Abstract
Liver is a central metabolic organ, which is sensitive to heat stress. Liver damage affects animals' health and endangers the livestock and poultry industry. This study aimed to investigate the mechanism of chronic heat stress-induced liver damage in broiler chickens. Broilers were divided into 3 treatments: normal control group (NOR, 22°C), heat stress group (HS, 32°C) and pair-feeding group (PF, 22°C) for a 7-d and 14-d trial. The results showed that 7 d heat exposure caused microvesicular steatosis and reduced glutamine synthetase activity in broiler liver (P < 0.05). After 14 d of heat exposure, heat stress caused vacuolar degeneration and apoptosis in the liver; elevated liver relative weight and liver glutaminase activity as well as plasma ammonia level (P < 0.05). Additionally, heat stress enhanced GRP78 protein expression and the mRNA expressions of endoplasmic reticulum (ER) stress responses genes and apoptosis-related genes in broiler liver after 14 d of heat exposure (P < 0.05). In conclusion, chronic heat stress triggered ER stress-induced apoptosis and caused liver damage, which may compromise ammonia detoxification in broiler liver.
Key words: heat stress, broiler, endoplasmic reticulum stress, liver apoptosis
INTRODUCTION
With the increased severity of global warming (Stillman, 2019), heat stress has become an increasingly prevalent environmental stressor that threatens human and animals health (Arifwidodo and Chandrasiri, 2020; Chauhan et al., 2021), and it occurs when body heat production exceeds the heat lost to surrounding environment. Modern commercial broilers are especially vulnerable to heat stress under high ambient temperature, owing to their restrictive heat loss capacity, high metabolic rates, high heat production, hypoplasia of sweat gland, and intensive genetic selection (Deeb and Cahaner, 2002; Nawab et al., 2018). Heat stress impairs welfare and growth performance of broiler, and causes enormous economic loss in commercial broiler industry (Mohammadi, 2021; Hamidi et al., 2022).
Liver is pivotal organ of metabolic activity, which performs essential cellular functions containing the balance of energy metabolism, biosynthesis of vitamins and minerals, and ammonia detoxification (Schliess et al., 2014). Elevated blood flow transfers from the hepato-splanchnic region to respiratory muscles and superficial body tissues to accelerate heat dissipation and decrease body temperature under heat stress, therefore, liver is more sensitive to heat stress (Hai et al., 2006; Crandall et al., 2008). It has been reported that heat stress caused liver fat accumulation and inflammation, and impaired liver function in broiler (Lu et al., 2019; Chen et al., 2021a).
As the crucial organelle, endoplasmic reticulum (ER) is in charge of protein maturation and correct folding, calcium homostasis, as well as the detoxification of certain drugs (Ron and Walter, 2007). ER stress occurs when accumulation of unfolded and misfolded proteins in ER. Consequently, the cells activate dynamic signaling pathway, called the unfolded protein response (UPR) containing three specific ER-resident protein sensors: the activating transcription factor 6 (ATF6), the inositol-requiring enzyme-1 (IRE1), and double-stranded RNA-activated protein kinase (PKR)-like ER kinase (PERK) (Schröder and Kaufman, 2005). These sensors bind to glucose regulated protein 78 (GRP78) in order to maintain the inactive state under normal physiological status (Jie et al., 2017). When ER stress occurs, PERK is separated from GRP78 and activated by oligomerization and autophosphorylation, which subsequently suppresses mRNA translation and induces severe protein load in ER by phosphorylating eukaryotic translation initiation factor 2 alpha (EIF2A) (Xu et al., 2005). After departing from GRP78, IRE1, and ATF6 are activated and engage in facilitating protein folding, decreasing protein synthesis, and elevating protein degradation (Han and Kaufman, 2016). Under persistent or excessive ER stress, the UPR is unable to reestablish protein homeostasis, subsequently induces cell apoptosis (Zinszner et al., 1998). Growing evidence manifests that heat stress could trigger hepatocyte ER stress (Xu et al., 2011).
Liver damage is common pathological basis of various liver diseases, and ER stress inevitably appears in liver damage (Liu et al., 2017). It was reported that the suppression of EIF2A alleviated carbon tetrachloride (CCl4)-induced liver damage in mice and human hepatocyte cell line LO2 (Tang et al., 2020). Similarly, Borkham-Kamphorst et al. found that long-term repetitive injection of CCl4 aggravated ER stress and UPR then caused liver cell apoptosis in lipocalin 2 null mice (Borkham-Kamphorst et al., 2020). Additionally, selenium deficiency-induced and nickel chloride-induced liver damage were achieved by activation of ER stress in chickens (Yao et al., 2015; Guo et al., 2016). Collectively, ER stress plays a critical role in triggering liver damage.
However, the mechanism of liver damage due to heat stress in broilers and the role of ER stress in liver damage remain to be elucidated. Therefore, the aim of this study was to explore whether heat stress induces broiler liver damage and its mechanism.
MATERIALS AND METHODS
Animals and Treatment Design
Animals were raised in accordance with institutional guidelines, and all procedures were performed according to the guidelines of the Animal Care Committee of Nanjing Agricultural University, (Nanjing, China). Two hundred one-day-old male broilers (Arbor Acres) were obtained from a commercial hatchery and housed according to commercial management from 1 to 27 d of age.
At 28 d of age, 144 broiler chicks were randomly allotted to 3 treatment groups, in which there were 6 replicates (cages) and 8 birds per replicate (cage) for each group. The birds in normal control (NOR) group were maintained at 22°C, and feed and water were available ad libitum; chickens in heat stress (HS) group were housed at 32°C and received ad libitum feeding and watering; broilers in pair-feeding (PF) group were raised at 22°C and the amount of feed offered to PF broilers was equal to the amount of feed consumedption of HS group on the previous day. The PF group was designed to demonstrate that the detrimental effect of heat stress is independent of decreased feed intake. The relative humidity of all treatments was maintained at 55 ± 5% for 2 wk. The composition and nutrient content of the basal diets are shown in Table 1.
