Abstract
This study aimed to investigate the effect of chronic heat stress on oxidative stress in liver of broilers. In our study, chickens were randomly allocated to control 1 group (control 7 d), heat stress 1 group (HS1, 7 d), control 2 group (control 14 d) and heat stress 2 group (HS2, 14 d), with 30 replicates in each group. Broilers in heat stress groups exposed 8 h/day heat stress (35 ± 2°C) for 7 or 14 consecutive days, and the rest of time per day were kept at 23 ± 2℃ the same as control group broilers. Growth performance and the liver tissues were collected for histological observation and detection of organ index and liver redox status. The serum indicators (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) related to liver injury were determined. Moreover, Nrf2-related genes and protein expression levels in liver were measured. The results showed that in heat stress group broilers the body weight gain, feed conversion ratio, liver weight, and liver index were decreased, inflammatory cells infiltration in liver, and serum AST level was enhanced, compared with control group broilers. Moreover, the hepatic malondialdehyde (MDA) and superoxide dismutase (SOD) level were increased after 1 wk of heat stress. Nrf2, Sqstm1/p62, HO-1, and NQO1 mRNA expressions in the liver of broilers were decreased by heat stress. P62 and p-p62 protein expressions were significantly up-regulated, but Nrf2 and keap1 protein level was decreased in heat stress group broilers as compared to control group. The mRNA expression levels of Beclin1, LC3-I, LC3-II were down-regulated significantly with heat stress for 1 wk. The mRNA expression level of mTOR up-regulated after 2 wk of heat stress. In conclusion, heat stress induced liver injury of broilers by down-regulating Nrf2-keap1 signaling pathway and autophagy.
Key words: heat stress, broilers, liver, oxidative stress, Nrf2
INTRODUCTION
Heat stress (HS) impairs humans and animals’ health, which results in great economic loses in livestock industry and public health care. Moreover, the negative effects of HS will be more obvious with the aggravation of global warming (Chen et al., 2021). It's well known that HS increases reactive oxygen species (ROS) formation, which leads to oxidative injury and antioxidant capacity damage (Song et al., 2018). Oxidative injury can directly or indirectly induce the biomacromolecules (DNA, proteins, and lipids) damage and further cause cell dysfunction and even tissues injury (Cheng et al., 2017b), and was regarded as one of the most pesky issues in the modern poultry industry (Gessner et al., 2017). It's reported that HS can induce oxidative damage in different species of livestock, such as broilers (Nanto-Hara et al., 2021), pigs (Liu et al., 2016), and ducks (Yang et al., 2021). During the past few decades, the consumption of poultry meat increased consecutively in global market. Owing to its relatively low cost and high nutritive value, chicken meat has become one of the most popular muscle food (Petracci et al., 2015). With the increasing demand, poultry production requires fast growing broiler chickens to fulfill the meat market. So genes in broilers are constantly improving to meet the demand for meat quantity, and the selected broiler breeds are extremely sensitive to oxidative stress. There are lots of reports linking the suppression of growth performance with oxidant damage in heat-stressed birds (Kikusato et al., 2021). So, the effect of heat stress on oxidative stress in broiler chickens is very important in poultry industry.
High environmental temperature induces oxidative damage in liver tissues of broilers, which further disturbs lipid metabolism (Emami et al., 2020). When broilers are subjected to heat stress, the free radicals increase, whereas the activities of antioxidant enzymes and free radical scavenging ability decrease (Miao et al., 2020). Nuclear factor erythroid 2-related factor 2 (Nrf-2) has been recognized as a critical regulator of cellular redox homeostasis, which can protect cells against oxidative damage (Hu et al., 2020). Upon oxidative stress, the adapter protein Sqstm1/p62 interacts with the Nrf2, then modulating the expression and function of Nrf2. Nrf2 in the nucleus can bind to conserved antioxidant response elements (AREs) in the promoter regions of target genes, and regulate the activities of antioxidant enzymes such as glutathione peroxidase (GPx) (Liang et al., 2018). The Nrf2/Kelch-like ECH associated protein 1 (Nrf2-Keap1) pathway is involved in cellular responses to environmental and oxidative stress. Activation of the Nrf2-Keap1 pathway increases antioxidant response element-related molecules, including glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) (Zhang et al., 2018).
However, how heat stress affected liver and the mechanism of heat stress on redox homeostasis in broilers remains unclear. In this study, we sought to investigate the cellular enzyme activity and biomarker changes to identify the mechanisms that heat stress on redox homeostasis in the liver of broilers.
