance to explore ways to reduce or slow down the rapid increase in the prevalence of obesity worldwide. One of the challenges is to improve adherence to recommended lifestyle changes, preferably before individuals are diagnosed with noncommunicable diseases. In this study, average class attendance decreased by 18% for those who did now download the application, while average class attendance increased by 3% over the treatment course among those who used the mobile application. Hence, adding a mobile application to a structured treatment might increase participants’ acceptability of treatment and subsequent chances of improved outcomes.
When individuals in the intervention group were compared with controls in an ITT analysis we found significant differences in weight reduction between the groups. The per-protocol analyses found even stronger differences between the groups. The participants in the intervention group were 2.8 times more likely than the controls to achieve a 5% weight loss. Also, the difference observed in participants’ BMI reduction was statistically significant between the groups in both the ITT and per-protocol analyses, which is notable given the small number of participants in this study and the resulting small statistical power to detect differences.
Using only a mobile application may be a cost-effective solution to help people lose weight and thereby reduce the prevalence of diseases such as prediabetes and type 2 diabetes. We did, however, not test using the app without interacting with a coach as various studies have shown that the use of weight loss apps without coaching does not result in significant weight loss. For instance, in a study of 212 primary care patients, a mobile-only intervention with a popular weight loss app (MyFitnessPal), but without in-person or remote interactions with a coach, did not produce significant weight change (−0.003 kg) in six months compared to usual care (+0.272 kg). 13 Actual weights and weight loss % were not reported making it difficult to compare their results with our results but only 18% of their intervention group, versus 16% of their control group managed to reduce their weight by 6 lbs (2.7 kg) or more. 13 Also, a study of 107 overweight/obese adults participating in an eight-week intervention using an attentive eating app in combination with dietary advice but lacking personal interaction, did not result in more weight loss compared to dietary advice alone (1.2 kg vs 1.1 kg). 20
It appears to be difficult for people to adhere to using a mobile application by itself without a personal connection with a lifestyle coach, remote or otherwise. Analysis of user data from over one million users of a popular weight loss app showed that, on average, users engaged with the app for only 29 days, suggesting it will be difficult for the majority of people to achieve weight loss with an app alone. 21 With these findings in mind, recent updates to our mobile platform have added options for structured remote coach interactions with participants.
Various studies that provide interaction with a coach, either in-person or remote, do result in weight loss. For example, one study followed 187 participants in a digital-only, 12-month-long, web-based program (Prevent), without in-person sessions but offering regular remote interactions with a coach. Participants in the study achieved an average loss of 4.7% of baseline body weight after one year and maintained a weight loss of 4.2% after two years. 22 In our four-month program, participants who downloaded the app (treated per-protocol) achieved a weight loss of 3.6%. Longer follow-up is needed to determine how much long-term weight loss can be achieved. In a larger study involving 833 overweight and obese subjects that were either provided with a weight loss app for three months plus one counselling session on diet and physical activity, or the counselling session alone, BMI was found to be reduced, but only in women. 23
In another study, three groups participating in a 12-week-long intervention: a traditional in-person weight loss program (conventional group), a mobile-only program (b-SLIM), and a combination of an in-person and mobile program (“combi group”) were compared (102 overweight or obese adults in total). The study revealed a trend that the combination group did better than the mobile-only group (19% more participants in the combination group achieved at least 5% weight loss when compared to the mobile-only group (P = 0.06)). In contrast to our findings, however, no significant difference was found between the combination group (in-person and mobile program) and the conventional, in-person only group. 24 We hypothesize this may be due to design and functional differences between the apps used in the two studies, perhaps including more content and the gamified and behavioral-economics-style nudging in the app used in this study, which may have been missing in the other one. Alternatively, a slightly longer intervention duration, as was the case with this study, may have been needed to achieve a significant difference in weight loss.