Table 1.
Composition and nutrient content of the basal diets.
| Ingredients (%) | Calculated nutrient levels (%) | ||
|---|---|---|---|
| Corn | 62.07 | ME3 (MJ/kg) | 13.19 |
| Soybean meal | 23.00 | Crude protein | 19.60 |
| Corn gluten meal1 | 6.00 | Calcium | 0.95 |
| Soybean oil | 4.00 | Available phosphorus | 0.39 |
| Limestone | 1.20 | Lysine | 1.05 |
| Dicalcium phosphate | 2.00 | Methionine | 0.42 |
| L-lysine | 0.35 | Methionine+ cysteine | 0.76 |
| DL-methionine | 0.08 | ||
| Salt | 0.30 | ||
| Premix2 | 1.00 | ||
Crude protein content was 600 g·kg−1.
The premix provided per kilogram of diet: retinyl acetate for vitamin A, 12,000 IU; cholecalciferol for vitamin D3, 2,500 IU; DL-α-tocopheryl acetate forvitamin E, 20 IU; menadione sodium bisulfate, 1.3 mg; thiamin, 2.2mg; riboflavin, 8.0 mg; nicotinamide, 40 mg; choline chloride, 400 mg;calcium pantothenate, 10 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; Fe (from ferroussulfate), 80 mg; Cu (from copper sulfate), 8.0 mg; Mn (frommanganese sulfate), 110 mg; Zn (from zinc sulfate), 60 mg; I (fromcalcium iodate), 1.1 mg; Se (from sodium selenite), 0.3 mg.
ME, metabolizable energy.
Sample Collection
After 7 and 14 d of heat stress, a total of 36 birds (2 birds per replicate) close to the replicate average weight were stunned by an electric shock (50 V, alternating current; 400 Hz for 5 s) and euthanized by exsanguination. Plasma was collected and stored at −25°C. The whole liver was excised and weighed for calculating the relative weight of live. The 3 g liver tissue was sampled and cooled in liquid nitrogen for subsequent analysis. A part of liver (1 cm × 1 cm × 1 cm cube) was fixed in 4% paraformaldehyde for subsequent analysis.
Liver Biochemical Parameters and Blood Ammonia
The activities of glutamate dehydrogenase (GDH), glutaminase (GLS), and glutamine synthetase (GS) in the liver and plasma ammonia level were assessed by standard kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with manufacturer's certificate.
Hepatic Histology
The fixed liver tissue was dehydrated in the following changes of 75, 85, 90, 95, and 100% ethanol, and then equilibrated in xylene for the process of paraffin embedding. After dewaxing, liver tissue section (3 µm thick) was stained with hematoxylin and eosin (H&E) (Wuhan Servicebio Technology Co. Ltd., Wuhan, China). Morphological features of liver tissue sections were observed and captured using light microscopy (Olympus BX50, Tokyo, Japan) with digital camera (Olympus XC10).
Terminal Deoxynucleotidyl Transferase Mediated-dUPT Nick End Lableing Staining
To assess the degree of apoptosis in the liver tissue, terminal deoxynucleotidyl transferase mediated-dUPT nick end lableing (TUNEL) kit (Vazyme Biotech Co. Ltd., Nanjing, China) was processed in accordance with manufacturer's protocols. Briefly, the liver paraffin sections were treated with proteinase K at 37°C for 25 min after de-waxing, and subsequently incubated at 37°C for 2 h in the present with terminal deoxynucleotidyl transferase 2′-deoxyuridin 5′-triphosphate mixture. After washing with phosphate buffered saline, the sections were treated with 4′,6-diamidino-2-phenylindoledihydrochloride (DAPI) under dark condition for 10 min at room-temperature. The sections mounted by antifade mounting medium were observed and captured for assessing the degree of apoptosis in the liver tissue via fluorescencemicroscope. Liver apoptotic rate was expressed as the ratio of apoptotic cells to total number of cells.
Quantitative Real-Time PCR
The separation of total RNA was performed using RNAiso Plus reagent (TAKARA BIO INC, Nojihigashi, Japan). According to manufacturer's protocol, RNA concentrations and purity were assessed using an ultramicrospectrophotometer (Thermo Scientific, Wilmington, DE), and then the concentration of RNA was adjusted to 500 ng/µL. Reverse transcription was carried out using HiScript Ⅲ qRT SuperMix kit (Vazyme Biotech Co. Ltd.), as manufacturer's recommendations.
The gene expressions of resulting cDNA were detected using Quantitative Real-Time PCR on ABI PRISM 7500 (Applied Biosystems, Foster City, CA). The reaction was carried out in a 20-µL reaction system containing 10 µL ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co. Ltd.), 8.2 µL of RNase-free dH2O, 0.4 µL of each primer, and 1 µL of cDNA. The details of primer sequences were shown in Table 2. The PCR procedure was performed by steps as follows: 95°C for 30 s, with 40 cycles of 95°C for 5s and 60°C for 30 s, 95°C for 15 s, 60°C for 1 min, terminated by 95°C for 15 s. The value of target gene expression was normalized to that of GAPDH and calculated using 2−△△Ct method (Livak and Schmittgen, 2001).
Table 2.