MATERIALS AND METHODS
Animals and Experimental Design
All animals work was carried out in accordance with the guidelines for the care and use of experimental animals established by the Ministry of Science and Technology of the People's Republic of China (Approval number: 2006-398) and was approved by the Laboratory Animal Management Committee of Foshan University.
A total of 120 two-wk-old Ma chickens were provided by Foshan Nanhai Poultry Corporation (Foshan, China). Ma chickens are a pure line of local Qing Yuan Ma Chickens. All broilers were acclimatized in a room with a controlled temperature at 23 ± 2°C, 12 h light/dark cycles. Broilers had free access to standard water and diet. After 1 wk of acclimation, the broilers were randomly divided into 4 groups: control 1 group (Control 7 d), control 2 group (Control 14 d), heat stress 1 group (HS1, heat stress for 7 d) and heat stress 2 group (HS2, heat stress for 14 d), with 30 replicates in each group following the body weight. The control group broilers were kept at 23 ± 2℃, and the humidity control as about 70%. Broilers in heat stress group were exposure to 35 ± 2°C with 8 h/d (8:00–16:00 every day) for 7 or 14 consecutive days (at 21-day-old), and the rest of 26 h/d were kept at 23 ± 2℃ like control group broilers. The body weight and feed consumed amount were recorded every day. Then the average weight gain (WG), feed intake, and feed conversion ratio were analyze and used the data we recorded. After the last day of heat stress, blood samples were obtained from jugular vein and used to determine alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Then, broilers sacrificed by CO2 inhalation, and liver tissues were collected for subsequent testing.
Growth Performance and Sample Collection
Feed intake and body weight per broiler was recorded every day. The average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR, FCR = ADFI/ADG) which were calculated. On the 7th and 14th d after heat exposure, broilers were sacrificed with CO2 inhalation, and liver tissues were collected for subsequent testing.
Serum ALT and AST Activity Analysis
The serum samples were separated from the whole blood by centrifugation 12,000 × g, 10 min at 4°C, and then the serum was harvested for measurement of ALT and AST levels by following the instructions on the commercial kits. ALT Assay Kit (105-000442-00) and the AST Assay Kit (105-000443-00) were provided by Shenzhen Mindray Bio-Medical Electronics Co., Ltd (Mindray, Shenzhen, China).
Pathological Changes and the Organ Index of Liver
Complete liver was photographed to observe the changes of pathological injury during the slaughter of broilers. Complete liver was taken from broilers, washed with PBS, dried with filter paper, weighed and then recorded for calculating liver index. Liver index = liver weight (g)/body weight (g) ×100%.
The other liver tissues were taken to observe the pathological changes by hematoxylin and eosin (H&E) staining. Tissues were fixed with 4% paraformaldehyde for 48 h and dehydrated with ethanol, and then the tissue was embedded in paraffin. The liver sections were cut into slices (5 μm), mounted on microscopic slides, and stained with HE. Sections were then observed under an optical microscope (Bio-Rad). Five to 10 fields of at least 2 sections per animal and 3 animals per experimental group were evaluated.
Liver Redox Status Measurement
The lipid peroxidation and antioxidants levels in livers were measured to evaluate the liver redox status of heat stressed broilers. The hepatic total superoxide dismutase assay kit (A001-3-2) and malondialdehyde (MDA) assay kit (A003-1-2) were provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The redox status was measured using the kits according to the manufacturer's instructions.
Western Blot Analysis
Liver tissues were homogenized with a lysis buffer containing RIPA and phosphatase-inhibitors on ice. Protein concentration was quantificated by BCA kit (ThermoFisher Scientific) after centrifuged at 13,000 r/min at 4℃ for 15 min. Thirty microgram proteins were subjected to 10% SDS-PAGE electrophoresis and transferred to polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 3% BSA for 2 h and then immunoblotted with primary antibodies (Nrf2, 12721; Keap1, 8047; p62, 5114; p-p62, 95697; β-actin, 4970; Cell Signaling Technology, Danvers, MA) diluted with 3% BSA for 2 h at room temperature. The membranes were incubated with secondary antibodies (Anti-rabbit IgG, 7074; Cell Signaling Technology, Danvers, MA), followed by visualized with the Chemiluminescence System. And the gray level of the protein expression was analyzed by using the software, ImageJ.