The gamification in our mobile application allows for the incorporation of an element of competition. Competition has been shown to be a powerful tool to make people change their behavior and may even be more powerful than social support.25,26 For example, in a recent study in the USA, 602 overweight and obese adults were given a wearable step counter and were assigned to a support, a collaborative, or a competition group. In that study, the individuals in the competition group achieved on average the largest increase in their daily step count. 27 As such, incorporating competition, either against oneself or as a team or solo against others, may be an interesting option to explore in future interventions delivered via a mobile application.
Our results, as well as the above findings, are gradually building knowledge about different methods of using technology to support lifestyle change programs—such as augmenting conventional in-person programs with technology versus remotely delivering programs, with or without the support of human coaches. While many agree that technology offers a compelling way of expanding the reach of effective programs, more research is needed on the relative advantages of the different forms of delivery for these programs.
The strengths of our study include a controlled design and relatively high rates of participation and completion of the intervention. Study limitations include a relatively small, self-selected sample, with a large majority of female participants. While this limits generalizability to the general population, it approximates real-world populations in lifestyle change programs.21,28-30 Another limitation is the relatively short study duration. While this also approximates many real-world programs, it calls for a larger and longer-term follow-up study.
Conclusions
The study results suggest that weight loss and attendance in a structured lifestyle change program may be enhanced by the addition of a mobile digital therapeutic platform. Larger, controlled studies are warranted to follow-up these findings over a longer period and to continue exploring different methods of using technology to help achieve meaningful lifestyle change at scale.
Acknowledgments
This study was funded by the Icelandic Research Fund (Grant Number 141381051 to RB). Development of the mobile application was partially funded by the Icelandic Technology Development Fund (Grant Number 131619-0611) and the Swedish Bio-X Fund (Grant Number 2014-05214).
Footnotes
Abbreviations: BMI, body mass index; CI, confidence interval; ITT, intention-to-treat; OR, odds ratio; SD, standard deviation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Tryggvi Thorgeirsson is a founder and co-owner of SKH, Saemundur Oddsson is a founder and co-owner of SKH, Unnur Anna Valdimarsdóttir has no conflicts of interest, Jóhanna Eyrún Torfadóttir has no conflicts of interest, Anna Sigríður Ólafsdóttir has no conflicts of interest, Ragnar Grímur Bjarnason has no conflicts of interest, Hans-Olov Adami has no conflicts of interest, Ichiro Kawachi has no conflicts of interest, Thrudur Gunnarsdottir is a current employee of SKH, Erlendur Egilsson is a prior employee at a related company and a minority shareholder in SKH.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Icelandic Research Fund, Icelandic Technology Development Fund, Swedish Bio-X Fund.
ORCID iDs: Tryggvi Thorgeirsson  https://orcid.org/0000-0003-4963-6424
https://orcid.org/0000-0003-4963-6424
Thor Aspelund  https://orcid.org/0000-0002-7998-5433
https://orcid.org/0000-0002-7998-5433
References
- 1. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-2450. [DOI] [PubMed] [Google Scholar]
- 2. Banjare J, Bhalerao S. Obesity associated noncommunicable disease burden. Int J Health Allied Sci. 2016;5(2):81-87. [Google Scholar]
- 3. Mainous AG, 3rd, Tanner RJ, Baker R, Zayas CE, Harle CA. Prevalence of prediabetes in England from 2003 to 2011: population-based, cross-sectional study. BMJ Open. 2014;4(6):e005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029. [DOI] [PubMed] [Google Scholar]
- 5. International Diabetes Federation. IDF Diabetes Atlas. 8th ed; 2017. http://fmdiabetes.org/wp-content/uploads/2018/03/IDF-2017.pdf
- 6. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343-1350. [DOI] [PubMed] [Google Scholar]