Information of sequences of the oligonucleotide primers.
| Genes1 | Genbank number | Primer sequences (5′→to 3′ direction) | Product size (bp) |
|---|---|---|---|
| GAPDH | NM_204305 | Forward: GAGGGTAGTGAAGGCTGCTG | 113 |
| Reverse: CATCAAAGGTGGAGGAATGG | |||
| GRP78 | NM_205491.1 | Forward: TCCTGCTCCTCGTGGTGTCC | 148 |
| Reverse: CTCCTCTGGTGTTAGCCGATTCTG | |||
| PERK | XM_420868.6 | Forward:GTGGATGAGCAGGAGGCAATGATG | 150 |
| Reverse:ATCCTTAACCAGCCATGCAGAAGC | |||
| EIF2A | NM_001031323.2 | Forward: GCTGCGAGTCAGTAATGGGTATAA | 103 |
| Reverse: CTGCCAGGAAACTTGCCACA | |||
| ATF4 | NM_204880.2 | Forward: AATTGGCTCGCTGTGGACAG | 148 |
| Reverse: CGGTGGCTTCCAGATGTTCC | |||
| ATF6 | XM_422208 | Forward: GATTGTGGGCGTCACTTCTCG | 142 |
| Reverse: TGGGATGCCAATGTTAGCCTG | |||
| IRE1 | NM_001285499 | Forward: TGAGGGCAATGAGAAATAAGAAGC | 127 |
| Reverse: TGTAGGAGCAGGTGAGGGAAGC | |||
| XBP1 | NM_001006192 | Forward:GCGAGTCTACGGATGTGAAGGA | 140 |
| Reverse: TGTGGAGGTTGTCAGGAATGGT | |||
| CHOP | XM_015273173.2 | Forward:CAGGAAGAAGAGCTGGCCCCACT | 123 |
| Reverse:TGCTGTGCTCGCCGTGCTGT | |||
| Caspase-3 | XM_015276122.2 | Forward:ACAGCAAGCGAAGCAGTTTT | 115 |
| Reverse:TCACCTCTGAAAAGGCTGGT | |||
| Bcl-2 | NM_205339.2 | Forward:ATCGTCGCCTTCTTCGAGTT | 150 |
| Reverse:ATCCCATCCTCCGTTGTCCT |
Abbreviations: ATF4, Activated transcription factor 4; ATF6, Activated transcription factor 6; Bcl-2, B-cell CLL/lymphoma 2; CHOP, transcriptional factor C/EBP homologous protein; EIF2A, elongation initiation factor 2 alpha; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GRP78, glucose-regulated protein 78; IRE1, Inositol-requiring enzyme 1; PERK, protein kinase RNA-like ER kinase; XBP1, X-boxbinding protein 1.
Western Blot
The refrigerated liver tissue (approximately 30 mg) was homogenized in 300 µL strong RIPA buffer supplemented with 3 µL PMSF and 3 µL protease inhibitor, then the supernatant was gathered to extract total protein. Protein concentration was detected by the BCA assay reagent (Sangon Biotech Co., Ltd, Shanghai, China). Approximately 20 µg protein was subjected by 10 % SDS-PAGE gels and transferred onto a 0.45-µm PVDF membrane. The membrane was incubated with GRP78 antibody overnight (Cell signaling technology, Beverly, MA) after blocking with a 5% skim milk powder, and then incubated with HRP-linked secondary antibody (Cell signaling technology). The protein band was visualized by chemiluminescence using chemiluminescence imaging system (BIO-RAD, Richmond, California). The band intensity was calculated by Image J software.
Statistical Analysis
Statistical analysis of the data was carried out using oneway ANOVA Tukey's post hoc test via SPSS-statistical software version 19.0 (SPSS Inc, Chicago, IL). The data were presented as means ± standard errors and analyzed with the cage as a statistical unit (n = 6). Statistically significant was set at P < 0.05.
RESULTS
The Relative Weight of Liver and Plasma Ammonia
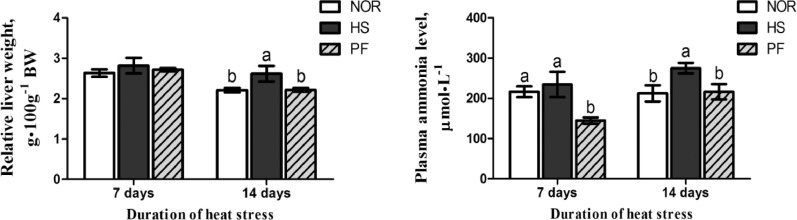
As presented in Figure 1, after 7 d of heat exposure, no significant difference was observed in the relative weight of liver among the 3 groups (P > 0.05). Plasma ammonia level in the PF group was significantly decreased in comparison with the NOR and PF groups (P < 0.05). Compared with those of the NOR and PF groups, 14 d of heat exposure significantly elevated the relative weight of liver and plasma ammonia level in the HS group (P < 0.05).
Figure 1.
Effect of chronic heat stress on the relative weight of liver and blood ammonia level in broiler. The data is presented as mean ± SE (n = 6). a,b-Different letters manifest that there was significant difference among three groups (P < 0.05). Abbreviations: HS, heat-stress group; NOR, normal control group; PF, pair-feeding group.
Liver Ammonia-Metabolizing Enzymes Activities
As shown in Table 3, after 7 d of heat exposure, broilers in the PF group exhibited significant reduction of liver GLS activity compared with that of the NOR and HS groups (P < 0.05), and liver GS activity in the HS group was significantly lower than that of the NOR group (P < 0.05). Additionally, 14 d of heat exposure significantly elevated liver GLS activity in the HS group compared to NOR and PF groups (P < 0.05).
Table 3.
Effect of chronic heat stress on ammonia-metabolizing enzymes activities in broiler liver.
| Items | Treatments1 |
P-values | ||
|---|---|---|---|---|
| NOR | HS | PF | ||
| After 7 days of heat exposure | ||||
| GDH (nmol/min/mg protein) | 45.25 ± 6.73 | 39.35 ± 3.06 | 33.53 ± 3.03 | 0.232 |
| GLS (nmol/min/mg protein) | 8.86 ± 0.31a | 9.67 ± 0.81a | 6.99 ± 0.47b | 0.013 |
| GS (µmol/h/mg protein) | 0.34 ± 0.02a | 0.24 ± 0.03b | 0.28 ± 0.01ab | 0.017 |
| After 14 days of heat exposure | ||||
| GDH (nmol/min/mg protein) | 25.94 ± 2.28 | 25.78 ± 2.24 | 31.21 ± 3.65 | 0.323 |
| GLS (nmol/min/mg protein) | 11.05 ± 0.92b | 13.89 ± 0.62a | 11.15 ± 0.65b | 0.026 |
| GS (µmol/h/mg protein) | 0.28 ±0.02 | 0.24 ± 0.03 | 0.20 ± 0.02 | 0.149 |
The data is presented as mean ± SE (n = 6).