Hepatic Gene Expression
Total RNA isolated from the liver using Trizol Reagent (Hlingene Corporation, Shanghai, China) and reversely transcribed through first Strand cDNA Synthesis kit (Takara, Tokyo, Japan). Then, quantitative real-time polymerase chain reaction using the TB Green Premix Ex Taq II (Takara, Tokyo, Japan) and a Roche LightCycler 480 system (Roche, Basel, Switzerland). Gene expressions were normalized using the expression of housekeeping gene β-actin (internal reference) and the relative fold changes were calculated using the 2–ΔΔCt method. All primers used for qRT-PCR are listed in Table 1.
Table 1.
The primers used in qPCR as Table 1 follows.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| Sqstm1/p62 | GACCCAGCCAAGACTACCAT | CAGAGGCATGTAGTTTCGGC |
| NFE2L2(Nrf2) | ATCACCTCTGCACCGAA | GCTTTCTCCCGCTCTTTCTG |
| NQO1 | CCCTCAAGAACCCCGAGT | GCCATCTCCATCTCGTAGACA |
| HO-1 | CTTCGCACAAGGAGTGTTAAC | CATCCTGCTTGTCCTCTCAC |
| mTOR | GAAGAGCTGATTCGGGTAG | ACCATTCTTGTGCCTCCATT |
| LC3I | TTACACCCATATCAGATTCTTG | ATTCCAACCTGTCCCTCA |
| LC3II | AGTGAAGTGTAGCAGGATGA | AAGCCTTGTGAACGAGAT |
| Beclin1 | CGTATGGCAACCACTCGTATT | TTATTGTCCCAGAAGAACCTCAG |
| β-actin | ACGTCTCACTGGATTTCGAGCAGG | TGCATCCTGTCAGCAATGCCAG |
Statistical Analysis
Data are expressed as means ± standard deviation. All data were analyzed by using SPSS 17.0 statistical software (SPSS, Chicago, IL). Statistical significance was determined by one-way analysis of variance (ANOVA). Student's t-test was used to examine the differences between 2 groups. Difference was considered statistically significant at P < 0.05.
RESULTS
Heat Stress Decreased Growth Performance of Broilers
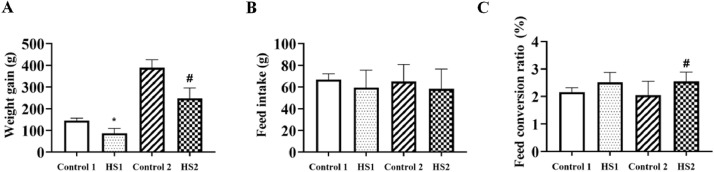
The data concerning the growth performance of broilers were shown in Figure1. The weight gain in the heat stress 1 and 2 groups was significantly decreased than that in the control 1 and 2 groups (P < 0.05, P < 0.05, respectively). The feed conversion ratio in the heat stress 2 group was obviously increased than that of the control 2 group (P < 0.05). The feed intake had no significance between control and heat stress group broilers. No mortality was observed in broilers throughout the experimental period.
Figure 1.
Effects of heat stress on growth performance of broilers. (A) The weight gain, (B) feed intake and (C) feed conversion ratio (FCR) in broilers. *P < 0.05 vs. control 1; #P < 0.05 vs. control 2 (n = 30).
Heat Stress Decreased Liver Index and Induced Liver Inflammatory Infiltration of Broilers
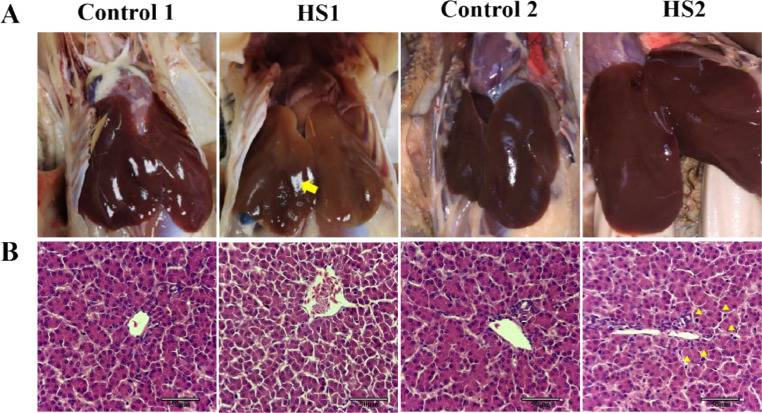
Body weight, liver weight, and liver index were measured (Table 2). Compared with control 2 group, heat stress for 2 wk significantly reduced body weight (P < 0.05), liver weight (P < 0.05), and liver index (P < 0.05). But, there were no significance between control 1 group and HS1 group among those 3 indicators above. After necropsy (Figure 2A) we observed that the texture of the liver surface in the control broilers were normal. Broilers exposure to heat stress for 1 or 2 wk showed necrosis points on the liver surface. Liver histological changes showed that no morphology alterations were found in liver of control group broilers. However, inflammatory infiltration was observed in heat stress group broilers. The results above showed that heat stress induced liver tissue damage.