- 7. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klasnja P, Pratt W. Healthcare in the pocket: mapping the space of mobile-phone health interventions. J Biomed Inform. 2012;45(1):184-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaffey D. Mobile marketing statistics 2016. Accessed August 2020. http://www.smartinsights.com/
- 10. Taylor K, Silver L. Smartphone ownership is growing rapidly around the world, but not always equally. 2019. https://www.pewresearch.org/global/2019/02/05/smartphone-ownership-is-growing-rapidly-around-the-world-but-not-always-equally/
- 11. Pagoto S, Schneider K, Jojic M, DeBiasse M, Mann D. Evidence-based strategies in weight-loss mobile apps. Am J Prev Med. 2013;45(5):576-582. [DOI] [PubMed] [Google Scholar]
- 12. Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laing BY, Mangione CM, Tseng CH, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients: a randomized, controlled trial. Ann Intern Med. 2014;161(10 suppl):S5-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Semper HM, Povey R, Clark-Carter D. A systematic review of the effectiveness of smartphone applications that encourage dietary self-regulatory strategies for weight loss in overweight and obese adults. Obes Rev. 2016;17(9):895-906. [DOI] [PubMed] [Google Scholar]
- 15. Thorgeirsson T, Kawachi I. Behavioral economics: merging psychology and economics for lifestyle interventions. Am J Prev Med. 2013;44(2):185-189. [DOI] [PubMed] [Google Scholar]
- 16. Kahneman D. Maps of bounded rationality: psychology for behavioral economics. Am Econ Rev. 2003;93(5):1449-1475. [Google Scholar]
- 17. Craighead LW. The Appetite Awareness Workbook. New Harbinger Publications, Inc.; 2006. [Google Scholar]
- 18. Vickers AJ, Altman DG. Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323(7321):1123-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whitelock V, Kersbergen I, Higgs S, Aveyard P, Halford JCG, Robinson E. A smartphone based attentive eating intervention for energy intake and weight loss: results from a randomised controlled trial. BMC Public Health. 2019;19(1):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Serrano KJ, Coa KI, Yu M, Wolff-Hughes DL, Atienza AA. Characterizing user engagement with health app data: a data mining approach. Transl Behav Med. 2017;7(2):277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sepah SC, Jiang L, Peters AL. Long-term outcomes of a Web-based diabetes prevention program: 2-year results of a single-arm longitudinal study. J Med Internet Res. 2015;17(4):e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomez-Marcos MA, Patino-Alonso MC, Recio-Rodriguez JI, et al. Short- and long-term effectiveness of a smartphone application for improving measures of adiposity: a randomised clinical trial—EVIDENT II study. Eur J Cardiovasc Nurs. 2018;17(6):552-562. [DOI] [PubMed] [Google Scholar]
- 24. Hurkmans E, Matthys C, Bogaerts A, Scheys L, Devloo K, Seghers J. Face-to-face versus mobile versus blended weight loss program: randomized clinical trial. JMIR Mhealth Uhealth. 2018;6(1):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Brackbill D, Yang S, Becker J, Herbert N, Centola D. Support or competition? How online social networks increase physical activity: a randomized controlled trial. Prev Med Rep. 2016;4:453-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawachi I. It’s all in the game—the uses of gamification to motivate behavior change. JAMAIntern Med. 2017;177(11):1593-1594. [DOI] [PubMed] [Google Scholar]
- 27. Patel MS, Small DS, Harrison JD, et al. Effectiveness of behaviorally designed gamification interventions with social incentives for increasing physical activity among overweight and obese adults across the United States: the STEP UP randomized clinical trial. JAMA Intern Med. 2019;179(12):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grunseit AC, Bohn-Goldbaum E, Crane M, et al. Participant profile and impacts of an Aboriginal healthy lifestyle and weight loss challenge over four years 2012-2015. Aust N Z J Public Health. 2019;43(4):328-333. [DOI] [PubMed] [Google Scholar]
- 29. Drozek D, DeFabio A, Amstadt R, Dogbey GY. Body Mass Index change as a predictor of biometric changes following an intensive lifestyle modification program. Adv Prev Med. Published online March 24, 2019. doi: 10.1155/2019/8580632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verheijden MW, Jans MP, Hildebrandt VH, Hopman-Rock M. Rates and determinants of repeated participation in a web-based behavior change program for healthy body weight and healthy lifestyle. J Med Internet Res. 2007;9(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]