Different superscript letters manifest that there was significant difference among three groups (P < 0.05).
Liver Histopathological Analysis
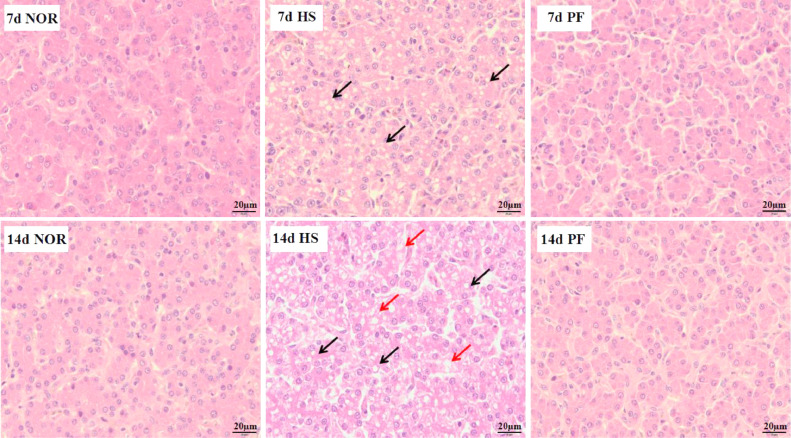
H&E staining was employed to illustrate changes in liver histopathology and results were shown in Figure 2. After 7 d of heat exposure, microvesicular steatosis occurred in the HS group characterized by hepatocytes containing amounts of fat droplets, and there were no obvious pathological changes in the NOR and PF groups. After 14 d of heat exposure, severe vacuolar degeneration of the hepatocyte was observed in the HS group, and no obvious pathological changes were found in the NOR and PF groups.
Figure 2.
Effect of chronic heat stress on the histopathological changes in broiler liver (H&E stain; scale bar: 50 µm). Abbreviations: 7d NOR, 7 d of heat exposure, normal control group; 14d NOR, 14 d of heat exposure, normal control group; 7d HS, 7 d of heat exposure, heat stress group; 14d HS, 14 d of heat exposure, heat stress group; 7d PF, 7 d of heat exposure, pair-feeding group; 14d PF, 14 d of heat exposure, pair-feeding group.
Liver Gene Expressions and Protein Expression
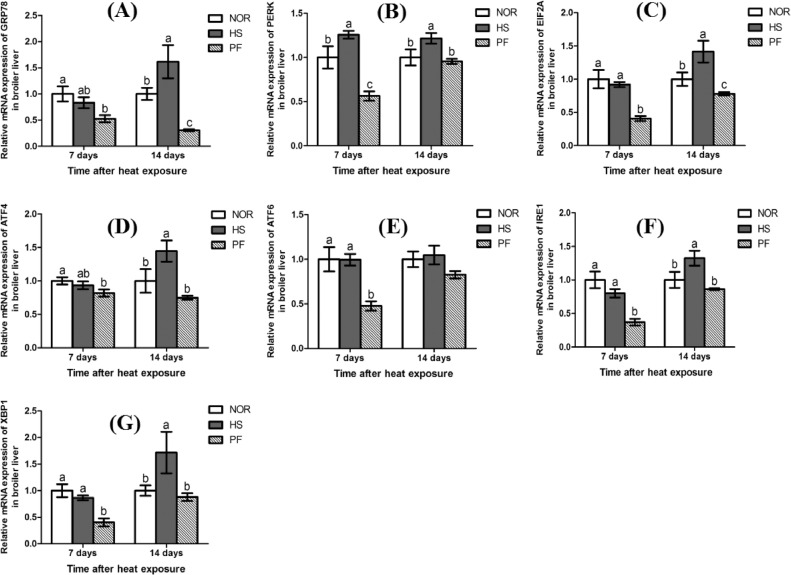
As shown in Figure 3, after 7 d of heat exposure, birds in the HS group exhibited higher level of the mRNA expression of PERK than that of the NOR and PF groups (P < 0.05), the RNA expressions of EIF2A, ATF6, IRE1, and spliced X-box binding protein 1 (XBP1) in the PF group were significantly lower than those of the other 2 groups (P < 0.05). Additionally, after 7 d of heat exposure, the RNA expressions of GRP78 and activating transcription factor 4 (ATF4) in the PF group were significantly lower than those of the NOR group (P < 0.05). Compared with the NOR and PF groups, birds in the HS group exhibited higher levels of the mRNA expressions of GRP78, PERK, EIF2A, ATF4, IRE1, and XBP1 in broiler liver after 14 d of heat exposure (P < 0.05).
Figure 3.
Effect of chronic heat stress on the mRNA expressions of GRP78 (A), PERK (B), EIF2A (C), ATF4 (D), ATF6 (E), IRE1 (F), XBP1 (G) in broiler liver. The data is presented as mean ± SE (n = 6). a-c-Different letters manifest that there was significant difference among three groups (P < 0.05). Abbreviations: ATF4, activated transcription factor 4; ATF6, activated transcription factor 6; EIF2A, elongation initiation factor 2 alpha; GRP78, glucose-regulated protein 78; HS, heat-stress group; NOR, normal control group; IRE1, Inositol-requiring enzyme 1; PERK, protein kinase RNA-like ER kinase; PF, pair-feeding group; XBP1, X-box binding protein 1.