Table 2.
Effects of heat stress on liver index of broilers.
| Group | Body weight (g) | Liver weight (g) | Liver index (%) |
|---|---|---|---|
| Control 1 | 497.73 ± 24.40 | 12.24 ± 1.49 | 2.15 ± 0.15 |
| HS1 | 439.76 ± 34.00 | 10.78 ± 0.78 | 2.52 ± 0.33 |
| Control 2 | 724.11 ± 65.07 | 20.30 ± 1.40 | 2.05 ± 0.45 |
| HS2 | 592.00 ± 26.88# | 13.56 ± 1.22# | 2.55 ± 0.31# |
P < 0.05 vs. control 2.
Figure 2.
Effects of heat stress on liver index and histological changes of broilers. (A) Liver necropsy and (B) histological changes. Yellow arrow represents necrosis points. Yellow triangle represents inflammatory infiltration.
Effects of Heat Stress on Serum Enzymatic Activity and the Balance of Hepatic Redox Status of Broilers
ALT and AST are important serological indexes reflecting liver injury. As shown in Table 3, compared with control 1 or 2 groups, serum AST levels both in heat stress 1 and 2 wk groups were significantly enhanced (P < 0.01 and P < 0.01, respectively), but the ALT level had no change between control and heat stress group broilers. Intracellular antioxidant enzymes protect biological macromolecules from oxidative stress. In this study, we measured the activities of antioxidant enzyme superoxide dismutase and the malondialdehyde level. Compared with the control 1 group, exposure to heat stress 1 wk alone significantly increased MDA production (P < 0.05) and enhanced SOD activity (P < 0.05). However, HS2 group did not induce more severe lipid peroxidation and SOD activity compared to control 2 group (P > 0.05).
Table 3.
Effects of heat stress on serum enzymatic activity and liver redox status of broilers.
| Group | ALT (U/L) | AST (U/L) | MDA (nmol/mgprot) | SOD (U/mgprot) |
|---|---|---|---|---|
| Control 1 | 2.43 ± 0.15 | 198.68 ± 10.72 | 0.64 ± 0.13 | 128.56 ± 6.17 |
| HS1 | 2.62 ± 0.29 | 237.83 ± 10.90⁎⁎ | 0.97 ± 0.09⁎⁎ | 142.38 ± 5.87* |
| Control 2 | 2.58 ± 0.24 | 218.57 ± 10.94 | 0.71 ± 0.12 | 112.73 ± 2.37 |
| HS2 | 2.61 ± 0.26 | 252.57 ± 16.56## | 0.83 ± 0.04 | 104.53 ± 8.55 |
Serum ALT and AST levels were measured by IFCC rate method. Liver MDA and SOD level were measured in broilers.
P < 0.05,
P < 0.01 vs. control 1.
P < 0.01 vs. control 2.
Heat Stress Inhibited the Gene Expressions of Hepatic Redox Related Genes of Broilers
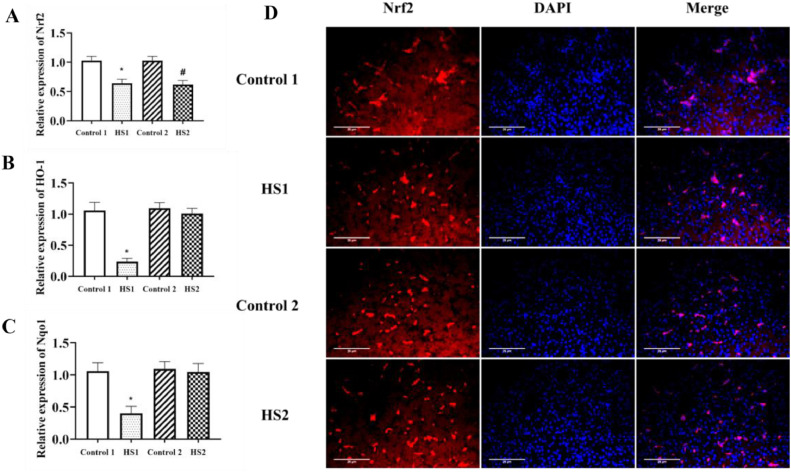
As shown in Figure 3, compared with the control 1 and 2 groups, heat stress for 1 and 2 wk decreased the hepatic mRNA expressions of Nrf2 (P < 0.05, P < 0.05, respectively) in the HS1 and HS2 group broilers. Broilers in the HS1 groups had lower HO-1 (heme oxygenase-1) and NQO1 (the detoxification enzyme NADPH quinone oxidoreductase 1, NQO1) mRNA expression levels (P < 0.05, P < 0.05, respectively) in the liver compared with the control 1 group. Moreover, heat stress for 2 wk had no effect on HO-1 and NQO1 mRNA levels, when compared with the control 2 group broilers.