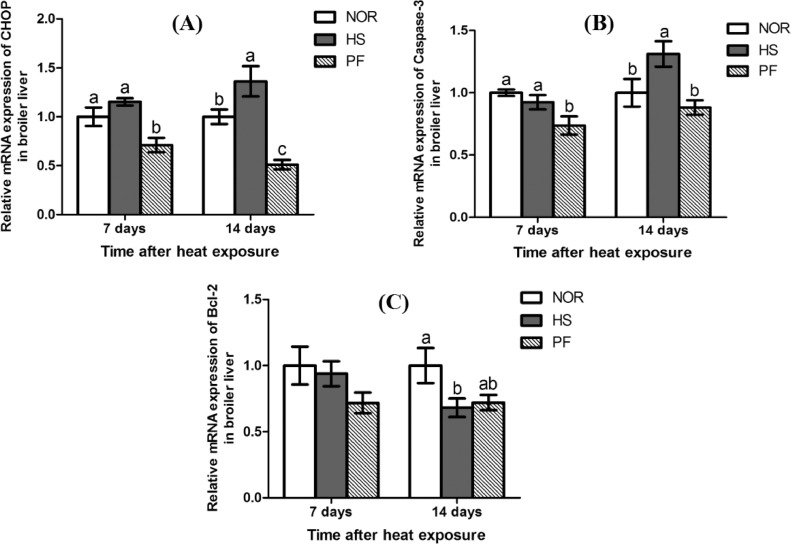
The mRNA expressions of liver apoptosis associated genes were shown in Figure 4. Broilers in the PF group exhibited a significant reduction in the mRNA expressions of C/EBP homologous protein (CHOP) and Caspase-3 in comparison with the other 2 groups in the liver after 7 d of heat exposure (P < 0.05). The mRNA expressions of CHOP and Caspase-3 in the HS group were significantly higher than those of the other 2 groups in broiler liver (P < 0.05), and the liver Bcl-2 mRNA expression in the HS group was significantly lower than that of the NOR group after 14 d of heat exposure (P < 0.05).
Figure 4.
Effect of chronic heat stress on the mRNA expressions of CHOP (A), Caspase3 (B), and Bcl-2 (C) in broiler liver. Abbreviations: CHOP, transcriptional factor C/EBP homologous protein; Caspase-3, caspase-3; Bcl-2, B-cell CLL/lymphoma 2; HS, heat-stress group; NOR, normal control group; PF, pair-feeding group. The data is presented as mean ± SE (n = 6). a-c-Different letters manifest that there was significant difference among three groups (P < 0.05).
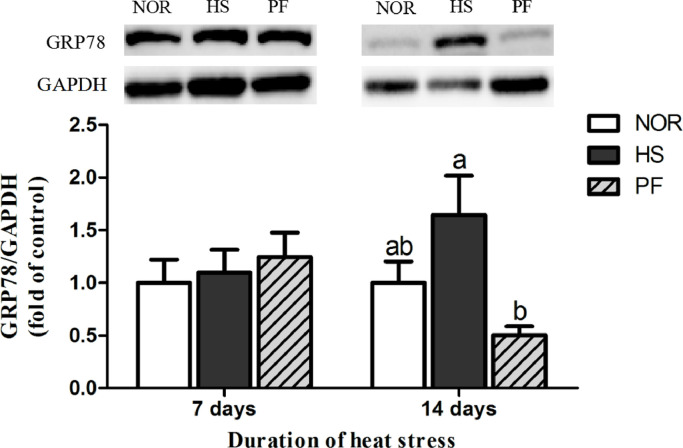
The GRP78 protein expression in broiler liver was shown in Figure 5. There was no significant difference in protein expression of liver GRP78 between 3 groups after 7 d of heat exposure (P > 0.05). After 14 d of heat exposure, compared with that of the NOR group, the GRP78 protein expression showed an elevating trend (P = 0.089) in the HS group, and the GRP78 protein expression in the HS group was higher than that of the PF group (P < 0.05).
Figure 5.
Effect of chronic heat stress on the protein expressions of GRP78 in broiler liver. Abbreviations: GRP78, glucose-regulated protein 78; HS, heat-stress group; NOR, normal control group; PF, pair-feeding group. The data is presented as mean ± SE (n = 6). a,b-Different letters manifest that there was significant difference among three groups (P < 0.05).
TUNEL Analysis
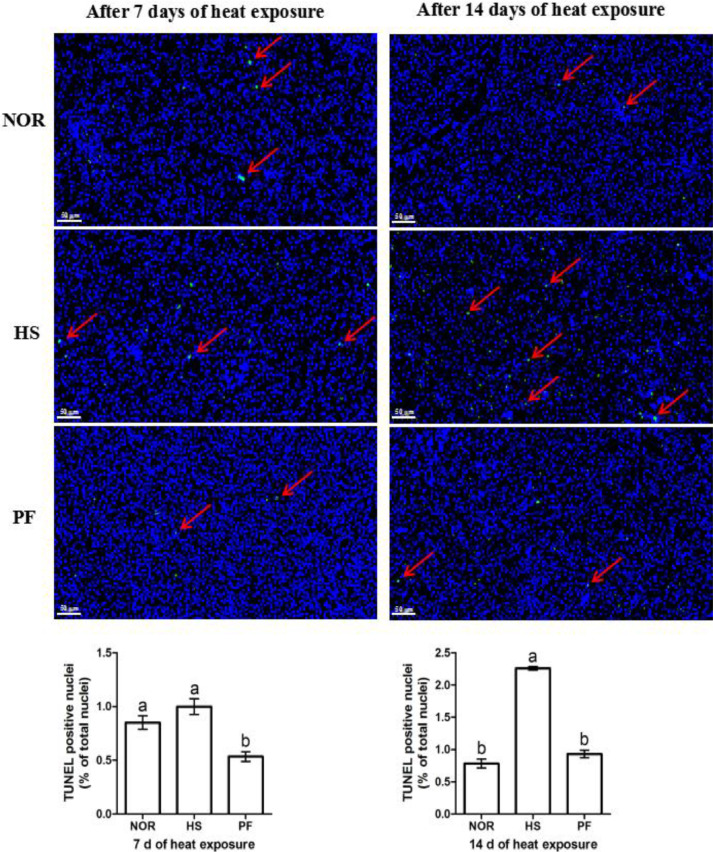
Liver apoptotic rate was detected by TUNEL assay and shown in Figure 6. After 7 d of heat exposure, liver apoptotic rate in the PF group was significantly lower than that of the NOR and HS groups (P < 0.05). Compared with NOR and PF groups, birds in the HS group exhibited higher liver apoptotic rate after 14 d of heat exposure (P < 0.05).