Figure 3.
Effects of heat stress on hepatic redox related genes expressions in liver of broilers. (A) Nrf2, (B) HO-1 and (C) Nqo1 genes expressions in liver of broilers were measured by qRT-PCR. (D) Nrf2 protein expression in liver of broilers was measured by immunofluorescence histochemistry. *P < 0.05 vs. control 1; #P < 0.05 vs. control 2.
In Figure 3D, the red staining indicated positive expression of Nrf2 and the blue staining indicated nucleus. Compared with the control group 1 and 2 groups, Nrf2 positive expressions were decreased in the liver of HS1 and HS2 group broilers. The decline slowed in heat stress for 2 than for 1 wk.
Heat Stress Enhanced the Expressions of p62 and it's Phosphorylation in Liver of Broilers
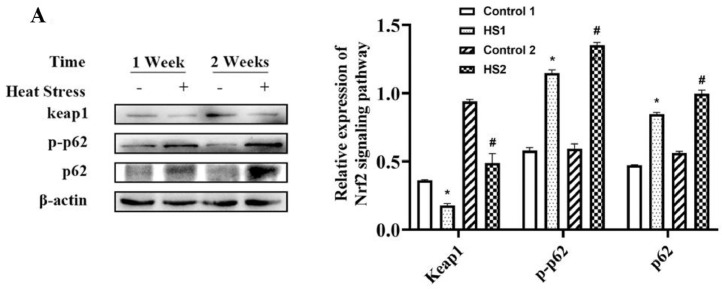
As shown in Figure 4, compared with the control group, heat stress for 1 and 2 wk lowered the liver keap1 protein expressions (P < 0.05, P < 0.05, respectively). When compared with the control 1 and 2 group broilers, heat stress for 1 and 2 wk also had higher liver of p-p62 (P < 0.05, P < 0.05, respectively) and p62 (P < 0.05, P < 0.05, respectively) protein expressions in the HS1 and HS2 group broilers.
Figure 4.
Effects of heat stress on liver p62 expression of broilers. Proteins expressions in liver of broilers were measured by western blotting. *P < 0.05 vs. control 1; #P < 0.05 vs. control 2.
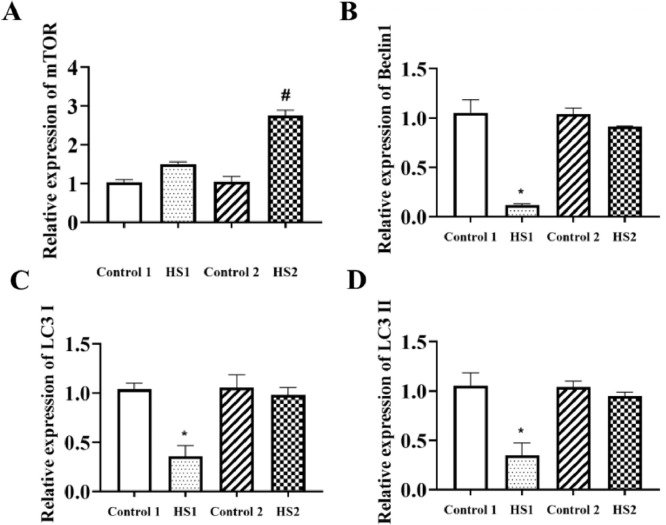
Heat Stress Inhibited the Gene Expressions of Hepatic Autophagy Related Genes of Broilers
The results in Figure 5 expressed that compared with the control 2 group, mTOR gene level was enhanced in the liver of HS2 group broilers (P < 0.05). Compared with control 1 group, heat stress for 1 wk significantly reduced Beclin1, LC3I, and LC3II gene levels (P < 0.05, P < 0.05, P < 0.05, respectively). The change of above 3 gene levels had no significance between control 2 and HS2 group broilers.
Figure 5.
Effects of heat stress on hepatic autophagy related genes levels in broilers. Gene expressions in liver of broilers were measured by RT-PCR. *P < 0.05 vs. control 1; #P < 0.05 vs. control 2.
DISCUSSION
Exceed thermoneutral zone temperature range, irreversible, and potentially lethal thermoregulatory events could take place in poultry (Al-Zghoul et al., 2015). Such events lead to numerous complications involving impaired growth rates, decreased meat production and lower feed intakes, that inevitably cause greater economic losses. The results of this study suggested that heat stress had negative effects on growth performance in broilers, by decreasing the average body weight gain and increasing the feed conversion ratio. Avian species are particularly susceptible to heat stress, especially liver. Chronic heat stress can decrease liver index and other immunity organs index and cellular immunity would also lowered (Chen et al., 2020). In this study, heat stress for 2 wk decreased liver weight, liver index, and enhanced serum AST level of broilers. Liver histological changes indicated that heat stress induced inflammatory infiltration in liver of heat stressed broilers. The results above indicate that heat stress impaired liver growth and induced liver injury in broilers, the mechanism is still unclear.
Heat stress causes oxidative stress in liver tissues of laying hens which can affect production performance (Zhao et al., 2021). Oxidative stress, which is characterized with a major shift in the cellular redox balance. MDA, a lipid peroxidation substance, is widely known to be a marker of oxidative stress in tissues (Cheng et al., 2017a). Lan reported that after cycle heat stress (34℃ with 8 h/d), the intestinal GSH-Px activity was decreased and MDA level was increased in yellow-feather broilers (Lan et al., 2020). In this study, heat stress for 1 wk raised MDA level, and SOD level also enhanced. The increased level in MDA indicated oxidative damage was observed in liver of heat stressed broilers. Nevertheless, MDA and SOD level had no change after heat stress for 2 wk. These results indicated an increase in oxidative stress together with an increase in antioxidant enzymes levels during one week of heat stress. This increase in antioxidant enzyme activities has been considered to be a protective response to oxidative stress. In conclusion, exposure of broilers to chronic heat stress resulted in disrupted oxidative and antioxidative balance.
As a key transcription factor in antioxidant system, Nrf-2, regulates antioxidant defense that protects cells from various stimuli via up-regulating the related the expression of several antioxidants such as SOD, GSH-PX and catalase, thus alleviating stress reaction (Mahanty et al., 2017). When activated, Nrf2 is translocated to the nucleus to bind with target genes, such as the glutathione synthesis enzyme glutamate cysteine ligase (catalytic subunit Gclc) and the detoxification enzyme NADPH quinone oxidoreductase 1 (Nqo1) (Osama et al., 2020). The findings of this study indicated that Nrf2 gene expression was significantly decreased in broilers after heat stress for 1 and 2 wk. Moreover, HO-1 and Nqo1 mRNA expression levels in HS1 group broilers were obviously lower than that of in control 1 group. But heat stress for 2 wk had no influence to HO-1 and Nqo1 gene expressions. The results above suggested that with the extension of heat stress exposure, the antioxidant capacity was gradually enhanced and the poor redox status of the broilers exposure with heat stress was associated with the decline of Nrf2 mRNA expression level.
It's reported that redox homeostasis changes are related with the expression and function of transcription factors such as Nrf2 and Kelch-like ECH-associated protein 1 (Keap-1), which regulates the activities of antioxidant enzymes (Liang et al., 2018). The Nrf2-Keap1 pathway is a critical regulator of antioxidative stress responses, and p62 is known to regulates this pathway by suppressing Keap1-Nrf2 interaction. Under resting conditions, Keap1 binds to Nrf2 so as to sequester Nrf2 in the cytoplasm, where it is ubiquitinated and degraded via the proteasomal pathway (Yamamoto et al., 2008). Upon oxidative stress, the adapter protein Sqstm1/p62 interacts with Keap1to disrupt the interaction of Nrf2-Keap1. Nrf2 is subsequently translocated to the nucleus to induce expression of the transcription of antioxidative genes (Deng et al., 2020). In our study heat stress led to a significant increase in protein levels of p62 and phosphorylation of p62 but decreased Keap1 in liver of broilers exposured with heat stress for 1 and 2 wk. Nrf2, HO-1 and Nqo1 gene expressions were significantly decreased in broilers after heat stress. Upon heat stress, increased p62 can competitive bind with Keap1 to release Nrf2 from Nrf2-Keap1 interaction. However, the antioxidative genes level expressions of broilers were not enhanced. The possible mechanisms need to be further explored.