Figure 6.
Effect of chronic heat stress on liver apoptotic rate in broiler (TUNEL assay, × 200). Abbreviations: HS, heat-stress group; NOR, normal control group; PF, pair-feeding group. Normal cell nucleus is blue, and apoptotic cells was dyed green. The data is presented as mean ± SE (n = 6). a,b-Different letters manifest that there was significant difference among three groups (P < 0.05).
DISCUSSION
Over the last decade, the global surface temperature has been increased by approximately 1.2°C and it is forecasted to increase by 1.5°C between 2030 and 2052, which will aggravate the problem of heat stress (Stillman, 2019). Heat stress can induce the decrease of feed intake in order to decrease body metabolic heat production in chicken (Song et al., 2012; Hamidi et al., 2022). Broilers characterized by feather coverage, high heat production rate and absence of sweat glands are more vulnerable to heat stress. Numerous evidences manifested that negative effects caused by heat stress is independent of the reduction in feed intake in broilers (Lu et al., 2017; Lu et al., 2019). Liver is prone to damage during heat stress, due to the 22% decrease in the liver blood flow (Wolfenson et al., 1981). Chen et al. found that diquat increased the relative liver weight and caused liver damage in broiler chickens, and this phenomenon was also observed in heat-stressed broiler (Hosseini-Vashan et al., 2019; Chen et al., 2021b), which is in similarity to our findings. In the present study, after 7 d of heat exposure, the relative liver weight of HS group increased by approximately 7% when compared with the NOR group although the difference was not significant. In addition, 14 d of heat exposure significantly increased the relative liver weight compared to the NOR and PF groups, and no significant difference was found between the NOR and PF groups. This abnormal liver weight caused by heat stress may be explained as follows. First, heat stress accelerates the accumulation of fat in the liver (Lu et al., 2019). Second, heat stress triggers compensatory hypertrophy of liver in order to make up for decline in liver function of heat-stressed broiler. Additionally, we also investigated the organizational changes in broiler liver by H&E staining. The results showed that 7 d of heat exposure caused microvesicular steatosis in the liver characterized by the presence of a large number of fat droplets, and amounts of fat droplets and vacuolar degeneration were captured in broiler liver slide in the HS group after 14 d of heat exposure. In addition, there were no obvious pathological features in the liver slides of NOR and PF groups, which indicated that elevation of the relative liver weight and liver damage was induced only by the high temperature based on the same feed consumption between the HS and PF groups.
As a crossroad of peripheral metabolism and highly secretory tissue, liver possesses abundant ER responsible for synthesis of secretory proteins and the transmission of stress signaling (Ji and Kaplowitz, 2006; Rutkowski, 2019). Liver damage is associated with ER stress (Ji et al., 2011). ER stress activates dynamic signaling pathway called UPR, and then triggers ATF6, IRE1, and PERK pathways to restore ER homeostasis for ensuring cell survival. Our results showed that the mRNA expressions of ER stress responses in the PF group were significantly lower than those of the NOR and HS groups after 7 d of heat exposure. This was probably because of the insufficient feed intake in the PF group, which might induce lipolysis to supply energy by liver gluconeogenesis. Therefore, the lipid deposition in broiler liver of the PF group was lower, and the extent of liver damage and stress was lower (Poljicak-Milas et al., 2003; Rodrigues et al., 2011; Pan et al., 2014). After 14 d of heat exposure, the mRNA expressions of ER stress responses and GRP78 protein expression of the HS group were significantly higher than those of the NOR and PF groups, which clearly suggested that liver ER stress was only triggered by the high temperature based on the same feed consumption between the HS and PF groups. Triglyceride deposition is critical causation of ER stress. Previous research indicated that triglyceride treatment induced HepG2 cells ER stress (Kim et al., 2007). In the present study, we observed that a large number of fat droplets were deposited in broiler liver, this might be the reason of liver ER stress during heat stress, which remains to be further explored.
Cells are unable to alleviate persistent and severe ER stress and succumbed to death through the activation of CHOP (Marciniak et al., 2004; Choi and Song, 2020). CHOP triggers cell apoptosis via downregulating anti-apoptotic Bcl-2 expression and upregulating caspase-3 (Puthalakath et al., 2007; Hsu et al., 2018). In the present study, 7 d of heat exposure did not affect the mRNA expressions of CHOP and caspase-3 as well as the TUNEL positive nuclei in the liver, but those indicators in the PF group were significantly lower than those of the NOR and HS groups, which was probably resulted from low level of ER stress in the PF group. After 14 d of heat exposure, heat stress increased the mRNA expressions of CHOP and caspase-3, and the mRNA expressions of CHOP and caspase-3 in the HS group were higher than those of the PF group in broiler liver based on the same feed intake, which suggested that elevation of liver apoptosis was only caused by the high temperature rather than reduction of feed intake. Elevation of hepatocyte apoptosis triggers acute liver damage and induces in chronic liver diseases (Malhi and Gores, 2008). The data presented thus far indicated that chronic heat stress enhanced ER stress-induced liver apoptosis and induced in liver damage. We also employed the TUNEL assay to assess the impact of heat stress on the extent of liver apoptosis, and found that heat stress increased the TUNEL positive nuclei in broiler liver after 14 d of heat exposure, which further proved the above viewpoint.
Amino acids metabolism promotes ammonia production. In order to avoid excessive ammonia-induced intoxication, the ammonia is transferred to liver for detoxification. In mammals, ammonia detoxification is primarily achieved by urea formation. However, birds characterized by the absence of carbamoyl phosphate synthetase I are unable to synthesize urea (Smith and Campbell, 1988). Hence, GS is main enzyme for ammonia detoxification. Liver damage reduces the distribution of GS staining and GS activity in the liver, which is due to its strict perivenous localization (Gebhardt and Mecke, 1983; Schöls et al., 1990). On the contrary, liver damage induces the elevation of GLS activity. When liver damage occurs, the elevation of GLS activity increases glutaminolysis to activate hepatic stellate cell in order to accelerate liver regeneration (Jiang et al., 2017). In the present study, 7 days of heat exposure did not affect liver GLS activity, but liver GLS activity in the PF group was lower than that of the other 2 groups. This was probably ascribed to insufficient energy supply in the PF group, which induced fat mobilization and decrease of liver fat content, the level of stress and damage in the liver is low (Poljicak-Milas et al., 2003; Rodrigues et al., 2011; Pan et al., 2014). In this study, we found that 7 d of heat exposure reduced GS activity in the liver, which might be due to liver damage. After 14 d of heat exposure, heat stress increased GLS activity in the liver and increased plasma ammonia level in comparison with the NOR and PF groups, which suggested that the reduction of liver ammonia detoxification and the increase of plasma ammonia was only caused by the high temperature based on the same feed consumption between the HS and PF groups.
CONCLUSIONS
This study demonstrated that chronic heat stress triggered ER stress-induced apoptosis in broiler liver, subsequently induced in liver damage, which might be responsible for impairment of liver ammonia detoxification ability and the elevation of plasma ammonia level. These findings have important practical significance in exploiting reasonable effective means to alleviate liver damage and improve health in broilers under high ambient temperature.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (32072780, 31872374), the National Key Research and Development Program of China (2016YFD 0500501), and the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2022]).
DISCLOSURES
None of the authors has any conflicts of interest to declare.
REFERENCES
- Arifwidodo S.D., Chandrasiri O. Urban heat stress and human health in Bangkok. Thailand Environ. Res. 2020;185 doi: 10.1016/j.envres.2020.109398. [DOI] [PubMed] [Google Scholar]
- Borkham-Kamphorst E., Haas U., Van de Leur E., Trevanich A., Weiskirchen R. Chronic carbon tetrachloride applications induced hepatocyte apoptosis in lipocalin 2 null mice through endoplasmic reticulum stress and unfolded protein response. Int. J. Mol. Sci. 2020;21:5230. doi: 10.3390/ijms21155230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S.S., Rashamol V.P., Bagath M., Sejian V., Dunshea F.R. Impacts of heat stress on immune responses and oxidative stress in farm animals and nutritional strategies for amelioration. Int. J. Biometeorol. 2021;65:1231–1244. doi: 10.1007/s00484-021-02083-3. [DOI] [PubMed] [Google Scholar]
- Chen Y., Cheng Y., Wen C., Zhou Y. Protective effects of dietary mannan oligosaccharide on heat stress-induced hepatic damage in broilers. Environ. Sci. Pollut. Res. 2021;27:29000–29008. doi: 10.1007/s11356-020-09212-2. [DOI] [PubMed] [Google Scholar]
- Chen Y.P., Gu Y.F., Zhao H.R., Zhou Y.M. Dietary squalene supplementation alleviates diquat-induced oxidative stress and liver damage of broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.A, Song C.H. Insights into the role of endoplasmic reticulum stress in infectious diseases. Front. Immunol. 2020;10:3147. doi: 10.3389/fimmu.2019.03147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall C.G., Wilson T.E., Marving J., Vogelsang T.W., Kjae A., Hesse B., Secher N.H. Effects of passive heating on central blood volume and ventricular dimensions in humans. J. Physiol. 2008;586:293–301. doi: 10.1113/jphysiol.2007.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb N., Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002;81:293–301. doi: 10.1093/ps/81.3.293. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Mecke D. Heterogeneous distribution of glutamine synthetase among rat liver parenchymal cells in situ and in primary culture. Embo J. 1983;2:567–570. doi: 10.1002/j.1460-2075.1983.tb01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Cui H., Fang J., Zuo Z., Deng J., Wang X., Zhao Z.L., Chen K., Deng J. Nickel chloride (NiCl2) in hepatic toxicity: apoptosis, G2/M cell cycle arrest and inflammatory response. Aging. 2016;8:3009–3027. doi: 10.18632/aging.101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai L., Decuypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Hamidi O., Chamani M., Ghahri H., Sadeghi A.A., Malekinejad H., Palangi V. Effects of supplemental chromium nanoparticles on ifn-γ expression of heat stress broilers. Biol. Trace Elem. Res. 2022;200:339–347. doi: 10.1007/s12011-021-02634-0. [DOI] [PubMed] [Google Scholar]
- Han J., Kaufman R.J. The role of er stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016;57:1329–1338. doi: 10.1194/jlr.R067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Vashan S.J., Safdari-Rostamabad M., Piray A.H., Sarir H. The growth performance, plasma biochemistry indices, immune system, antioxidant status, and intestinal morphology of heat-stressed broiler chickens fed grape (vitis vinifera) pomace. Anim. Feed Sci. Technol. 2019;259 [Google Scholar]