Autophagy is a crucial homeostasis mechanism that under adverse conditions (Dikic and Elazar., 2018). Under certain stress, defensive mechanisms are often insufficient to completely avoid cellular damage, autophagy is crucial for the repair and removal of damaged components (Navarro-Yepes et al., 2014). Beclin1 is a well-known autophagy initiation factor, which triggers the related protein including Atg to promote autophagosome formation (Sun et al., 2019). When autophagy is activated, LC3 is cleaved to proteolytic derived LC3-II, which is necessary for the autophagosomes formation. P62, an autophagy adaptor protein, can bind to LC3-II and be selectively degraded by autophagy (Kang et al., 2011). So, degradation of p62 is another marker for monitoring autophagy. In our study Beclin1, LC3-I, and LC3-II gene expressions were detected to estimate the effects of heat stress on autophagy. Results from this study revealed that Beclin1, LC3-I and LC3-II gene levels were obviously decreased while p62 protein level was significantly enhanced after heat stress, suggesting a decrease in autophagic activity. P62 plays an important role in the regulation of autophagy and Nrf2 activity, and acts as a bridge linking the 2 access. In this study, increased p62 did not induce Nrf2 translocating to the nucleus to transcript antioxidative genes. So, autophagy may play a role in Nrf2 expression and activation, and this hypothesis requires further testing in the future.
In conclusion, heat stress could induce oxidative stress and autophagy dysfunction in liver, which result in liver damage of broilers.
Acknowledgments
Funding: This project was supported by Natural Science Foundation of Guangdong Province, China [Grant No. 2017A030310607], Guangdong Science and Technology Department, China [Grant No. 2015A040404048]. Guangdong Province Department of Agriculture and Rural Affairs [Grant No. 2019KJ119]. Guangdong Province Department of education [Grant No. 2017GCZX006].
Disclosures
The authors declare that they have no competing interests. The author confirms that the work described has not been published before and its publication has been approved by all co-authors.
References
- Al-Zghoul M.B., El-Bahr S.M., Al-Rukibat R.K., Dalab A.E.S., Althnaian T.A., Al-Ramadan S.Y. Biochemical and molecular investigation of thermal manipulation protocols during broiler embryogenesis and subsequent thermal challenge. BMC Vet. Res. 2015;11:292–300. doi: 10.1186/s12917-015-0609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Cheng Y., Wen C., Zhou Y. Protective effects of dietary mannan oligosaccharide on heat stress-induced hepatic damage in broilers. Environ. Sci. Pollut. Res. Int. 2020;27:29000–29008. doi: 10.1007/s11356-020-09212-2. [DOI] [PubMed] [Google Scholar]
- Chen S., Yong Y., Ju X. Effect of heat stress on growth and production performance of livestock and poultry: mechanism to prevention. J. Therm. Biol. 2021;99 doi: 10.1016/j.jtherbio.2021.103019. [DOI] [PubMed] [Google Scholar]
- Cheng K., Song Z.H., Zheng X.C., Zhang H., Zhang J.F., Zhang L.L., Zhou Y.M., Wang T. Effects of dietary vitamin E type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poult. Sci. 2017;96:1159–1166. doi: 10.3382/ps/pew336. [DOI] [PubMed] [Google Scholar]
- Cheng K., Zhang M., Huang X., Zheng X., Song Z., Zhang L., Wang T. An evaluation of natural and synthetic vitamin E supplementation on growth performance and antioxidant capacity of broilers in early age. Can. J. Anim. Sci. 2017;98:187–193. [Google Scholar]
- Deng Z., Lim J., Wang Q., Purtell K., Wu S., Palomo G.M., Tan H., Manfredi G., Zhao Y., Peng J., Hu B., Chen S., Yue Z. ALS-FTLD-linked mutations of SQSTM1/p62 disrupt selective autophagy and NFE2L2/NRF2 anti-oxidative stress pathway. Autophagy. 2020;16:917–931. doi: 10.1080/15548627.2019.1644076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I., Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Jung U., Voy B., Dridi S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. 2020;10:35. doi: 10.3390/antiox10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner D.K., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017;101:605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- Hu H., Dai S., Li J., Wen A., Bai X. Glutamine improves heat stress-induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2-related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020;99:1454–1461. doi: 10.1016/j.psj.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R., Livesey K.M., Zeh H.J., Lotze M.T., Tang D. HMGB1 as an autophagy sensor in oxidative stress. Autophagy. 2011;7:904–906. doi: 10.4161/auto.7.8.15704. [DOI] [PubMed] [Google Scholar]
- Kikusato M., Xue G., Pastor A., Niewold T.A., Toyomizu M. Effects of plant-derived isoquinoline alkaloids on growth performance and intestinal function of broiler chickens under heat stress. Poult. Sci. 2021;100:957–963. doi: 10.1016/j.psj.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R., Li Y., Chang Q., Zhao Z. Dietary chitosan oligosaccharides alleviate heat stress-induced intestinal oxidative stress and inflammatory response in yellow-feather broilers. Poult. Sci. 2020;99:6745–6752. doi: 10.1016/j.psj.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M., Wang Z., Li H., Cai L., Pan J., He H., Wu Q., Tang Y., Ma J., Yang L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018;115:315–328. doi: 10.1016/j.fct.2018.03.029. [DOI] [PubMed] [Google Scholar]
- Liu F., Cottrell J.J., Furness J.B., Rivera L.R., Kelly F.W., Wijesiriwardana U., Pustovit R.V., Fothergill L.J., Bravo D.M., Celi P., Leury B.J., Gabler N.K., Dunshea F.R. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016;101:801–810. doi: 10.1113/EP085746. [DOI] [PubMed] [Google Scholar]
- Mahanty A., Mohanty S., Mohanty B.P. Dietary supplementation of curcumin augments heat stress tolerance through upregulation of nrf-2-mediated antioxidative enzymes and hsps in Puntius sophore. Fish. Physiol. Biochem. 2017;43:1131–1141. doi: 10.1007/s10695-017-0358-z. [DOI] [PubMed] [Google Scholar]
- Miao Q., Si X., Xie Y., Chen L., Liu Z., Liu L., Tang X., Zhang H. Effects of acute heat stress at different ambient temperature on hepatic redox status in broilers. Poult. Sci. 2020;99:4113–4122. doi: 10.1016/j.psj.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanto-Hara F., Ohtsu H., Yamazaki M., Hirakawa T., Sato K., Murakami H. Effects of dietary brown rice on the growth performance, systemic oxidative status, and splenic inflammatory responses of broiler chickens under chronic heat stress. J. Poult. Sci. 2021;58:154–162. doi: 10.2141/jpsa.0200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Yepes J., Burns M., Anandhan A., Khalimonchuk O., del Razo L.M., Quintanilla-Vega B., Pappa A., Panayiotidis M.I., Franco R. Oxidative stress, redox signaling, and autophagy: cell death versus survival. Antioxid. Redox Signal. 2014;21:66–85. doi: 10.1089/ars.2014.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osama A., Zhang J., Yao J., Yao X., Fang J. Nrf2: a dark horse in Alzheimer's disease treatment. Ageing Res. Rev. 2020;64 doi: 10.1016/j.arr.2020.101206. [DOI] [PubMed] [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. Worlds Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Sun Y., Cai Y., Zang Q.S. Cardiac autophagy in sepsis. Cells. 2019;8:141. doi: 10.3390/cells8020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Suzuki T., Kobayashi A., Wakabayashi J., Maher J., Motohashi H., Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Luo P., Chen S.J., Deng Z.C., Fu X.L., Xu D.N., Tian Y.B., Huang Y.M., Liu W.J. Resveratrol sustains intestinal barrier integrity, improves antioxidant capacity, and alleviates inflammation in the jejunum of ducks exposed to acute heat stress. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhuang Y., Shi Y., Xu Z., Zhou C., Guo L., Liu P., Wu C., Hu R., Hu G., Guo X., Xu L. Effects of N-acetyl-l-cysteine on heat stress-induced oxidative stress and inflammation in the hypothalamus of hens. J. Therm. Biol. 2021;98 doi: 10.1016/j.jtherbio.2021.102927. [DOI] [PubMed] [Google Scholar]
- Zhang J.F., Bai K.W., Su W.P., Wang A.A., Zhang L.L., Huang K.H., Wang T. Curcumin attenuates heatstress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]