- Hsu H.Y., Lin T.Y., Hu C.H., Shu D.T.F., Lu M.K. Fucoidan upregulates TLR4/CHOP-mediated caspase-3 and PARP activation to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Cancer Lett. 2018;432:112–120. doi: 10.1016/j.canlet.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Ji C., Kaplowitz N. ER stress: can the liver cope? J. Hepatol. 2006;45:321–333. doi: 10.1016/j.jhep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ji C., Kaplowitz N., Mo Y.L., Kao E., Petrovic L.M., Lee A.S. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229–239. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Mohammed G., Ke L., Huang Y., Chang N., Jie F., Fengtian H., Liying L., Shizhong B., Wen X., Xiaochao M., Song L. Regulation of hepatic stellate cell proliferation and activation by glutamine metabolism. Plos One. 2017;12 doi: 10.1371/journal.pone.0182679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie W., Lee J., Liem D., Ping P. Hspa5 gene encoding hsp70 chaperone bip in the endoplasmic reticulum. Gene. 2017;618:14–23. doi: 10.1016/j.gene.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.S., Jeong S.K., Kim H.R., Kim D.S., Chae S.W., Chae H.J. Effects of triglyceride on ER stress and insulin resistance. Biochem. Biophys. Res. Commun. 2007;363:140–145. doi: 10.1016/j.bbrc.2007.08.151. [DOI] [PubMed] [Google Scholar]
- Liu Y., Pan X., Li S., Yu Y., Chen J., Yin J., Li G. Endoplasmic reticulum stress restrains hepatocyte growth factor expression in hepatic stellate cells and rat acute liver failure model. Chem.-Biol. Interact. 2017;277:43–54. doi: 10.1016/j.cbi.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Livak K.J, Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X.F., Ma B.B., Zhang L., Li J.L., Jiang Y. Increased fat synthesis and limited apolipoprotein b cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019;98:3695–3704. doi: 10.3382/ps/pez056. [DOI] [PubMed] [Google Scholar]
- Malhi H., Gores G.J. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak S.J., Yun C.Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H.P., Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi F. Effect of different levels of clove (syzygium aromaticum L.) essential oil on growth performance and oxidative/nitrosative stress biomarkers in broilers under heat stress. Trop. Anim. Health Prod. 2021;53:1–7. doi: 10.1007/s11250-020-02517-x. [DOI] [PubMed] [Google Scholar]
- Nawab A., Ibtisham F., Li G., Kieser B., Wu J., Liu W., Zhao Y., Nawab Y., Li K., Xiao M., An L. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018;78:131–139. doi: 10.1016/j.jtherbio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Pan Y.E., Liu Z.C., Chang C.J., Huang Y.F., Lai C.Y., Walzem R.L., Chen S.E. Feed restriction ameliorates metabolic dysregulation and improves reproductive performance of meat-type country chickens. Anim. Reprod. Sci. 2014;151:229–236. doi: 10.1016/j.anireprosci.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Poljicak-Milas N., Susnjic M., Marenjak T.S., Milinkovic-Tur S., Stojevic Z. Effect of fasting on hepatic and renal gluconeogenic enzyme activities in ducklings. Vet. Arh. 2003;73:153–165. [Google Scholar]
- Puthalakath H., O'Reilly L.A., Gunn P., Lee L., Kelly P.N., Huntington N.D., Hughes P.D., Michalak E.M., McKimm-Breschkin J., Motoyama N., Gotoh T., Akira S., Bouillet P., Strasser A. ER stress triggers apoptosis by activating bh3-only protein bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Rodrigues L., Crisóstomo J., Matafome P., Louro T., Nunes S., Seiça R. Dietary restriction improves systemic and muscular oxidative stress in type 2 diabetic goto–kakizaki rats. J. Physiol. Biochem. 2011;67:613–619. doi: 10.1007/s13105-011-0108-0. [DOI] [PubMed] [Google Scholar]
- Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rutkowski D.T. Liver function and dysfunction-a unique window into the physiological reach of ER stress and the unfolded protein response. FEBS. J. 2019;286:356–378. doi: 10.1111/febs.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliess F., Hoehme S., Henkel S.G., Ghallab A., Driesch D., Böttger J., Guthke R., Pfaff M., Hengstler J.G., Gebhardt R., Häussinger D., Drasdo D., Zellmer S. Integrated metabolic spatial-temporal model for the prediction of ammonia detoxification during liver damage and regeneration. Hepatology. 2014;60:2040–2051. doi: 10.1002/hep.27136. [DOI] [PubMed] [Google Scholar]
- Schöls L., Mecke D., Gebhardt R. Reestablishment of the heterogeneous distribution of hepatic glutamine synthetase during regeneration after ccl4-intoxication. Histochemistry. 1990;94:49–54. doi: 10.1007/BF00266789. [DOI] [PubMed] [Google Scholar]
- Schröder M., Kaufman R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Smith D.D, Campbell J.W. Distribution of glutamine synthetase and carbamoyl-phosphate synthetase I in vertebrate liver. Proc. Natl. Acad. Sci. U. S. A. 1988;85:160–164. doi: 10.1073/pnas.85.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Liu L., Sheikhahmadi A. Effect of heat exposure on gene expression of feed intake regulatory peptides in laying hens. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/484869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman J.H. Heat waves, the new normal: Summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology. 2019;34:86–100. doi: 10.1152/physiol.00040.2018. [DOI] [PubMed] [Google Scholar]
- Tang Y.J., Chen H., Yi Y., Chen G.M., He Y.H. Inhibition of eif2α dephosphorylation protects hepatocytes from apoptosis by alleviating er stress in acute liver injury. BioMed Res. Int. 2020;2020:1–16. doi: 10.1155/2020/2626090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenson D., Frei Y.F., Snapir N., Berman A. Heat stress effects on capillary blood flow and its redistribution in the laying hen. Pfluegers Arch. 1981;390:86–93. doi: 10.1007/BF00582717. [DOI] [PubMed] [Google Scholar]
- Xu C., Bailly-Maitre B., Reed J.C. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Gupta S., Hu W., McGrath B.C., Cavener D.R. Hyperthermia induces the ER stress pathway. PLoS One. 2011;6:e23740. doi: 10.1371/journal.pone.0023740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L., Du Q., Yao H., Chen X., Zhang Z., Xu S. Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. BioMetals. 2015;28:255–265. doi: 10.1007/s10534-014-9819-3. [DOI] [PubMed] [Google Scholar]
- Zinszner H., Kuroda M., Wang X.Z., Batchvarova N., Ron D. Chop is implicated in programmed cell death in response to impaired function of the endoplasmicreticulum. Genes. Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]